Abstract
Age-related hearing loss, presbycusis, can be thought of, in part, as a slow progressive peripheral deafferentation. Previous studies suggest that certain deficits seen in presbycusis may partially result from functional loss of the inhibitory neurotransmitter glycine in dorsal cochlear nucleus (DCN). The present study assessed age-related behavioral gap detection changes and neurochemical changes of postsynaptic glycine receptor (GlyRs) subunits and their anchoring protein gephyrin in fusiform cells of young (7-11 month) and aged (28-33 month) Fischer Brown Norway (FBN) rats. Aged rats showed significantly (20-30 dB) elevated auditory brainstem-evoked response thresholds across all tested frequencies and worse gap detection ability compared to young FBN rats. In situ hybridization and quantitative immunocytochemistry were used to measure GlyR subunit message and protein levels. There were significant age-related increases in the α1 subunit message with significant age-related decreases in α1 subunit protein. Gephyrin message and protein showed significant increases in aged DCN fusiform cells. The pharmacologic consequences of these age-related subunit changes were assessed using [3H] strychnine binding. In support of the age-related decrease of α1 subunit protein levels in DCN, there was a significant age-related decrease in the total number of GlyR binding sites with no significant change in affinity.
These age-related changes may reflect an effort to re-establish a homeostatic balance between excitation and inhibition impacting on DCN fusiform cells by down-regulation of inhibitory function in the face of an age-related loss of peripheral input. Age-related decrease in presynaptic glycine release results in altered subunit composition and this may correlate with loss of temporal coding of the aged fusiform cell in DCN. The previously reported role for gephyrin in retrograde intracellular receptor subunit trafficking could contribute to the α1 decrease in the face of increased message.
Keywords: presbycusis, aging, dorsal cochlear nucleus, auditory, glycine receptor, gephyrin
Age-related hearing loss, presbycusis, is defined as a bilaterally, progressive symmetrical sensorineural hearing loss associated with difficulty in central auditory processing, which affects approximately 30–35 percent of the population between 65 and 75 years of age and 40–50 percent of people older than 75 years (NIDCD report, 2006). The most common type of presbycusis occurs at the high frequencies and is caused by hair cell degeneration at the basal end of the cochlea (Keithley et al., 1982; Spongr et al., 1997; Pichora-Fuller and Souza, 2003) which can be induced/exacerbated by lifetime noise exposure, ototoxic agents and heredity.
As the first nucleus in the central auditory pathway and part of the cochlear nucleus complex, dorsal cochlear nucleus (DCN) is thought to have a role in temporal coding of envelope frequencies, signal detection in noise, echo suppression, and responding to spectral localization cues (Rhode and Greenberg, 1994; Frisina et al., 1994; Joris and Smith, 1998; Spirou et al., 1999; Oertel and Young, 2004; Reiss and Young, 2005). Human studies indicate age-related changes in temporal activity and cortical processing between young and aged subjects with normal hearing (Schneider et al., 1994; Snell, 1997; Ostroff et al., 2003). These data suggested that age-related hearing loss alters temporal response properties in the central auditory system, which may be related to age-related changes regulating excitatory and inhibitory neurotransmission (Tremblay et al., 2002, 2003; Caspary et al., 1995; Palombi and Caspary, 1996). DCN is organized into three layers: a deep layer that primarily receives acoustic nerve inputs; a superficial layer that receives input from non-auditory as well as descending auditory structures forming a cerebellar-like network; and a middle layer containing fusiform, cartwheel and granule cells. Fusiform cells comprise the major output cells of the DCN, projecting primarily to the contralateral inferior colliculus (Beyerl, 1978; Paloff and Usunoff, 1992; Zhang and Oertel, 1994). Fusiform cells receive focused glycinergic input from vertical cells in DCN (Rhode, 1999) and less narrowly tuned inhibitory projections from glycinergic D-multipolar cells in the ventral cochlear nucleus (VCN) (Saint-Marie et al., 1991; Kolston et al., 1992; Doucet et al., 1999). DCN rodent aging studies show decreased glycine immunostaining (Willott et al., 1997) along with an age-related reduction in cochlear nucleus (CN) glycine levels (Banay-Schwartz et al., 1989). Caspary and colleagues (Caspary et al., 2005; Schatteman et al., 2008) showed significant age-related functional changes in the response properties of fusiform cells, suggestive of a selective down regulation of glycinergic inhibitory processing in the DCN.
Glycine is the primary inhibitory neurotransmitter in DCN and glycine receptors (GlyRs) are made up of subunits which form pentameric protein complexes constituting a ligand-gated ion channel permeable to chloride ions (Langosch et al., 1990). The nature of the subunit composition impacts the physiology and the pharmacology of GlyRs (Betz et al., 1999; Legendre, 2001). GlyRs are traditionally believed to be heteromeric, composed of three α and two β subunits (3α2β) (Betz et al., 1999; Legendre, 2001). A recent stoichiometric study using co-expression of wild type α or β subunits with α1β tandem constructs, suggested a 2α3β subunit stoichiometry (Grudzinska et al., 2005). Alpha subunits are able to assemble into functional homomeric GlyRs which may predominant during development (Legendre, 2001). The α1 subunits are widespread in brainstem and spinal cord including the brainstem central auditory pathway (Lynch, 2004). The α2 subunits are not only abundant during embryonic development (Sato et al., 1992) but to some extent persist into adulthood (Danglot et al., 2004). The α3 subunit distribution resembles α1 and is expressed at a lower level during development (Malosio et al., 1991; Lynch, 2004). Gephyrin, a tubulin-binding protein, binds to the cytoplasmic loop of the β subunits and is essential for GlyR clustering at postsynaptic membrane sites (Meier et al., 2000). In addition to age-related changes, inhibitory amino acid receptors have been shown to undergo plastic changes with partial deafferentation (Krenning et al., 1998; Caspary et al., 1999; Fukuoka et al., 1998). The present study examined age-related changes in the makeup and pharmacology of the GlyR. Fusiform cell glycine receptor subunits and gephyrin message and protein levels were assessed. Quantitative receptor binding autoradiography was used to assess receptor function.
Method
Subjects
Subjects were male young adult (7-11 months of age; 40) and aged (28-33 months of age; 36) Fischer Brown Norway (FBN) rats from Harlan Sprague-Dawley Inc. (Indianapolis, IN), under a contract through the Office of Biological Resources of the National Institute on Aging. Rats were generally housed at Southern Illinois University (SIU) and used under protocols approved by the SIU Laboratory Animal Care Committee.
Auditory brainstem response
The degree of age-related hearing loss was measured by auditory brainstem response (ABR) for both left and right ears. ABR testing was conducted in a double-wall sound-attenuation chamber using an Intelligent Hearing Systems (Miami, FL) high-frequency system. Subdermal electrodes were inserted posterior to each pinna and an apex reference electrode was placed at the dorsal cranial midline, with the ground electrode in the animal's hind leg. ABR thresholds were obtained for clicks and 5 msec tone bursts presented at a rate of 50/sec. Tone bursts (4, 10, 16, 20, 24 and 32 kHz) were gated using a Blackman envelope (2.5 msec rise/decay, 0 msec plateau). Evoked potentials were averaged over 1024 sweeps. Amplifier gain was set at 200X and waveforms were filtered using a 100-3000 Hz bandpass filter.
Gap detection testing
Testing was conducted using the Kinder Scientific startle reflex hardware and software customized for this application by the manufacturer (Kinder Behavioral Testing Systems, Poway, CA). Sixteen young and 15 aged FBN rats were tested using the equipment with similar procedures described to Turner et al. (2006). Briefly, animals were tested inside a sound-attenuating box with background noise presented through one speaker (Vifa XT25TG30-04) and the startle stimuli presented through a second speaker (Powerline CTS KSN-1005) mounted in the ceiling of the testing chamber, 15 cm above the animal. A clear polycarbonate animal holder with slits cut for sound passage was suspended above the floor allowing the animal to turn around freely while minimizing excessive movement. A piezo transducer plate was attached to the animal holder and provided a measure of the startle force applied by the animal. An adjustable-height roof was set to a level so that the animal was unable to rear up; a behavior that adds variability to the startle response. The background signal in the chamber consisted of 75 dB SPL broadband noise (BBN) calibrated using a cloth model rat with a Bruel and Kjaer Pulse System via a ½ inch free field microphone (Bruel and Kjaer Model 4191-A). Baseline noise levels in the test chamber (with background test noise turned off) were measured below 20 dB SPL in the 4-40 kHz range. The session began with a 2-min acclimation period followed by 2 startle-only trials (noise burst at 115 dB SPL, 20 msec in duration) to habituate the startle response to a more stable baseline. These 2 data points were not used in the analysis. The remainder of the session consisted of additional startle-only trials pseudo-randomly mixed with gap trials. Gap trials consisted of startle stimuli preceded by 100 ms by one of eight different gaps (10 trials each) of variable duration (1, 2, 3, 4, 5, 10, 15 and 50 ms) and were shaped with a 0.1 ms rise/ fall time. The presence of a detectable gap in the background inhibits the subsequent startle reflex amplitude in a reliable manner. If the animal cannot detect a gap, startle reflex amplitude is no different than the control (no gap, startle only) condition. Startle testing does not cause a temporary or permanent threshold shift in mice (Willott and Turner, 1999). Using this behavioral equipment and stimulus settings, pilot experiments revealed no pre-to-post ABR threshold shifts either immediately or 1 week post startle testing (Turner et al., 2006).
Frequency division of the DCN
Previous studies using c-fos expression and electrophysiological techniques delineated the tonotopicity gradient along the mediolateral axis of the DCN in rats and cats. Low frequencies were found laterally with high frequencies represented medially (Rose et al., 1959; Rose, 1960; Yajima and Hayashi, 1989; Rouiller et al., 1992; Friauf, 1992; Saint Marie et al., 1999). Based on these data, the DCN was divided into three parts, a most medial third, a middle third and a most lateral third of DCN, representing high, middle and lower frequency areas respectively (Fig. 1; Ryan et al., 1988; Yajima and Hayashi, 1989; Saint Marie et al., 1999).
