Summary
The control of cellular signaling cascades is of utmost importance in regulating the immune response. Exquisitely precise protein-protein interactions and chemical modification of substrates by enzymatic catalysis are the fundamental components of the signals that alert immune cells to the presence of a foreign antigen. In particular, the phosphorylation events induced by protein kinase activity must be spatially and temporally regulated by specific interactions to maintain a normal and effective immune response. High resolution structures of many protein kinases along with supporting biochemical data are providing significant insight into the intricate regulatory mechanisms responsible for controlling cellular signaling. The Tec family kinases are immunologically important kinases for which regulatory details are beginning to emerge. This review focuses on bringing together structural insights gained over the years to develop an understanding of how domain interactions both within the Tec kinases and between the Tec kinases and other signaling molecules control immune cell function.
Keywords: signaling proteins, Tec kinases, Itk, Btk, regulation, Src homology domains
Introduction
It can be said that protein kinases control the currency of signal transduction. Protein kinases catalyze the transfer of the γ-phosphate of adenosine triphosphate (ATP) to specific amino acid side chains creating a cascade of chemical information that elicits a cellular response. The creation of transiently phosphorylated amino acid side chains directs specific protein interactions and controls catalytic activity within the complexity of the cell. It is therefore not unexpected that precise regulation of kinase activity is vital in maintaining normal cellular function and that loss of specificity and/or loss of catalytic control can profoundly affect cell fate. Exquisitely controlled protein interactions and conformational changes within the multi-domain structures of these enzymes are emerging as the switches that control kinase activity (1–8).
Of the 518 protein kinases identified in humans, ~17% are tyrosine kinases that can be broadly classified into receptor tyrosine kinases and non-receptor tyrosine kinases (9). The Src family kinases and the Tec family kinases constitute the two largest families of non-receptor tyrosine kinases (10–12). The well-studied Src kinase family includes nine different proteins that mediate signaling in a number of different cell types (13–15). The regulation of Src activity and Src-mediated signaling has been extensively explored and provides a point of comparison for other tyrosine kinase families. Our interest has focused on the Tec kinases, enzymes that are primarily expressed in hematopoietic cells where they function downstream of several immune cell receptors (10, 11, 16).
The Tec family of non-receptor tyrosine kinases consists of five members: interleukin-2 (IL-2)-inducible T-cell kinase (Itk), Bruton’s tyrosine kinase (Btk), Tec, Txk, and Bmx (bone marrow tyrosine kinase gene on chromosome X) (10, 17). The critical role of these immunological tyrosine kinases is underscored by the fact that mutations within Btk are associated with a severe B-cell immunodeficiency, X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency (XID) in mice (18). XLA afflicts around 1 in 200,000 individuals and is characterized by an increased susceptibility to bacterial infections (18). The physiological roles of the Tec kinases have been extensively reviewed elsewhere and are not explored here (10–12, 16, 19–21). Instead, we focus on the structural features of the Tec kinases with an emphasis on gaining insight into how conformational switches and domain interactions control Tec kinase activity during signaling.
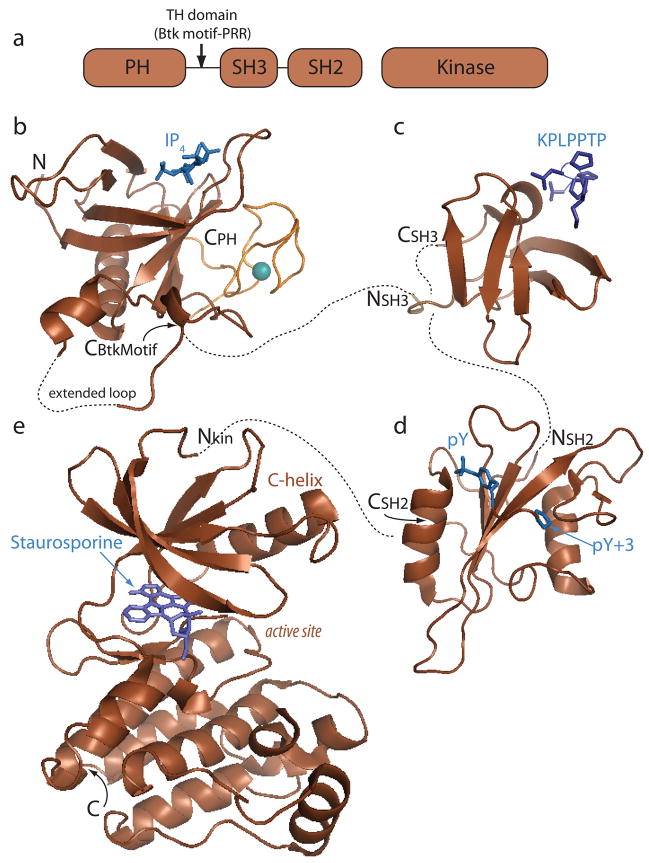
The domain architecture of the Tec kinases (Fig. 1A) is similar to that of the Src family kinases. Both kinase families contain a single Src homology 3 (SH3) domain and Src homology 2 (SH2) domain followed by the catalytic kinase domain [also referred to as a Src homology 1 (SH1) domain] (10). Unlike the Src kinases, the Tec kinases (with the exception of Bmx), also contain a pleckstrin homology (PH) domain followed by a Tec homology (TH) domain at the amino-terminus. The TH domain consists of a region unique to the Tec kinases (the Btk motif) followed by one or more proline-rich regions. Another significant difference between the Tec and Src kinases is that the Tec kinases lack the key regulatory tyrosine that is present in the C-terminal tail of Src kinases (10). Since the identification of the first mammalian Tec kinase member (Tec) 18 years ago (22), the absence of a Src-like regulatory tail (and the concomitant absence of the critical tyrosine residue) has suggested that the Tec kinase regulatory apparatus differs significantly from that of the related Src kinases (23). From this standpoint, a number of investigators have probed the structural features of the Tec kinases with the goal of defining the interactions and conformational switches that control Tec kinase signaling and subsequent immune cell function.
Fig. 1. Tec family kinase domain structures.
(A) The Tec kinases consist of a pleckstrin homology (PH) domain, a Tec homology (TH) domain that contains both the Btk motif and a proline-rich region (PRR), SH3, SH2, and kinase domains. (B-E) Individual domain structures. Ligands for each domain are shown in blue. Dotted lines connect the N- and C-termini of consecutive domains. A structure of a full length Tec kinase is not available, and so the precise spatial relationship between domains is not known. (B) Crystal structure of the PH-TH region of Btk (pdb: 1b55) complexed with inositol-1,3,4,5-tetrakisphosphate (IP4). The Btk motif (orange) consists of an extended loop structure that is held in place by a coordinated zinc ion (teal sphere). (C) Itk SH3 domain bound to a proline-rich ligand (pdb: 1awj). The proline-rich peptide ligand (KPLPPTP) adopts a left handed helical conformation and contacts the SH3 domain binding groove via hydrophobic and electrostatic interactions. (D) Itk SH2 domain bound to a phosphotyrosine-containing peptide ligand (pdb: 2etz). Classical SH2 ligand binding consists of a two-pronged interaction involving a phosphorylated tyrosine side chain (pY) contacting a basic binding pocket on the SH2 domain that contains at least one conserved arginine and a ligand residue three positions removed from the phosphotyrosine (pY+3) that contacts a specificity pocket on the SH2 domain. (E) Structure of the Itk kinase domain bound to staurosporine, an inhibitor that binds to the ATP binding site (pdb: 1snu). The kinase domain consists of two lobes, the smaller N-terminal lobe and the larger C-terminal lobe. Catalysis occurs in the binding cleft (labeled active site) between these two lobes. All structures in this and other figures were generated using PyMOL (134).
With a structure of a full-length Tec family kinase still eluding crystallographers, we begin the discussion of Tec kinase structure with an overview of the individual domains. Numerous structures have been reported for each of the individual Tec kinase domains (Fig. 1B-E). PDB entries can be found for the Btk PH domain (pdb: 1btk, 1b55, 2z0p, 1bwn) (24–26); the Btk motif is included in these PH domain structures but has also been solved separately for Itk and Bmx (pdb: 2e6i and 2ys2). The SH3 domains of Itk, Btk, and Tec have each been solved (pdb: 1qly, 1awx, 2rna, 1awj, 2yuq, 1gl5) (27–30); SH2 domains structures for Itk, Btk, Bmx, and Txk are reported (pdb: 2etz, 2dm0, 2ge9, 2ekx, 1luk, 1lun) (31, 32). Structures for the isolated kinase domains of Btk and Itk are known (pdb: 1k2p, 1snx, 1sm2, 1snu) (33, 34). These modular domains are found in large numbers of signaling proteins and the general functions of each isolated domain have been well characterized. In the following paragraphs, we provide a brief description of each domain and describe classical ligand-binding functions. Information about how each of these domains participates in immune cell signaling is summarized as well. In the second part of this review, we discuss recent molecular level work aimed at elucidating how these modular domains act in concert to control Tec kinase activity and specificity during signaling.
Overview of the individual Tec kinase domains
PH domain
The structure and function of the PH domain have been reviewed (35–38). In contrast to the lipid modified Src kinases that are constitutively present on cellular membranes, Tec kinases (with the exception of Txk) are transiently recruited to the cell membrane via their N-terminal PH domain upon receptor activation (39–42). PH domains are lipid-binding domains consisting of ~120 amino acids (37, 43). Although PH domains share limited amino acid sequence homology, their structural folds are strikingly similar (37). The crystal structure of the isolated Btk PH domain R28C mutant as well as the structures of the Btk wildtype and a gain-of-function E41K PH domain mutant in complex with inositol 1,3,4,5-tetrakisphosphate (IP4) have been solved (24, 25). The Btk PH domain structure is similar to other PH domains and consists of a seven-stranded β-sandwich connected by loops of variable length at one end and capped off by a C-terminal α-helix at the other (25) (Fig. 1B). One notable feature of the Btk PH domain structure is the presence of a long insertion in the loop connecting β-strands 5 and 6. This extended loop is not well defined in the Btk PH domain crystal structure nor is it present in the other Tec family members. The Btk motif consists of another long loop that folds back onto itself and packs against β-strands 5–7 of the PH domain. The motif is stabilized by a zinc ion that is coordinated by three conserved cysteines and one histidine residue (25).
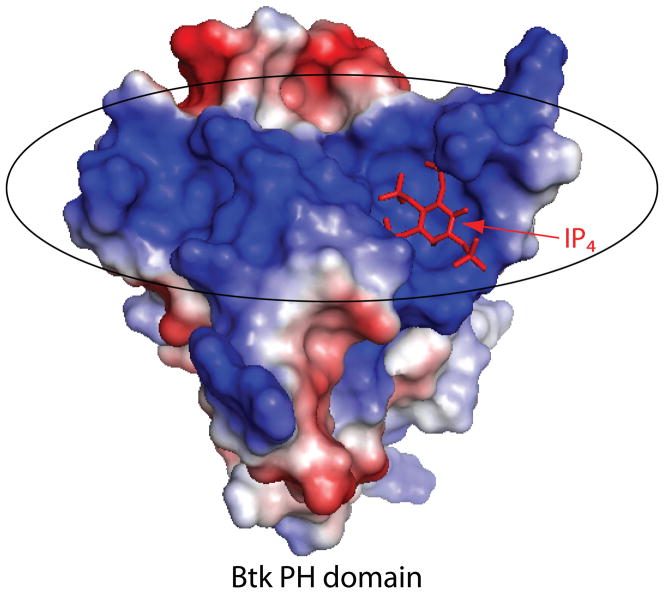
The lipid-binding properties of the Btk and Itk PH domain have been extensively studied in vitro (39, 44–46). Both show a preference for D-3 inositol phosphate lipids, with a Kd of 40 nM measured for the binding of the Btk PH domain to IP4 (39, 44). Similar to other PH domains, the Btk PH domain is electrostatically polarized (Fig. 2) with positively charged residues in the β1-β2 and β3-β4 loops forming a charged surface that mediates binding of IP4 (25). Deletion of the Tec kinase PH domain or mutations in the PH domain that disrupt lipid binding abolish translocation to the cell membrane (44, 47). Another piece of evidence that the PH domain plays an important role in signaling comes from the numerous XLA-causing mutations that have been identified in the Btk PH domain of XLA-afflicted patients (18). Most of these XLA-causing mutations either directly disrupt inositol lipid binding or change the electrostatic properties of the lipid-binding site (24, 25).
