Abstract
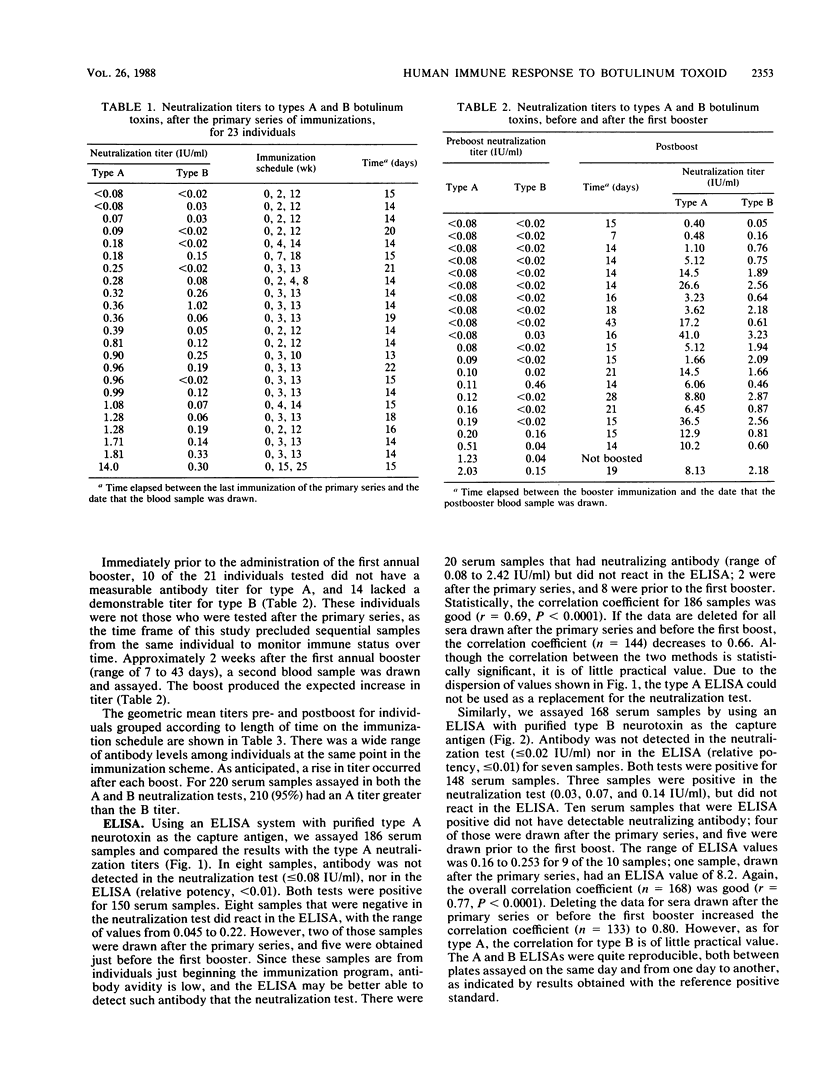
To determine the immune status of persons receiving botulinum pentavalent (ABCDE) toxoid and to evaluate the effectiveness of the vaccine, we surveyed immunized individuals for neutralizing antibodies to type A and to type B botulinum toxins. After the primary series of three immunizations administered at 0, 2, and 12 weeks, 21 of 23 persons tested (91%) had a titer for type A that was greater than or equal to 0.08 international units (IU)/ml, and 18 (78%) had a titer for type B of greater than or equal to 0.02 IU/ml. (One international unit is defined as the amount of antibody neutralizing 10,000 mouse 50% lethal doses of type A or B botulinum toxin). Just before the first annual booster, 10 of 21 (48%) and 14 of 21 (67%) people lacked a detectable titer for type A and for type B, respectively. After the first booster, all individuals tested had a demonstrable titer to both types A and B. Of 77 persons who had previously received from one to eight boosts of the toxoid, 74 (96%) had an A titer of greater than or equal to 0.25 IU/ml and would not require an additional booster, according to the recommendations of the Centers for disease Control. However, only 44 of 77 (57%) had a B titer of greater than or equal to 0.25 IU/ml. In each group by booster number, even the group having had eight boosts, at least one person would require reimmunization on the basis of B titer. There was a wide range of antibody levels among individuals at the same point in the immunization scheme. Results from an enzyme linked immunosorbent assay, with purified type A or type B neurotoxin as the capture antigen, were compared with neutralization test results on 186 serum samples for type A and 168 samples for type B. Statistically, the correlation coefficients for results from the two assays were high (r = 0.69, P < 0.0001, for type A and r = 0.77, P < 0.0001, for type B). However, due to the wide dispersion of values obtained, using enzyme-linked immunosorbent assay results to predict neutralizing antibody levels is unwarranted.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOWMER E. J. PREPARATION AND ASSAY OF THE INTERNATIONAL STANDARDS FOR CLOSTRIDIUM BOTULINUM TYPES A, B, C, D AND E ANTITOXINS. Bull World Health Organ. 1963;29:701–709. [PMC free article] [PubMed] [Google Scholar]
- Boroff D. A., Meloche H. P., Dasgupta B. R. Amino Acid Analysis of the Isolated and Purified Components from Crystalline Toxin of Clostridium botulinum Type A. Infect Immun. 1970 Nov;2(5):679–680. doi: 10.1128/iai.2.5.679-680.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock S. L., Walls K. W. Evaluation of some of the parameters of the enzyme-linked immunospecific assay. J Infect Dis. 1977 Oct;136 (Suppl):S279–S285. doi: 10.1093/infdis/136.supplement_2.s279. [DOI] [PubMed] [Google Scholar]