Figure 1.
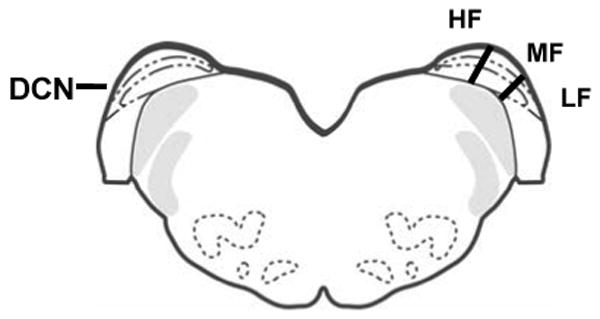
Cartoon of frontal section through rat brainstem showing approximate tonotopic frequency divisions of DCN used in the present study. HF = high frequency; MF = middle frequency; LF = low frequency.
In situ hybridization and autoradiography
Thirteen rats (seven young adult and six aged) were used for in situ hybridization experiments; each replication included one young and one aged animal for processing. Animals were decapitated and their brains were rapidly removed and immediately frozen on powdered dry ice and stored at −80°C until use. Serial coronal sections (16-μm thick) from bregma −10.52 to −11.6 were cut at −20°C on a cryostat (Leica CM1850 Microsystems, Nussloch GmbH, Germany) and thaw-mounted on Superfrost/Plus slides (Fisher Scientific, St. Louis, MO, USA). Tissue preparation and hybridization procedures were performed as described previously (Milbrandt et al., 1997; Krenning et al., 1998; Ling et al., 2005). In situ hybridization was performed with 3′ 35S-labelled oligonucleotide probes designed to detect GlyR subunits (α1-3) and gephyrin. Oligonucleotide probe sequences are detailed in Table 1.
Table 1.
Oligonucleotide probe sequences of GlyR α subunits and gephyrin for in situ hybridization
| Probes | Sequence 5'- 3' |
|---|---|
| GlyR α1 (Malosio et al., 1991) | 5'-GTT GGC ACC CTT GAC AGA GAT GCC ATC CTT GGC TTG CAG GCA GGC-3' |
| GlyR α2 (Malosio et al., 1991) | 5'-CTT TTG GGG GTT GCG GAA GTG GGT TGG CAG GTG TAG CCT TGA CAG-3' |
| Gly Rα3 (Malosio et al., 1991) | 5'-GGC AGT GAA GCT GAG CCG ACT CTC CCT CAC CTC ATC ATC CGT GTC-3' |
| Gephyrin (Kirsch et al., 1993) | 5'-CCT TCA ATA TCC AAA GTT GCA AAT GTT GTT GGC AAG CCT GGC TTC-3' |
Finally, sections were dipped in Kodak NTB-2 photographic emulsion (VWR, West Chester, PA, USA) and stored at 4°C for 1 or 2 weeks (depending on the probe and labeling). Sections were developed in Kodak D-19 (Eastman-Kodak, Rochester, NY, USA) and counterstained with thionin. No labeling was observed in the two extra sections processed with control hybridization buffer using an unlabeled oligonucleotide probe.
Autoradiographs (corresponding to 12 or 14 sections per animal) were digitized using a CCD camera. Hybridization signals (silver grains) were quantified with a computerized image analysis system (CoolSnap monochrome digital camera interfaced with a Nikon Microphot light microscope and coupled to an MCID imaging system with Elite 6.0 software). An average of 15-30 fusiform cells was measured from each section. The numbers of grains over the fusiform cell soma were expressed as density of silver grains/100 μm2, and as such were not influenced by changes in neuron size and/or neuronal shape. Density values were corrected by subtracting background labeling obtained from five random areas located off the tissue sections of each slide (Milbrandt et al., 1997; Krenning et al., 1998).
GlyR subunits and gephyrin protein levels
Immunocytochemistry
The procedure used for quantitative immunocytochemistry was based on those published by Ling et al. (2005) and Abbott et al. (1994). Briefly, four rats from each young and aged group were anesthetized with a mixture of ketamine (105 mg/kg body wt. i.p.) and xylazine (7 mg/kg body wt. i.p.) and transcardially perfused with 500 ml of fixative containing 4% paraformaldehyde in PBS buffer (pH 7.4). The brains were removed, dehydrated in 20% sucrose and stored at −80°C until use.
Cryostat frozen sections (16 μm) were cut and blocked in 1.5% normal donkey serum in 0.1 M PBS for 30 min, then transferred to purified primary antibody diluted in blocking buffer (GlyR α1: polyclonal anti-goat primary antibody, 1:100; GlyR α2: polyclonal anti-rabbit primary antibody, 1:100; GlyR α3: polyclonal anti-rabbit primary antibody, 1:200; Gephyrin: polyclonal anti-rabbit primary antibody, 1:100; all from Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA). For each antibody, one extra slide was processed with the primary antibody omitted, as a control. In these sections, only background staining was observed. Sections were incubated for 1.5 hrs and then at 4°C overnight with agitation. ABC kits (Vector Laboratories, Burlingame, CA, USA) were used with a secondary biotinylated antibody. Sections were visualized by exposure to 3,3′-diaminobenzidine (DAB) in PBS for 1-3 min. Images were captured using a CoolSnap camera interfaced with a Leitz Diaplan microscope coupled to an ImagePro Plus imaging system. Images were captured at 10X including a calibration micrograph standard and each section contained an average of 30-40 fusiform cells. Relative optical density (ROD) for each DCN fusiform cell soma was measured using NIH Scion Image. ROD is an average measure of density/unit area, therefore, independent of age-related changes in neuronal size or shape. Estimates of protein expression levels were based on the findings of Huang et al. (1996). Using an antibody for a GABAA receptor β2/3 subunit, Huang et al. (1996) found a strong, positive linear relationship between immunostaining intensity and antigen concentration estimating that this method could detect protein level changes of less than 10% reflected in immunostaining intensity in brain sections. Notably, fusiform cell bodies are oriented more horizontally in the high frequency region of DCN than in the middle and low frequency regions. All values were corrected by subtracting background obtained from the measurements of negatively stained strial fibers within the deep DCN.
[3H]Strychnine binding
Four young and four aged rats were decapitated and the brains were stored at −80°C until sectioned. Strychnine binding sites were examined using a modified protocol of Milbrandt and Caspary (1995). Tissue sections (16 μm) were pre-washed twice in 50 mM Na-K phosphate buffer for 25 min and incubated with 0, 2, 4, 8, 12, 16, 32 nM [3H] strychnine (23–25 Ci/mmol, New England Nuclear, Boston, MA, USA) in 50 mM Na-K buffer for 20 min. Non-specific binding was determined by incubating companion slides with [3H] strychnine and 10 mM glycine. Incubation was terminated by a quick dip in 50 mM Na-K phosphate buffer followed by a 2 min wash in the same buffer twice and one quick dip in distilled water, then air dried. Sections were apposed to tritium sensitive phosphor screens (PerkinElmer Life and Analytical Sciences, Waltham, MA) with calibrated autoradiographic [3H]-Tritium standard (American Radiolabeled Chemicals Inc. St. Louis, MO, USA) for 3 days. Images were scanned using a Cyclone Storage Phosphor System and quantified by using OptiQuant image analysis software (version 3.10). Average optical density was determined by taking multiple density readings from the area of interest. There were no significant density differences between low, high and middle frequency regions, therefore, the combined DCN results are presented. The commercial standards have been previously calibrated against known amounts of tritium and protein by the American Radiolabeled Chemicals Inc. Use of these standards allows for the conversion of areal optical density to fmol/mg protein. Saturation data were determined by using a Scatchard analysis. Kd and Bmax values were determined for each animal and averaged for each age group. Images were imported and analyzed in GraphPad Prism (version 4.0). Specific binding was determined by subtracting nonspecific binding from total binding.
For better resolution of images, the slides were apposed to Tritium Hyperfilm (GE Healthcare, Piscataway, NJ, USA) for 4-5 weeks at 4°C. The films were later developed in Kodak D19 (Eastman-Kodak, Rochester, NY, USA) for 4 minutes at room temperature, stopped in 1% acetic acid, fixed in Kodak rapid fixer, washed, and air dried. Films were placed on a light box, and digitized images were captured by using a CCD video camera.
Statistical analysis
Data were imported to Excel spreadsheets (Excel; Microsoft Corp, Redmond, WA, USA) and assessed for integrity and distributional characteristics. Descriptive statistics and graphical depictions were used to determine if transformations and/or adjustments were necessary to meet the assumptions of the inferential statistical procedures employed. Bonferroni corrections were used to ensure that experiment-wise Type I error rates did not exceed the stated alpha level p<0.05. Statistical procedures were implemented within SPSS 14.0 (SPSS, Chicago, IL, USA). Statistical differences in ABR's between young and aged groups were determined by a mixed effects analysis of variance (ANOVA) with repeated measures. For each gap duration, data from gap detection yielded a single value expressed as a percentage (mean response in gap trials/ mean response in startle-only trials). ANOVAs were used to compare differences in gap detection between age groups. Differences between age groups for in situ and immunochemistry protein data were evaluated using factorial ANOVAs.