Fig. 2. Btk PH domain: electrostatic potential.
IP4 (shown in red sticks) bound to the Btk PH domain. The PH domain surface is colored to indicate electrostatic potential; the large concentration of positive charge (indicated in blue and circled) coincides with the IP4 binding site.
The localization and activity of the Tec family kinases are dependent on the activity of the lipid kinase phosphoinositide 3-kinase (PI3K) (39, 41, 42). PI3K generates phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] by the phosphorylation of phosphatidylinositol 4,5-bisphosphate. Decreasing the intracellular levels of PtdIns(3,4,5)P3 by the overexpression of phosphatases, such as the inositol 5-phosphatase SHIP (SH2-domain-containing inositol-5-phosphatase), or the use of PI3K inhibitors (such as Wortmanin), decreases membrane recruitment of the Tec kinases (42, 48, 49). SHIP1 and SHIP2 have also been shown to be negative regulators of Tec, and a direct interaction between SHIP1 and SHIP2 and the Tec SH3 domain has been demonstrated (49). In a corresponding manner, the deficiency of the inositol 3-phosphatase PTEN (phosphatase and tensin homologue) in Jurkat cells leads to elevated levels of PtdIns(3,4,5)P3, which is responsible for the constitutive membrane localization of Itk in these cells (50).
Membrane association of the Tec kinases may not be a simple matter. A recent report by Huang et al. (51) has shown that membrane recruitment of Itk is dependent on cellular levels of the soluble inositol lipid IP4. Mice defective in IP3 3-kinase B (ItpkB), the enzyme that generates IP4 from IP3, have severe defects in the Itk signaling pathway and lack mature T cells (51). It is proposed that IP4 may bind to the Itk PH domain and induce a conformation that has a higher affinity for both IP4 and PtdIns(3,4,5)P3. The higher concentrations of PtdIns(3,4,5)P3 at the site of receptor activation would then displace IP4. An alternate hypothesis is based on the observation that the Itk PH domain oligomerizes (51). Binding of IP4 by one PH domain subunit could induce conformational changes in an associated PH domain that result in increased affinity for the headgroup of PtdIns(3,4,5)P3. Regardless of its effect on ligand binding, PH domain oligomerization itself is notable, and we discuss other possible functional consequences of Itk PH domain self-association below.
SH3 domain
The SH3 domain structure and function has been reviewed (52, 53). The SH3 fold consists of two three stranded β-sheets juxtaposed at right angles (Fig. 1C). Conserved aromatic residues within the primary sequence of the SH3 domain form a hydrophobic groove on the surface that binds proline-rich ligands (30, 54, 55). In particular, a conserved tryptophan residue protrudes into the aromatic binding cleft and plays a critical role in binding of proline-rich ligands. This tryptophan residue is the target for traditional loss of function SH3 domain mutation and examination of such a mutant in full length Itk showed normal catalytic activity in vitro (56). In contrast, Itk carrying the mutated SH3 domain does not permit IL-2 production in T cells (56). Together these data suggest that the role of the Itk SH3 domain is primarily in mediating protein-protein interactions rather than regulating catalytic activity. Although a variety of SH3 domain ligands have been identified for Tec kinases (57–61), the precise mechanism by which these interactions regulate in vivo function of Tec kinases remains unclear.
The SH3 domains of the Tec kinases also contain a conserved tyrosine residue (Y180 in Itk, Y223 in Btk) (56, 62–64) that is the site of autophosphorylation. Mutation of this tyrosine has been shown to have varying effects in vivo with the outcome appearing dependent on the assay system used. For example, the Btk Y223F mutation increases the oncogenic activity of a Btk E41K mutant, while the same Btk Y223F mutation was found to restore calcium signaling in a Btk-deficient B-cell line (62, 65). Similarly, the Itk Y180F mutant has been shown to only partially restore extracellular signal-regulated kinase (ERK) phosphorylation in Itk-deficient T cells (56). Autophosphorylation within the SH3 domain has no impact on the in vitro catalytic activity of the Tec kinases (56, 62, 66). Thus, the functional consequences of Tec kinase autophosphorylation in vivo are presumed to be due to altered ligand interacts rather than changes in intrinsic kinase activity (56, 66).
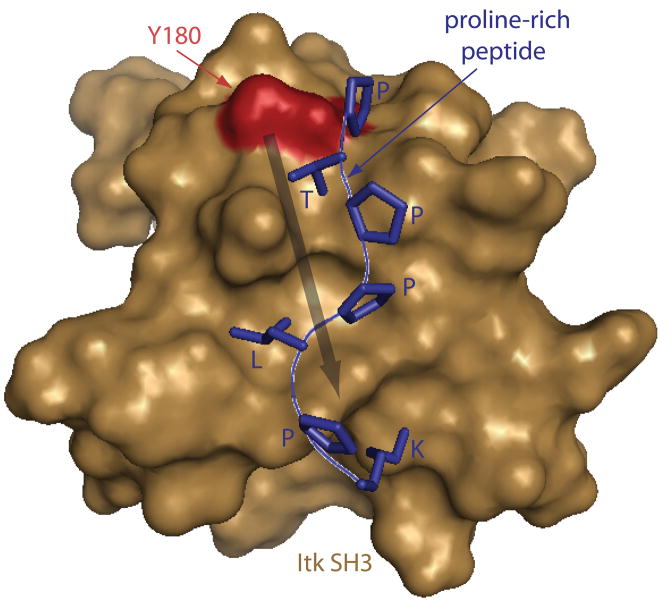
This model is consistent with the location of the autophosphorylation site within the aromatic binding pocket of the SH3 domain (Fig. 3). Itk Y180 sits at the edge of the SH3 proline-binding pocket, and nuclear magnetic resonance (NMR) chemical shift mapping of an Itk SH3 Y180E mutant (chosen to mimic the phosphorylated SH3 domain) shows chemical shift perturbations (indicative of structural perturbations) that extend well into the ligand-binding pocket of the SH3 domain (66)(Fig. 3). Consistent with structural perturbations on the ligand-binding pocket, the Itk SH3 Y180E mutant exhibits diminished affinity for a proline-rich ligand, while the affinity for a different, non-canonical ligand is increased (66). Autophosphorylation of the Btk SH3 domain also induces ligand-specific effects (59). Phosphorylated Btk SH3 domain leads to a decreased affinity for Wiskott-Aldrich syndrome protein (WASP), whereas binding to c-Cbl remains unchanged (59). Thus, autophosphorylation of the Tec SH3 domains seems to fine tune binding affinity in a ligand-specific manner with no direct effect on catalytic activity.
Fig. 3. Location of the autophosphorylation site within the SH3 ligand binding site.
The Itk SH3 domain bound to the classical proline rich peptide ligand (shown in blue sticks). Y180 (the Itk autophosphorylation site) is indicated in red on the brown SH3 domain surface. Y180 sits at the edge of the ligand-binding site and, when mutated to glutamate (to mimic phosphorylation), induces structural changes that extend into the ligand binding cleft (indicated with arrow). SH3 ligand binding affinities are altered by this phopho-mimicking mutation (66).
Adjacent to the SH3 domain in each of the Tec family kinases is at least one proline-rich sequence that corresponds to the classical SH3 ligand sequence (10, 54, 67). A number of SH3-containing interacting partners of the Tec kinases have been identified that likely bind to these proline stretches during signaling (68, 69). As well, SH3/proline interactions within the same molecule (either in an intramolecular or intermolecular sense) have been well studied among the Tec kinases. The first efforts along these lines involved the adjacent proline-rich region and SH3 domain of Itk (55). That work as well as a follow up study (70) show that the SH3/proline ligand interaction within Itk occurs exclusively in an intramolecular fashion. In contrast, the SH3/proline interaction within the nearly identical region of Txk/Rlk occurs only in the intermolecular sense (70). To complicate these observations further, Btk and Tec contain two consecutive proline-rich segments adjacent to their SH3 domain. The results of detailed NMR and biophysical studies of the Btk and Tec proline-proline-SH3 fragments reveal a complex equilibrium where the proline stretches interact both inter- and intra-molecularly with the SH3 domain (28, 71, 72).
These intra- and inter-molecular SH3/proline interactions could certainly compete with exogenous ligands to regulate the activity and localization of Tec kinases (55, 73). However, despite the enormous amount of attention paid to SH3 domains in general and the Tec family SH3 domains specifically, we still lack a precise understanding of how the SH3 domain integrates Itk and related kinases into their respective signaling cascades. As a final example of the complexity in these systems, we can consider the observed translocation of both Itk and Btk to the nucleus following receptor activation (74, 75). The SH3 domain appears to play a role as deletion of the SH3 domain disrupts the export of Btk from the nucleus, thereby causing nuclear sequestration (74).
SH2 domain
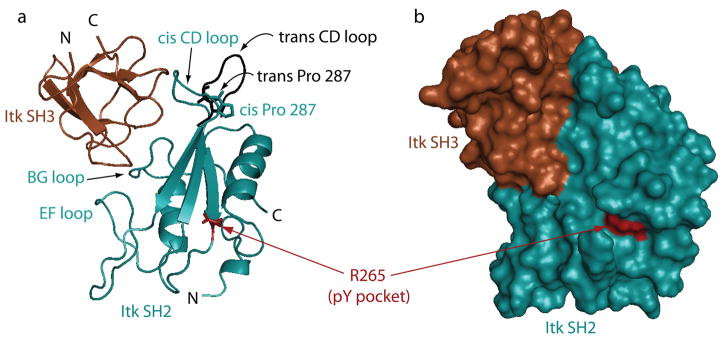
The SH2 domain structure and function have been reviewed (76–79). SH2 domains consists of ~100 amino acids and are well characterized phosphotyrosine peptide-binding modules (79). Like the numerous other SH2 structures solved, the Tec kinase SH2 domains fold into a centralβ-sheet that is flanked by two α-helices (Fig. 1D). The phosphopeptide ligand binds perpendicularly to the central β-sheet in a two-prong manner with the phosphotyrosine (pY) and the residue in the pY+3 position making critical contacts with the SH2 domain (31, 32, 80). Phosphopeptide binding is mediated by a conserved arginine residue (R265 in Itk) in the pY pocket of the SH2 domain, while ligand specificity is conferred by residues in the specificity pocket which coordinate the peptide residue in the pY+3 position (80) (Figs 1D and 4B).
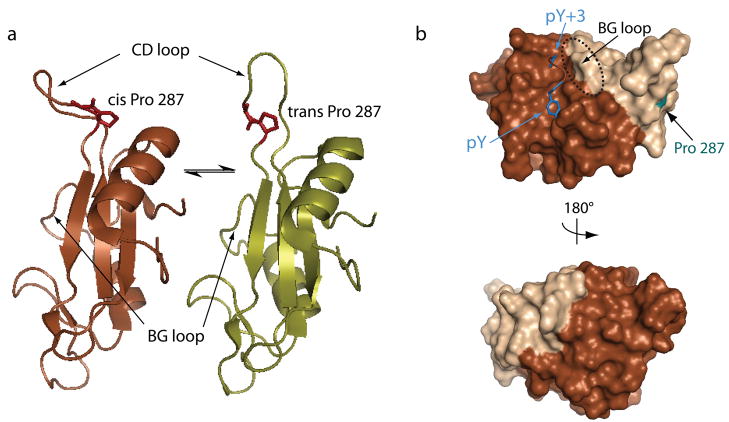
Fig. 4. Conformational heterogeneity in the Itk SH2 domain.
