Abstract
Emotional learning is necessary for individuals to survive and prosper. Once acquired, however, emotional associations are not always expressed. Indeed, the regulation of emotional expression under varying environmental conditions is essential for mental health. The simplest form of emotional regulation is extinction, in which conditioned responding to a stimulus decreases when the reinforcer is omitted. Two decades of research on the neural mechanisms of fear conditioning have laid the groundwork for understanding extinction. In this review, we summarize recent work on the neural mechanisms of extinction learning. Like other forms of learning, extinction occurs in three phases: acquisition, consolidation, and retrieval, each of which depends on specific structures (amygdala, prefrontal cortex, hippocampus), and molecular mechanisms (receptors and signaling pathways). Pharmacological methods to facilitate consolidation and retrieval of extinction, for both aversive and appetitive conditioning, are setting the stage for novel treatments for anxiety disorders and addictions.
Keywords: infralimbic cortex, amygdala, fear conditioning, glutamate receptors, bursting, PTSD
Introduction
Extinction of classical conditioning has been studied experimentally for almost a century, since Pavlov's classic study of appetitive conditioned responses in dogs (Pavlov, 1927). His observation that extinguished responding to a conditioned stimulus (CS) spontaneously recovers with the passage of time indicated that extinction does not erase the conditioned memory, but is a form of inhibition (Konorski, 1967; Pavlov, 1927). Since then, we have learned that extinguished responses can return following other types of manipulations such as a change in context or presentation of the unconditioned stimulus (Bouton, 1993; Rescorla and Heth, 1975). Such “uncovering phenomena” confirm that extinction is new learning and raise the question, what are the neural circuits of extinction learning and how do these interact with circuits mediating conditioning?
Early investigation of the neural mechanisms of extinction focused on the hippocampus, in accordance with the behavioral inhibition hypothesis of hippocampus popular at the time (Kimble, 1968; Gray, 1972; Rabe and Haddad, 1968). Following this period, psychological research on extinction continued (Bouton and Bolles, 1979; Rescorla, 1988), but neuroscientific investigations languished (Kimble and Kimble, 1970). The last decade, however, has seen a resurgence of interest in the neural mechanisms of this important form of learning. The reasons for this resurgence are probably numerous, but three factors stand out. First, impressive gains were made in deciphering the neural mechanisms of classical fear conditioning (LeDoux, 2000; Davis, 2000; Fendt and Fanselow, 1999), which provided an appropriate model system in which to study extinction. For this reason, the most complete understanding of extinction is in the fear system. Second, advances in psychological research on extinction started to converge with neuroscience research. For example, the discovery that extinction was context-specific paralleled the development of spatial mapping theories of the hippocampus (for a review, see Delamater, 2004). Third, and perhaps most importantly, there has been increased use of extinction-based exposure therapies for the treatment of anxiety disorders (Rothbaum and Schwartz, 2002; Wolpe, 1969; Barlow, 1990; Barad, 2005). Exposure therapy is highly effective (Foa, 2006), however, there is the possibility of improving the effectiveness and/or shortening the duration of treatment if extinction learning could be facilitated with pharmacological or other methods (Davis et al., 2006b). Here, we review the field of extinction research, emphasizing the phases of extinction learning and the structures involved. An excellent recent review focuses on molecular and pharmacological findings (Myers and Davis, 2007).
Where is “extinction memory”?
While it may be tempting to identify a single structure as the locus of extinction memory, it is more likely that extinction, like conditioning itself, is distributed across a network of structures. Extinction-related plasticity in each structure, however, may not serve identical roles. For example, plasticity in the amygdala may serve to inhibit fear expression, whereas plasticity in the hippocampus or prefrontal cortex may allow for contextual modulation of that inhibition. It is also possible that CS-responsiveness may be inhibited at various sites throughout the sensory processing stream, as suggested by metabolic mapping studies (Bruchey et al., 2007),
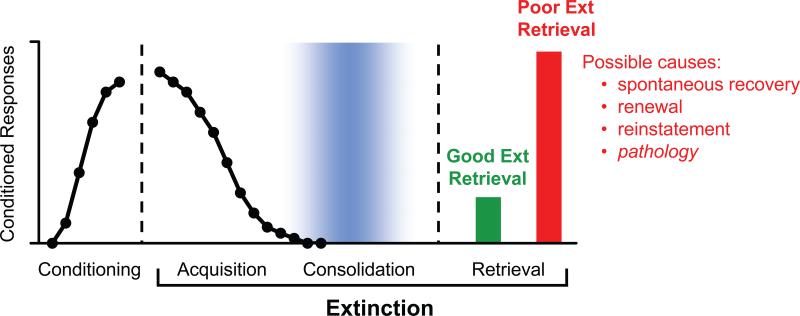
The involvement of a given structure or molecular process in extinction is likely to be determined by the particular phase of extinction learning in which the animal is engaged. Like other types of learning, extinction learning occurs in three phases: acquisition, consolidation, and retrieval (see Figure 1). Acquisition of extinction is the initial learning that occurs when conditioned responses are declining within an extinction training session. This is followed by a consolidation phase, lasting several hours, in which physiological and molecular processes stabilize a long-term memory for extinction. Subsequent to this, presentation of the extinguished CS triggers retrieval of extinction, as evidenced by low levels of conditioned responding. Poor retrieval of extinction is characterized by high levels of conditioned responding to the extinguished CS, reflecting expression of the original conditioning memory. Poor retrieval of extinction could be due to uncovering phenomena (e.g., renewal, reinstatement) or to a pathological process that prevents consolidation or recall of extinction. We will now outline what is known concerning the neural mechanisms of each of the three phases of extinction learning, focusing on extinction of conditioned fear. We will then review appetitive extinction, related brain imaging studies in humans, and the attempts to translate extinction research to the clinic.
Fig. 1.
Extinction learning occurs in three phases. Acquisition is characterized by a decrease in conditioned responses to the presentation of a CS without the US. Consolidation is a time-dependent process during which a long-term extinction representation is formed. Retrieval of extinction occurs at a later time, when the CS is re-presented. Good extinction retrieval is characterized by low levels of conditioned responses (green bar), whereas poor extinction retrieval is characterized by high levels of conditioned responses (red bar). Poor retrieval of extinction is normally observed following renewal, reinstatement, spontaneous recovery, or in pathological conditions characterized by extinction failure.
Acquisition of Extinction
Systemic studies
Systemic drug studies have attempted to identify the key molecules in the acquisition of extinction. The first molecule implicated in extinction was the N-methyl-D-aspartate receptor (NMDAr). Systemic administration of the NMDAr antagonist MK801 prevented extinction (Baker and Azorlosa, 1996; Cox and Westbrook, 1994), but because extinction was carried out over many days with few trials per day, it was not possible to distinguish impairments in acquisition vs. consolidation. When a massed extinction training design was used, it was observed that systemic NMDAr blockade (with CPP, (±)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid) prior to extinction training did not prevent acquisition of extinction, but did impair retrieval of extinction the following day (Santini et al., 2001; Suzuki et al., 2004). This suggests that NMDArs play a role in consolidation, rather than in acquisition, of extinction. More recently, however, it was shown that a selective antagonist of the Nr2B subunit of the NMDAr, ifenprodil, blocked acquisition of extinction within a session (Sotres-Bayon et al., 2007). The discrepancy in findings between ifenprodil and CPP is likely due to the higher affinity of ifenprodil for the Nr2B subunit, in contrast to the higher affinity of CPP for the Nr2A subunit (Lozovaya et al., 2004). In addition to having a higher affinity for the Nr2B subunit , ifenprodil does not impair expression of freezing like CPP (Sotres-Bayon et al., 2007), and is therefore a better tool for investigating extinction. Thus, it appears that NMDArs are necessary for the acquisition of extinction.
An equally robust blockade of extinction acquisition has been observed with systemic administration of the voltage-gated calcium channel (VGCC) antagonist nifedipine (Cain et al., 2002; Barad et al., 2004). Together with the NMDA findings, this suggests that calcium currents are required for the initial decrements in responding that occur during an extinction session. Calcium currents operating through Ca++/calmodulin-dependent protein kinase II (CaMKII) have been linked to short-term memory for other types of learning (Rodrigues et al., 2004; Irvine et al., 2005) and are thought to trigger receptor insertion and other local changes that can support memory acquisition. It is not known, however, if inhibitors of CaMKII (such as KN-62) prevent extinction. A recent study using inducible transgenic techniques showed that inhibition of protein kinase A (PKA) accelerated acquisition of fear extinction (Isiegas et al., 2006), suggesting that some kinase pathways may serve to inhibit extinction.
Other receptors implicated in the acquisition of extinction are cannabinoid and opioid receptors. Systemic administration of the opioid antagonist naloxone impaired within-session extinction of fear in rats (McNally and Westbrook, 2003). Acquisition of extinction was also slowed by blockade of the cannabinoid CB1 receptor (Marsicano et al., 2002; Varvel et al., 2005) and accelerated by CB1 agonists or cannabinoid reuptake inhibitors (Chhatwal et al., 2005a; Pamplona et al., 2006). Increasing levels of the endogenous cannabinoid anandamide appears to accelerate extinction of both fear and spatial memories (Varvel et al., 2007), suggesting that manipulating anandamide levels may be clinically useful. Thus, extinction-induced calcium currents may activate downstream kinases, which are modulated by endogenous cannabinoids (Cannich et al., 2004) to amplify extinction-related plasticity.