Results
1. Age-related threshold shifts
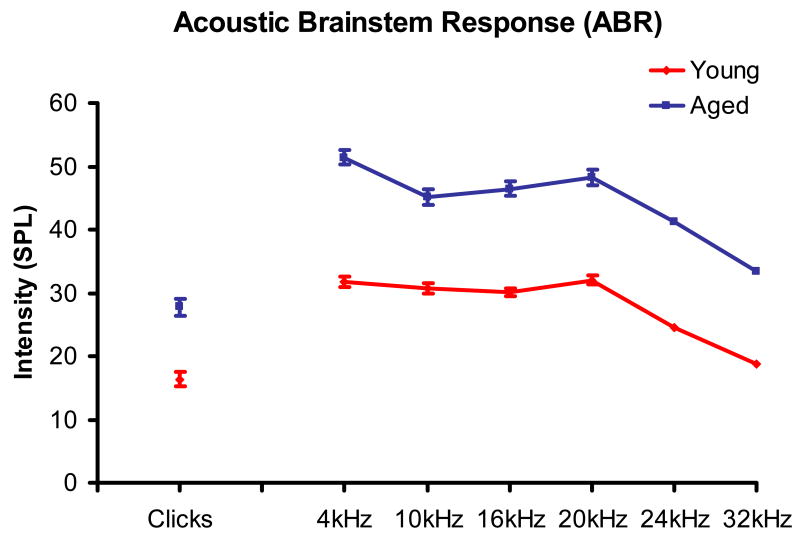
Data from 38 FBN rats are presented in this study. Clicks and six tone bursts (4, 10, 16, 20, 24 and 32 kHz) were used to obtain ABR thresholds. Young rat thresholds (n = 21) were measured at 7-8 months while aged rats (n = 17) were 28-29 months old (Fig. 2). Aged animals showed significantly elevated thresholds for all tones tested from 4-32 kHz and for clicks (p < 0.02) (Fig. 2) and these age-related threshold shifts were greatest at the lowest frequency measured, 4 kHz, likely reflecting previously described apical pattern of outer hair cell loss (Caspary and Turner, 2005).
Figure 2.
Age-related ABR threshold shift for young (7-8 months, n = 21) and aged (28-29 months, n = 17) FBN rats. Mean ABR thresholds are shown for six frequencies and clicks. Aged FBN rats showed significant threshold elevation across all pure tone frequencies and clicks. Error bars at 24 and 32 kHz are within the line width (p < 0.05).
2. Gap detection in young and aged FBN rats
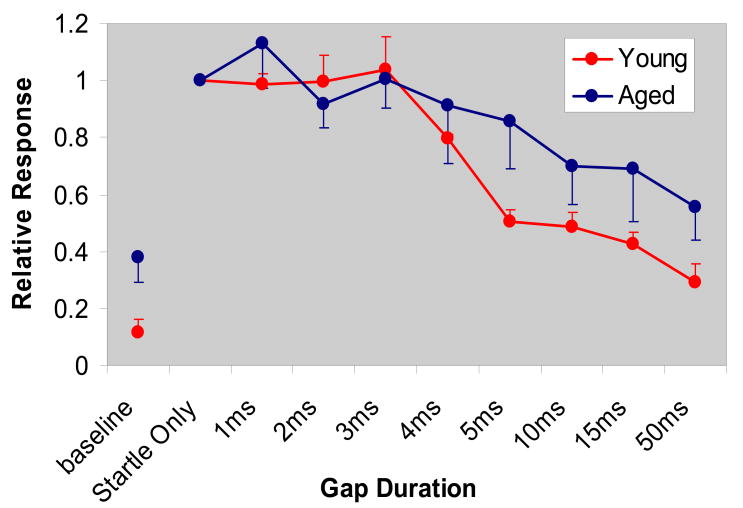
Baseline responses reflect the force applied by animals from normal movement when no startle stimulus is present (Fig. 3). For gap durations above 3 ms (gaps, which normal animals would be expected to hear), detection was significantly worse in aged FBN rats [Fig. 3. F (1, 58) = 5.07, p = 0.02]. Threshold, defined as a 20% reduction of baseline startle reflex, was approximately 4 ms in young rats and close to 10 ms in aged rats. Aged rats also exhibited a shallower slope as a function of gap width than young animals. Whereas young animals showed marked improvement in gap detection between 3-50 ms, improvements in aged animals across these same gap widths were more flat. Aged animals also exhibited greater variability in their gap response evidenced by the larger standard error bars.
Figure 3.
Response to various gap durations relative to startle only control condition with no gap present. Baseline score measures force applied by animals from normal movement when no startle stimulus is present. For gap durations above 3 ms, detection was significantly worse in aged FBN rats, p = 0.02. Aging appears to affect both the threshold for gap detection as well as the maximum level of inhibition possible. Threshold, defined as a 20% reduction from baseline startle reflex, was 4 ms in young and approximately 10 ms in aged FBN rats (n = 16, 15). Even for long gap latencies aged animals were not able to inhibit the startle reflex to the same extent as the young.
3. Age-related fusiform cell GlyR subunit and gephyrin message changes
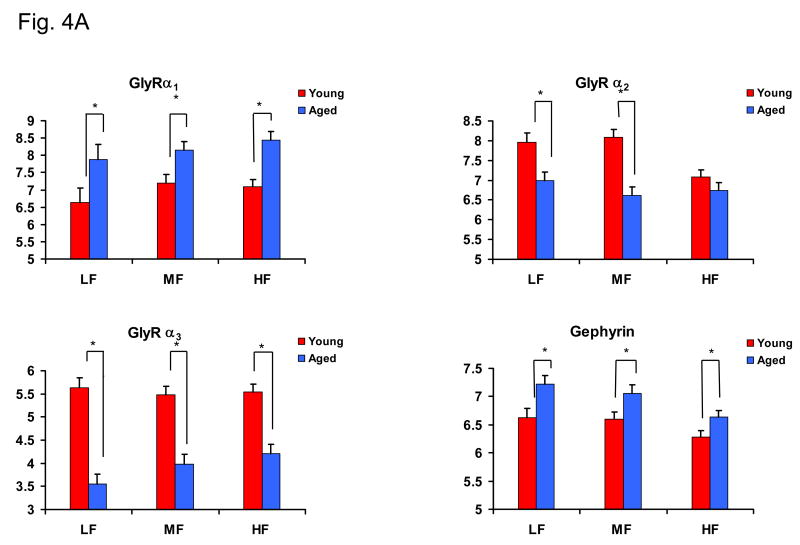
Distinct labeling indicating mRNA expression of GlyR α1–3 subunits were observed over DCN fusiform cells for all subjects (Fig. 4A, B; Table 2). Significant age-related increases in α1 message were found across the entire DCN frequency range (low, middle and high) (p < 0.05, Fig. 4A, B). Alpha2 message levels showed age-related decreases over fusiform cells within the low and middle frequency regions of DCN with no significant change observed over fusiform cells in the high frequency region (p < 0.05, Fig. 4A). Significant age-related decreases in α3 subunit message levels were observed throughout the fusiform cell layer (Fig. 4A). The scaffolding protein gephyrin, which is thought to anchor postsynaptic GlyRs synaptic clusters through its association with GlyR β subunits, showed significant age-related increases in message over fusiform cells across all frequency regions of the DCN (Fig. 4A, B).
Figure 4.

Age-related changes in DCN fusiform cell mRNA levels for GlyRs α1-3, and the anchoring protein gephyrin in the FBN rat. 4A) Bar graphs indicated differential age-related changes for GlyRs α1-3, and gephyrin messages measured by mean silver grain density/100μm2 in DCN fusiform cells. Aged rats showed a significant increase in GlyR α1 subunit message and gephyrin. Message levels for GlyR α2 significantly decreased in middle and low frequency areas in aged DCN, while GlyR α3 displayed an age-related decrease across all DCN frequency areas (*: p < 0.05, n = 7, 6). Error bars represent the standard error of the mean. LF = low frequency; MF = middle frequency; HF = high frequency. Clusters of silver grains represent hybridization of transcripts of GlyRs α1-3, and gephyrin with 35S-labeled selective oligonucleotide probes. 4B) Representative in situ hybridization images of stained fusiform cells from high frequency areas of DCN. Arrows point to fusiform cell soma. D=dorsal, M=medial, Scale bar = 5μm.
Table 2.
Age-related message and protein changes of GlyR α1-3 and gephyrin in DCNs of FBN rats.
| mRNA | α1 | α2 | α3 | gephyrin | |
| HF | ↑ | NS | ↓ | ↑ | |
| MF | ↑ | ↓ | ↓ | ↑ | |
| LF | ↑ | ↓ | ↓ | ↑ | |
| Protein | α1 | α2 | α3 | gephyrin | |
| HF | ↓ | ↓ | NS | ↑ | |
| MF | ↓ | ↓ | NS | ↑ | |
| LF | NS | NS | NS | NS |
Note: Arrow directions indicate significant up or down shift in aged group relative to young FBN rats. NS means no significant change.
4. Age-related fusiform cell GlyR subunit and gephyrin protein changes
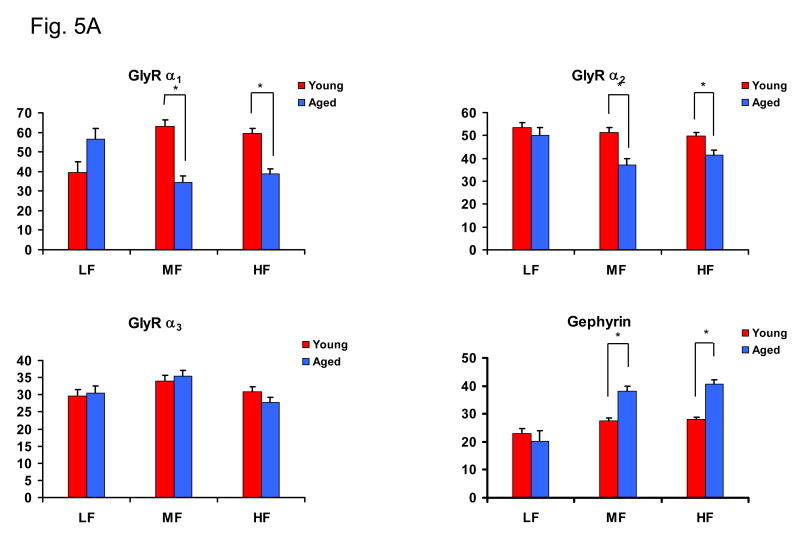
Age-related subunit protein changes were not necessarily consistent with, and in some cases dramatically different from, observed subunit message changes. GlyR α1 subunit immunopositive cells were evenly distributed throughout the middle layer of DCN (Fig. 5A, B). In contrast to the observed age-related message increases, there were significant age-related decreases in the relative optical density (ROD) of the GlyR subunit α1 immunolabeling in the middle and high frequency areas of DCN (p < 0.05, Fig. 5A, B). GlyR α2 subunit protein levels showed significant age-related decreases in middle and high frequency areas of DCN (p < 0.05 Fig. 5A). No significant GlyR α3 subunit protein changes were observed across DCN fusiform cells (Fig. 5A). Consistent with increased message changes, significant age-related increases in levels of the GlyR anchoring protein, gephyrin, was observed in middle and high frequency regions (p < 0.05, Fig. 5A, B).
Figure 5.
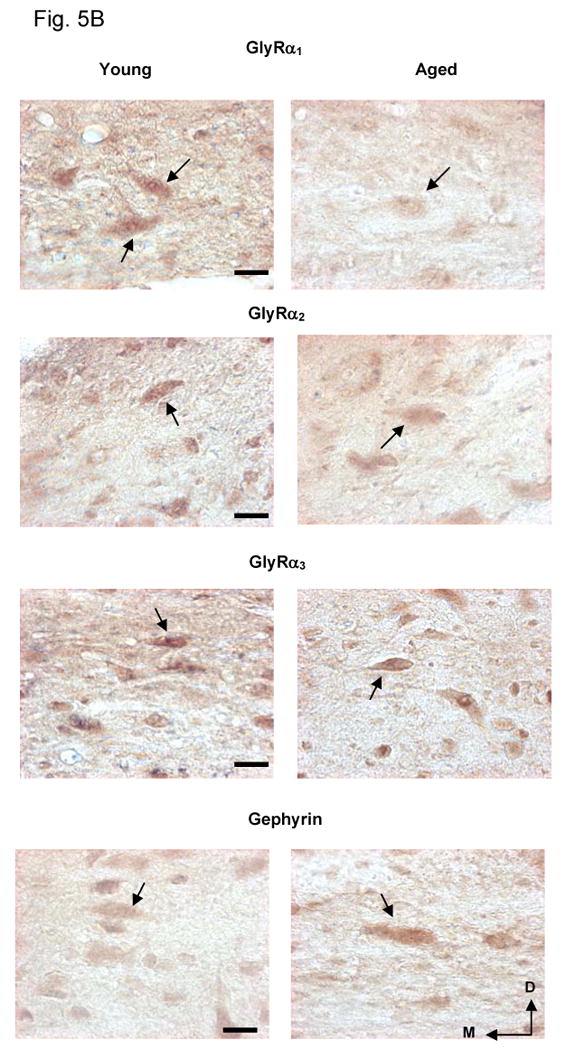
Age-related protein changes in DCN fusiform cells for GlyRs α1-3, and anchoring protein gephyrin in FBN rat. 5A) Relative optical density (ROD) over fusiform cells for GlyR α1-3, and gephyrin in young and aged rats. GlyR α1 and α2 subunit proteins showed significant age-related decreases in HF and MF areas of DCN while no significant age-related α3 subunit changes were observed. Gephyrin showed a significant age-related increases in HF and MF areas of DCN (*: p < 0.05, n = 4, 4). Error bars represent standard error of the mean. LF = low frequency; MF = middle frequency; HF = high frequency. 5B) Representative images of immuno-stained fusiform cells from high frequency area in DCN (40× magnification). Arrows point to fusiform cell somas. Images were captured using exacting parallel conditions and are representative of the quantitative data. Arrows point to fusiform cell soma. D=dorsal, M=medial, Scale bar = 5μm.
5. [3H] Strychnine binding
The integrity and pharmacology of GlyRs in the DCN of young and aged animals were assessed by a GlyR binding saturation analysis using the α1 subunit selective antagonist strychnine (Legendre, 2001; Kirsch, 2006). Levels of [3H] strychnine binding were high throughout the young adult DCN and particularly pronounced in the middle (fusiform) layer of DCN (Fig. 6B). Consistent with the previous data from Fischer 344 rats (Milbrandt and Caspary, 1995), no strychnine binding was observed in cerebellum. Saturation analysis (Fig. 6A) showed specific [3H] strychnine binding across a series of concentrations in young and aged DCN. Scatchard analyses revealed linear strychnine binding for both the young and aged group, reflecting single affinity binding sites. A significant age-related decrease in [3H] strychnine binding was observed in DCN (p < 0.05) of aged rats (33 mos) compared with young rats (11 mos; Fig. 6A). The total number of binding sites (Bmax) in young rats was higher than aged rats when the Bmax values were analyzed based on protein concentration. Scatchard plots obtained from saturation analysis revealed a Bmax = 3746 fmol/mg protein for the young rats and Bmax = 2619 fmol/mg protein for aged rats. No age-related change in the strychnine binding affinity to fusiform cell GlyRs was observed as indicated by the lack of change in the equilibrium dissociation constant (Kd). Kd was calculated from saturation isotherms of young and aged group data and showed no significant age-related slope change (Fig. 6A).
Figure 6.
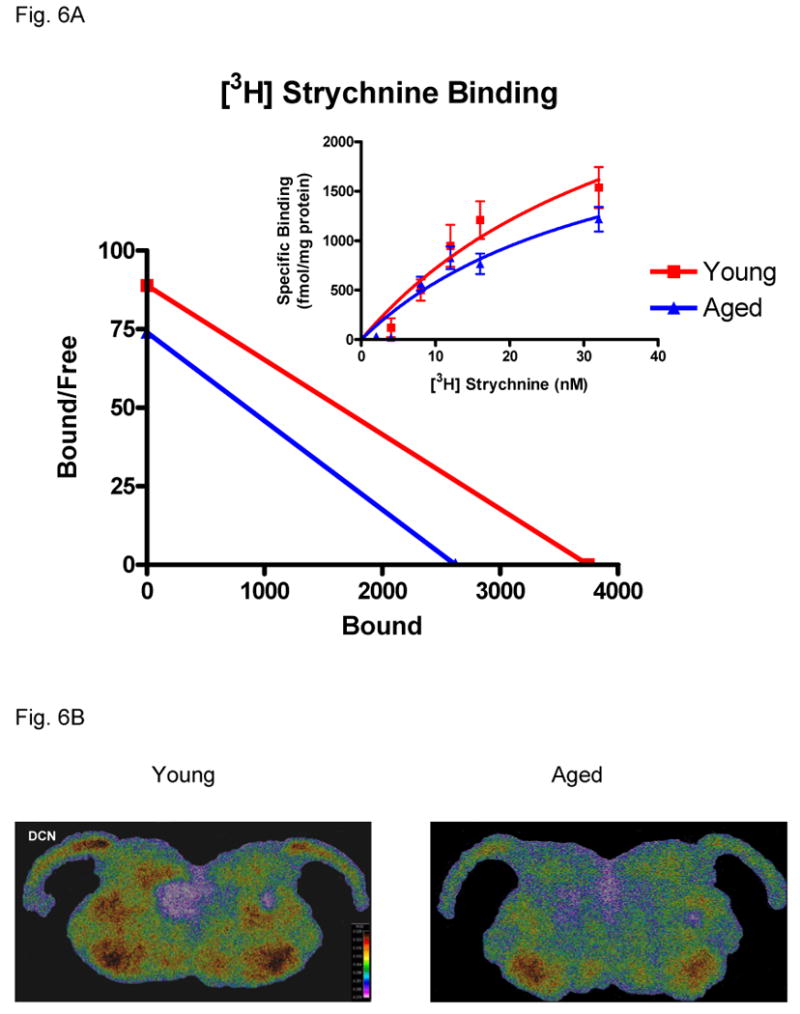
Age-related changes in strychnine binding between young and aged FBN rats. 6A) Scatchard plots of [3H] strychnine binding in DCN of young and aged groups. A significant age-related loss of strychnine binding sites (X intercept = Bmax; slope = Kd) is seen in aged FBN rats with no statistically significant change in affinity (p < 0.05, n = 4, 4). 6B) Color enhanced digitized images showing strychnine binding changes at 32 nM in DCN between young and aged FBN rats. The warm colors indicate higher levels of binding.
Discussion
In the present study, aged FBN rats exhibited significantly worse gap detection suggesting age-related deficits in temporal processing. The neurochemistry data showed age-related decreases of GlyR α1 protein level over fusiform cells in DCN, which was confirmed by the reduction of binding sites in aged animals. The present studies revealed disconnect between age-related GlyR α1 message and protein changes.
Age-related hypoxia and/or ischemia in the cochlea can cause excess release of glutamate leading to excitotoxicity which could cause age-related loss of auditory inputs triggering activity-dependent pre- and postsynaptic plastic changes in the central auditory pathway (Pujol et al., 1990; Puel et al., 2002; Mazurek et al., 2006). These changes may include altered neurotransmitter release, receptor composition (seen as receptor subunit changes) and modifications in receptor intracellular trafficking, insertion or location (Milbrandt and Caspary, 1995; Magnusson et al., 2002; Vela et al., 2003; Rissman et al., 2006, 2007; Caspary et al., 2008). Present findings support an age-related loss of normal adult fusiform cell glycinergic inhibitory neurotransmission due to altered postsynaptic receptor subunit number and/or composition, location and/or receptor function in DCN.
1. Age-related ABR threshold shifts
Consistent with previous findings (Caspary and Turner, 2005; Caspary et al., 2005) for FBN rats, aged FBN rats displayed significant, 20-30 dB, threshold shifts across all tested frequencies with the largest shift at the lowest frequency (4 kHz). The greater ABR threshold elevations are likely related to age-induced cochlear hair cell loss or dysfunction. Age-related hair cell loss has been confirmed in mice, rats, guinea pigs and humans (Nadol, 1979; Keithley and Feldman, 1982; Keithley et al., 1992; Ingham et al., 1999; Francis et al., 2003; Turner and Caspary, 2005). The spiral ganglion neurons (SGNs) of the auditory nerve have also been shown to degenerate with age (Otte et al., 1978; Keithley and Feldman, 1979; Keithley and Ryan, 1989, Keithley and Croskrey, 1990; Keithely et al., 1992; Dazert et al., 1996; Spoendlin and Schrott, 1988, Schrott et al., 1990; Stamataki et al., 2006). Keithly et al. (1992) reported that the greatest age-related SGN loss was found at cochlear apical turns of Brown Norway rats, consistent with age-related apical turn outer hair cell loss in FBN rats (Caspary and Turner, 2005). Recent research by Kujawa et al. (2008) reported adult mice with hearing recovery 16 weeks after sound exposure showed no loss of hair cells but significant loss of SGNs suggesting SGN levels do not necessarily reflect hair cell levels.
2. Age-related gap detection testing
Age-related changes of gap detection has been tested using humans, mice and gerbils (Schneider et al., 1994; Snell, 1997; Walton et al., 1997; Syka et al., 2002; Allen et al., 2003; Hamann et al., 2004). In our study, aged FBN rats exhibited significantly worse detection at gap durations above 3 ms (Fig. 3). Even with long gap latencies (50 ms), aged animals were unable to inhibit the startle reflex to the same extent as young animals. Recent research by our group and others have suggested that age-related poor gap detection may be related to the loss of temporal coding of the aged fusiform cell in DCN, which may be due to less tonic glycinergic inhibition on fusiform cells in aged animals (Banay-Schwartz et al., 1989; Willott et al., 1997; Caspary et al., 2005, 2006; Schatteman et al., 2008).
3. Age-related GlyR subunits message and protein changes
Age-related GlyR subunit protein data from the present study is consistent with previous GlyR binding studies, but reveals a striking disconnect between DCN age-related α1 subunit message and protein changes. In the present study, there was a significant age-related increase in the α1 subunit message expression across all three frequency regions of DCN, similar to that described for a preliminary Fischer 344 rat aging study (unpublished data), however protein level changes have not previously been examined. Significant age-related decreases in GlyR α1 protein with a significant increase in GlyR α1 message in fusiform cells was found in both high and middle frequency regions of DCN (Figs. 4A, 5A). This discordance between age-related inhibitory amino acid receptor subunit message and protein has been reported in different brain regions. There was a significant decrease in mRNA expression in the GABAA receptor α1 subunit without changes in protein expression or in [3H] zolpidem binding sites in cerebellum (Gutiérrez et al., 1997). Present findings suggest altered steps of receptor biosynthesis during aging, which may be related to functional changes in inhibitory transmission. The age-related loss of DCN GlyR subunit α1 in aged rats is likely responsible for the altered response properties of aged fusiform cells (Caspary et al., 2005; Schatteman et al., 2008) resulting from impaired glycinergic transmission onto fusiform cells. It is reasonable to speculate that there would be an abnormal age-related transcription mechanism and/or translational regulatory dysfunction. The previous findings of an age-related reduction of glycine tissue levels (Banay-Schwartz et al., 1989) suggest an age-related reduction in the presynaptic release of glycine onto postsynaptic GlyRs. This could result in a compensatory upregulation of the transcription machinery (Banay-Schwartz et al., 1989). In turn, this would result in more α1 mRNA. Age-related protein levels may not only correlate with synthesis, but possibly with post-translational protein modifications and protein degradation rates (Rattan et al., 1992; Rattan, 1996; Ryazanov and Nefsky, 2002). There is evidence that activities associated with proteasome and lysosomal pathways of protein degradation are reduced in senescent cells, and this defect may cause an intracellular accumulation of abnormal proteins (Dice, 1989; Cuervo and Dice, 2000; Friguet et al., 2000; Keller et al., 2000; Massey et al., 2006; Kiffin et al., 2007). Age-related deregulation could occur on the degradative side with changes in transport to endosomes or altered ubiquitin function. Abnormally functioning receptors might be removed from the membrane surface via endocytosis and internalized. An in vitro study by Huang et al. (2007) demonstrated that the endocytosis of homomeric α1 GlyRs was regulated by protein kinase C (PKC) and previous evidence showed that the quantity of activated PKC or their cellular distribution was deficient within aging cortex or hippocampus (Battaini et al., 1990, Battaini and Pascale, 1995, 2005; Colombo et al., 1997, Colombo and Gallagher, 2002; Pascale et al., 2007). It is possible that newly composed homomeric GlyRs in aged animals may not be effectively endocytosed, for aged animals, due to age-related functional deficit of PKC, which may reduce glycine release in DCN. These factors may ameliorate pathological symptoms of age-related hearing loss.
An in vitro study by Levi et al. (1998) showed strychnine induced GlyR inactivity led to a significant decrease in the number of cell surface receptor clusters on cultured spinal cord neurons. This suggested that an age-related loss of glycinergic inhibition could induce a change/redistribution of postsynaptic membrane GlyRs. The exact relationship between GlyR subunit changes and neural function will require electrophysiological experiments to characterize receptor function and electron microscopy to reveal the subcellular distribution of receptor subunits. Unlike the age-related discordance between GlyR α1 subunit message, protein levels and receptor binding in DCN, in aged anteroventral cochlear nucleus (AVCN), GlyR α1 subunit message and strychnine binding were reduced (Milbrandt and Caspary, 1995; Krenning et al., 1998). This age-related difference between AVCN and DCN is interesting since vertical cells provide a portion of the glycinergic input to both of these regions (Wickesberg and Oertel, 1988).
4. Age-related strychnine binding change
The significant age-related decreases of GlyR α1 and α2 protein staining of fusiform cells in middle and high frequency areas of DCN may correlate directly with the loss of strychnine binding sites (Fig. 6), consistent with previous rat and mouse aging studies (Frostholm and Rotter, 1985, 1986; Glendenning and Baker, 1988; Milbrandt and Caspary, 1995). Strychnine receptor binding is thought to be α1 sensitive and binds to the interface between α and β subunits (Young and Snyder, 1973, 1974; Betz et al., 1994; Schmieden and Betz, 1995; Legendre, 2001; Grudzinska et al., 2005). Thus, strychnine binding depends on the presence of these two subunits (Milbrandt and Caspary, 1995; Krenning et al., 1998). Consistent with Milbrandt and Caspary (1995), the present study finds significant age-related decreases in Bmax with little or no change in Kd which supports an age-related loss in the number of functional membrane receptors rather than changes in GlyR subunit composition.
Immunocytochemistry detects both assembled receptor subunits and non-assembled cytoplasmic subunits that are in the process of synthesis or degradation. High-affinity strychnine binding labels only fully assembled and functional receptors likely concentrated at synaptic sites. More specific changes regarding the number of surface receptors, their trafficking or subsynaptic localization remains to be investigated. Age-related pharmacologic receptor changes could offer a new target for treatment of presbycusis.
5. Age-related gephyrin change
The anchoring protein at glycinergic synapses, gephyrin, binds GlyR via the cytoplasmic loop of the β subunit (Kneussel et al., 1999; Kneussel and Betz, 2000; Moss and Smart, 2001) and tubulin via a tubulin-binding domain to stabilize postsynaptic GlyRs on the membrane (Kneussel et al., 1999; Kneussel and Betz, 2000; Kneussel, 2005). The present study finds that gephyrin up-regulation was reflected at the transcriptional level, as well as the translational and/or post-translational level in the high and middle frequency regions of DCN. Kirsch and Betz (1998) indicated membrane GlyR activation was required for gephyrin clustering to occur beneath developing postsynaptic sites. It is likely that age-related loss of SGNs results in decreased vertical cell excitation, which in turn would result in less glycine being released onto fusiform cells. This could explain, in part, the disinhibition observed in previous physiologic studies (Brozoski et al., 2002; Caspary et al., 2005). Previously described age-related loss of DCN glycine levels (Banay-Schawatz et al., 1989; Willott et al., 1997) suggests that postsynaptic GlyRs on fusiform cell membranes may not fully activate due to the insufficient glycine neurotransmitter in the synaptic cleft. This could underpin the altered receptor composition and possibly alter gephyrin intracellular aggregation/localization. Gephyrin could be involved in intracellular GlyR anterograde or retrograde transport (Maas et al., 2006; Kneussel and Loebrich, 2007; Fritschy et al., 2008). Maas et al (2006) demonstrated that loss of glycinergic inhibition caused removal of GlyRs from synaptic membranes by the gephyrin transport complex. Further work will be required to reveal the multiple roles of gephyrin in this aging system.
6. Age-related functional interpretation
Age-related hearing loss frequently results in a loss in the ability to discriminate speech signals, especially in noise. An age-related loss of glycinergic inhibition in DCN has been proposed as a possible cause (Milbrandt and Caspary, 1995; Krenning et al., 1998; Brozoski et al., 2002; Caspary et al., 2005, 2006; Bauer et al., 2008). As the main output neuron from DCN, fusiform cells receive inhibitory input from vertical cells and cartwheel cells while sending direct excitatory projections to the contralateral inferior colliculus (Caspary et al., 1987; Rhode, 1999; Davis and Young, 2000). Vertical cells project strong near-characteristic frequency (CF) glycinergic inhibition onto fusiform cells, thus shaping the neuron's response to narrowband stimuli (Voigt and Young, 1980, 1990; Caspary et al., 1987; Saint-Marie et al., 1991; Spirou et al., 1999). Recent studies from our lab have illustrated that fusiform cells in aged animals have higher maximum discharge rates to CF tones than those from young FBN rats suggesting a loss of glycinergic inhibition from vertical neurons in aged animals (Caspary et al., 2005). This loss of non-monotonic fusiform cell output likely alters temporal processing in the IC (Walton et al., 1998; Frisina, 2001). Previous studies demonstrated a loss of sound orientation accuracy when DCN output projections were surgically lesioned (Sutherland et al., 1998; May, 2000). Thus, the age-related changes of DCN fusiform outputs may also impact spatial auditory processing mechanisms.
In conclusion age-related slow partial deafferentation of acoustic nerve inputs appears to result in a compensatory down-regulation in the glycinergic activity of the DCN. Glycine receptor composition may modulate the response of fusiform cells near characteristic frequency and, therefore, may be involved in the reshaping of the DCN outputs. Our data and previous studies suggest that the age-related GlyR subunit makeup changes in both the DCN and AVCN may be one of the cellular mechanisms involved in age-related hearing loss. The present study found age-related changes in GlyR subunits in DCN. Moreover, the strychnine binding data suggests that less functional glycine receptors are anchored in the postsynaptic membranes. A better understanding of the properties of the aged glycine receptor could eventually lead to the development of selective pharmacotherapies for age-related hearing loss.
Acknowledgments
The authors thank Dr. Thomas Brozoski for his critical contributions to the manuscript and Patricia Jett, Diana Smith and Judith Bryan for help in proofing/editing the manuscript. This research was supported by NIH grant DC00151 to DMC.
Supporting grant info (NIH DC00151-25)
Abbreviations
- DCN
dorsal cochlear nucleus
- GlyR
glycine receptor
- FBN
Fischer Brown Norway
- NIDCD
National Institute on Deafness and Other Communication Disorders
- VCN
ventral cochlear nucleus
- CN
cochlear nucleus
- GlyRs
glycine receptors
- SIU
Southern Illinois University
- ABR
auditory brainstem response
- BBN
broadband noise
- PBS
phosphate buffered saline
- DAB
diaminobenzidine
- ROD
relative optical density
- ANOVA
analysis of variance
- SGNs
spiral ganglion neurons
- PKC
protein kinase C
- AVCN
anteroventral cochlear nucleus
- IC
inferior colliculus
- CF
characteristic frequency
- RLF
rate-level function
- DAS
dorsal acoustic striae
- IAS
intermediate acoustic striae
- LF
low frequency
- MF
middle frequency
- HF
high frequency
- D
dorsal
- M
medial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen PD, Burkard RF, Ison JR, Walton JP. Impaired gap encoding in aged mouse inferior colliculus at moderate but not high stimulus levels. Hear Res. 2003;186:17–29. doi: 10.1016/s0378-5955(03)00300-9. [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M. Changes with aging in the levels of amino acids in rat CNS structural elements II. Taurine and small neutral amino acids. Neurochem Res. 1989;14:563–570. doi: 10.1007/BF00964919. [DOI] [PubMed] [Google Scholar]
- Battaini F, Del Vesco R, Govoni S, Trabucchi M. Regulation of phorbol ester binding and protein kinase C activity in aged rat brain. Neurobiol Aging. 1990 Sep-Oct;11(5):563–566. doi: 10.1016/0197-4580(90)90118-j. [DOI] [PubMed] [Google Scholar]
- Battaini F, Elkabes S, Bergamaschi S, Ladisa V, Lucchi L, De Graan PN, Schuurman T, Wetsel WC, Trabucchi M, Govoni S. Protein kinase C activity, translocation, and conventional isoforms in aging rat brain. Neurobiol Aging. 1995 Mar-Apr;16(2):137–148. doi: 10.1016/0197-4580(94)00154-5. [DOI] [PubMed] [Google Scholar]
- Battaini F, Pascale A. Protein kinase C signal transduction regulation in physiological and pathological aging. Ann N Y Acad Sci. 2005 Dec;1057:177–192. doi: 10.1196/annals.1356.011. Review. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008 Aug 15;86(11):2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H, Kuhse J, Fischer M, Schmieden V, Laube B, Kuryatov A, Langosch D, Meyer G, Bormann J, Rundström N. Structure, diversity and synaptic localization of inhibitory glycine receptors. J Physiol Paris. 1994;88(4):243–248. doi: 10.1016/0928-4257(94)90087-6. Review. [DOI] [PubMed] [Google Scholar]
- Betz H, Kuhse J, Schmieden V, Laube B, Kirsch J, Harvey RJ. Structure and functions of inhibitory and excitatory glycine receptors. Ann N Y Acad Sci. 1999 Apr 30;868:667–676. doi: 10.1111/j.1749-6632.1999.tb11343.x. Review. [DOI] [PubMed] [Google Scholar]
- Beyerl BD. Afferent projections to the central nucleus of the inferior colliculus in the rat. Brain Res. 1978 Apr 28;145(2):209–223. doi: 10.1016/0006-8993(78)90858-2. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002 Mar 15;22(6):2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Pazara KE, Kössl M, Faingold CL. Strychnine alters the fusiform cell output from the dorsal cochlear nucleus. Brain Res. 1987 Aug 11;417(2):273–282. doi: 10.1016/0006-8993(87)90452-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol. 1995 May-Aug;30(34):349–360. doi: 10.1016/0531-5565(94)00052-5. Review. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABAA receptor subunit composition and function in rat auditory system. Neuroscience. 1999;93:307–312. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Turner JG. Comparison of two rat models of aging. In: Josef S, Michael MM, editors. Plasticity and Signal Representation in the Auditory System, Comparison of two rat models of aging. 2005. pp. 217–227. [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs. J Neurosci. 2005 Nov 23;25(47):10952–10959. doi: 10.1523/JNEUROSCI.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Hughes LF, Schatteman TA, Turner JG. Age-related changes in the response properties of cartwheel cells in rat dorsal cochlear nucleus. Hear Res. 2006 Jun-Jul;216-217:207–215. doi: 10.1016/j.heares.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008 Jun;211(Pt 11):1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo PJ, Wetsel WC, Gallagher M. Spatial memory is related to hippocampal subcellular concentrations of calcium-dependent protein kinase C isoforms in young and aged rats. Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):14195–14199. doi: 10.1073/pnas.94.25.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo PJ, Gallagher M. Individual differences in spatial memory among aged rats are related to hippocampal PKCgamma immunoreactivity. Hippocampus. 2002;12(2):285–289. doi: 10.1002/hipo.10016. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. When lysosomes get old. Exp Gerontol. 2000 Mar;35(2):119–131. doi: 10.1016/s0531-5565(00)00075-9. Review. [DOI] [PubMed] [Google Scholar]
- Danglot L, Rostaing P, Triller A, Bessis A. Morphologically identified glycinergic synapses in the hippocampus. Mol Cell Neurosci. 2004 Dec;27(4):394–403. doi: 10.1016/j.mcn.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol. 2000 Feb;83(2):926–940. doi: 10.1152/jn.2000.83.2.926. [DOI] [PubMed] [Google Scholar]
- Dazert S, Feldman ML, Keithley EM. Cochlear spiral ganglion cell degeneration in wild-caught mice as a function of age. Hear Res. 1996 Oct;100(12):101–106. doi: 10.1016/0378-5955(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Dice JF. Altered intracellular protein degradation in aging: a possible cause of proliferative arrest. Exp Gerontol. 1989;24(56):451–459. doi: 10.1016/0531-5565(89)90051-x. [DOI] [PubMed] [Google Scholar]
- Doucet JR, Ross AT, Gillespie MB, Ryugo DK. Glycine immunoreactivity of multipolar neurons in the ventral cochlear nucleus which project to the dorsal cochlear nucleus. J Comp Neurol. 1999;408:515–531. [PubMed] [Google Scholar]
- Francis HW, Ryugo DK, Gorelikow MJ, Prosen CA, May BJ. The functional age of hearing loss in a mouse model of presbycusis. II. Neuroanatomical correlates. Hear Res. 2003;183:29–36. doi: 10.1016/s0378-5955(03)00212-0. [DOI] [PubMed] [Google Scholar]
- Friauf E. Tonotopic Order in the Adult and Developing Auditory System of the Rat as Shown by c-fos Immunocytochemistry. Eur J Neurosci. 1992;4(9):798–812. doi: 10.1111/j.1460-9568.1992.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Friguet B, Bulteau AL, Chondrogianni N, Conconi M, Petropoulos I. Protein degradation by the proteasome and its implications in aging. Ann N Y Acad Sci. 2000 Jun;908:143–154. doi: 10.1111/j.1749-6632.2000.tb06643.x. Review. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Walton JP, Karcich KJ. Dorsal cochlear nucleus single neurons can enhance temporal processing capabilities in background noise. Exp Brain Res. 1994;102(1):160–164. doi: 10.1007/BF00232448. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hear Res. 2001 Aug;158(12):1–27. doi: 10.1016/s0378-5955(01)00296-9. Review. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008 May;31(5):257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Frostholm A, Rotter A. Glycine receptor distribution in mouse CNS: autoradiographic localization of [3H] strychnine binding sites. Brain Res Bull. 1985 Nov;15(5):473–486. doi: 10.1016/0361-9230(85)90038-3. [DOI] [PubMed] [Google Scholar]
- Frostholm A, Rotter A. Autoradiographic localization of receptors in the cochlear nucleus of the mouse. Brain Res Bull. 1986 Feb;16(2):189–203. doi: 10.1016/0361-9230(86)90033-x. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998 Oct;78(1):13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Baker BN. Neuroanatomical distribution of receptors for three potential inhibitory neurotransmitters in the brainstem auditory nuclei of the cat. J Comp Neurol. 1988 Sep 8;275(2):288–308. doi: 10.1002/cne.902750210. [DOI] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005 Mar 3;45(5):727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Gutiérrez A, Khan ZU, Miralles CP, Mehta AK, Ruano D, Araujo F, Vitorica J, De Blas AL. GABAA receptor subunit expression changes in the rat cerebellum and cerebral cortex during aging. Brain Res Mol Brain Res. 1997 Apr;45(1):59–70. doi: 10.1016/s0169-328x(96)00237-9. [DOI] [PubMed] [Google Scholar]
- Hamann I, Gleich O, Klump GM, Kittel MC, Strutz J. Age-dependent changes of gap detection in the Mongolian Gerbil (Meriones unguiculatus) JARO. 2004;5:49–57. doi: 10.1007/s10162-003-3041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Chen S, Tietz EI. Immunocytochemical detection of regional protein changes in rat brain sections using computer-assisted image analysis. J Histochem Cytochem. 1996 Sep;44(9):981–987. doi: 10.1177/44.9.8773563. [DOI] [PubMed] [Google Scholar]
- Huang R, He S, Chen Z, Dillon GH, Leidenheimer NJ. Mechanisms of homomeric alpha1 glycine receptor endocytosis. Biochemistry. 2007 Oct 16;46(41):11484–11493. doi: 10.1021/bi701093j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham NJ, Comis SD, Withington DJ, Ingham NJ, Comis SD, Withington DJ. Hair cell loss in the aged guinea pig cochlea. Acta Otolaryngol. 1999 Jan;119(1):42–47. doi: 10.1080/00016489950181918. [DOI] [PubMed] [Google Scholar]
- Joris PX, Smith PH. Temporal and binaural properties in dorsal cochlear nucleus and its output tract. J Neurosci. 1998;18(23):10157–10170. doi: 10.1523/JNEUROSCI.18-23-10157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley EM, Feldman ML. Spiral ganglion cell counts in an age-graded series of rat cochleas. J Comp Neurol. 1979 Dec 1;188(3):429–442. doi: 10.1002/cne.901880306. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Feldman ML. Hair cell counts in an age-graded series of rat cochleas. Hear Res. 1982 Dec;8(3):249–262. doi: 10.1016/0378-5955(82)90017-x. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Ryan AF, Woolf NK. Spiral ganglion cell density in young and old gerbils. Hear Res. 1989 Mar;38(12):125–133. doi: 10.1016/0378-5955(89)90134-2. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Croskrey KL. Spiral ganglion cell endings in the cochlear nucleus of young and old rats. Hear Res. 1990 Nov;49(13):169–177. doi: 10.1016/0378-5955(90)90103-v. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Ryan AF, Feldman ML. Cochlear degeneration in aged rats of four strains. Hear Res. 1992 May;59(2):171–178. doi: 10.1016/0378-5955(92)90113-2. [DOI] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Markesbery WR. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience. 2000;98(1):149–156. doi: 10.1016/s0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Hermann A, Kirsch J, Betz H. Hydrophobic interactions mediate binding of the glycine receptor beta-subunit to gephyrin. J Neurochem. 1999 Mar;72(3):1323–1326. doi: 10.1046/j.1471-4159.1999.0721323.x. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Betz H. Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations. J Physiol. 2000 May 15;525(Pt 1):1–9. doi: 10.1111/j.1469-7793.2000.t01-4-00001.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M. Postsynaptic scaffold proteins at non-synaptic sites. The role of postsynaptic scaffold proteins in motor-protein-receptor complexes. MBO Rep. 2005 Jan;6(1):22–27. doi: 10.1038/sj.embor.7400319. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Loebrich S. Trafficking and synaptic anchoring of ionotropic inhibitory neurotransmitter receptors. Biol Cell. 2007 Jun;99(6):297–309. doi: 10.1042/BC20060120. Review. [DOI] [PubMed] [Google Scholar]
- Kiffin R, Kaushik S, Zeng M, Bandyopadhyay U, Zhang C, Massey AC, Martinez-Vicente M, Cuervo AM. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci. 2007 Mar 1;120(Pt 5):782–791. doi: 10.1242/jcs.001073. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993 Dec 23-30;366(6457):745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998 Apr 16;392(6677):717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- Kirsch J. Glycinergic transmission. Cell Tissue Res. 2006 Nov;326(2):535–540. doi: 10.1007/s00441-006-0261-x. Review. [DOI] [PubMed] [Google Scholar]
- Kolston J, Osen KK, Hackney CM, Ottersen OP, Storm-Mathisen J. An atlas of glycine- and GABA-like immunoreactivity and colocalization in the cochlear nuclear complex of the guinea pig. Anat Embryol (Berl) 1992;186:443–465. doi: 10.1007/BF00185459. [DOI] [PubMed] [Google Scholar]
- Krenning J, Hughes LF, Caspary DM, Helfert RH. Age-related glycine receptor subunit changes in the cochlear nucleus of Fischer-344 rats. Laryngoscope. 1998;108:26–31. doi: 10.1097/00005537-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Kujawa SJ, Liberman MC. Noise-Induced Primary Neuronal Degeneration in Ears with Complete Threshold Recovery. Abstract 760 of the Association for Research in Otolaryngology (ARO) meeting 2008.2008. [Google Scholar]
- Langosch D, Becker CM, Betz H. The inhibitory glycine receptor: a ligand-gated chloride channel of the central nervous system. Eur J Biochem. 1990 Nov 26;194(1):1–8. doi: 10.1111/j.1432-1033.1990.tb19419.x. [DOI] [PubMed] [Google Scholar]
- Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001 May;58(56):760–793. doi: 10.1007/PL00000899. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévi S, Vannier C, Triller A. Strychnine-sensitive stabilization of postsynaptic glycine receptor clusters. J Cell Sci. 1998 Feb;111(Pt 3):335–345. doi: 10.1242/jcs.111.3.335. [DOI] [PubMed] [Google Scholar]
- Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132(4):1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004 Oct;84(4):1051–1095. doi: 10.1152/physrev.00042.2003. Review. [DOI] [PubMed] [Google Scholar]
- Maas C, Tagnaouti N, Loebrich S, Behrend B, Lappe-Siefke C, Kneussel M. Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. J Cell Biol. 2006 Jan 30;172(3):441–451. doi: 10.1083/jcb.200506066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Brain Res Mol Brain Res. 2002 Feb 28;99(1):40–45. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- Malosio ML, Marquèze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991 Sep;10(9):2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey AC, Kiffin R, Cuervo AM. Autophagic defects in aging: looking for an ‘emergency exit’? Cell Cycle. 2006 Jun;5(12):1292–1296. doi: 10.4161/cc.5.12.2865. [DOI] [PubMed] [Google Scholar]
- May BJ. Role of the dorsal cochlear nucleus in the sound localization behavior of cats. Hear Res. 2000 Oct;148(12):74–87. doi: 10.1016/s0378-5955(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Mazurek B, Haupt H, Georgiewa P, Klapp BF, Reisshauer A. A model of peripherally developing hearing loss and tinnitus based on the role of hypoxia and ischemia. Med Hypotheses. 2006;67(4):892–899. doi: 10.1016/j.mehy.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Meier J, Meunier-Durmort C, Forest C, Triller A, Vannier C. Formation of glycine receptor clusters and their accumulation at synapses. J Cell Sci. 2000 Aug;113(Pt 15):2783–2795. doi: 10.1242/jcs.113.15.2783. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Caspary DM. Age-related reduction of [3H]strychnine binding sites in the cochlear nucleus of the Fischer 344 rat. Neuroscience. 1995 Aug;67(3):713–719. doi: 10.1016/0306-4522(95)00082-t. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Hunter C, Caspary DM. Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol. 1997 Mar 17;379(3):455–465. doi: 10.1002/(sici)1096-9861(19970317)379:3<455::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001 Apr;2(4):240–250. doi: 10.1038/35067500. Review. [DOI] [PubMed] [Google Scholar]
- Nadol JB. Electron microscopic findings in presbycusic degeneration of the basal turn of the human cochlea, Otolaryngol. Head Neck Surg. 1979;87:818–836. doi: 10.1177/019459987908700617. [DOI] [PubMed] [Google Scholar]
- Ostroff JM, McDonald KL, Schneider BA, Alain C. Aging and the processing of sound duration in human auditory cortex. Hear Res. 2003 Jul;181(12):1–7. doi: 10.1016/s0378-5955(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004 Feb;27(2):104–110. doi: 10.1016/j.tins.2003.12.001. Review. [DOI] [PubMed] [Google Scholar]
- Otte J, Schunknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope. 1978 Aug;88(8 Pt 1):1231–1246. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- Paloff AM, Usunoff KG. Projections to the inferior colliculus from the dorsal column nuclei. An experimental electron microscopic study in the cat. J Hirnforsch. 1992;33(6):597–610. [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. GABA inputs control discharge rate primarily within frequency receptive fields of inferior colliculus neurons. J Neurophysiol. 1996 Jun;75(6):2211–2219. doi: 10.1152/jn.1996.75.6.2211. [DOI] [PubMed] [Google Scholar]
- Pascale A, Amadio M, Govoni S, Battaini F. The aging brain, a key target for the future: the protein kinase C involvement. Pharmacol Res. 2007 Jun;55(6):560–569. doi: 10.1016/j.phrs.2007.04.013. Review. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Souza PE. Effects of aging on auditory processing of speech. Int J Audiol. 2003 Jul;42 2:2S11–16. Review. [PubMed] [Google Scholar]
- Puel JL, Ruel J, Guitton M, Wang J, Pujol R. The inner hair cell synaptic complex: physiology, pharmacology and new therapeutic strategies. Audiol Neurootol. 2002 Jan-Feb;7(1):49–54. doi: 10.1159/000046864. [DOI] [PubMed] [Google Scholar]
- Pujol R, Rebillard G, Puel JL, Lenoir M, Eybalin M, Recasens M. Glutamate neurotoxicity in the cochlea: a possible consequence of ischaemic or anoxic conditions occurring in ageing. Acta Otolaryngol Suppl. 1990;476:32–36. doi: 10.3109/00016489109127253. [DOI] [PubMed] [Google Scholar]
- Rattan SI, Derventzi A, Clark BF. Protein synthesis, posttranslational modifications, and aging. Ann N Y Acad Sci. 1992 Nov 21;663:48–62. doi: 10.1111/j.1749-6632.1992.tb38648.x. Review. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Synthesis, modifications, and turnover of proteins during aging. Exp Gerontol. 1996 Jan-Apr;31(12):33–47. doi: 10.1016/0531-5565(95)02022-5. Review. [DOI] [PubMed] [Google Scholar]
- Reiss LA, Young ED. Spectral edge sensitivity in neural circuits of the dorsal cochlear nucleus. J Neurosci. 2005 Apr 6;25(14):3680–3691. doi: 10.1523/JNEUROSCI.4963-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode WS, Greenberg S. Encoding of amplitude modulation in the cochlear nucleus of the cat. J Neurophysiol. 1994;71:1797–1825. doi: 10.1152/jn.1994.71.5.1797. [DOI] [PubMed] [Google Scholar]
- Rhode WS. Vertical cell responses to sound in cat dorsal cochlear nucleus. J Neurophysiol. 1999;82:1019–1032. doi: 10.1152/jn.1999.82.2.1019. [DOI] [PubMed] [Google Scholar]
- Rissman RA, Nocera R, Fuller LM, Kordower JH, Armstrong DM. Age-related alterations in GABA(A) receptor subunits in the nonhuman primate hippocampus. Brain Res. 2006 Feb 16;1073-1074:120–130. doi: 10.1016/j.brainres.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Rissman RA, De Blas AL, Armstrong DM. GABA(A) receptors in aging and Alzheimer's disease. J Neurochem. 2007 Nov;103(4):1285–1292. doi: 10.1111/j.1471-4159.2007.04832.x. Review. [DOI] [PubMed] [Google Scholar]
- Rose JE, Galambos R, Hughes JR. Microelectrode studies of the cochlear nuclei of the cat. Bull Johns Hopkins Hosp. 1959 May;104(5):211–251. [PubMed] [Google Scholar]
- Rouiller EM, Wan XS, Moret V, Liang F. Mapping of c-fos expression elicited by pure tones stimulation in the auditory pathways of the rat, with emphasis on the cochlear nucleus. Neurosci Lett. 1992 Sep 14;144(12):19–24. doi: 10.1016/0304-3940(92)90706-d. [DOI] [PubMed] [Google Scholar]
- Rose JE, Galambos R, Hughes J. Organization of frequency sensitive neurons in the cochlear nuclear complex of the cat. In: Rasmussan GC, Windle WF, editors. Neuronal mechanisms of the auditory and vestibular systems. Springfield, IL: Thomas; 1960. pp. 116–136. [Google Scholar]
- Ryan AF, Furlow Z, Woolf NK, Keithley EM. The spatial representation of frequency in the rat dorsal cochlear nucleus and inferior colliculus. Hear Res. 1988 Nov;36(23):181–189. doi: 10.1016/0378-5955(88)90060-3. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Nefsky BS. Protein turnover plays a key role in aging. Mech Ageing Dev. 2002 Jan;123(23):207–213. doi: 10.1016/s0047-6374(01)00337-2. Review. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Tohyama M. Regional distribution of cells expressing glycine receptor alpha 2 subunit mRNA in the rat brain. Brain Res. 1992 Sep 11;590(12):95–108. doi: 10.1016/0006-8993(92)91085-s. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Benson CG, Ostapoff EM, Morest DK. Glycine immunoreactive projections from the dorsal to the anteroventral cochlear nucleus. Hear Res. 1991 Jan;51(1):11–28. doi: 10.1016/0378-5955(91)90003-r. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Luo L, Ryan AF. Effects of stimulus frequency and intensity on c-fos mRNA expression in the adult rat auditory brainstem. J Comp Neurol. 1999 Feb 8;404(2):258–270. doi: 10.1002/(sici)1096-9861(19990208)404:2<258::aid-cne9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schatteman TA, Hughes LF, Caspary DM. Aged-related loss of temporal processing: Altered responses to amplitude modulated tones in rat dorsal cochlear nucleus. Neuroscience. 2008 Jun 12;154(1):329–337. doi: 10.1016/j.neuroscience.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V, Betz H. Pharmacology of the inhibitory glycine receptor: agonist and antagonist actions of amino acids and piperidine carboxylic acid compounds. Mol Pharmacol. 1995 Nov;48(5):919–927. [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK, Kowalchuk D, Lamb M. Gap detection and the precedence effect in young and old adults. J Acoust Soc Am. 1994;95:980–991. doi: 10.1121/1.408403. [DOI] [PubMed] [Google Scholar]
- Schrott A, Stephan K, Spoendlin H. Auditory brainstem response thresholds in a mouse mutant with selective outer hair cell loss. Eur Arch Otorhinolaryngol. 1990;247(1):8–11. doi: 10.1007/BF00240940. [DOI] [PubMed] [Google Scholar]
- Snell KB. Age-related changes in temporal gap detection. J Acoust Soc Am. 1997;101:2214–2220. doi: 10.1121/1.418205. [DOI] [PubMed] [Google Scholar]
- Spirou GA, Davis KA, Nelken I, Young ED. Spectral integration by type II interneurons in dorsal cochlear nucleus. J Neurophysiol. 1999 Aug;82(2):648–663. doi: 10.1152/jn.1999.82.2.648. [DOI] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Am Jun. 1997;101(6):3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Schrott A. The spiral ganglion and the innervation of the human organ of Corti. Acta Otolaryngol. 1988 May-Jun;105(56):403–410. doi: 10.3109/00016488809119493. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Schrott A. Quantitative evaluation of the human cochlear nerve. Acta Otolaryngol Suppl. 1990;470:61–9. doi: 10.3109/00016488909138358. discussion 69-70. [DOI] [PubMed] [Google Scholar]
- Stamataki S, Francis HW, Lehar M, May BJ, Ryugo DK. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear Res. 2006 Nov;221(12):104–118. doi: 10.1016/j.heares.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Sutherland DP, Glendenning KK, Masterton RB. Role of acoustic striae in hearing: discrimination of sound-source elevation. Hear Res. 1998 Jun;120(12):86–108. doi: 10.1016/s0378-5955(98)00056-2. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Mazelova J, Druga R. Gap detection threshold in the rat before and after auditory cortex ablation. Hear Res. 2002;172:151–159. doi: 10.1016/s0378-5955(02)00578-6. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Aging alters the neural representation of speech cues. Neuroreport. 2002;13:1865–1870. doi: 10.1097/00001756-200210280-00007. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol. 2003 Jul;114(7):1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006 Feb;120(1):188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Vela J, Gutierrez A, Vitorica J, Ruano D. Rat hippocampal GABAergic molecular markers are differentially affected by ageing. J Neurochem. 2003 Apr;85(2):368–377. doi: 10.1046/j.1471-4159.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- Voigt HF, Young ED. Evidence of inhibitory interactions between neurons in dorsal cochlear nucleus. J Neurophysiol. 1980 Jul;44(1):76–96. doi: 10.1152/jn.1980.44.1.76. [DOI] [PubMed] [Google Scholar]
- Voigt HF, Young ED. Cross-correlation analysis of inhibitory interactions in dorsal cochlear nucleus. J Neurophysiol. 1990 Nov;64(5):1590–1610. doi: 10.1152/jn.1990.64.5.1590. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, Ison JR, O'Neill WE. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J Comp Physiol. 1997;181:161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O'Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci. 1998 Apr 1;18(7):2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickesberg RE, Oertel D. Tonotopic projection from the dorsal to the anteroventral cochlear nucleus of mice. J Comp Neurol. 1988 Feb 15;268(3):389–399. doi: 10.1002/cne.902680308. [DOI] [PubMed] [Google Scholar]
- Willott JF. Effects of aging, hearing loss, and anatomical location on thresholds of inferior colliculus neurons in C57BL/6 and CBA mice. J Neurophysiol. 1986 Aug;56(2):391–408. doi: 10.1152/jn.1986.56.2.391. [DOI] [PubMed] [Google Scholar]
- Willott JF, Milbrandt JC, Bross LS, Caspary DM. Glycine immunoreactivity and receptor binding in the cochlear nucleus of C57BL/6J and CBA/CaJ mice: effects of cochlear impairment and aging. J Comp Neurol. 1997;385:405–414. [PubMed] [Google Scholar]
- Willott JF, Turner JG. Prolonged exposure to an augmented acoustic environment ameliorates age-related auditory changes in C57BL/6J and DBA/2J mice. Hear Res. 1999;135(12):78–88. doi: 10.1016/s0378-5955(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Yajima Y, Hayashi Y. Response properties and tonotopical organization in the dorsal cochlear nucleus in rats. Exp Brain Res. 1989;75(2):381–389. doi: 10.1007/BF00247945. [DOI] [PubMed] [Google Scholar]
- Young AB, Snyder SH. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AB, Snyder SH. The glycine synaptic receptor: evidence that strychnine binding is associated with the ionic conductance mechanism. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4002–4005. doi: 10.1073/pnas.71.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Neuronal circuits associated with the output of the dorsal cochlear nucleus through fusiform cells. J Neurophysiol. 1994 Mar;71(3):914–930. doi: 10.1152/jn.1994.71.3.914. [DOI] [PubMed] [Google Scholar]