(A) The Itk SH2 domain structure reveals a single proline residue (Pro 287) that interconverts between the cis and trans imide bond conformations in solution. Pro 287 is located at the base of a large loop (the CD loop using standard SH2 domain nomenclature). In the trans imide bond containing SH2 conformer (right), the CD loop is extended and flexible; in contrast, the CD loop in the cis structure (left) is more rigid and adopts a conformation that bends toward the body of the SH2 domain (32). The differences in loop flexibility between the cis and trans structures are evident in independent dynamics measurements using NMR spectroscopy (32). (B) The conformational heterogeneity induced by prolyl isomerization in the Itk SH2 domain causes structural differences (as measured by NMR) across nearly half of the domain surface. The lighter shading represents SH2 residues that give rise to doubled resonances in NMR spectra of the domain due to slow interconversion between the cis and trans peptide bond between residues 286 and 287. Doubled resonances are indicative of two different structural environments for each particular residue in the cis and trans structures. Darker surface are those regions that are unaffected by prolyl isomerization. It is noteworthy that classical phosphopeptide binding is affected by isomerization (89). On the top structure, the bound phospholigand is included, and it is clear that the pY binding pocket is not affected by proyl isomerization in the CD loop. The BG loop that makes up one side of the pY+3 pocket (circled), however, is influenced by prolyl isomerization explaining how phospholigand binding discriminates between the two structures despite its distance from Pro 287 (labeled and highlighted in cyan) (80).
The Syk family kinases create phosphorylation sites on SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) and linker for activation of T cells (LAT) for binding of the Tec SH2 domains (81–84). The interaction between the Itk SH2 domain and the phospho-SLP-76/LAT complex is required for proper Itk activity in vivo (68, 85, 86). The traditional loss-of-function mutation within the Itk SH2 domain [mutation of the conserved arginine (R265) responsible for binding the ligand phosphotyrosine] therefore impedes membrane localization and subsequent activation (85). Thus, the PH domain-mediated membrane recruitment described above, while necessary for Tec kinase activation, is not sufficient (40, 85). The SH2 domain plays an equally critical role.
The structures of the isolated Tec kinase SH2 domains have been thoroughly examined (31, 32). The Itk SH2 domain is unique among the Tec family SH2 domains, as it adopts two distinct conformational states (Fig. 4). The observed conformational heterogeneity is due to cis/trans prolyl isomerization about a single imide bond within the domain (32), a structural phenomenon that is still somewhat unique among folded proteins (87). Prolyl isomerization arises from the nearly equal energies of the cis and trans imide bond conformers. Isomerization is typically slow (msec to sec) due to the energetic barrier of interconversion (on the order of 14–24 kcal/mol) (88).
For short, proline-containing peptides, cis/trans prolyl isomerization is nearly always observed because of the lack of restraints on the flexible polypeptide chain. Instead, the prolyl imide bonds in most folded proteins are restricted (by the tertiary structure) into either the trans or cis conformations; the lower energy trans imide bond conformer predominates in structures of folded proteins. Thus, the proline-induced conformational heterogeneity within the folded Itk SH2 domain is of considerable interest from a structural biology point of view. Finally, the fact that NMR spectroscopy was used to solve the solution structure of the Itk SH2 domain greatly facilitated identification of prolyl isomerization in this system. In solution, the polypeptide chain readily samples an ensemble of conformations including cis/trans imide bond conformations. Given the preponderance of protein structures solved by x-ray crystallography in the protein database, we should question whether prolyl isomerization is a more common structural feature of proteins than currently recognized. Is protein crystallization itself impeding our ability to fully appreciate important dynamical features of the protein backbone?
The structural consequences of prolyl isomerization in the Itk SH2 domain are not limited to the local sequence surrounding the proline. In fact, prolyl isomerization around the single imide bond induces structural differences across nearly half of the domain surface (Fig. 4B). This extensive effect is likely responsible for the fact that the two imide bond conformers of the Itk SH2 domain exhibit different ligand binding properties (89). The SH2 domain containing the trans imide bond conformer has a higher affinity for the classical phosphotyrosine-containing SH2 ligand than does the cis imide bond conformer. The structure of the trans Itk SH2 domain complexed with a phosphotyrosine peptide (80) suggests that the preferential binding of the Itk SH2 trans conformer arises from the pre-organized nature of the specificity pocket (BG loop) in the trans form compared to the cis conformer (80). It is interesting to note that the phosphopeptide ligand is some distance from the proline switch (Fig. 4B) and that the structure of the phosphotyrosine-binding pocket itself is unaffected by prolyl isomerization. Thus, phosphopeptide discrimination of trans versus cis likely arises from differential contacts in the pY+3 specificity pocket.
What then is the role of the cis imide bond containing SH2 conformer? Early work identified a novel ligand for the Itk SH2 domain (90), and subsequent analysis clarified that the cis imide bond conformer mediates the interaction (89). The novel SH2 ligand is the Itk SH3 domain; Itk SH3 binds with higher affinity to the cis Itk SH2 domain than the trans (89). The interaction between the Itk SH2 and SH3 domains is phosphotyrosine independent and does not involve a canonical proline-rich ligand. Thus, both the SH3 and SH2 binding partners in this complex exploit recognition elements that do not fit the well-defined ligand-binding properties of these common domains. The structure of the Itk SH2 domain in the cis conformer complexed with the Itk SH3 domain has recently been solved (Severin A, Fulton DB, Andreotti AH, manuscript in preparation, pdb code: 2k79) and reveals how the cis imide bond containing SH2 domain is the preferred ligand for the SH3 domain (Fig. 5). The conformationally heterogeneous proline does not make direct contact to the SH3 domain but rather stabilizes the large CD loop structure in the SH2 domain to make extensive contacts with the Itk SH3 domain. This structure combined with that of the trans SH2 domain complexed with phosphopeptide (80) reveal how a prolyl switch can alter a protein interaction surface to achieve selectivity (90). Again, it remains to be determined how many other examples of this type of molecular recognition exist, and a first step will be to accurately predict or determine the presence of proline-driven conformational switches in other folded proteins.
Fig. 5. The non-canonical Itk SH3/Itk SH2 interaction.
(A) The Itk SH3 domain (brown) binds to the Itk SH2 domain (cyan) via the conserved SH3 binding site despite the absence of a classical proline rich ligand sequence in the Itk SH2 domain. Three SH2 loops (EF, BG, and CD) make contact to the SH3 domain and the CD loop in particular is pre-organized for binding the SH3 domain by the cis imide bond at Pro 287. The trans imide bond-containing SH2 conformer is shown in black (the CD loop is shown while the rest of the trans SH2 structure is omitted for clarity). The conformation of the CD loop in the trans structure does not permit contacts between this part of the SH2 domain and the SH3 binding surface. The conserved arginine in the phosphotyrosine (pY) binding pocket is indicated in red and is sterically accessible in the SH3/SH2 complex. (B) Surface rendering of the same view of the SH3/cisSH2 complex.
The functional significance of prolyl isomerization within Itk is of great interest (91). It is discussed further below, but it is notable that Itk is the only member of the Tec family that contains the conformationally heterogeneous proline in the SH2 domain. As well, Itk is the only kinase in the family that does not self-associate in an intermolecular fashion via the SH3/proline-region interaction; instead the cisSH2/SH3 interaction occurs inter-molecularly, while the proline-SH3 region of Itk exclusively associates intra-molecularly. In fact, the first study to examine Itk SH3 domain accessibility (55) predates characterization of the Itk SH3/SH2 interaction, and so those early data can now be better interpreted. In addition to the proline/SH3 intramolecular interaction, the interaction of the Itk SH3 domain with the cis Itk SH2 domain also masks the SH3-binding cleft and would affect binding of exogenous ligands to the Itk SH3 domain. Thus, all of the Tec kinases form inter-molecularly associated species, albeit through distinct domain-domain interactions. We revisit the importance of intermolecular Tec kinase self-association again in the context of catalytic control.
Kinase domain
The kinase domain structure and function have been reviewed (3, 23,92). The kinase domain contains the catalytic center of the kinase molecule. Tyrosine kinase domains have a conserved structure consisting of a small N-terminal lobe composed of five β strands and one prominent helix: the C-helix (3, 93) (Fig. 1E). The larger C-terminal lobe consists mainly of helices. ATP binds in a deep cleft between the two lobes of the kinase. The activation loop runs between the two lobes of the kinase domain and provides a platform for the binding of the peptide substrate (3, 94, 95). Kinase activation is accompanied by a tyrosine phosphorylation step that induces large conformational changes in the activation loop (3). For the Tec family kinases Itk and Btk, activation is achieved by phosphorylation of the activation loop tyrosines Y511 and Y551, respectively (96, 97), by Src family kinases (10, 98). Based on work in other kinases, the activation loop is collapsed into the active site and blocks access to the substrate prior to this activating phosphorylation step. Upon phosphorylation of the activation loop tyrosine, the loop moves away from the active site permitting the substrate to bind. This is accompanied by an inward movement of the C-helix that enables a conserved glutamate (E445 in Btk) on the C-helix to form an ion pair with and orient a conserved lysine (K430 in Btk) within the N-terminal lobe of the kinase (3) (Fig. 6).
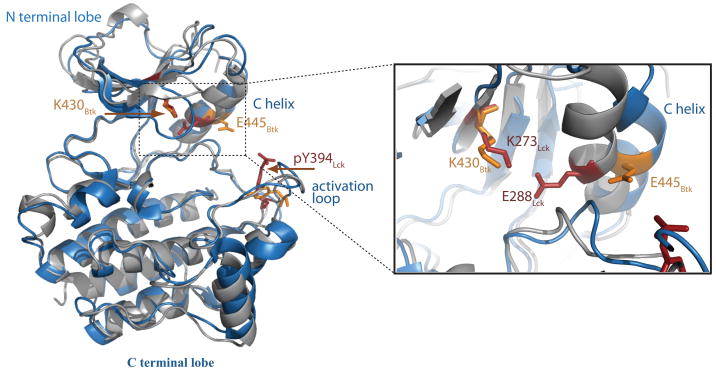
Fig. 6. Tec family kinase domain structure and active site residues.
Superposition of the unphosphorylated kinase domain of Btk (1k2p) in blue with the phosphorylated kinase domain of Lck (3lck) in grey. Yellow side chains are those of Btk and red side chains are from Lck. The conserved activation loop tyrosine is phosphorylated within Lck (labeled pY394) and unphosphorylated in the Btk structure. The active site details are shown in the expanded view on the right. The position of the conserved lysine within each kinase domain and the glutamate on the C-helix is indicated. Activation of the kinase requires the critical ion pair between lysine and glutamate and the position of the C-helix determines the distance between these side chains.
In the crystal structure of the unphosphorylated, inactive kinase domain of Btk (34), the activation loop adopts an unexpected, extended conformation that does not block the active site but instead is rather similar to that of the activated Src family kinase Lck (34) (Fig. 6). However, in the same structure, the C-helix is not optimally positioned, consistent with the ‘inactive’ state of the Btk kinase domain. The distance between the key Btk residues E445 and K430 is 10 A, unlike the 3 A distance found for the corresponding pair in active kinases (34) (Fig. 6). Btk E445 instead forms a hydrogen bond with the side chain of R544, which prevents the formation of the E445:K430 hydrogen bond that in turn prevents repositioning of the C-helix. Phosphorylation on Btk Y551 is thought to engage R544 in an interaction with the phosphate group of pY551, which then releases E445 to interact with K430 and form an active kinase conformation. In fact, this hydrogen bond switch has been proposed as a general mechanism for the activation of Tec kinases (34).