- Dezfulian M., Bartlett J. G. Detection of Clostridium botulinum type A toxin by enzyme-linked immunosorbent assay with antibodies produced in immunologically tolerant animals. J Clin Microbiol. 1984 May;19(5):645–648. doi: 10.1128/jcm.19.5.645-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfulian M., Bitar R. A., Bartlett J. G. Kinetics study of immunological response to Clostridium botulinum toxin. J Clin Microbiol. 1987 Jul;25(7):1336–1337. doi: 10.1128/jcm.25.7.1336-1337.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIOCK M. A., CARDELLA M. A., GEARINGER N. F. STUDIES ON IMMUNITY TO TOXINS OF CLOSTRIDIUM BOTULINUM. IX. IMMUNOLOGIC RESPONSE OF MAN TO PURIFIED PENTAVALENT ABCDE BOTULINUM TOXIOD. J Immunol. 1963 May;90:697–702. [PubMed] [Google Scholar]
- FIOCK M. A., DEVINE L. F., GEARINGER N. F., DUFF J. T., WRIGHT G. G., KADULL P. J. Studies on immunity to toxins of Clostridium botulinum. VIII. Immunological response of man to purified bivalent AB botulinum toxoid. J Immunol. 1962 Mar;88:277–283. [PubMed] [Google Scholar]
- Hagenaars A. M., van Delft R. W., Nagel J. Comparison of ELISA and toxin neutralization for the determination of tetanus antibodies. J Immunoassay. 1984;5(1-2):1–11. doi: 10.1080/01971528408062995. [DOI] [PubMed] [Google Scholar]
- Hatheway C. H., Snyder J. D., Seals J. E., Edell T. A., Lewis G. E., Jr Antitoxin levels in botulism patients treated with trivalent equine botulism antitoxin to toxin types A, B, and E. J Infect Dis. 1984 Sep;150(3):407–412. doi: 10.1093/infdis/150.3.407. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Melville-Smith M. E., Seagroatt V. A., Watkins J. T. A comparison of enzyme-linked immunosorbent assay (ELISA) with the toxin neutralization test in mice as a method for the estimation of tetanus antitoxin in human sera. J Biol Stand. 1983 Apr;11(2):137–144. doi: 10.1016/s0092-1157(83)80038-9. [DOI] [PubMed] [Google Scholar]
- Rubin L. G., Dezfulian M., Yolken R. H. Serum antibody response to Clostridium botulinum toxin in infant botulism. J Clin Microbiol. 1982 Oct;16(4):770–771. doi: 10.1128/jcm.16.4.770-771.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. J., Siegel L. S. Purification of type E botulinum neurotoxin by high-performance ion exchange chromatography. Anal Biochem. 1986 Jul;156(1):213–219. doi: 10.1016/0003-2697(86)90175-2. [DOI] [PubMed] [Google Scholar]
- Shone C., Appleton N., Wilton-Smith P., Hambleton P., Modi N., Gatley S., Melling J. In vitro assays for botulinum toxin and antitoxins. Dev Biol Stand. 1986;64:141–145. [PubMed] [Google Scholar]