Basolateral amygdala
Studies on the neurobiology of extinction have been driven by the well-documented circuitry of conditioned fear. The basolateral amygdala (BLA) associates sensory and shock-related inputs and influences central nucleus output neurons, which drive fear expression through descending projections (Pare et al., 2004; Davis, 2006; Phelps and LeDoux, 2005). A single site of extinction acquisition, however, has been difficult to pinpoint, perhaps because acquisition is distributed across several structures. Indeed, within-session declines in neural conditioned responses have been observed throughout the fear conditioning circuit (Quirk et al., 1996; Olds et al., 1972; Ben Ari and Le Gal, 1974), and it has been argued that extinction may be a habituation-like process (Kamprath and Wotjak, 2004) likely to manifest itself throughout the system. While there may be many sites of extinction-related plasticity, the important question is where is plasticity necessary for the acquisition of extinction?
Because lesions of BLA eliminate freezing, it is not practical to use lesions to assess the role of BLA in extinction. Lesions restricted to the basal nuclei of the amygdala, however, do not block the acquisition of conditioned freezing (Nader et al., 2001), and also do not prevent extinction (Anglada-Figueroa and Quirk, 2005; Sotres-Bayon et al., 2004). This suggests that the basal nuclei are not necessary for extinction, however, it is possible that other structures may have assumed the function of the basal areas. Local infusion of pharmacological agents is a more useful way to study the role of the BLA in the acquisition of extinction. In fact, the BLA was the first structure implicated in extinction, because local infusion of NMDAr antagonists and kinase inhibitors prevented extinction (Falls et al., 1992; Lu et al., 2001; Lin et al., 2003b). In these studies, however, within-session extinction was not assessed and it was therefore not possible to distinguish an effect of the blockers on acquisition vs. consolidation processes.
Infusion of muscimol (an inactivating agent) into BLA reduced fear expression during extinction, but did not impair extinction learning as evidenced by normal retrieval of extinction the following day (Akirav et al., 2006b). This would appear to suggest that BLA processing is not required for extinction acquisition, although in that study, levels of extinction in controls were very low. Herry and coworkers recently showed that blockade of mitogen-activated protein kinase (MAPk) activity in BLA completely prevented within-session extinction, and that extinction increased levels of pMAPk in BLA (Herry et al., 2006). It is not yet known if VGCCs in the BLA are necessary for extinction acquisition, but local blockade of NMDArs (Sotres-Bayon et al., 2007) or metabotropic glutamate receptors (Kim et al., 2007) in the BLA were recently shown to impair the acquisition of extinction. Cannabinoids modulate glutamatergic and GABAergic transmission in BLA (Azad et al., 2003), as well as BLA kinase activity (Cannich et al., 2004), but it has yet to be determined if cannabinoid activity in BLA is necessary for extinction. Thus, it is now becoming clear that acquisition of extinction is mediated by calcium-triggered cascades within the BLA, and these may be modulated by metabotropic glutamate receptors and endogenous cannabinoids.
Periaqueductal Gray
The ventrolateral periaqueductal gray (vlPAG) is important for the expression of fear responses (De Oca et al., 1998; LeDoux et al., 1988) and is rich in opioid receptors (Atweh and Kuhar, 1983). McNally and coworkers recently suggested that vlPAG opioids are necessary for extinction acquisition. They have shown that blocking μ-opioid receptors with naloxone in the vlPAG prevented acquisition of extinction (McNally et al., 2004b; McNally et al., 2005). Because naloxone also facilitated acquisition of conditioning (McNally et al., 2004a), the authors proposed that opioids signal the current associative strength of the target CS, which is used to calculate the error term in classical learning theory (Rescorla and Wagner, 1972). This is the first theory of extinction acquisition linked to both neuroanatomy and learning theory. A challenge for this model, however, is to determine how opioid signals in the vlPAG communicate the error signal to the amygdala or other sites where conditioning and extinction–related plasticity occurs. In a general sense, extinction of conditioned fear may involve opioids because the omission of an expected shock may be rewarding. This would imply that opioid systems may play different roles in extinction of appetitive vs. aversive conditioning.
Consolidation of Extinction
Like other forms of learning, extinction acquisition is followed by a consolidation phase. Extinction consolidation is supported by two sets of findings: 1) pharmacological agents administered prior to extinction training do not interfere with extinction acquisition but render the animal unable to recall extinction at a later time (i.e., intact within-session extinction but deficient between-session extinction), and 2) pharmacological agents administered shortly after extinction training (during the consolidation phase) render the animal unable to recall extinction at a later time. For pre-extinction infusions, it is important to rule out state-dependent learning effects of the drug. This is not a problem for post-training administration of drugs. Consolidation processes could involve activation of molecular cascades triggered by acquisition-induced events or, more interestingly, neuronal activity that initiates during the post-training period to strengthen extinction memory (Routtenberg and Rekart, 2005; Wittenberg and Tsien, 2002; McGaugh, 2000).
Basolateral Amygdala
As a site of initial acquisition of extinction, it might be expected that the BLA is also a site of extinction consolidation. Augmenting BLA activity after extinction with the GABA-A antagonist bicuculline facilitated extinction in a norepinephrine-dependent manner (Berlau and McGaugh, 2006). This suggests that post-training activity in the amygdala is involved in extinction (but see Akirav et al., 2006b). Extinction is known to involve several kinase pathways in the amygdala such as MAPk (Lu et al., 2001; Herry et al., 2006) and PI-3 kinase (Lin et al., 2003b), as well as immediate early genes cFos and EGR-1 (Herry and Mons, 2004). In each case, interfering with the given pathway prevented consolidation of extinction. Protein synthesis in the BLA is also necessary for extinction (Lin et al., 2003b), suggesting that extinction of fear is similar to other forms of extinction learning that rely on protein synthesis for the formation of long-term memory (Berman and Dudai, 2001; Vianna et al., 2001; Pedreira and Maldonado, 2003). However, extinction also activates the phosphatase calcineurin in the BLA, leading to a reversal of conditioning-induced phosphorylation of the transcription factor CREB (Lin et al., 2003a). Thus, dephosphorylation of CREB could drive some erasure of original fear memory in the BLA. This finding does not conflict with the existence of uncovering phenomena in extinction, which requires that the conditioned memory is maintained in some, but not necessarily all, structures. Recent behavioral data indicate that extinction may indeed erase conditioning, especially when extinction is initiated within minutes of conditioning (Myers et al., 2006). Extinction-induced erasure may be a remnant from early stages of development, as extinction of fear in 16-day-old rats results in erasure of conditioning whereas extinction in 23-day-old rats leaves conditioning intact (Kim and Richardson, 2007a; Kim and Richardson, 2007b).
Recent studies suggest that extinction training leads to structural changes in BLA synapses. Two hours after extinction training, mRNA for the GABA receptor binding protein gephyrin is upregulated (Chhatwal et al., 2005b). This has the effect of clustering GABA-A receptors in the synaptic cleft for maximal inhibition. At this same time point, mRNA for the neurotrophic factor BDNF is upregulated in BLA (Chhatwal et al., 2006). Importantly, rats with lentiviral-induced reduction in BDNF receptors in the BLA can extinguish normally within the session, but are unable to recall extinction the following day (Chhatwal et al., 2006), consistent with a role of BLA BDNF in consolidation of extinction. Structural changes following extinction are also suggested by a recent report showing that inhibition of the cell-adhesion molecule PSA-NCAM in the BLA had no effect on within-session extinction, but strengthened extinction memory (Markram et al., 2007). Cell adhesion molecules, which stabilize synaptic morphology, are thought to oppose plasticity (Bonfanti, 2006). The same study showed that PSA-NCAM levels in BLA were increased following conditioning, suggesting that conditioning induces morphological changes that oppose extinction. Thus, it appears that extinction-induced calcium currents in BLA trigger molecular cascades and morphological changes responsible for stabilizing extinction memory.
Prefrontal Cortex
One of the earliest observations regarding the neural mechanisms of extinction was that lesions of the ventral medial prefrontal cortex (vmPFC) impaired extinction of conditioned fear (Morgan et al., 1993). This study was prompted by earlier findings showing that monkeys with lesions of orbitofrontal cortex showed perseverative response tendencies in extinction (for a review see Sotres-Bayon et al., 2006). The vmPFC can modulate fear expression through descending projections to the amygdala, as well as to the amygdala's targets in the brainstem and hypothalamus. A role for the infralimbic region (IL) of the vmPFC in consolidation was suggested by the observation that rats with lesions of IL could acquire extinction within a session, but had difficulty retrieving extinction the following day (Quirk et al., 2000). Similar findings were observed in other studies employing lesions of vmPFC (Lebron et al., 2004; Morgan et al., 2003; Weible et al., 2000; Fernandez, 2003), but other studies found no effect (Gewirtz et al., 1997; Garcia et al., 2006; Farinelli et al., 2006) (see Table 1 for summary of vmPFC lesion studies).
Table 1.
Effects of ventromedial prefrontal cortex manipulations on extinction retrieval
| Method | Task | Timing | Retrieval | Reference | |
|---|---|---|---|---|---|
| Lesions | |||||
| electrolytic | cued fc | pre-cond | impaired | Morgan et al., 1993 | |
| electrolytic | cued fc | pre-cond | impaired | Morgan and Ledoux, 1995 | |
| electrolytic | cued fc | pre-cond | no effect | Gewirtz et al., 1997 | |
| electrolytic | cued fc | pre-cond | impaired | Quirk et al., 2000 | |
| aspiration | eyeblink | pre-cond | impaired | Weible et al., 2000 | |
| electrolytic | cued fc | pre-ext | no effect | Morgan et al., 2003 | |
| 6-OHDA | context fc | pre-cond | impaired | Fernandez Espejo, 2003 | |
| electrolytic | cued fc | pre-cond | impaired | Lebron et al., 2004 | |
| excitotoxic: ibotenic acid | food | pre-cond | impaired | Rhodes and Killcross, 2004 | |
| electrolytic | cued fc | pre-cond | no effect | Farinelli et al., 2006 | |
| electrolytic | cued fc | pre-cond | no effect | Garcia et al., 2006 | |
| excitotoxic: ibotenic acid | food | pre-cond | impaired | Rhodes and Killcross, 2007 | |
| Infusions | |||||
| muscarinic antagonist: scopalamine | food | pre-ext | impaired | Maruki et al., 2003 | |
| protein synthesis inhibitor: anisomycin | cued fc | pre-ext | impaired | Santini et al., 2004 | |
| MAPk inhibitor: PD098059 | cued fc | post-ext | impaired | Hugues et al., 2004, 2006 | |
| D4 antagonist: L-741,741 | cued fc | pre-ext | impaired | Pfeiffer and Fendt, 2006 | |
| inactivation: tetrodotoxin | cued fc | pre-ext | impaired | Sierra-Mercado et al., 2006 | |
| inactivation: muscimol | cued fc | pre-ext | enhanced | Akirav et al., 2006a | |
| protein synthesis inhibitor: anisomycin | CTA | pre-ext | impaired | Akirav et al., 2006b | |
| NMDA antagonist: AP5 | CTA | pre-ext | no effect | Akirav et al., 2006b | |
| PKA inhibitor: Rp-cAMPS | cued fc | pre-ext | impaired | Mueller and Quirk, 2006 | |
| serine/threonine kinase inhibitor: H-7 | cued fc | pre-test | impaired | Holahan and Routtenberg, 2007 | |
| NMDA antagonist: CPP | cued fc | pre-ext | impaired | Burgos-Robles et al., 2007 | |
| NMDA antagonist: CPP | cued fc | post-ext | impaired | Burgos-Robles et al., 2007 | |
| Stimulation | |||||
| vmPFC microstimulation | cued fc | ext | enhanced | Milad and Quirk, 2002 | |
| thalamic inputs to vmPFC | cued fc | pre-ext | enhanced | Herry and Garcia, 2002 | |
| vmPFC microstimulation | cued fc | ext | enhanced | Milad et al., 2004 | |
| hippocampal inputs to vmPFC | cued fc | post-ext | enhanced | Farinelli et al., 2006 | |
cond, conditioning; CTA, conditioned taste aversion; ext, extinction; fc, fear conditioning; food, operant response for food
Because permanent lesions can lead to recovery of function by other structures (Anglada-Figueroa and Quirk, 2005), local infusion of inactivating agents is a more reliable method of assessing the role of a structure in extinction. Accordingly, infusion studies of IL show more consistent findings than lesion studies (see Table 1). IL infusions of the Na+ channel blocker TTX (Sierra-Mercado et al., 2006), NMDAr antagonist CPP (Burgos-Robles et al., 2007), PKA inhibitor (Mueller and Quirk, 2006), or protein synthesis blocker anisomycin (Santini et al., 2004) do not impair acquisition of extinction, but lead to impaired retrieval of extinction the following day. Control procedures for anisomycin (Santini et al., 2004) and CPP (Santini et al., 2001) rule out state-dependent learning effects as an explanation for the deficits. A consolidation role of the vmPFC is further suggested by recent findings that infusion of a MAPk inhibitor (Hugues et al., 2004; Hugues et al., 2006) or NMDAr antagonist (Burgos-Robles et al., 2007) immediately after extinction training (but not 2 or 4 hrs after) impaired subsequent retrieval of extinction. This is further evidence that infusion effects are not due to state-dependent learning, and suggests that consolidation of extinction involves initiation of molecular cascades during the post-training period. This is similar to other forms of learning where post-training NMDAr activity is required for consolidation (McDonald et al., 2005; de Lima et al., 2005; Shimizu et al., 2000).
As molecular signatures of extinction consolidation in vmPFC begin to emerge, evidence suggests that there are also physiological signatures. In the hours after extinction training, there is potentiation of evoked potentials (Farinelli et al., 2006; Herry and Garcia, 2002), and neuronal tone responses (Milad and Quirk, 2002) in the IL. In both cases, the degree of potentiation was correlated with the amount of extinction in a subsequent retrieval test. More recently, it has been shown that high-frequency bursting of IL neurons shortly after extinction predicts retrieval of extinction the following day (Burgos-Robles et al., 2007) (see Figure 2). NMDA receptors are required for both IL bursting and consolidation of extinction (Burgos-Robles et al., 2007), suggesting that bursting may trigger calcium currents in IL necessary for stabilizing extinction memory (Quirk et al., 2006). Consolidation of extinction can be strengthened by manipulations that augment IL function, such as 1) long-term potentiation of thalamic (Herry and Garcia, 2002) or hippocampal (Farinelli et al., 2006) inputs, 2) local microstimulation (Milad et al., 2004; Milad and Quirk, 2002), 3) systemic administration of a metabolic enhancer (Gonzalez-Lima and Bruchey, 2004), 4) systemic administration of histone deacetylase inhibitors (Bredy et al., 2007), and 5) local infusion of an AMPA receptor potentiator (Zushida et al., 2007) (see Table 1).
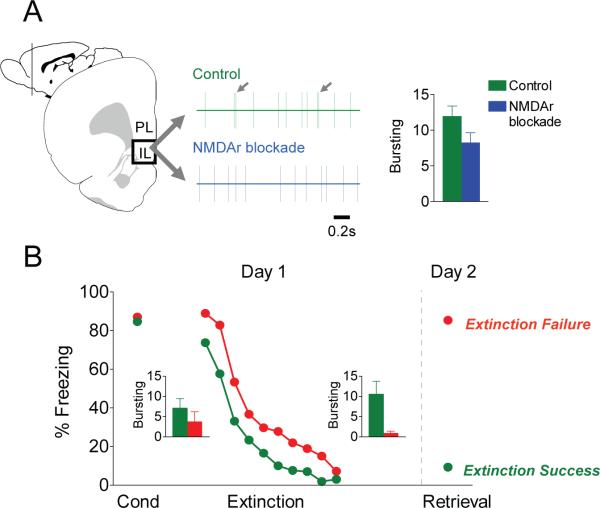
Fig. 2.
Consolidation of extinction involves NMDAr-mediated bursting in infralimbic (IL) cortex. A) Action potentials from a single IL neuron before and after systemic injection of CPP, a competitive antagonist the NMDA receptor. CPP did not change the firing rate, but reduced high-frequency bursting, as evidenced by short interspike intervals (20−30 ms). B) Rats were conditioned to freeze to a tone paired with a shock, and then extinguished (tone alone). The following day, two thirds of the rats showed good retrieval of extinction (extinction success), whereas one third were unable to retrieve extinction (extinction failure). Prior to extinction, these two groups showed equivalent bursting in IL (bar graph insets), but 30 minutes after extinction, there was significantly less bursting in the extinction failure group. Thus, post-training IL bursting predicts extinction success and is a physiological signature of extinction consolidation (modified from Burgos-Robles et al., 2007).
Hippocampus
The role of the hippocampus in consolidation of extinction has been extensively studied in two rodent paradigms in which the hippocampus is also required for conditioning: inhibitory avoidance and contextual fear conditioning. In inhibitory avoidance, the rat learns to refrain from stepping down onto an electrified floor. The advantage of this task is that extinction can be learned in a single trial, thereby facilitating the examination of post-training treatments. Using this paradigm, Izquierdo, Cammorata and colleagues have implicated numerous molecular processes within the hippocampus in the consolidation of extinction. These include NMDArs, MAPks, PKA, SRC tyrosine kinases, gene expression, and protein synthesis (Rossato et al., 2006; Bevilaqua et al., 2005; Vianna et al., 2003; Szapiro et al., 2003; Vianna et al., 2001). Interestingly, many of these processes are also involved in conditioning and/or recall of the avoidance memory, but some are unique to extinction (Cammarota et al., 2005). For contextual fear extinction, the MAPk cascade in the hippocampus is necessary (Fischer et al., 2007), as is actin rearrangement (Fischer et al., 2004). Consistent with findings in the amygdala (Chhatwal et al., 2006), lentiviral inactivation of BDNF in the hippocampus impairs consolidation of fear extinction in a cued fear conditioning paradigm (Heldt et al., 2007). Thus, the hippocampus appears to be essential for consolidation of extinction, especially in tasks such as inhibitory avoidance, which require the hippocampus for conditioning.
Retrieval of Extinction
As discussed above, the retrieval of extinction involves the expression of an inhibitory memory, and is highly context-specific. Accordingly, retrieval of extinction would be expected to activate inhibitory networks, as well as the hippocampus. These retrieval circuits are beginning to be understood for extinction of conditioned fear. Understanding retrieval of extinction is clinically important, because anxiety disorders and relapse of drug abuse are thought to be caused by a failure to retrieve an extinction memory generated in extinction-based treatment (Rothbaum and Davis, 2003; Rauch et al., 2006; Kalivas et al., 2006).