The crystal structures of the phosphorylated and unphosphorylated forms of the Itk kinase domain in complex with the inhibitor staurosporine have also been solved (33). Interestingly, the Itk kinase domain crystallized in the same conformation in both the phosphorylated and unphosphorylated forms of the kinase (33). The authors call into question the activating role of phosphorylation on Itk Y511 as well as the activation mechanism proposed earlier for Tec kinases based on the unphosphorylated Btk kinase structure (34). However, inhibitor binding has been shown to cause considerable rearrangements within the active site of other kinases (93, 99), and in fact, the distance between the conserved lysine within the N-terminal lobe (Itk K390) and conserved glutamate on the C-helix (Itk E405) is ~ 7 A in both Itk structures (33). It is possible therefore that the conformation observed for the Itk kinase domain is a consequence of staurosporine binding rather than an indication that the active site of this particular kinase follows a different set of rules to achieve activation. Moreover, the role of the non-catalytic domains adjacent to the kinase domain needs to be considered to fully understand the mechanisms of regulation at work within the Tec kinase family.
The regulatory domains in context: their role in activation
When the Tec kinases were first identified (22, 100–104), it was immediately obvious that they lack the C-terminal regulatory tail that plays such a critical role in controlling the catalytic activity of the Src kinases (10). Another difference between the Src and Tec kinases emerged from detailed biochemical analyses; enzymatic assays using a panel of Itk, Btk and Tec constructs revealed that the isolated Tec family kinase domains are far less active than the corresponding full-length enzymes (33, 105, 106). In contrast, deletion of the regulatory SH3 and SH2 domains from the Src kinase yields a significantly more active kinase domain (23, 107, 108). How then do the non-catalytic domains of Itk and the other Tec kinases exert this positive influence on the activity of the neighboring kinase domain? Posed in a different way, why are the isolated Tec family kinase domains almost inactive toward their substrates?
Without a structure of any full-length Tec kinase we need to look to related molecules for which structural data exist. In addition to the Src and Tec kinases, the C-terminal Src kinase (Csk) shares the SH3-SH2-kinase domain cassette. Csk also lacks the C-terminal regulatory tail of the Src kinases (109), and, like the Tec family, the isolated kinase domain of Csk is 100 times less active than the full-length enzyme containing the SH3 and SH2 domains (110). These similarities with the Tec kinase family suggest that Csk may serve as a useful model for understanding Tec kinase regulation.
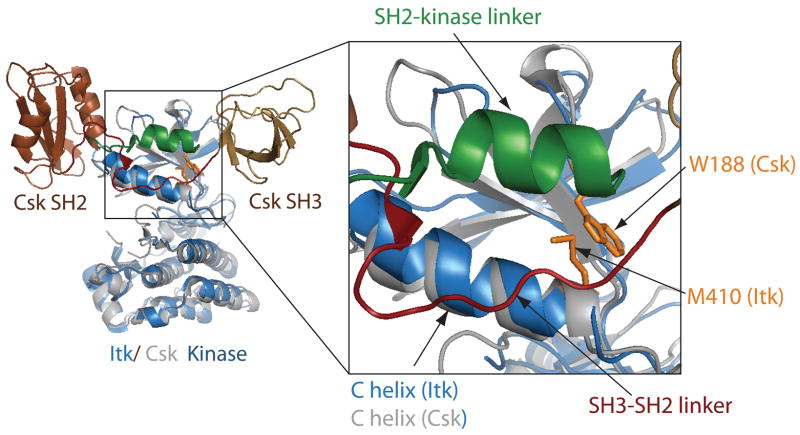
A crystal structure of full-length, active Csk is available and shows that the SH3 and SH2 domains sit at the top of the N-terminal lobe of the kinase domain (109) (Fig. 7). The linkers between the SH3 and SH2 domains and between the SH2 and Csk kinase domains run nearly parallel to each other and make extensive contacts with the N-terminal lobe of the Csk kinase domain (Fig. 7). The C-helix adopts the classical ‘active’ kinase configuration in this structure likely stabilized by contacts between the N-terminal kinase lobe and the parallel linkers (109). Indeed, a tryptophan residue in the linker between the SH2 and kinase domains of Csk plays a critical role in stabilizing the active state. Mutation of this single Trp residue inactivates the Csk kinase (109). The SH3 and SH2 domains (and the intervening linker regions) become uncoupled from the kinase domain in this mutant, permitting the C-helix to move outward disrupting side chain contacts within the active site that are necessary for maintaining activity (110–115).
Fig. 7. Comparison of the full length Csk structure with the Itk kinase domain structure.
Superposition of the Itk kinase domain (blue) with full-length active Csk (1snx and 1k9a, respectively). The Csk kinase domain is gray, and the Csk SH3 and SH2 domains are colored brown and labeled. The linker between Csk SH3 and SH2 domains is red and the Csk SH2-kinase linker region is colored green. The expanded view shows the details of Csk activation by the SH3-SH2 and SH2-kinase linkers. Side chains that stabilize the active conformation of the C-helix [M410 (of Itk) and W188 (of Csk)] are shown in gold sticks. The position of the Itk W355 is predicted to be in the same location as that of Csk W188.
The available crystal structures of the Tec family kinase domains do not contain any of these regulatory components; neither SH3 nor SH2 domain is present nor are the intervening linkers. In particular, the SH2/kinase domain linker region that contains the conserved tryptophan residue is absent in the Itk constructs used for crystallization. Thus, using available structures we cannot assess structural similarities and differences between Itk and Csk. Nevertheless, an overlay of the Itk structures that are available with the Csk structure suggests a mutational strategy to pin down the regulatory mechanism of Itk (106).
Like Csk, the tryptophan residue in the linker preceding the Itk kinase domain is required to couple the Itk regulatory domains to the kinase domain. Point mutation of this conserved Trp (W355 in Itk) reduces the activity of full-length Itk, Tec, and Btk to the low level associated with the isolated kinase domains in each case (106). Itk also contains a methionine at position 410 that is present in the Itk structure and is uniquely conserved within the Tec family kinases. The structural superposition with Csk (Fig. 7) suggests that Met 410 at the end of the C-helix makes direct contact with the critical tryptophan side chain and thus stabilizes the active conformation of the C-helix. Indeed, mutation of Met 410 in the context of full length Itk abolishes kinase activity, suggesting a critical role for this side chain as well (106). Thus, interactions between the Itk linker and the N-terminal kinase lobe likely impinge on the C-helix stabilizing Itk in the active conformation. Removing the stabilizing contacts [either by point mutation of critical residues (106) or by wholesale deletion of the linkers and regulatory domains of Itk (105, 106)] leads to a complete loss of catalytic activity as the C-helix reverts back to its inactive orientation. Thus, a structural picture of the active configuration of the Tec kinases is beginning to emerge. The SH2-kinase linker region is particularly important and must be available to make specific contacts with the N-terminal lobe of the kinase domain. This activation mechanism is consideredagain after discussion of possible mechanisms that serve to inactivate Tec kinase activity.
Substrate recognition: how is specificity controlled?
Once activated, the Tec kinases phosphorylate a tyrosine within their own SH3 domain as well as exogenous downstream substrates. Recognition of appropriate substrates in the context of complex signaling pathways remains an area of active research especially for the protein tyrosine kinases (116, 117). While localization of proteins in distinct regions of the cell can restrict access to certain substrates, it is not sufficient to explain the specificity of these enzymes in vivo (118–120).
The crystal structures of kinases in complex with peptide substrates have shed considerable light on the mechanism by which kinases recognize their substrates (116, 121). The peptide substrate binds to the C-terminal lobe of the kinase forming an anti-parallel β sheet with the activation loop (116, 121) (Fig. 8). Some kinases have exhibited sequence preferences for their substrates with selective residues at the P 1, P+1, and P+3 positions relative to the substrate tyrosine (116). The insulin receptor kinase (Irk), for example, has a substrate sequence preference of YMXM (122). The crystal structure of the Irk in complex with a peptide substrate containing this sequence shows that the methionine residues are located in hydrophobic pockets on the surface of the Irk kinase domain (Fig. 8), thereby explaining its strict sequence preference (121). However, not all kinases exhibit such strict sequence requirements. Instead, substrate specificity can be achieved via remote substrate docking interactions (119).
Fig. 8. Peptide substrate recognition.
A detailed view of the active site of insulin receptor kinase (Irk) shows a peptide substrate, containing the sequence YMNM (orange) forming an anti-parallel β sheet with the activation loop of the kinase (labeled and indicated by dashed box). The kinase is colored green and depicted with ribbons. The tyrosine (Y) and two methionines (M) of the peptide substrate are labeled. The Irk active site also contains the non-hydrolyzable ATP analog adenylyl diphosphate (AMP-PNP), depicted with sticks and colored red. The C-helix of the N-terminal kinase lobe is labeled.
By definition, substrate docking occurs when a specific region on the substrate (remote from the site to be phosphorylated) binds to the kinase domain on a site distinct from the active site (119). This mechanism, though commonly described for serine/threonine kinases, has been less commonly described for the tyrosine kinases. It has been suggested that one reason for the difference is that serine/threonine kinases evolved to develop docking sites as they sometimes lack interaction modules such as the SH3 and SH2 domains that are often present in tyrosine kinases (118). That said, a specific docking interaction has been described for the tyrosine kinase Csk (123–125), and it is in fact likely that most kinases make use of docking interactions to ensure specificity.
Our attention was drawn to the possibility that the Tec tyrosine kinases might also exploit a substrate docking mechanism based on simple sequence comparison of the two established Itk substrates in T cells. As already described, Itk autophosphorylates Y180 within its own SH3 domain. As well, Itk phosphorylates a specific tyrosine residue (Y783) in the exogenous substrate phospholipase Cγ1 (PLCγ1). The Y180 autophosphorylation site is contained with the LVIALY180DYQTN sequence, and the sequence surrounding the Y783 phosphorylation site in PLCγ1 is RNPGFY783VEANP. It is immediately evident that local sequence similarities are lacking (particularly at the P+1 and P+3 positions; the P 1 positions are both hydrophobic but for PLCγ1 it is aromatic), and so the Itk kinase active site must achieve specificity for these target tyrosines by an alternative means.
Another significant clue that the Tec kinases employ a substrate docking mechanism to achieve specific substrate phosphorylation comes from the surprising observation that none of the full length Itk, Tec, or Btk enzymes phosphorylate the autophosphorylation sites within their isolated SH3 domains in vitro (Y180, Y187 and Y223 for Itk, Tec and Btk respectively) (126). Efficient phosphorylation is only observed when these substrates contain both the SH3 domain (which carries the target tyrosine residue) and the neighboring SH2 domain (126). In complete agreement with these observations, phosphorylation of the PLCγ1 substrate by Itk follows the same pattern. Phosphorylation efficiency for Y783 increases dramatically for PLCγ1 substrates that include the PLCγ1 SH2 domain adjacent to the target tyrosine at position 783 (126). Detailed examination of this phenomenon reveals that the SH2 domain serves a specific docking role for Tec kinase substrate phosphorylation (126). The SH2 domains of Itk substrates bind directly to the Itk kinase domain. This binding is independent of classical pY binding (mutation of the conserved arginine in the pY pocket has no impact on the docking interaction or substrate phosphorylation) but docking rather involves a distinct face of the SH2 domain. Moreover, Itk substrate phosphorylation is efficiently inhibited by the presence of free SH2 domain in the kinase assay (126).
To fully understand the Tec kinase docking mechanism, these biochemical data (126) need to be translated into a molecular level understanding of just how kinase domain and SH2 domain come together to facilitate and control target phosphorylation. Clearly, in this vein, a high-resolution structure of the docked complex will be invaluable. In lieu of such a structure, results of mutagenesis experiments can guide model building to more fully characterize the docking motifs on both the enzyme and substrate.
Two protein surfaces need to be identified; the Itk kinase domain presents a docking site that binds to a specific surface of the Itk and PLCγ1 SH2 domains. The kinase docking sites may or may not be the same for each substrate. For both, however, there is evidence that binding occurs outside of the kinase active site, as binding of the isolated SH2 domains to the Itk kinase domain does not affect the phosphorylation kinetics of a short peptide substrate (126). This suggests that SH2 docking onto the Itk kinase domain surface does not impede access to the active site. The SH2 side of each kinase substrate interaction has been identified by direct mutagenesis (Min L, Joseph RE, Andreotti AH, unpublished data). For both the Itk SH2 domain and the PLCγ1 SH2 domain, a cluster of basic residues is necessary to mediate substrate phosphorylation. Mutation of specific SH2 residues that are remote from the site of phosphorylation in each substrate (and outside of the classical pY binding pockets) leads to complete loss of Y180 or Y783 phosphorylation by Itk.
Given these emerging clues, we can begin to visualize how the Tec kinases achieve specificity via docking interactions with the SH2 domains of their substrates (Figs 9 and 10). The basic nature of the SH2 binding site prompted examination of the surface exposed residues on the Itk kinase domain. A cluster of acidic side chains is evident on the N-terminal lobe of the kinase domain. Simple juxtaposition of the PLCγ1 SH2 domain and the Itk kinase domain that places the charged surfaces together shows that the C-terminus of the PLCγ1 SH2 domain points directly toward the kinase active site (Fig. 9). Since Y783 is located about 20 residues beyond the C-terminus of the SH2 domain, one can readily envision how this docking arrangement could facilitate appropriate placement of this target tyrosine into the Itk active site. As well, this model would be consistent with the data that show SH2 binding to the Itk kinase domain does not inhibit phosphorylation of a short peptide substrate; the Itk active site is not obstructed by the SH2 binding (126). Another feature of this docking model is that the phospholigand-binding pocket of the SH2 domain of PLCγ1 remains accessible for ligand binding; this is consistent with biochemical data showing that the phosphotyrosine binding pocket is not involved in the SH2/kinase docking interaction (126, Min L, Joseph RE, Andreotti AH, unpublished data).
Fig. 9. Itk substrate recognition model: PLC.
γ1. A direct interaction between the Itk kinase domain and the SH2 domain of PLCγ1 mediates specific phosphorylation of Y783 in PLCγ1. The docking interface consists of a cluster of basic residues on the PLCγ1 SH2 domain that do not overlap with the classical phosphotyrosine-binding functions of the SH2 domain and an as yet undetermined surface on the Itk kinase domain. We speculate docking occurs on the N-terminal lobe (labeled N-lobe) based on a potentially complementary cluster of acidic residues in this region. The Itk kinase domain is depicted here as a surface representation and the PLCγ1 SH2 domain structure (pdb 2pld) is show with ribbons (brown). SH2 interface residues identified by mutation are indicated in blue. SH2-bound phosphopeptide is shown (cyan) and would be compatible with the proposed docking model. The C-terminus of the PLCγ1 SH2 structure is labeled (C), and the model suggests that Y783 would be positioned into the active site as indicated by the arrow.
Fig. 10. Itk autophosphorylation model.
(A) The Itk kinase domain (1snx), depicted in brown ribbons, was superimposed with the Irk structure (1irk). As already shown in Fig. 8, the Irk structure includes a peptide substrate and the non-hydrolyzable ATP analog AMP-PNP. For clarity, only the Itk kinase domain structure, AMP-PNP (in blue), and the Irk peptide substrate (in orange) are shown. Next, the side chain of the Itk autophosphorylation tyrosine (Y180 shown in red) within the Itk SH3 domain was superimposed with the substrate tyrosine of the YMNM peptide in the Irk structure. The Itk SH3 domain (in light brown) was not further adjusted within the active site. The proximity of the βA strand of the SH3 domain and the Irk peptide substrate is evident in the model. The activation loop is not defined in the Itk kinase domain structure. The predicted Itk SH2 domain docking site is circled. (B) Surface rendering of the same model shown in (A). The excellent fit of the Itk SH3 domain in the active site cleft of Itk is evident in this representation. As in (A), the ATP analog is depicted in blue and Y180 in the SH3 domain is red.
The SH2-mediated docking interaction that leads to autophosphorylation of Y180 within Itk can be considered separately. For this substrate, the target tyrosine is embedded in the confines of the SH3 domain tertiary structure. In an effort to generate a model of Y180 docked into the active site of the Itk kinase domain, a different kinase/substrate structure was used as a template (66). The Itk kinase domain was superimposed with the Irk structure [solved in complex with a tyrosine containing peptide substrate and the non-hydrolyzable ATP analog, adenylyl imidodiphosphate (AMP-PNP)] (Fig. 8). The Y180 side chain within the Itk SH3 domain was then aligned with the peptide substrate tyrosine (Fig. 10). In this simple model, the SH3 domain fits extremely well into the Itk kinase active site (Fig. 10B). Moreover, like the Irk substrate and other kinase substrate complexes for which structures have been solved (116, 121), the β strand in the SH3 domain encompassing Itk Y180 (βA using standard SH3 domain nomenclature) runs anti-parallel to the Itk kinase activation loop (Fig. 10A).
Biochemical data show that this SH3 domain does not get phosphorylated on Y180 without a direct docking interaction between the adjacent SH2 domain and the Itk kinase domain (126). Moreover, Itk autophosphorylation occurs 'in cis'; the Itk kinase domain mediates phosphorylation of its own SH3 domain rather than an SH3 domain from another Itk molecule (66). Building on this information and the available enzyme/substrate complex model (Fig. 10), we can consider that the Itk SH2 domain is covalently linked to the C-terminus of the Itk SH3 domain and to the N-terminus of the Itk kinase domain via the linker that makes activating contacts across the back of the N-terminal kinase lobe (106)(Fig. 7). These covalent restraints together with the observation that the Itk SH2 domain interacts directly with the Itk kinase domain allows us to suggest that the Itk SH2 domain docks onto the N-terminal lobe of the kinase domain (Fig. 10). The resulting quaternary arrangement of the Itk SH3-SH2-casette would be distinct from that previously observed for other tyrosine kinases such as Src. The model also predicts that the Itk regulatory domains exhibit sufficient flexibility to adopt the docked conformation required for autophosphorylation. Prior to and post autophosphorylation, the Itk SH3 domain must move away from the kinase active site.
The proposed docking sites for substrate SH2 domains illustrated in Fig 9 and 10 need to be considered in light of data suggesting that, like Csk (Fig. 7), the SH2 domain of Itk is situated just above the active site and in contact with the N-terminal kinase lobe when Itk is active. During Itk autophosphorylation, we wonder whether the Itk SH2 domain serves two simultaneous roles, both activating the Itk kinase domain and positioning the SH3 domain into the active site. After autophosphorylation, the SH3 domain would swing out of the active site, and the SH2 domain would remain in its activating position. In considering Itk-mediated phosphorylation of the exogenous substrate PLCγ1, the same question needs to be answered. Does the PLCγ1 SH2 domain dock onto the Itk kinase domain in a manner that allows simultaneous activating interactions between Itk kinase domain and Itk SH2 domain? Perhaps the PLCγ1 SH2 domain docks onto a shared surface of the Itk SH2 and kinase domains in a manner that maintains the active Itk conformation (6). Or alternatively, does the PLCγ1 SH2 domain transiently displace the Itk SH2 domain keeping Itk in its active conformation during catalysis? Confirmation of these various models awaits further mutagenesis and structure determination of multi-domain fragments or full-length Tec kinases. Also, the C-terminal lobe of the Itk kinase domain has not been ruled out as the substrate-docking site; indeed the other tyrosine kinase for which substrate docking has been described is Csk (123–125). In that case, the substrate (Src) docks onto the larger C-terminal lobe to position the Src tail into the Csk active site (125).
Inactivating the Tec kinases the ‘off switch’
Of equal importance to the activation mechanisms of protein kinases is negative regulation. Catalytic activity must be negatively regulated both prior to and following activation. Layers of regulatory interactions often exist to achieve tight control of catalytic activity (23). While sequestering the activators of Tec kinases at the membrane will ensure that Tec kinase activation occurs only after the generation of lipid second messengers (39, 42), it does not preclude the existence of additional modes of negative regulation.
All of the Tec kinases exhibit intermolecular self-association via their non-catalytic regulatory domains. Btk, Tec, and Txk fragments self-associate through classical SH3/proline interactions (28, 70, 71), while for Itk, the SH3 and SH2 domains interact in a manner that mediates intermolecular self-association (90). In addition to SH3-mediated intermolecular self-association, the Itk PH domain also interacts with itself in an intermolecular fashion (51), and the structure of the Btk PH domain reveals a dimeric arrangement (25). The molecular details of PH domain self-association in solution are not yet known, but it seems likely that intermolecular contacts across the entire regulatory region of the Tec kinases serve to stabilize the self-associated form. We next consider whether these intermolecular domain interactions might impact the activation status of the kinase domain itself.
In considering the functional implications of intermolecular domain interactions among the Tec kinases, the observations of domain fragment interactions (28, 51, 70, 71, 90) must first be extended to full-length protein. Along these lines, full length Itk readily co-immunoprecipitates with itself (51, 91). As well, Itk clustering at the T-cell receptor has been observed upon T-cell activation (127). Building on these observations, we tested in vitro catalytic activity as a function of Itk enzyme concentration. Notably, we find that for increasing concentrations of purified Itk, in vitro kinase activity does not increase linearly, as would be expected for a monomeric enzyme, but kinase activity instead is diminished as concentration increases suggesting that intermolecular self-association might negatively influence Itk function (Min L, Andreotti AH, unpublished observations). Further experiments have indicated that disrupting the SH3/SH2 interaction in the context of full-length Itk results in a simple linear increase in activity with increasing enzyme concentration. Thus, we can suggest that formation of self-associated species or Itk clusters via intermolecular regulatory domain interactions leads to a decrease in catalytic activity.
Mechanistically, several scenarios emerge from this hypothesis. A simple model for Itk autoinhibition would invoke steric blockage of the catalytic site upon intermolecular self-association. In fact, small-angle X-ray scattering analysis of full-length unphosphorylated (inactive) Btk shows a linear arrangement for the regulatory domains (128). Such an extended conformation could promote oligomerization and maintain Tec kinases in an inactive state by blocking access to the kinase active site. However, as already discussed, the isolated Itk kinase domain exhibits little or no catalytic activity by itself (105, 106), suggesting the possibility of a complementary or perhaps alternative autoinhibition mechanism. Intermolecular self-association of the Itk regulatory domains might compete with the arrangement of the regulatory domains in the active state of the kinase. In essence, intermolecular interactions between the PH, SH3, and SH2 domains of distinct Itk molecules could disfavor activating interactions between the regulatory domains of Itk and the N-terminal lobe of the kinase domain within the same molecule. The result would be similar to that observed upon deletion of the non-catalytic domains; intermolecular regulatory domain interactions pry the SH2 domain and SH2-kinase linker region away from the Itk kinase domain; the C-helix reverts to the inactive conformation and catalytic activity ceases.
The stoichiometry of Itk self-association is not known, but several clues now point to oligomerization rather than a simple monomer/dimer equilibrium. When first characterized by NMR spectroscopy (90), the SH3-SH2-containing fragment of Itk was considered primarily dimeric. This conclusion was based on measured linewidths and NMR diffusion data, both of which would have been weighted toward a dimeric species rather than larger aggregates, given the inherent line broadening and disappearance of the NMR signal with increasing molecular weight. More recent work using native gels clearly shows multiple bands consistent with multiple self-associating species for both the SH3-SH2 fragment of Itk and the full-length molecule (Joseph RE, Min L, Andreotti AH, unpublished observation). As well, the recently solved structure of the SH3/SH2 interaction is consistent with formation of oligomers rather than a simple head-to-tail dimer. The C-terminus of the SH3 domain and the N-terminus of the SH2 domain (location of adjacent SH2 and SH3 domains in the single polypeptide chains, respectively) protrude on opposite sides of the complex structure (Fig. 5). Thus, even given full flexibility in the SH3-SH2 linker region, it is difficult to visualize a head to tail dimer arrangement for this region of Itk.
Mounting evidence points to intermolecular association as a regulatory mode for Itk. Whether this structural characteristic predominates in resting cells and/or whether intermolecular contacts are responsible for turning Itk activity off following membrane proximal signaling is not yet clear. While the inter-molecular interaction between the Itk SH3 domain and the Itk SH2 domain can mediate oligomerization, the pY binding pocket remains accessible in the Itk SH3/SH2 complex structure (Fig. 5). It is therefore possible that the engagement of the Itk SH2 domain with activating phospholigands could disrupt the Itk SH3/SH2-mediated oligomer favoring Itk activation. A point mutant designed to mimic autophosphorylation in the Itk SH3 domain (Y180E) leads to a higher affinity for the Itk SH2 domain. Keeping in mind that this is a mutant protein and not a tyrosine-phosphorylated protein, it is nevertheless worth considering that autophosphorylation in the Itk SH3 domain might trigger downregulation by favoring intermolecular domain associations between different Itk molecules.
Another regulatory layer likely inhibiting the function of the Tec kinases involves association with exogenous proteins. For example, SAB (SH3 domain-binding protein that preferentially associates with Btk) and IBtk (inhibitor of Btk) are two proteins that inhibit the activity of Btk via a direct interaction with the Btk SH3 or PH domains, respectively (129, 130). For Itk, one of the first notions that emerged upon characterization of prolyl isomerization in the SH2 domain is the possible involvement of the peptidyl prolyl isomerases in regulating ligand binding and/or catalytic activity. The peptidyl prolyl isomerases (PPIases) consist of a class of enzymes that catalyze the interconversion between cis and trans imide bond conformers and have been most widely characterized for their action on short peptide substrates (131, 132).
The extent to which the PPIases, cyclophilin A (CypA) or FKBP (FK506 binding protein), directly interact with the Itk SH2 domain was originally examined using NMR spectroscopy (133). Spectral changes were observed upon addition of CypA but not FKBP to the Itk SH2 domain and initial interpretation of the data suggested that CypA catalyzes the slow cis/trans isomerization based on line broadening (133). Analysis since that time revealed that the NMR linewidth changes were due instead to paramagnetic impurities associated with the CypA sample used. Line broadening was reversed upon addition of ethylene diamene tetraacetic acid to the NMR sample. Even so, chemical shift perturbations in the Itk SH2 domain do arise upon addition of CypA, indicating the presence of an interaction in the NMR tube.
Both biochemically and in T cells, Itk function is regulated by the peptidyl prolyl isomerase CypA (91, 133). Specifically, Itk autophosphorylation is diminished in vitro in the presence of CypA (133), and increased immunoglobulin E levels are seen in CypA knockout mice, indicative of increased Itk signaling (91). Further functional data suggest that the Itk/SLP-76 interaction is enhanced in CypA-depleted cells and that CypA association with Itk increases Itk oligomerization (91). All of these pieces of published data point to a regulatory interaction between Itk and CypA, yet mechanistic insight into just how these proteins interact and alter signaling remains elusive.
Further observations on CypA and Itk function are allowing us to generate testable hypotheses regarding the manner in which CypA modulates Itk signaling. In a simple in vitro kinase assay using purified Itk, we find that CypA has no effect on phosphorylation kinetics of a short peptide substrate. In contrast, in vitro phosphorylation of a protein substrate is diminished in the presence of CypA (Xu R, Andreotti AH, unpublished observations), consistent with the decreased autophosphorylation of Itk observed in the presence of CypA (133). These findings suggest that CypA does not alter the kinase activity of Itk per se but might rather inhibit accessibility to physiological substrates. Whether CypA directly affects the substrate docking mechanism we have described above or elicits an effect on protein substrates by another means remains to be determined.
Bringing the conformational snapshots together
Structural biology plays an important role in elucidating regulatory mechanisms of signaling proteins. Ultimately, structural approaches combined with biochemistry, genetics, and cell biological approaches will lead to a full appreciation of the interplay between the intra- and intermolecular interactions that control Tec kinase function. In this review, we have brought together the available pieces of structural knowledge to begin to make sense of the complex regulatory features of this family of immunological kinases. The salient features of the preceding figures and discussion are summarized in Fig. 11.
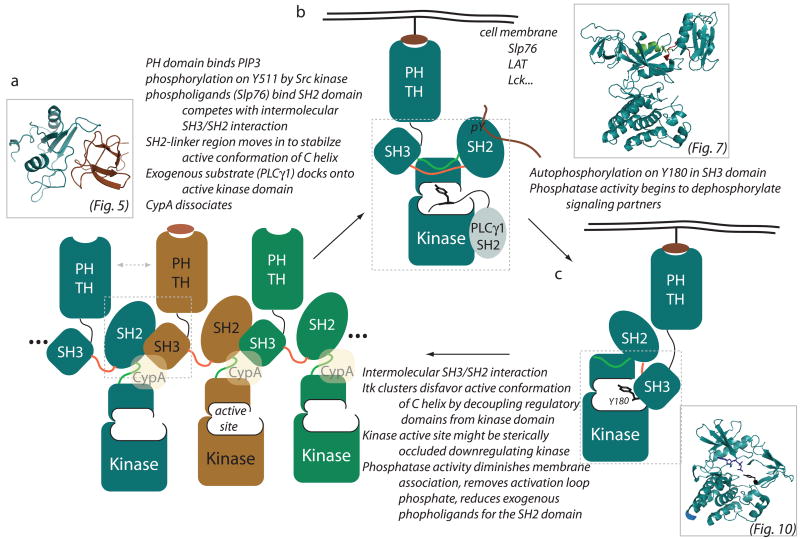
Fig. 11. Conformational snapshots of Itk signaling.
Cartoon depiction of the Itk regulatory apparatus. (A) Intermolecular self-association of the Tec family regulatory domains suggest that these kinases might cluster or oligomerize within the cell. For Itk, the intermolecular interactions consist of the SH3/SH2 complex [structure shown in inset (see also Fig. 5); corresponding region is boxed on cartoon] as well as biochemical data showing PH domain self-association (indicated by double headed dotted arrow). Building from the SH3/SH2 structure, a cartoon representation of oligomerized Itk is shown. Individual molecules of Itk are colored differently (blue, brown, green), and the possibility of larger clusters is indicated on both sides. The SH2-SH3 linker region is colored red, and the SH2-kinase linker is green (corresponding to color scheme in Fig. 7). The kinase active site is labeled on one subunit of the oligomer, and CypA is included to represent its putative regulatory interaction with Itk. The orange oval bound to one of the PH domains indicates the possible role of IP4 in regulating Itk signaling. (B) A number of activating signals upon receptor engagement have been well described and are listed above the arrow going from (A) to (B). The cartoon representation of Itk in (B) then depicts the possible conformation and location of active Itk. A monomeric Itk is shown, but the actual aggregation state of Itk at the membrane (whether still partially or wholly self-associated) is not known. PH domain binding to phospholipids generated by PI3K brings Itk to the membrane where the enzyme is co-localized with activating signals from Slp76, LAT and Lck. Phospholigand bound to the SH2 domain is shown. The SH3-SH2-kinase region is boxed and the corresponding Csk structure after which the cartoon is modeled is shown in the inset (see also Fig. 7). Biochemical data suggest that Tec kinases (like Csk) are positively regulated by their regulatory domains; in particular the SH2 and SH2-kinase linker regions. This linker (shown in green) packs against the small lobe of the kinase positioning the C-helix appropriately for catalytic activity to commence. Substrate recognition via SH2 docking onto the Itk kinase domain is indicated for the PLCγ1 substrate of Itk (gray). Location of substrate docking on the Itk kinase domain in not known and is therefore intentionally shown in a position that is different from that depicted in Fig. 9. (C) In cis autophosphorylation of Y180 in the Itk SH3 domain is depicted. The structural model of Y180 phosphorylation (based on the Irk crystal structure complexed with a short peptide substrate; see Fig. 10) is shown in the inset and the corresponding kinase/SH3 portion of the cartoon is boxed. Intramolecular SH2 docking is required for autophosphorylation. The possibility that phosphorylation on Y180 might shift Itk toward the self-association state is discussed in the text.
The non-catalytic regulatory domains of Itk and family members participate in a number of canonical and non-canonical interactions that control both activation and sequestration of Tec kinase activity during signaling. One of the first non-classical interactions identified for Itk involves the intermolecular interaction between the SH3 and SH2 domain. This protein-protein interaction makes use of the unusual cis prolyl imide bond conformation in the SH2 domain and appears to contribute to intermolecular clustering of full length Itk (Fig. 11A). Biochemical data indicate that increased Itk clustering leads to a decrease in catalytic activity. As well, mutation of the conformationally heterogeneous proline in Itk to a residue that cannot access the cis imide bond conformation leads to an increase in IL-4 production in wild type mice (91), possibly due to a shift in equilibrium away from the self-associated state. Thus, we propose that the inactive state of Itk consists, at least in part, of self-associated molecules that either serve to decouple the regulatory domains from the kinase domain or sterically block the kinase active site or both. Furthermore, the negative regulatory effect of CypA might be to stabilize the self-associated form or block access to substrate or both (Fig. 11A).
Crystals structures of the isolated Tec family kinase domains along with extensive kinetic characterization of mutants and deletion fragments suggest that like the Csk tyrosine kinase, the SH3-SH2 cassette and adjacent linker regions of the Tec kinases are required to stabilize the active state of the kinase domain (Fig. 11B). Triggering the switch between the resting state and this activated state could be PH domain association with membrane lipids, binding of transient phospholigands to the SH2 domain (this competes directly with the SH3/SH2 intermolecular interaction), and of course phosphorylation of the Tec kinase activation loop tyrosine by Src family kinases. Upon activation, the Tec kinases engage the SH2 domain of their target substrates (via a non-canonical interaction interface) to achieve specificity in the phosphorylation reaction. It will be interesting to ascertain just how the Itk kinase domain, for example, is activated by its adjacent regulatory region and at the same time docks onto the SH2 domain of downstream substrates.
In addition to downstream Tec substrates that carry the activation signal forward, the Tec kinases autophosphorylate a conserved tyrosine in their own SH3 domain (Fig. 11C). In putting these pieces of information into context, it is interesting to note that Wilcox and Berg (56) show that the Itk Y180F mutant can still be phosphorylated on Y511 in Sf9 cells, yet the Itk Y511F mutant is not phosphorylated on Y180. These data, perhaps not surprisingly, suggest that Y511 phosphorylation (Itk activation) occurs prior to autophosphorylation. How then does autophosphorylation fit into the signaling cascade? Autophosphorylation clearly serves to fine tune ligand binding of the SH3 domain. Another possibility is that phosphorylation of Y180 in Itk is a step toward inactivation of Itk. The enhanced affinity between the Itk SH3 and SH2 domains that might result from phosphorylation on Y180 would shift the ensemble of Itk molecules toward the self-associated state. As well, in cis SH2-mediated substrate docking required to autophosphorylate the SH3 domain might displace exogenous ligands. Certainly additional factors will be at work as the Tec kinases are ‘turned off’, such as phosphatase activity leading to the disappearance of the phospholigands that bind the SH2 domain, diminished membrane association due to the action of lipid phosphatases, and dephosphorylation of the activation loop tyrosine.
The next step to advance our knowledge in this area will be crystallization of multidomain fragments and/or the full-length Tec kinases. High-resolution structures of members of the Tec kinases as well as enzyme/substrate complexes of Tec kinases are eagerly awaited. These structures will be key to testing the proposed activation models and for unraveling the precise mechanisms underlying Tec kinase regulation.
Acknowledgments
The authors thank Dr. D. Bruce Fulton, Dr. Lie Min, Dr. Leslie Berg, Dr. Wenfang Wu, Dr. Pamela Schwartzberg, Andrew Severin, and Ruo Xu for stimulating discussions on Tec family kinase regulation. Funding for this work comes from the NIH (NIAID) and the Roy J. Carver Charitable Trust.
References
- 1.Cowan-Jacob SW, et al. The crystal structure of a c-Src complex in an active conformation suggests possible steps in c-Src activation. Structure. 2005;13:861–871. doi: 10.1016/j.str.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Deindl S, Kadlecek TA, Brdicka T, Cao X, Weiss A, Kuriyan J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell. 2007;129:735–746. doi: 10.1016/j.cell.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–282. doi: 10.1016/s0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 4.Nagar B, et al. Organization of the SH3-SH2 unit in active and inactive forms of the cAbl tyrosine kinase. Mol Cell. 2006;21:787–798. doi: 10.1016/j.molcel.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol. 1997;7:777–785. doi: 10.1016/s0959-440x(97)80146-7. [DOI] [PubMed] [Google Scholar]
- 6.Filippakopoulos P, et al. Structural coupling of SH2-kinase domains links Fes and Abl substrate recognition and kinase activation. Cell. 2008;134:793–803. doi: 10.1016/j.cell.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan B, et al. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol. 2003;5:155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- 8.Latour S, et al. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat Cell Biol. 2003;5:149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- 9.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 10.Schwartzberg PL, Finkelstein LD, Readinger JA. TEC-family kinases: regulators of T- helper-cell differentiation. Nat Rev Immunol. 2005;5:284–295. doi: 10.1038/nri1591. [DOI] [PubMed] [Google Scholar]
- 11.Felices M, Falk M, Kosaka Y, Berg LJ. Tec kinases in T cell and mast cell signaling. Adv Immunol. 2007;93:145–184. doi: 10.1016/S0065-2776(06)93004-1. [DOI] [PubMed] [Google Scholar]
- 12.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 13.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 14.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 15.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 16.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T- cell development. Nat Rev Immunol. 2007;7:479–485. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 17.Smith CI, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. Bioessays. 2001;23:436–446. doi: 10.1002/bies.1062. [DOI] [PubMed] [Google Scholar]
- 18.Vihinen M, Mattsson PT, Smith CI. Bruton tyrosine kinase (BTK) in X-linked agammaglobulinemia (XLA) Front Biosci. 2000;5:D917–928. doi: 10.2741/vihinen. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Rodriguez J, Readinger JA, Viorritto IC, Mueller KL, Houghtling RA, Schwartzberg PL. Tec kinases, actin, and cell adhesion. Immunol Rev. 2007;218:45–64. doi: 10.1111/j.1600-065X.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 20.Mihara S, Suzuki N. Role of Txk, a member of the Tec family of tyrosine kinases, in immune-inflammatory diseases. Int Rev Immunol. 2007;26:333–348. doi: 10.1080/08830180701690835. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt U, Boucheron N, Unger B, Ellmeier W. The role of Tec family kinases in myeloid cells. Int Arch Allergy Immunol. 2004;134:65–78. doi: 10.1159/000078339. [DOI] [PubMed] [Google Scholar]
- 22.Mano H, Ishikawa F, Nishida J, Hirai H, Takaku F. A novel protein-tyrosine kinase, tec, is preferentially expressed in liver. Oncogene. 1990;5:1781–1786. [PubMed] [Google Scholar]
- 23.Engen JR, et al. Structure and dynamic regulation of Src-family kinases. Cell Mol Life Sci. 2008;65:3058–3073. doi: 10.1007/s00018-008-8122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baraldi E, et al. Structure of the PH domain from Bruton's tyrosine kinase in complex with inositol 1,3,4,5-tetrakisphosphate. Structure. 1999;7:449–460. doi: 10.1016/s0969-2126(99)80057-4. [DOI] [PubMed] [Google Scholar]
- 25.Hyvonen M, Saraste M. Structure of the PH domain and Btk motif from Bruton's tyrosine kinase: molecular explanations for X-linked agammaglobulinaemia. EMBO J. 1997;16:3396–3404. doi: 10.1093/emboj/16.12.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murayama K, et al. Crystal structure of the Bruton's tyrosine kinase PH domain with phosphatidylinositol. Biochem Biophys Res Commun. 2008 doi: 10.1016/j.bbrc.2008.09.055. in press. [DOI] [PubMed] [Google Scholar]
- 27.Hansson H, et al. Solution structure of the SH3 domain from Bruton's tyrosine kinase. Biochemistry. 1998;37:2912–2924. doi: 10.1021/bi972409f. [DOI] [PubMed] [Google Scholar]
- 28.Pursglove SE, Mulhern TD, Mackay JP, Hinds MG, Booker GW. The solution structure and intramolecular associations of the Tec kinase SRC homology 3 domain. J Biol Chem. 2002;277:755–762. doi: 10.1074/jbc.M108318200. [DOI] [PubMed] [Google Scholar]
- 29.Severin A, Fulton DB, Andreotti AH. Murine Itk SH3 domain. J Biomol NMR. 2008;40:285–290. doi: 10.1007/s10858-008-9231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzeng SR, Lou YC, Pai MT, Jain ML, Cheng JW. Solution structure of the human BTK SH3 domain complexed with a proline-rich peptide from p120cbl. J Biomol NMR. 2000;16:303–312. doi: 10.1023/a:1008376624863. [DOI] [PubMed] [Google Scholar]
- 31.Huang KC, Cheng HT, Pai MT, Tzeng SR, Cheng JW. Solution structure and phosphopeptide binding of the SH2 domain from the human Bruton's tyrosine kinase. J Biomol NMR. 2006;36:73–78. doi: 10.1007/s10858-006-9064-3. [DOI] [PubMed] [Google Scholar]
- 32.Mallis RJ, Brazin KN, Fulton DB, Andreotti AH. Structural characterization of a proline-driven conformational switch within the Itk SH2 domain. Nat Struct Biol. 2002;9:900–905. doi: 10.1038/nsb864. [DOI] [PubMed] [Google Scholar]
- 33.Brown K, et al. Crystal structures of interleukin-2 tyrosine kinase and their implications for the design of selective inhibitors. J Biol Chem. 2004;279:18727–18732. doi: 10.1074/jbc.M400031200. [DOI] [PubMed] [Google Scholar]
- 34.Mao C, Zhou M, Uckun FM. Crystal structure of Bruton's tyrosine kinase domain suggests a novel pathway for activation and provides insights into the molecular basis of X-linked agammaglobulinemia. J Biol Chem. 2001;276:41435–41443. doi: 10.1074/jbc.M104828200. [DOI] [PubMed] [Google Scholar]
- 35.Cozier GE, Carlton J, Bouyoucef D, Cullen PJ. Membrane targeting by pleckstrin homology domains. Curr Top Microbiol Immunol. 2004;282:49–88. doi: 10.1007/978-3-642-18805-3_3. [DOI] [PubMed] [Google Scholar]
- 36.Lemmon MA. Pleckstrin homology (PH) domains and phosphoinositides. Biochem Soc Symp. 2007:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemmon MA, Ferguson KM. Molecular determinants in pleckstrin homology domains that allow specific recognition of phosphoinositides. Biochem Soc Trans. 2001;29:377–384. doi: 10.1042/bst0290377. [DOI] [PubMed] [Google Scholar]
- 38.Lemmon MA, Ferguson KM, Abrams CS. Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 2002;513:71–76. doi: 10.1016/s0014-5793(01)03243-4. [DOI] [PubMed] [Google Scholar]
- 39.August A, Sadra A, Dupont B, Hanafusa H. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci USA. 1997;94:11227–11232. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li T, Rawlings DJ, Park H, Kato RM, Witte ON, Satterthwaite AB. Constitutive membrane association potentiates activation of Bruton tyrosine kinase. Oncogene. 1997;15:1375–1383. doi: 10.1038/sj.onc.1201308. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Wahl MI, Eguinoa A, Stephens LR, Hawkins PT, Witte ON. Phosphatidylinositol 3-kinase-gamma activates Bruton's tyrosine kinase in concert with Src family kinases. Proc Natl Acad Sci USA. 1997;94:13820–13825. doi: 10.1073/pnas.94.25.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang WC, Ching KA, Tsoukas CD, Berg LJ. Tec kinase signaling in T cells is regulated by phosphatidylinositol 3-kinase and the Tec pleckstrin homology domain. J Immunol. 2001;166:387–395. doi: 10.4049/jimmunol.166.1.387. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson KM, et al. Structural basis for discrimination of 3-phosphoinositides by pleckstrin homology domains. Mol Cell. 2000;6:373–384. doi: 10.1016/s1097-2765(00)00037-x. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda M, Kojima T, Kabayama H, Mikoshiba K. Mutation of the pleckstrin homology domain of Bruton's tyrosine kinase in immunodeficiency impaired inositol 1,3,4,5-tetrakisphosphate binding capacity. J Biol Chem. 1996;271:30303–30306. doi: 10.1074/jbc.271.48.30303. [DOI] [PubMed] [Google Scholar]
- 45.Kojima T, Fukuda M, Watanabe Y, Hamazato F, Mikoshiba K. Characterization of the pleckstrin homology domain of Btk as an inositol polyphosphate and phosphoinositide binding domain. Biochem Biophys Res Commun. 1997;236:333–339. doi: 10.1006/bbrc.1997.6947. [DOI] [PubMed] [Google Scholar]
- 46.Salim K, et al. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 47.Varnai P, Rother KI, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J Biol Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]
- 48.Scharenberg AM, et al. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomlinson MG, Heath VL, Turck CW, Watson SP, Weiss A. SHIP family inositol phosphatases interact with and negatively regulate the Tec tyrosine kinase. J Biol Chem. 2004;279:55089–55096. doi: 10.1074/jbc.M408141200. [DOI] [PubMed] [Google Scholar]
- 50.Shan X, et al. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol. 2000;20:6945–6957. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang YH, et al. Positive regulation of Itk PH domain function by soluble IP4. Science. 2007;316:886–889. doi: 10.1126/science.1138684. [DOI] [PubMed] [Google Scholar]
- 52.Kaneko T, Li L, Li SS. The SH3 domain--a family of versatile peptide- and protein- recognition module. Front Biosci. 2008;13:4938–4952. doi: 10.2741/3053. [DOI] [PubMed] [Google Scholar]
- 53.Li SS. Specificity and versatility of SH3 and other proline-recognition domains: structural basis and implications for cellular signal transduction. Biochem J. 2005;390:641–653. doi: 10.1042/BJ20050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu H, Chen JK, Feng S, Dalgarno DC, Brauer AW, Schreiber SL. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994;76:933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- 55.Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL. Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature. 1997;385:93–97. doi: 10.1038/385093a0. [DOI] [PubMed] [Google Scholar]
- 56.Wilcox HM, Berg LJ. Itk phosphorylation sites are required for functional activity in primary T cells. J Biol Chem. 2003;278:37112–37121. doi: 10.1074/jbc.M304811200. [DOI] [PubMed] [Google Scholar]
- 57.Bunnell SC, Henry PA, Kolluri R, Kirchhausen T, Rickles RJ, Berg LJ. Identification of Itk/Tsk Src homology 3 domain ligands. J Biol Chem. 1996;271:25646–25656. doi: 10.1074/jbc.271.41.25646. [DOI] [PubMed] [Google Scholar]
- 58.Matsushita M, et al. Identification and characterization of a novel SH3-domain binding protein, Sab, which preferentially associates with Bruton's tyrosine kinase (BtK) Biochem Biophys Res Commun. 1998;245:337–343. doi: 10.1006/bbrc.1998.8420. [DOI] [PubMed] [Google Scholar]
- 59.Morrogh LM, Hinshelwood S, Costello P, Cory GO, Kinnon C. The SH3 domain of Bruton's tyrosine kinase displays altered ligand binding properties when auto-phosphorylated in vitro. Eur J Immunol. 1999;29:2269–2279. doi: 10.1002/(SICI)1521-4141(199907)29:07<2269::AID-IMMU2269>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 60.Garcon F, Ghiotto M, Gerard A, Yang WC, Olive D, Nunes JA. The SH3 domain of Tec kinase is essential for its targeting to activated CD28 costimulatory molecule. Eur J Immunol. 2004;34:1972–1980. doi: 10.1002/eji.200324777. [DOI] [PubMed] [Google Scholar]
- 61.Cory GO, Lovering RC, Hinshelwood S, MacCarthy-Morrogh L, Levinsky RJ, Kinnon C. The protein product of the c-cbl protooncogene is phosphorylated after B cell receptor stimulation and binds the SH3 domain of Bruton's tyrosine kinase. J Exp Med. 1995;182:611–615. doi: 10.1084/jem.182.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park H, et al. Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity. 1996;4:515–525. doi: 10.1016/s1074-7613(00)80417-3. [DOI] [PubMed] [Google Scholar]
- 63.Kashiwakura J, et al. Evidence of autophosphorylation in Txk: Y91 is an autophosphorylation site. Biol Pharm Bull. 2002;25:718–721. doi: 10.1248/bpb.25.718. [DOI] [PubMed] [Google Scholar]
- 64.Nore BF, et al. Identification of phosphorylation sites within the SH3 domains of Tec family tyrosine kinases. Biochim Biophys Acta. 2003;1645:123–132. doi: 10.1016/s1570-9639(02)00524-1. [DOI] [PubMed] [Google Scholar]
- 65.Kurosaki T, Kurosaki M. Transphosphorylation of Bruton's tyrosine kinase on tyrosine 551 is critical for B cell antigen receptor function. J Biol Chem. 1997;272:15595–15598. doi: 10.1074/jbc.272.25.15595. [DOI] [PubMed] [Google Scholar]
- 66.Joseph RE, Fulton DB, Andreotti AH. Mechanism and functional significance of Itk autophosphorylation. J Mol Biol. 2007;373:1281–1292. doi: 10.1016/j.jmb.2007.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng S, Chen JK, Yu H, Simon JA, Schreiber SL. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994;266:1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- 68.Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 69.Yang W, Malek SN, Desiderio S. An SH3-binding site conserved in Bruton's tyrosine kinase and related tyrosine kinases mediates specific protein interactions in vitro and in vivo. J Biol Chem. 1995;270:20832–20840. doi: 10.1074/jbc.270.35.20832. [DOI] [PubMed] [Google Scholar]
- 70.Laederach A, Cradic KW, Fulton DB, Andreotti AH. Determinants of intra versus intermolecular self-association within the regulatory domains of Rlk and Itk. J Mol Biol. 2003;329:1011–1020. doi: 10.1016/s0022-2836(03)00531-x. [DOI] [PubMed] [Google Scholar]
- 71.Laederach A, et al. Competing modes of self-association in the regulatory domains of Bruton's tyrosine kinase: intramolecular contact versus asymmetric homodimerization. Protein Sci. 2002;11:36–45. doi: 10.1110/ps.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansson H, Okoh MP, Smith CI, Vihinen M, Hard T. Intermolecular interactions between the SH3 domain and the proline-rich TH region of Bruton's tyrosine kinase. FEBS Lett. 2001;489:67–70. doi: 10.1016/s0014-5793(00)02438-8. [DOI] [PubMed] [Google Scholar]
- 73.Hao S, August A. The proline rich region of the Tec homology domain of ITK regulates its activity. FEBS Lett. 2002;525:53–58. doi: 10.1016/s0014-5793(02)03066-1. [DOI] [PubMed] [Google Scholar]
- 74.Mohamed AJ, Vargas L, Nore BF, Backesjo CM, Christensson B, Smith CI. Nucleocytoplasmic shuttling of Bruton's tyrosine kinase. J Biol Chem. 2000;275:40614–40619. doi: 10.1074/jbc.M006952200. [DOI] [PubMed] [Google Scholar]
- 75.Perez-Villar JJ, O'Day K, Hewgill DH, Nadler SG, Kanner SB. Nuclear localization of the tyrosine kinase Itk and interaction of its SH3 domain with karyopherin alpha (Rch1alpha) Int Immunol. 2001;13:1265–1274. doi: 10.1093/intimm/13.10.1265. [DOI] [PubMed] [Google Scholar]
- 76.Bradshaw JM, Waksman G. Molecular recognition by SH2 domains. Adv Protein Chem. 2002;61:161–210. doi: 10.1016/s0065-3233(02)61005-8. [DOI] [PubMed] [Google Scholar]
- 77.Schlessinger J, Lemmon MA. SH2 and PTB domains in tyrosine kinase signaling. Sci STKE. 2003;2003:RE12. doi: 10.1126/stke.2003.191.re12. [DOI] [PubMed] [Google Scholar]
- 78.Pawson T. SH2 and SH3 domains in signal transduction. Adv Cancer Res. 1994;64:87–110. doi: 10.1016/s0065-230x(08)60835-0. [DOI] [PubMed] [Google Scholar]
- 79.Marengere LE, Pawson T. Structure and function of SH2 domains. J Cell Sci Suppl. 1994;18:97–104. doi: 10.1242/jcs.1994.supplement_18.14. [DOI] [PubMed] [Google Scholar]
- 80.Pletneva EV, Sundd M, Fulton DB, Andreotti AH. Molecular details of Itk activation by prolyl isomerization and phospholigand binding: the NMR structure of the Itk SH2 domain bound to a phosphopeptide. J Mol Biol. 2006;357:550–561. doi: 10.1016/j.jmb.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 81.Takata M, et al. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bubeck Wardenburg J, et al. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol Chem. 1996;271:19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 83.Hashimoto S, et al. Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK--functional significance of Btk-SH2 domain in B-cell antigen receptor-coupled calcium signaling. Blood. 1999;94:2357–2364. [PubMed] [Google Scholar]
- 84.Su YW, Zhang Y, Schweikert J, Koretzky GA, Reth M, Wienands J. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur J Immunol. 1999;29:3702–3711. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 85.Ching KA, Grasis JA, Tailor P, Kawakami Y, Kawakami T, Tsoukas CD. TCR/CD3-Induced activation and binding of Emt/Itk to linker of activated T cell complexes: requirement for the Src homology 2 domain. J Immunol. 2000;165:256–262. doi: 10.4049/jimmunol.165.1.256. [DOI] [PubMed] [Google Scholar]
- 86.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci USA. 2007;104:6638–6643. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andreotti AH. Native state proline isomerization: an intrinsic molecular switch. Biochemistry. 2003;42:9515–9524. doi: 10.1021/bi0350710. [DOI] [PubMed] [Google Scholar]
- 88.Christoph Grathwohl KW. Nmr studies of the rates of proline cis-tran isomerization in oligopeptides. Biopolymers. 1981;20:2623–2633. [Google Scholar]
- 89.Breheny PJ, Laederach A, Fulton DB, Andreotti AH. Ligand specificity modulated by prolyl imide bond Cis/Trans isomerization in the Itk SH2 domain: a quantitative NMR study. J Am Chem Soc. 2003;125:15706–15707. doi: 10.1021/ja0375380. [DOI] [PubMed] [Google Scholar]
- 90.Brazin KN, Fulton DB, Andreotti AH. A specific intermolecular association between the regulatory domains of a Tec family kinase. J Mol Biol. 2000;302:607–623. doi: 10.1006/jmbi.2000.4091. [DOI] [PubMed] [Google Scholar]
- 91.Colgan J, et al. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21:189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 92.Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 93.Lamers MB, Antson AA, Hubbard RE, Scott RK, Williams DH. Structure of the protein tyrosine kinase domain of C-terminal Src kinase (CSK) in complex with staurosporine. J Mol Biol. 1999;285:713–725. doi: 10.1006/jmbi.1998.2369. [DOI] [PubMed] [Google Scholar]
- 94.Williams JC, et al. The 2.35 A crystal structure of the inactivated form of chicken Src: a dynamic molecule with multiple regulatory interactions. J Mol Biol. 1997;274:757–775. doi: 10.1006/jmbi.1997.1426. [DOI] [PubMed] [Google Scholar]
- 95.Yamaguchi H, Hendrickson WA. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature. 1996;384:484–489. doi: 10.1038/384484a0. [DOI] [PubMed] [Google Scholar]
- 96.Heyeck SD, Wilcox HM, Bunnell SC, Berg LJ. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J Biol Chem. 1997;272:25401–25408. doi: 10.1074/jbc.272.40.25401. [DOI] [PubMed] [Google Scholar]
- 97.Rawlings DJ, et al. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 98.Afar DE, Park H, Howell BW, Rawlings DJ, Cooper J, Witte ON. Regulation of Btk by Src family tyrosine kinases. Mol Cell Biol. 1996;16:3465–3471. doi: 10.1128/mcb.16.7.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prade L, Engh RA, Girod A, Kinzel V, Huber R, Bossemeyer D. Staurosporine-induced conformational changes of cAMP-dependent protein kinase catalytic subunit explain inhibitory potential. Structure. 1997;5:1627–1637. doi: 10.1016/s0969-2126(97)00310-9. [DOI] [PubMed] [Google Scholar]
- 100.Haire RN, Ohta Y, Lewis JE, Fu SM, Kroisel P, Litman GW. TXK, a novel human tyrosine kinase expressed in T cells shares sequence identity with Tec family kinases and maps to 4p12. Hum Mol Genet. 1994;3:897–901. doi: 10.1093/hmg/3.6.897. [DOI] [PubMed] [Google Scholar]
- 101.Tamagnone L, et al. BMX, a novel nonreceptor tyrosine kinase gene of the BTK/ITK/TEC/TXK family located in chromosome Xp22.2. Oncogene. 1994;9:3683–3688. [PubMed] [Google Scholar]
- 102.Siliciano JD, Morrow TA, Desiderio SV. Itk, a T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proc Natl Acad Sci USA. 1992;89:11194–11198. doi: 10.1073/pnas.89.23.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vetrie D, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 104.Heyeck SD, Berg LJ. Developmental regulation of a murine T-cell-specific tyrosine kinase gene, Tsk. Proc Natl Acad Sci USA. 1993;90:669–673. doi: 10.1073/pnas.90.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hawkins J, Marcy A. Characterization of Itk tyrosine kinase: contribution of noncatalytic domains to enzymatic activity. Protein Expr Purif. 2001;22:211–219. doi: 10.1006/prep.2001.1447. [DOI] [PubMed] [Google Scholar]
- 106.Joseph RE, Min L, Andreotti AH. The linker between SH2 and kinase domains positively regulates catalysis of the Tec family kinases. Biochemistry. 2007;46:5455–5462. doi: 10.1021/bi602512e. [DOI] [PubMed] [Google Scholar]
- 107.Veillette A, Caron L, Fournel M, Pawson T. Regulation of the enzymatic function of the lymphocyte-specific tyrosine protein kinase p56lck by the non-catalytic SH2 and SH3 domains. Oncogene. 1992;7:971–980. [PubMed] [Google Scholar]
- 108.Kato JY, Takeya T, Grandori C, Iba H, Levy JB, Hanafusa H. Amino acid substitutions sufficient to convert the nontransforming p60c-src protein to a transforming protein. Mol Cell Biol. 1986;6:4155–4160. doi: 10.1128/mcb.6.12.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ogawa A, et al. Structure of the carboxyl-terminal Src kinase, Csk. J Biol Chem. 2002;277:14351–14354. doi: 10.1074/jbc.C200086200. [DOI] [PubMed] [Google Scholar]
- 110.Sun G, Budde RJ. Mutations in the N-terminal regulatory region reduce the catalytic activity of Csk, but do not affect its recognition of Src. Arch Biochem Biophys. 1999;367:167–172. doi: 10.1006/abbi.1999.1253. [DOI] [PubMed] [Google Scholar]
- 111.Lin X, Ayrapetov MK, Lee S, Parang K, Sun G. Probing the communication between the regulatory and catalytic domains of a protein tyrosine kinase, Csk. Biochemistry. 2005;44:1561–1567. doi: 10.1021/bi048142j. [DOI] [PubMed] [Google Scholar]
- 112.Lin X, Wang Y, Ahmadibeni Y, Parang K, Sun G. