Abstract
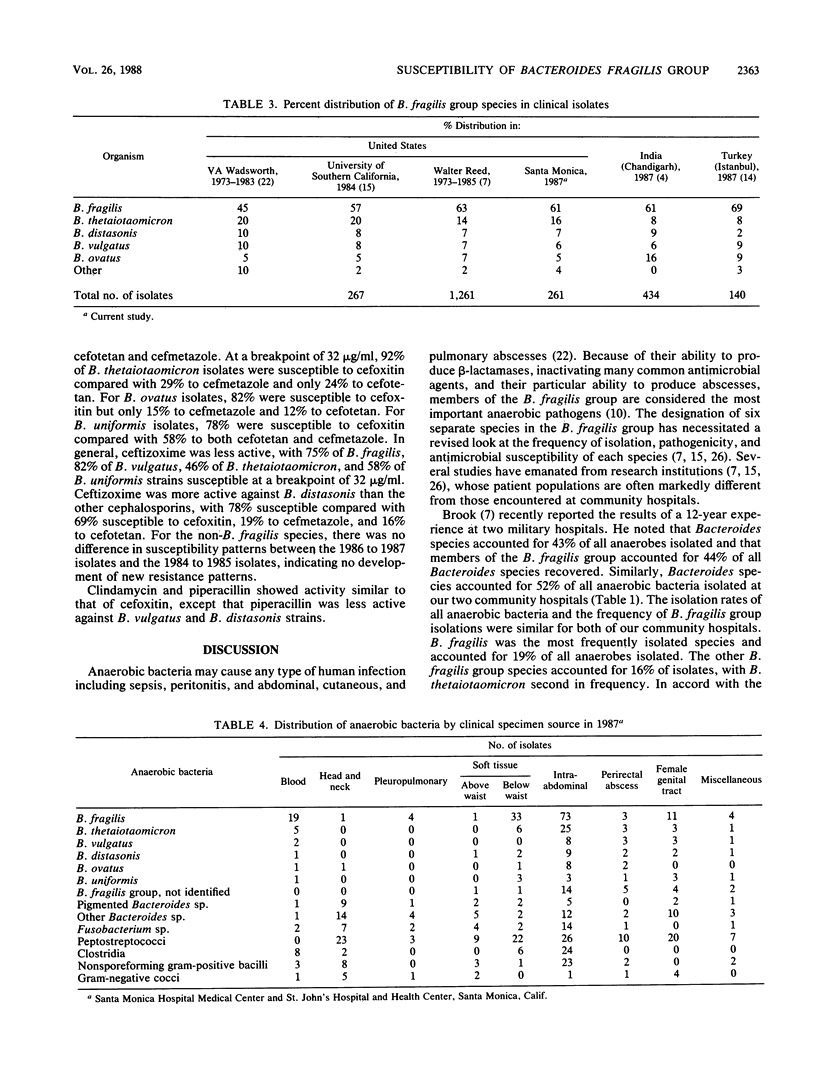
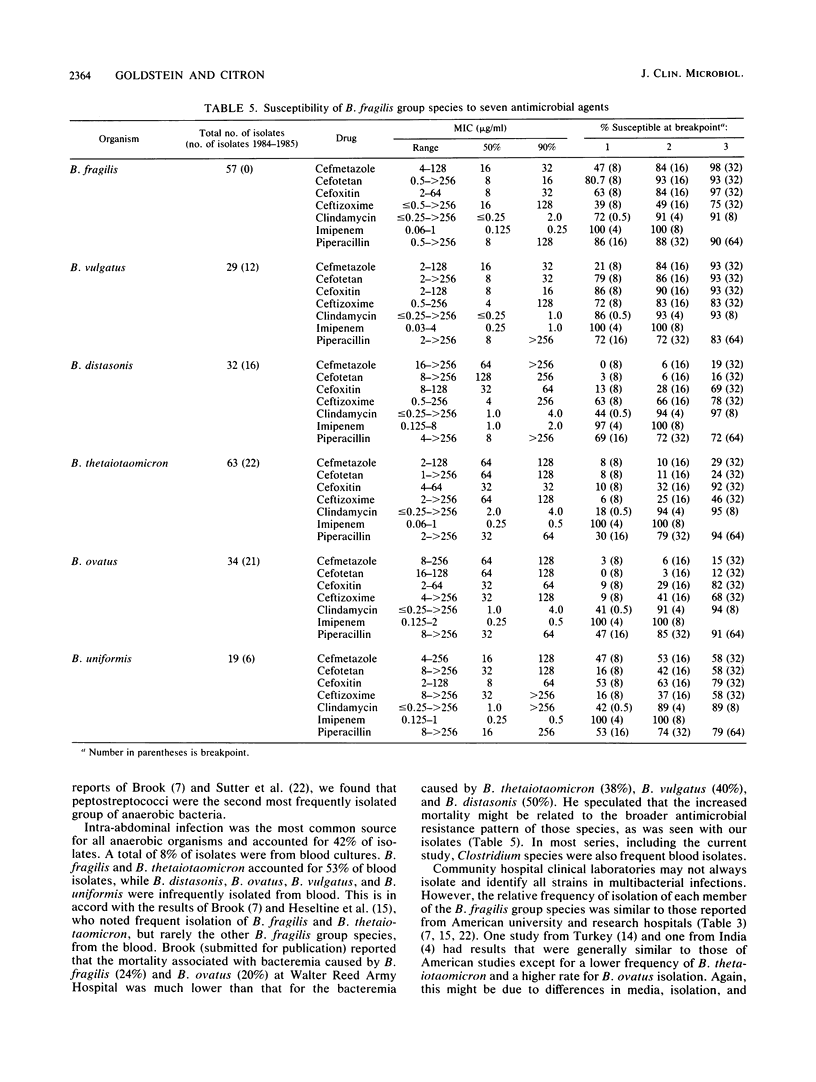
The six species of the Bacteroides fragilis group are potent pathogens and commonly have different susceptibility patterns. We determined the relative annual isolation rate of anaerobic bacteria and the susceptibility of B. fragilis group species isolated during 1987 at two community hospitals. The relative frequencies of isolation of 261 strains were as follows: B. fragilis, 61%; B. thetaiotaomicron, 17%; B. distasonis, 7%; B. vulgatus, 6%; B. ovatus, 5%; and B. uniformis, 4%. A total of 234 recent clinical isolates were tested against cefmetazole, cefotetan, cefoxitin, ceftizoxime, clindamycin, imipenem, and piperacillin by a brucella agar dilution method. Imipenem was the most active agent tested with all but three isolates (two B. vulgatus and one B. distasonis) susceptible to less than 2 micrograms/ml. Of the cephalosporins tested, cefoxitin, cefotetan, and cefmetazole were relatively equal against B. fragilis, with 93 to 98% of strains susceptible to 32 micrograms/ml. Ceftizoxime was less active, with an MIC for 90% of strains tested of 128 micrograms/ml and only 75% of isolates susceptible to 32 micrograms/ml. Against B. ovatus, B. vulgatus, B. thetaiotaomicron, and B. uniformis, cefoxitin showed a two- to threefold-superior activity compared with that of cefotetan and cefmetazole. In general, ceftizoxime was much less active, except against B. distasonis, for which 78% of isolates were susceptible to 32 micrograms/ml compared with 68% for cefoxitin, 19% for cefmetazole, and 16% for cefotetan. Clindamycin and piperacillin showed activity similar to that of cefoxitin, except piperacillin was less active versus B. vulgatus and B. distasonis. We therefore suggest that clinical laboratories determine the species of B. fragilis group isolates as well as perform susceptibility studies on the isolates. Clinicians should be aware that while B. fragilis is the most frequent isolate, 38% of isolates are from other, more resistant B. fragilis group species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Sanders C. V. Antibiotic- and method-dependent variation in susceptibility testing results of Bacteroides fragilis group isolates. J Clin Microbiol. 1987 Dec;25(12):2317–2321. doi: 10.1128/jcm.25.12.2317-2321.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge K. E., Sanders C. V., Janney A., Faro S., Marier R. L. Comparison of the activities of penicillin G and new beta-lactam antibiotics against clinical isolates of Bacteroides species. Antimicrob Agents Chemother. 1984 Sep;26(3):410–413. doi: 10.1128/aac.26.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew J. H., Greenwood D. Susceptibility of cefotetan and Sch 34343 to beta-lactamases produced by strains of Bacteroides that hydrolyse cefoxitin or imipenem. J Antimicrob Chemother. 1987 May;19(5):591–595. doi: 10.1093/jac/19.5.591. [DOI] [PubMed] [Google Scholar]
- Bourgault A. M., Harding G. K., Smith J. A., Horsman G. B., Marrie T. J., Lamothe F. Survey of anaerobic susceptibility patterns in Canada. Antimicrob Agents Chemother. 1986 Nov;30(5):798–801. doi: 10.1128/aac.30.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. Recovery of anaerobic bacteria from clinical specimens in 12 years at two military hospitals. J Clin Microbiol. 1988 Jun;26(6):1181–1188. doi: 10.1128/jcm.26.6.1181-1188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornick N. A., Jacobus N. V., Gorbach S. L. Activity of cefmetazole against anaerobic bacteria. Antimicrob Agents Chemother. 1987 Dec;31(12):2010–2012. doi: 10.1128/aac.31.12.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Malamy M. H., Tally F. P. Beta-lactamase-mediated imipenem resistance in Bacteroides fragilis. Antimicrob Agents Chemother. 1986 Nov;30(5):645–648. doi: 10.1128/aac.30.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Tally F. P. Bacteroides fragilis: current susceptibilities, mechanisms of drug resistance, and principles of antimicrobial therapy. Drug Intell Clin Pharm. 1986 Jul-Aug;20(7-8):567–573. doi: 10.1177/106002808602000712. [DOI] [PubMed] [Google Scholar]
- Drulak M. W., Chow A. W. Comparative in vitro activity of ceftizoxime, cefoperazone, and cefoxitin against anaerobic bacteria. Antimicrob Agents Chemother. 1981 Nov;20(5):683–685. doi: 10.1128/aac.20.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. R., Phillips I. The antibiotic sensitivity of the Bacteroides fragilis group in the United Kingdom. J Antimicrob Chemother. 1987 Oct;20(4):477–488. doi: 10.1093/jac/20.4.477. [DOI] [PubMed] [Google Scholar]
- Goldstein E. J., Kwok Y. Y., Sutter V. L. Absence of tolerance to cefoxitin in anaerobic bacteria. Antimicrob Agents Chemother. 1981 Jul;20(1):146–148. doi: 10.1128/aac.20.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J. Comparative activities of 13 beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jun;21(6):925–934. doi: 10.1128/aac.21.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J. P., Venezio F. R., DiVincenzo C. A., Shatzer K. L. Activity of newer beta-lactam agents against clinical isolates of Bacteroides fragilis and other Bacteroides species. Antimicrob Agents Chemother. 1987 Dec;31(12):2002–2004. doi: 10.1128/aac.31.12.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I., King A., Shannon K., Warren C. Cefotetan: in-vitro antibacterial activity and susceptibility to beta-lactamases. J Antimicrob Chemother. 1983 Jan;11 (Suppl):1–9. doi: 10.1093/jac/11.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Stratton C. W., Weeks L. S., Aldridge K. E. Comparison of kill-kinetic studies with agar and broth microdilution methods for determination of antimicrobial activity of selected agents against members of the Bacteroides fragilis group. J Clin Microbiol. 1987 Apr;25(4):645–649. doi: 10.1128/jcm.25.4.645-649.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Barry A. L., Wilkins T. D., Zabransky R. J. Collaborative evaluation of a proposed reference dilution method of susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1979 Oct;16(4):495–502. doi: 10.1128/aac.16.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Emmerman J., Randall E., Zabransky R. J., Birk R. J. Establishment of MICs of moxalactam for control and reference anaerobic organisms in agar dilution and microdilution techniques. Antimicrob Agents Chemother. 1985 Mar;27(3):424–426. doi: 10.1128/aac.27.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Cuchural G. J., Jr, Jacobus N. V., Gorbach S. L., Aldridge K., Cleary T., Finegold S. M., Hill G., Iannini P., O'Keefe J. P. Nationwide study of the susceptibility of the Bacteroides fragilis group in the United States. Antimicrob Agents Chemother. 1985 Nov;28(5):675–677. doi: 10.1128/aac.28.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler H. M., Finegold S. M. Antimicrobial resistance in Bacteroides. J Antimicrob Chemother. 1987 Feb;19(2):143–146. doi: 10.1093/jac/19.2.143. [DOI] [PubMed] [Google Scholar]
- Wexler H. M., Harris B., Carter W. T., Finegold S. M. Six-year retrospective survey of the resistance of Bacteroides fragilis group species to clindamycin and cefoxitin. Diagn Microbiol Infect Dis. 1986 Mar;4(3):247–253. doi: 10.1016/0732-8893(86)90104-5. [DOI] [PubMed] [Google Scholar]
- Zabransky R. J., Randall E., Sutter V. L., Birk R. J., Westenfelder G., Emmerman J., Ghoneim A. T. Establishment of minimum inhibitory concentrations of cefoperazone for control and reference anaerobic organisms. J Clin Microbiol. 1983 Apr;17(4):711–714. doi: 10.1128/jcm.17.4.711-714.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