Inhibitory networks in the amygdala
Extinction-induced activation of inhibitory networks suggests the involvement of the inhibitory neurotransmitter GABA in expression of extinction. An early study showed that facilitation of GABA-A activity with systemic injection of an inverse agonist of the benzodiazepine receptor (FG 7142) “reinstated” conditioned fear after extinction, consistent with a failure to retrieve extinction (Harris and Westbrook, 1998). Importantly, FG 7142 had no effect on fear expression prior to extinction, suggesting that activation of GABAergic systems is somewhat specific to extinction. Efforts to localize this effect to the amygdala have proved difficult because inhibition of GABA-A receptors in the amygdala can lead to seizures. Perhaps for this reason, no prior study has examined the effect of GABA-A antagonists (such as bicuculline) on the retrieval of extinction. Several groups have shown that facilitating GABAergic transmission with the GABA-A agonist muscimol in the BLA reduces fear expression (Blair et al., 2005; Muller et al., 1997; Muller and Fendt, 2006), but this simply confirms lesion studies showing that BLA is essential for expression of conditioned fear. Additional experiments are clearly needed. For example, it would be interesting to know if extinguished fear responses are more dependent on BLA GABA-A receptors than low fear levels due to partial conditioning (e.g., Jami and Barad, 2004).
Within the amygdala, there are well defined circuits for inhibition. These include local inhibitory neurons within the BLA and central nucleus of the amygdala, as well as the islands of GABAergic neurons situated between these two structures known as the intercalated (ITC) cells. ITC cells receive input from BLA as well as several cortical sites (Pare and Smith, 1998; McDonald et al., 1996), and inhibit central nucleus output neurons (Pare and Smith, 1993; Royer et al., 1999). In a similar manner, paracapsular ITC cells surround the BLA and inhibit BLA neurons (Marowsky et al., 2005). Thus, ITC cells can be seen as an “off switch” for the amygdala that is activated by cortical input. ITC cells show NMDAr-dependent LTP and LTD following high frequency stimulation of BLA inputs (Royer and Pare, 2002) and could serve as a site of extinction memory. In addition to ITC cells, NMDAr-dependent LTP has also been observed in inhibitory neurons in LA following high-frequency stimulation of thalamic inputs (Bauer and LeDoux, 2004). Thus, extensive local inhibition within the amygdala keeps the firing rate of BLA and central neurons low (Quirk et al., 1995; Collins and Pare, 2000; Goosens et al., 2003), and could serve as a substrate for expressing and storing extinction (Pare et al., 2004).
Cortical control of amygdala inhibition
Cortical inputs to the amygdala provide a mechanism by which contextual, temporal, and mnemonic factors can regulate fear expression. Amygdala ITC cells receive a strong projection from the IL mPFC in both rodents (McDonald et al., 1996) and primates (Chiba et al., 2001; Ghashghaei and Barbas, 2002). During extinction retrieval, IL activity is potentiated and is correlated with the extent of extinction retrieval (Milad and Quirk, 2002; Barrett et al., 2003; Herry and Garcia, 2002). Potentiated IL output could inhibit amygdala output via activation of ITC cells (Maren and Quirk, 2004; Pare et al., 2004). There are several lines of support for this model. Electrical stimulation of IL reduces the responsiveness of central nucleus output neurons to BLA stimulation (Quirk et al., 2003), and chemical stimulation of IL activates cFos in ITC neurons (Berretta et al., 2005). Electrical stimulation of IL reduces conditioned fear and strengthens extinction memory (Vidal-Gonzalez et al., 2006; Milad and Quirk, 2002; Milad et al., 2004), whereas infusion of a broad spectrum kinase inhibitor into mPFC prevents retrieval of extinction (Holahan and Routtenberg, 2007) (see Table 1).
During extinction, some neurons in LA (Repa et al., 2001) and auditory cortex (Quirk et al., 1997) continue to show conditioned responses, despite reduced fear. Extinction-induced inhibition of fear expression at the level of ITC cells, which are downstream from these areas, would effectively prevent fear signals from exiting the BLA (see Figure 3). Projections from vmPFC might also activate inhibitory interneurons directly within the BLA or in the pericapsular ITC cells to dampen neuronal responses to conditioned stimuli (Rosenkranz and Grace, 2002; Rosenkranz et al., 2003; Marowsky et al., 2005). However, anatomical (Smith et al., 2000) and physiological (Likhtik et al., 2005) findings suggest that vmPFC inputs to BLA are largely excitatory. In addition to the vmPFC, the entorhinal cortex and subiculum project strongly to ITC cells (Canteras and Swanson, 1992; McDonald and Mascagni, 1997), and could participate in regulation of fear responses in extinction. Consistent with this, recent findings have implicated the entorhinal cortex in extinction of inhibitory avoidance (Bevilaqua et al., 2006).
Fig. 3.
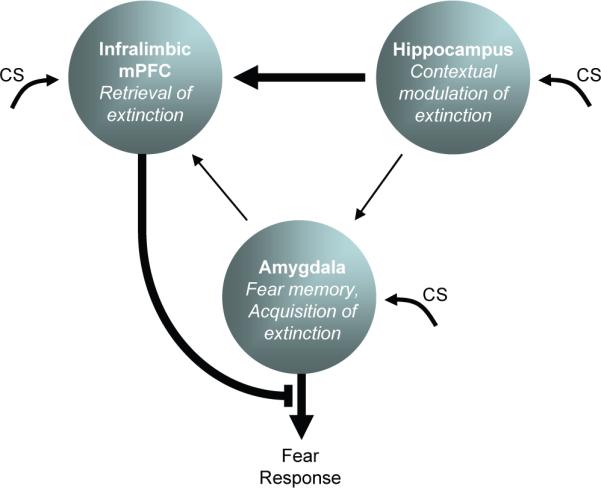
Extinction learning and expression relies on a network of three structures. The amygdala stores both conditioning and extinction memories. CS information enters the amygdala, hippocampus, and infralimbic cortex. The infralimbic cortex integrates CS information with contextual information from the hippocampus in order to determine extinction retrieval. In the extinction context, the infralimbic cortex inhibits amygdala output to reduce fear. Outside the extinction context, amygdala output is uninhibited.
While inhibitory influences of mPFC have been emphasized, recent findings suggest that the prelimbic (PL) mPFC excites fear expression. Pharmacological inactivation of PL reduces conditioned fear expression (Corcoran and Quirk, 2007a; Blum et al., 2006), and microstimulation of PL increases conditioned fear expression (Vidal-Gonzalez et al., 2006). During fear conditioning, PL and IL neurons show opposite response patterns (Gilmartin and McEchron, 2005), and bursting in PL neurons is correlated with acquisition of conditioned fear (Laviolette et al., 2005). Similar to extinction, this prefrontal system also is responsible for reducing fear under conditions where the stressor is controllable (Baratta et al., 2007). PL can augment fear expression via projections to the basal nucleus of the amygdala (Vertes, 2004), which was recently shown to be critical for expression of conditioned fear (Anglada-Figueroa and Quirk, 2005). Thus, PL and IL exert bidirectional control over fear expression, and both likely play a role in extinction retrieval.
Contextual Regulation
Extinguished responses are “renewed” in contexts other than where extinction occurred. The dependence of extinction retrieval on contextual factors suggests a key role of the hippocampus in the retrieval of extinction (see Figure 3). Initial studies examining the effect of lesions of the hippocampus on renewal found no effect (Frohardt et al., 2000; Wilson et al., 1995), but a more recent study revealed a deficit (Ji and Maren, 2005). This difference may be due to the exact renewal paradigm used (e.g., ABA vs. ABC) (Bouton et al., 2006), or the possibility of recovery of function by other structures. A clearer picture is emerging from studies using pharmacological inactivation. Inactivating the hippocampus prior to extinction retrieval prevented renewal (i.e., fear was lower than controls) (Corcoran and Maren, 2004; Corcoran and Maren, 2001; Hobin et al., 2006). Inactivating hippocampus prior to extinction training lead to poor retrieval of extinction the following day (i.e., fear was higher than controls) (Corcoran et al., 2005). This suggests that activity in the hippocampus is necessary for the renewal of fear in a non-extinction context, and that plasticity in the hippocampus (or its targets) is necessary for retrieval of extinction in an extinction context. Interestingly, similar results have been observed with inactivation of the mPFC (Sierra-Mercado et al., 2006), suggesting that the mPFC may be an important target of the hippocampus for contextual modulation of extinction retrieval (Hobin et al., 2003; Corcoran and Quirk, 2007b).
Support for an amygdala locus of action in the contextual modulation of extinction retrieval comes from work of Maren and colleagues, who showed that the responses of LA neurons to conditioned tones were modulated by context after extinction (Hobin et al., 2003). LA tone responses were reduced in the extinction context, compared to a non-extinction context, but there was no contextual modulation of neuronal responses to stimuli that had not been extinguished. A more recent study from this group extended the finding by showing that contextual modulation of LA activity requires the hippocampus (Maren and Hobin, 2007). Similar studies combining unit recording, pharmacological inactivation, and behavioral analyses will be needed to understand the neural mechanisms of contextual modulation of extinction.
Imaging of Extinction in Humans
An important goal of extinction research is to translate rodent findings to humans for future clinical applications. While previous human imaging studies focused solely on acquisition of conditioned fear (Buchel and Dolan, 2000; LaBar et al., 1998), more recent studies have focused on extinction. Following the animal literature, new study designs are allowing researchers to distinguish between extinction acquisition vs. extinction retrieval (Rauch et al., 2006; Delgado et al., 2006) by examining subjects both during extinction training as well as 24 hours later. Paralleling rat findings that the amygdala is necessary for acquisition of extinction, several groups observed amygdala activation during extinction training (Knight et al., 2004; Milad et al., 2007b; Gottfried and Dolan, 2004; Phelps et al., 2004). It is important to note that this activation was observed mid-extinction training, likely reflecting extinction learning rather than simply recall of conditioning. Indeed, in one study, these two processes appear to activate different parts of the amygdala (Knight et al., 2004).
During extinction retrieval (24 h after extinction training), several studies have reported significant activation of the vmPFC (Phelps et al., 2004; Kalisch et al., 2006; Milad et al., 2007b). Furthermore, Milad and coworkers observed that the amount of extinction retrieved was highly correlated with vmPFC activity (Milad et al., 2007b) and vmPFC thickness (Milad et al., 2005). These findings validate the preclinical rodent models of extinction retrieval, and suggest that the vmPFC may be a good target for clinical interventions. Abnormalities in functional connectivity between the prefrontal cortex and amygdala during emotional processing have been reported in humans carrying the short “S” allele for the serotonin transporter gene (Pezawas et al., 2005; Heinz et al., 2005) and in mutant mice lacking this gene (Wellman et al., 2007), suggesting that there may be a genetic component underlying individual variability in extinction.
In addition to the vmPFC, a network of interconnected structures is emerging that could serve to regulate fear expression. Recent work suggests that the supragenual anterior cingulate may be a functional homologue of the rodent PL (Hariri and Holmes, 2006). This more dorsal region shows structural and functional correlations with acquisition of conditioned fear (Milad et al., 2007a), and is overactive in carriers of the serotonin transporter “S” allele (Pezawas et al., 2005). The hippocampus is also activated during extinction retrieval in studies that manipulate context (Kalisch et al., 2006; Milad et al., 2007b) suggesting that a prefrontal-hippocampal network is involved in contextual modulation of extinction. Although one would expect such a network to inhibit the amygdala, one study found no correlation between vmPFC and amygdala during extinction retrieval (Kalisch et al., 2006) while two others found a positive correlation (Phelps et al., 2004; Milad et al., 2007b). Increased activity in the amygdala might represent activation of local inhibitory interneurons, which would be difficult to distinguish from activation of output neurons. Nevertheless, a striking convergence exists between rodent and human literatures on extinction retrieval, and suggests that extinction mechanisms, like fear learning itself, are highly conserved across species.
Consistent with the idea that post-traumatic stress disorder (PTSD) is caused by a failure to consolidate and retrieve memory for extinction, these same areas appear to be dysfunctional in PTSD. Subjects with PTSD show reduced vmPFC volume and activity, together with increased activity in the amygdala (Bremner, 2006; Shin et al., 2006; Liberzon and Martis, 2006). A recent meta-analysis showed that the prefrontal areas deficient in PTSD correspond to the same areas implicated in extinction (Milad et al., 2006). The hippocampus also shows decreased volume and activity in PTSD (Bremner, 2006; Shin et al., 2006; Gilbertson et al., 2002), consistent with the hypothesis that contextual modulation of extinction is compromised. Thus, optimal functioning in the hippocampal-prefrontal-amygdala network may be critical for normal emotional regulation, and may even determine certain personality traits (Rauch et al., 2005; Quirk and Beer, 2006; Hariri et al., 2006).
Extinction of Appetitive Responses
Relative to fear extinction, there are few studies on the neural mechanisms of appetitive extinction. The available evidence, however, indicates that appetitive extinction also involves the BLA and vmPFC. Classic work in monkeys (Weiskrantz, 1956) and more recent work in rats (Burns et al., 1999) has shown that lesions of the BLA impair extinction of conditioned responding for food rewards, suggesting that the BLA is necessary for acquisition of extinction in appetitive tasks. A similar finding was recently reported for pharmacological inactivation of the caudal BLA (McLaughlin and Floresco, 2007). In apparent contrast to these findings, BLA lesions in monkeys enhanced extinction of an appetitive instrumental response (Izquierdo and Murray, 2005). In that study, however, BLA lesions also impaired expression of the conditioned response at the start of extinction, making it difficult to interpret extinction deficits. As with conditioned fear, the central nucleus of the amygdala is necessary for expression of conditioned appetitive responses, acquired through both classical (Lee et al., 2005) and instrumental (Knapska et al., 2006) conditioning. Thus, there is good agreement across affective modalities as to the involvement of the amygdala in extinction.
The vmPFC and orbital cortex were originally implicated in extinction in early studies examining extinction of appetitive instrumental behaviors in monkeys (Butter et al., 1963; Sotres-Bayon et al., 2006). More recent studies in rodents using classical appetitive conditioning have re-examined the effects of vmPFC lesions. Similar to conditioned fear, lesions of vmPFC did not impair within-session extinction, but impaired retrieval of extinction the following day, as evidenced by increased spontaneous recovery (Rhodes and Killcross, 2004) and increased renewal (Rhodes and Killcross, 2007). Consistent with these findings, infusions of the muscarinic antagonist scopalamine into the vmPFC prior to extinction of lever pressing for food left within-session extinction intact, but impaired extinction retrieval the following day (Maruki et al., 2003). Extinction of appetitive behavior triggers norepinephrine efflux in vmPFC (Mingote et al., 2004), which could explain the deficits in extinction retrieval following forebrain depletion of norepinephrine (Mason and Iversen, 1977). A role of the vmPFC in retrieval of appetitive extinction suggests that vmPFC may modulate return of drug-seeking behavior following extinction (Kalivas et al., 2006).
Clinical Implications
Anxiety disorders are among the most commonly diagnosed mental health problems (Breslau et al., 2004), and are often treated with extinction-based exposure therapies (Foa, 2006; Hermans et al., 2005; Garakani et al., 2006). In patients with PTSD, deficits in fear extinction are observed (Peri et al., 2000; Orr et al., 2000), and are thought to contribute to the persistence of this disorder (Charney et al., 1993). Therefore, overcoming these deficits by enhancing current therapeutic treatments with pharmacological adjuncts could accelerate and strengthen extinction (Anderson and Insel, 2006). A number of pharmacological agents have been shown to enhance extinction in animals, and translational studies in humans are beginning to bear fruit.
In rodents, extinction of fear is enhanced by several classes of systemically applied drugs (see Table 2). With respect to monoaminergic systems, the dopamine D2 receptor antagonist sulpiride (Ponnusamy et al., 2005) and the α2-adrenoceptor antagonist yohimbine (Cain et al., 2004; Morris and Bouton, 2007) facilitate extinction. A general metabolic enhancer, methylene blue, has also been shown to facilitate fear extinction (Wrubel et al., 2007; Gonzalez-Lima and Bruchey, 2004), likely by enhancing extinction-induced activity in the vmPFC. The best studied extinction facilitator is the NMDAr partial agonist D-cycloserine (DCS), which has been shown to accelerate and strengthen extinction of fear in several laboratories (Weber et al., 2007; Woods and Bouton, 2006; Mao et al., 2006; Lee et al., 2006; Parnas et al., 2005; Walker et al., 2002; Ledgerwood et al., 2003). Intracerebral infusions indicate that the site of action of DCS is in the BLA (Walker et al., 2002; Ledgerwood et al., 2003), in agreement with the infusions of NMDAr antagonists in the BLA (Falls et al., 1992; Sotres-Bayon et al., 2007). DCS may also act in IL, which is a site of NMDAr-dependent consolidation of extinction (Burgos-Robles et al., 2007). With respect to appetitive learning, DCS facilitated extinction of drug-seeking behavior in rats (Botreau et al., 2006), suggesting that DCS could be used in conjunction with extinction-based treatments for addiction.
Table 2.
Pharmacological enhancers of extinction (systemic)
| Drug | Action | Reference | |
|---|---|---|---|
| Preclinical | |||
| DCS | partial NMDAr agonist | Walker et al., 2002 | |
| methylene blue | metabolic enhancer | Gonzalez-Lima and Bruchey, 2004 | |
| yohimbine | noradrenergic α2r antagonist | Cain et al., 2004 | |
| sulpiride | dopamine D2r antagonist | Ponnusamy et al., 2005 | |
| AM-404 | cannabinoid reuptake inhibitor | Chhatwal et al., 2005 | |
| WIN 55,212−2 | cannabinoid receptor agonist | Pamplona et al., 2006 | |
| dexamethasone | glucocorticoid receptor agonist | Yang et al., 2006 | |
| PEPA | AMPA receptor potentiator | Zushida et al., 2007 | |
| Clinical | |||
| DCS | partial NMDAr agonist | Ressler et al., 2004 | |
| cortisol | endogenous glucocorticoid | Soravia et al., 2006 | |
In humans, DCS has already been shown to augment therapeutic responses to therapies for acrophobia (Ressler et al., 2004) and social anxiety (Hofmann et al., 2006), suggesting that it may be useful as an adjunct to exposure therapy. Recent studies, however, found no effect of DCS on therapy for spider phobia (Guastella et al., 2007b) or on fear extinction itself (Guastella et al., 2007a). Other possible limitations of DCS have been recently documented in rodents, including CS nonspecificity and tolerance following repeated administration of DCS (Ledgerwood et al., 2005; Parnas et al., 2005). Thus, while initially promising, additional clinical trials with DCS are needed to determine its efficacy as an adjunct to therapy.
Since the discovery of DCS, other drugs have been shown to enhance extinction in rodents, and might be useful in humans (see Table 2). These include AM404, an inhibitor of endocannabinoid breakdown and reuptake (Chhatwal et al., 2005a), RB101(S), an inhibitor of enkephalin-degrading enzymes (McNally, 2005), and PEPA, a potentiator of AMPA receptors (Zushida et al., 2007). A particularly exciting new avenue of study involves the glucocorticoids, which have been recently shown to facilitate fear extinction in rats (Yang et al., 2007; Yang et al., 2006). It has been known for some time that PTSD sufferers have reduced circulating levels of cortisol (Yehuda, 2001), suggesting that corticosteroids may have a protective effect. In fact, repeated cortisol treatments administered prior to exposure therapy augmented the therapeutic response in social phobia and spider phobia (Soravia et al., 2006). Moreover, patients with spider phobia continued to express reduced fear during exposure therapy 48 hours following treatment with cortisol (Soravia et al., 2006), suggesting that extinction consolidation was enhanced. Thus, the use of glucocorticoid treatments to enhance therapeutic outcomes warrants further study.
Conclusions
Neuroscientific research on extinction has advanced rapidly over the past decade, uncovering the neural mechanisms that regulate this form of learning. The processes of acquisition, consolidation and retrieval of extinction require the interplay of several key structures, including the basolateral amygdala, infralimbic prefrontal cortex, and hippocampus. Parallel findings are emerging from studies of extinction of appetitive responses, suggesting that this neural circuitry is generalizable. Of particular importance is the determination of the mechanisms regulating extinction consolidation and retrieval. Pharmacological agents that facilitate extinction consolidation and retrieval could serve as adjuncts to cognitive behavioral therapy for anxiety disorders and addiction, offering a novel treatment strategy for enhancing therapeutic outcome.
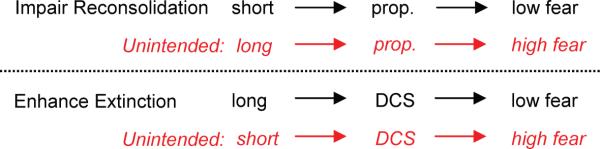
BOX 1: EXTINCTION VS. RECONSOLIDATION.
Extinction involves reactivation of the conditioning memory. An increasing number of studies over the past 7 years indicates that reactivation of a memory initiates a “reconsolidation” process necessary for maintenance of the conditioning memory (Nader et al., 2000; Tronson and Taylor, 2007; Dudai, 2002). Reconsolidation requires many of the same cellular processes as extinction, such as protein synthesis, NMDArs, β-adrenergic receptors, PKA and MAPk (for reviews, see Miller and Sweatt, 2006; Alberini, 2005). This raises the question: which process predominates in an extinction session, and how might they interact? Converging findings from conditioned fear studies in several species suggest that the process that predominates depends on the duration of the re-exposure to the conditioned stimulus (Sangha et al., 2003; Pedreira and Maldonado, 2003; Eisenberg et al., 2003; Suzuki et al., 2004). If re-exposure is very short (without accompanying extinction), reconsolidation will predominate and blockers will cause low levels of fear (impaired reconsolidation). If re-exposure is long enough to induce extinction, extinction will predominate and blockers will cause high levels of fear (impaired extinction). Thus, an extinction session may initially trigger reconsolidation, but this leads to consolidation of extinction as the session progresses. It should be noted, however, that reconsolidation processes can occur despite extinction, suggesting that these two processes can occur independently of one another (Duvarci et al., 2006).
From a clinical perspective, both reconsolidation and extinction could be pharmacologically manipulated to reduce the exaggerated fear responses seen in anxiety disorders. The intent would be to impair reconsolidation or facilitate extinction. Extinction can be facilitated with the NMDAr partial agonist D-cycloserine (DCS; see main text), while reconsolidation can be impaired with the β-adrenergic receptor blocker propranolol (Debiec and LeDoux, 2004). Both of these drugs are in various stages of clinical testing (Davis et al., 2006a; Brunet et al., 2007). However, the interaction between reconsolidation and extinction could result in undesirable effects, depending on the duration of re-exposure (see Box 1 table). With a short re-exposure, DCS was recently shown to increase fear in rats, presumably by strengthening reconsolidation (Lee et al., 2006). Similarly, propranolol was shown to impair extinction of conditioned fear in mice (Cain et al., 2004), resulting in high fear. Thus, the duration of exposure must be carefully coordinated with the drug used and the particular memory process that is being targeted.
BOX 2: EXTINCTION AND STRESS.
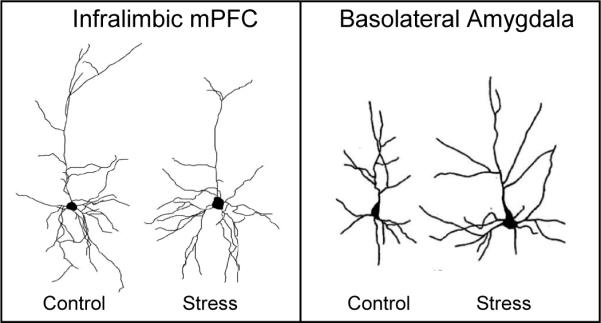
Does stress impair extinction? This is obviously an important question since many mental disorders are compounded by high levels of chronic stress, which could impede extinction-based therapies. Recent morphological evidence suggests that stress may impair extinction. Chronic stress (daily restraint over a period of 7−20 days) decreases dendritic branching and spine count in the hippocampus (McEwen, 2001) and mPFC (Radley et al., 2004; Cook and Wellman, 2004; Brown et al., 2005; Radley et al., 2006), while at the same time increasing dendritic branching and spine count in the BLA (Mitra et al., 2005; Vyas et al., 2002; Vyas et al., 2006) (see Box 2 figure). This pattern of effects would be expected to increase conditioning and impair extinction. Accordingly, chronic stress has been reported to impair recall of extinction (Miracle et al., 2006), but, because the stress was induced prior to conditioning, it was not possible to distinguish the effects of stress on conditioning vs. extinction. Morphological analysis of prefrontal alterations has been limited to the prelimbic (PL) mPFC, even though the infralimbic (IL) mPFC is the structure more implicated in extinction. Thus, additional studies are needed that focus on IL, and induce stress after conditioning, but prior to extinction. In this regard, a recent study showed that three days of forced swim stress induced dendritic retraction specific to IL, and impaired the acquisition of extinction (Izquierdo et al., 2006). Inset: infralimbic cells adapted from Izquierdo et al. (2006) and basolateral amygdala cells adapted from Vyas et al. (2002).
Duration of CS re-exposure determines treatment outcome
| Intent | CS exposure | Drug | Outcome |
|---|---|---|---|
| |||
Acknowledgements
The authors would like to thank Kevin Corcoran and Anthony Burgos-Robles for comments on the manuscript. GJQ is supported by NIH grants MH058883 and GM008239; DM is supported by a FQRNT (Quebec, Canada) postdoctoral fellowship.
Footnotes
Disclosure/Conflict of Interest
The authors declare no competing financial interests. GJQ is supported by NIH grants MH058883 and GM008239; DM is supported by a FQRNT (Quebec, Canada) postdoctoral fellowship.
References
- Akirav I, Khatsrinov V, Vouimba RM, Merhav M, Ferreira G, Rosenblum K, Maroun M. Extinction of conditioned taste aversion depends on functional protein synthesis but not on NMDA receptor activation in the ventromedial prefrontal cortex. Learn Mem. 2006a;13:254–258. doi: 10.1101/lm.191706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006b;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Anderson KC, Insel TR. The promise of extinction research for the prevention and treatment of anxiety disorders. Biol Psychiatry. 2006;60:319–321. doi: 10.1016/j.biopsych.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atweh SF, Kuhar MJ. Distribution and physiological significance of opioid receptors in the brain. Br Med Bull. 1983;39:47–52. doi: 10.1093/oxfordjournals.bmb.a071789. [DOI] [PubMed] [Google Scholar]
- Azad SC, Eder M, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Mem. 2003;10:116–128. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Barad M, Blouin AM, Cain CK. Like extinction, latent inhibition of conditioned fear in mice is blocked by systemic inhibition of L-type voltage-gated calcium channels. Learn Mem. 2004;11:536–539. doi: 10.1101/lm.78304. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH. Long-term outcome for patients with panic disorder treated with cognitive-behavioral therapy. J Clin Psychiatry. 1990;51(Suppl A):17–23. [PubMed] [Google Scholar]
- Barrett D, Shumake J, Jones D, Gonzalez-Lima F. Metabolic mapping of mouse brain activity after extinction of a conditioned emotional response. J Neurosci. 2003;23:5740–5749. doi: 10.1523/JNEUROSCI.23-13-05740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE. Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala. J Neurosci. 2004;24:9507–9512. doi: 10.1523/JNEUROSCI.3567-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ari Y, Le Gal IS. Lateral amygdala unit activity: II. Habituating and non-habituating neurons. Electroencephalogr Clin Neurophysiol. 1974;37:463–472. doi: 10.1016/0013-4694(74)90087-x. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilaqua LR, Bonini JS, Rossato JI, Izquierdo LA, Cammarota M, Izquierdo I. The entorhinal cortex plays a role in extinction. Neurobiol Learn Mem. 2006;85:192–197. doi: 10.1016/j.nlm.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Bevilaqua LR, da Silva WN, Medina JH, Izquierdo I, Cammarota M. Extinction and reacquisition of a fear-motivated memory require activity of the Src family of tyrosine kinases in the CA1 region of the hippocampus. Pharmacol Biochem Behav. 2005;81:139–145. doi: 10.1016/j.pbb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Blair HT, Sotres-Bayon F, Moita MA, LeDoux JE. The lateral amygdala processes the value of conditioned and unconditioned aversive stimuli. Neuroscience. 2005;133:561–569. doi: 10.1016/j.neuroscience.2005.02.043. [DOI] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80:129–164. doi: 10.1016/j.pneurobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8:445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Peterson EL, Poisson LM, Schultz LR, Lucia VC. Estimating post-traumatic stress disorder in the community: lifetime perspective and the impact of typical traumatic events. Psychol Med. 2004;34:889–898. doi: 10.1017/s0033291703001612. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Shumake J, Gonzalez-Lima F. Network model of fear extinction and renewal functional pathways. Neuroscience. 2007;145:423–437. doi: 10.1016/j.neuroscience.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. Journal of Psychiatric Research. 2007 doi: 10.1016/j.jpsychires.2007.05.006. in press. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [This paper demonstrates the necessity of NMDA receptor-mediated plasticity in the infralimbic cortex for consolidation of extinction and associated burst firing.] [DOI] [PubMed] [Google Scholar]
- Burns LH, Everitt BJ, Robbins TW. Effects of excitotoxic lesions of the basolateral amygdala on conditional discrimination learning with primary and conditioned reinforcement. Behav Brain Res. 1999;100:123–133. doi: 10.1016/s0166-4328(98)00119-3. [DOI] [PubMed] [Google Scholar]
- Butter CM, Mishkin M, Rosvold HE. Conditioning and extinction of a food-rewarded response after selective ablation of frontal cortex in Rhesus monkeys. Exp Neurol. 1963;7:65–75. doi: 10.1016/0014-4886(63)90094-3. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. L-type voltage-gated calcium channels are required for extinction, but not for acquisition or expression, of conditional fear in mice. J Neurosci. 2002;22:9113–9121. doi: 10.1523/JNEUROSCI.22-20-09113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Barros DM, Vianna MR, Izquierdo LA, Medina JH, Izquierdo I. Retrieval and the extinction of memory. Cell Mol Neurobiol. 2005;25:465–474. doi: 10.1007/s10571-005-4009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem. 2004;11:625–632. doi: 10.1101/lm.77904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005a;30:516–524. doi: 10.1038/sj.npp.1300655. [Increasing endogenous levels of cannabinoids was shown to dose-dependently facilitate extinction, and may have clinical applicability.] [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005b;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pare D. Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−). Learn Mem. 2000;7:97–103. doi: 10.1101/lm.7.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [Using temporary inactivation, this study demonstrated that hippocampal processing during extinction is necessary for contextual recall of extinction the following day, similar to mPFC (see Sierra-Mercado et al., 2006).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007a;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007b;12:200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Cox J, Westbrook RF. The NMDA receptor antagonist MK-801 blocks acquisition and extinction of conditioned hypoalgesic responses in the rat. Q J Exp Psychol B. 1994;47:187–210. [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The Amygdala. Oxford University Press; 2000. pp. 213–288. [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Barad M, Otto M, Southwick S. Combining pharmacotherapy with cognitive behavioral therapy: traditional and new approaches. J Trauma Stress. 2006a;19:571–581. doi: 10.1002/jts.20149. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006b;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Laranja DC, Bromberg E, Roesler R, Schroder N. Pre- or post-training administration of the NMDA receptor blocker MK-801 impairs object recognition memory in rats. Behav Brain Res. 2005;156:139–143. doi: 10.1016/j.bbr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- De Oca BM, DeCola JP, Maren S, Fanselow MS. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J Neurosci. 1998;18:3426–3432. doi: 10.1523/JNEUROSCI.18-09-03426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Delamater AR. Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Q J Exp Psychol B. 2004;57:97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Molecular bases of long-term memories: a question of persistence. Curr Opin Neurobiol. 2002;12:211–216. doi: 10.1016/s0959-4388(02)00305-7. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Mamou CB, Nader K. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. Eur J Neurosci. 2006;24:249–260. doi: 10.1111/j.1460-9568.2006.04907.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fernandez EE. Prefrontocortical dopamine loss in rats delays long-term extinction of contextual conditioned fear, and reduces social interaction without affecting short-term social interaction memory. Neuropsychopharmacology. 2003;28:490–498. doi: 10.1038/sj.npp.1300066. [DOI] [PubMed] [Google Scholar]
- Fischer A, Radulovic M, Schrick C, Sananbenesi F, Godovac-Zimmermann J, Radulovic J. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiol Learn Mem. 2007;87:149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci. 2004;24:1962–1966. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB. Psychosocial therapy for posttraumatic stress disorder. J Clin Psychiatry. 2006;67(Suppl 2):40–45. [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, Bouton ME. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behav Neurosci. 2000;114:227–240. doi: 10.1037//0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med. 2006;73:941–949. [PubMed] [Google Scholar]
- Garcia R, Chang CH, Maren S. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn Mem. 2006;13:14–17. doi: 10.1101/lm.60406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Falls WA, Davis M. Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medial prefrontal cortex in rats. Behav Neurosci. 1997;111:712–726. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Bruchey AK. Extinction memory improvement by the metabolic enhancer methylene blue. Learn Mem. 2004;11:633–640. doi: 10.1101/lm.82404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Hobin JA, Maren S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: mnemonic code or fear bias? Neuron. 2003;40:1013–1022. doi: 10.1016/s0896-6273(03)00728-1. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Gray JA. Effects of septal driving of the hippocampal theta rhythm on resistance to extinction. Physiol Behav. 1972;8:481–490. doi: 10.1016/0031-9384(72)90333-2. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of d-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007a;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007b;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology (Berl) 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [In humans, carriers of the short allele for the serotonin transporter show amygdala hyper-reactivity to emotionally provocative stimuli, and have abnormal connectivity between the prefrontal cortex and amygdala.] [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Dirikx T, Vansteenwegen D, Baeyens F, Van den BO, Eelen P. Reinstatement of fear responses in human aversive conditioning. Behav Res Ther. 2005;43:533–551. doi: 10.1016/j.brat.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Luthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [This is the first study to unequivocally demonstrate that the acquisition of extinction depends on the basolateral amygdala.] [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Pollack MH, Otto MW. Augmentation treatment of psychotherapy for anxiety disorders with D-cycloserine. CNS Drug Rev. 2006;12:208–217. doi: 10.1111/j.1527-3458.2006.00208.x. [In an important replication of Ressler et al. (2004), the authors show that the NMDA receptor partial agonist D-cycloserine facilitates exposure therapy for social anxiety disorder.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan MR, Routtenberg A. Post-translational synaptic protein modification as substrate for long-lasting, remote memory: an initial test. Hippocampus. 2007;17:93–97. doi: 10.1002/hipo.20245. [DOI] [PubMed] [Google Scholar]
- Hugues S, Chessel A, Lena I, Marsault R, Garcia R. Prefrontal infusion of PD098059 immediately after fear extinction training blocks extinction-associated prefrontal synaptic plasticity and decreases prefrontal ERK2 phosphorylation. Synapse. 2006;60:280–287. doi: 10.1002/syn.20291. [DOI] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn Mem. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Vernon J, Giese KP. AlphaCaMKII autophosphorylation contributes to rapid learning but is not necessary for memory. Nat Neurosci. 2005;8:411–412. doi: 10.1038/nn1431. [DOI] [PubMed] [Google Scholar]
- Isiegas C, Park A, Kandel ER, Abel T, Lattal KM. Transgenic inhibition of neuronal protein kinase A activity facilitates fear extinction. J Neurosci. 2006;26:12700–12707. doi: 10.1523/JNEUROSCI.2743-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [This is the first study to examine the effects of stress on morphology of IL neurons, and how these changes affect extinction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami SA, Barad M. Expression of fear extinction in mice depends on increased GABA-A receptor activity in basolateral amygdala. Soc Neurosci Abstr. 2004 Program Number 328.7. [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol Interv. 2006;6:339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Wotjak CT. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Mem. 2004;11:770–786. doi: 10.1101/lm.86104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Park H, Song B, Hong I, Geum D, Shin K, Choi S. Blockade of amygdala metabotropic glutamate receptor subtype 1 impairs fear extinction. Biochem Biophys Res Commun. 2007;355:188–193. doi: 10.1016/j.bbrc.2007.01.125. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem. 2007a;88:48–57. doi: 10.1016/j.nlm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci. 2007b;121:131–139. doi: 10.1037/0735-7044.121.1.131. [These authors demonstrate that extinction in young rats (16 days old) erases fear memory, whereas extinction in older rats (23 days old) forms an inhibitory memory.] [DOI] [PubMed] [Google Scholar]
- Kimble DP. Hippocampus and internal inhibition. Psychol Bull. 1968;70:285–295. doi: 10.1037/h0026470. [DOI] [PubMed] [Google Scholar]
- Kimble DP, Kimble RJ. The effect of hippocampal lesions on extinction and “hypothesis” behavior in rats. Physiol Behav. 1970;5:735–738. doi: 10.1016/0031-9384(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Knapska E, Walasek G, Nikolaev E, Neuhausser-Wespy F, Lipp HP, Kaczmarek L, Werka T. Differential involvement of the central amygdala in appetitive versus aversive learning. Learn Mem. 2006;13:192–200. doi: 10.1101/lm.54706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative Activity of the Brain. University of Chicago Press; 1967. [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. J Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Leu TH, Chang WC, Wang ST, Gean PW. Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci. 2003a;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci. 2003b;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze TS, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao SC, Hsiao YH, Gean PW. Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace. J Neurosci. 2006;26:8892–8899. doi: 10.1523/JNEUROSCI.0365-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn Mem. 2007;14:318–324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Markram K, Lopez Fernandez MA, Abrous DN, Sandi C. Amygdala upregulation of NCAM polysialylation induced by auditory fear conditioning is not required for memory formation, but plays a role in fear extinction. Neurobiol Learn Mem. 2007;87:573–582. doi: 10.1016/j.nlm.2006.11.007. [The polysialylated form of neural cell adhesion molecules impaired extinction learning, whereas cleavage of this molecule in the amygdala enhanced extinction.] [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di M,V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Maruki K, Izaki Y, Akema T, Nomura M. Effects of acetylcholine antagonist injection into the prefrontal cortex on the progress of lever-press extinction in rats. Neurosci Lett. 2003;351:95–98. doi: 10.1016/j.neulet.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Mason ST, Iversen SD. Effects of selective forebrain noradrenaline loss on behavioral inhibition in the rat. J Comp Physiol Psychol. 1977;91:165–173. doi: 10.1037/h0077311. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Projections of the lateral entorhinal cortex to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1997;77:445–459. doi: 10.1016/s0306-4522(96)00478-2. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Hong NS, Craig LA, Holahan MR, Louis M, Muller RU. NMDA-receptor blockade by CPP impairs post-training consolidation of a rapidly acquired spatial representation in rat hippocampus. Eur J Neurosci. 2005;22:1201–1213. doi: 10.1111/j.1460-9568.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Floresco SB. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience. 2007;146:1484–1494. doi: 10.1016/j.neuroscience.2007.03.025. [DOI] [PubMed] [Google Scholar]
- McNally GP. Facilitation of fear extinction by midbrain periaqueductal gray infusions of RB101(S), an inhibitor of enkephalin-degrading enzymes. Behav Neurosci. 2005;119:1672–1677. doi: 10.1037/0735-7044.119.6.1672. [DOI] [PubMed] [Google Scholar]
- McNally GP, Lee BW, Chiem JY, Choi EA. The midbrain periaqueductal gray and fear extinction: opioid receptor subtype and roles of cyclic AMP, protein kinase A, and mitogen-activated protein kinase. Behav Neurosci. 2005;119:1023–1033. doi: 10.1037/0735-7044.119.4.1023. [DOI] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G. Blocking, unblocking, and overexpectation of fear: a role for opioid receptors in the regulation of Pavlovian association formation. Behav Neurosci. 2004a;118:111–120. doi: 10.1037/0735-7044.118.1.111. [DOI] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of pavlovian fear conditioning. J Neurosci. 2004b;24:6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behav Neurosci. 2003;117:1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role of the human dorsal anterior cingulate cortex in expression of learned fear. Biol Psychiatry. 2007a doi: 10.1016/j.biopsych.2007.04.032. in press. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007b doi: 10.1016/j.biopsych.2006.10.011. in press. [A clear demonstration that, in humans, the prefrontal cortex and hippocampus are co-activated during recall of extinction, and that the magnitude of this activation is correlated with the strength of the extinction memory.] [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Amnesia or retrieval deficit? Implications of a molecular approach to the question of reconsolidation. Learn Mem. 2006;13:498–505. doi: 10.1101/lm.304606. [DOI] [PubMed] [Google Scholar]
- Mingote S, de Bruin JP, Feenstra MG. Noradrenaline and dopamine efflux in the prefrontal cortex in relation to appetitive classical conditioning. J Neurosci. 2004;24:2475–2480. doi: 10.1523/JNEUROSCI.4547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav Brain Res. 2003;146:121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci. 2007;121:501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Mueller D, Quirk GJ. Memory for extinction of conditioned fear requires PKA activity in the ventromedial prefrontal cortex, but is only partially dependent on mRNA synthesis. Soc Neurosci Abstr. 2006 Program Number 464.3. [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Muller M, Fendt M. Temporary inactivation of the medial and basolateral amygdala differentially affects TMT-induced fear behavior in rats. Behav Brain Res. 2006;167:57–62. doi: 10.1016/j.bbr.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [A thorough and exhaustive review of the literature on the molecular mechanisms of fear extinction.] [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem. 2001;8:156–163. doi: 10.1101/lm.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Olds J, Disterhoft JF, Segal M, Kornblith CL, Hirsh R. Learning centers of rat brain mapped by measuring latencies of conditioned unit responses. J Neurophysiol. 1972;35:202–219. doi: 10.1152/jn.1972.35.2.202. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212−2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 2006;188:641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. Intrinsic circuitry of the amygdaloid complex: common principles of organization in rats and cats. Trends Neurosci. 1998;21:240–241. doi: 10.1016/s0166-2236(98)01240-5. [DOI] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned Reflexes. Oxford University Press; 1927. [Google Scholar]
- Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pfeiffer UJ, Fendt M. Prefrontal dopamine D4 receptors are involved in encoding fear extinction. Neuroreport. 2006;17:847–850. doi: 10.1097/01.wnr.0000220142.29413.6f. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, Repa JC, Li XF, LeDoux JE. Emotional memory: a search for sites of plasticity. Cold Spring Harb Symp Quant Biol. 1996;61:247–257. [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe A, Haddad RK. Effect of selective hippocampal lesions in the rat on acquisition, performance, and extinction of bar pressing on a fixed ratio schedule. Exp Brain Res. 1968;5:259–266. doi: 10.1007/BF00235901. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process. 1975;1:88–96. [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [The first demonstration that D-cycloserine can facilitate exposure therapy in humans.] [DOI] [PubMed] [Google Scholar]
- Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex enhance renewal of extinguished appetitive Pavlovian responding. Eur J Neurosci. 2007;25:2498–2503. doi: 10.1111/j.1460-9568.2007.05486.x. [DOI] [PubMed] [Google Scholar]
- Rhodes SE, Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;11:611–616. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Farb CR, Bauer EP, LeDoux JE, Schafe GE. Pavlovian fear conditioning regulates Thr286 autophosphorylation of Ca2+/calmodulin-dependent protein kinase II at lateral amygdala synapses. J Neurosci. 2004;24:3281–3288. doi: 10.1523/JNEUROSCI.5303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Lima RH, Medina JH, Izquierdo I, Cammarota M. On the participation of hippocampal p38 mitogen-activated protein kinase in extinction and reacquisition of inhibitory avoidance memory. Neuroscience. 2006;143:15–23. doi: 10.1016/j.neuroscience.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Schwartz AC. Exposure therapy for posttraumatic stress disorder. Am J Psychother. 2002;56:59–75. doi: 10.1176/appi.psychotherapy.2002.56.1.59. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Rekart JL. Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci. 2005;28:12–19. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Sangha S, Scheibenstock A, Lukowiak K. Reconsolidation of a long-term memory in Lymnaea requires new protein and RNA synthesis and the soma of right pedal dorsal 1. J Neurosci. 2003;23:8034–8040. doi: 10.1523/JNEUROSCI.23-22-08034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena DO, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D. Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol. 2000;416:496–508. [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci U S A. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301316. in press. [The first study to demonstrate that NMDA receptors in the basolateral amygdala mediate the acquisition of extinction.] [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179:863–872. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Igaz LM, Coitinho AS, Medina JH, Izquierdo I. Memory extinction requires gene expression in rat hippocampus. Neurobiol Learn Mem. 2003;79:199–203. doi: 10.1016/s1074-7427(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc Natl Acad Sci U S A. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R. Effects of d-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav Neurosci. 2000;114:1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Brooks DC, Bouton ME. The role of the rat hippocampal system in several effects of context in extinction. Behav Neurosci. 1995;109:828–836. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]
- Wittenberg G, Tsien J. An emerging molecular and cellular framework for memory processing by the hippocampus. Trends Neurosci. 2002;25:501. doi: 10.1016/s0166-2236(02)02231-2. [DOI] [PubMed] [Google Scholar]
- Wolpe J. Basic principles and practices of behavior therapy of neuroses. Am J Psychiatry. 1969;125:1242–1247. doi: 10.1176/ajp.125.9.1242. [DOI] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- Wrubel KM, Barrett D, Shumake J, Johnson SE, Gonzalez-Lima F. Methylene blue facilitates the extinction of fear in an animal model of susceptibility to learned helplessness. Neurobiol Learn Mem. 2007;87:209–217. doi: 10.1016/j.nlm.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Ro LS, Wo YY, Lu KT. Glutamate NMDA receptors within the amygdala participate in the modulatory effect of glucocorticoids on extinction of conditioned fear in rats. Neuropsychopharmacology. 2007;32:1042–1051. doi: 10.1038/sj.npp.1301215. [Glucocorticoid receptor agonists were shown to facilitate consolidation of extinction in rats, suggesting clinical application.] [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [Enhancing AMPA receptor signaling in the mPFC facilitated extinction.] [DOI] [PMC free article] [PubMed] [Google Scholar]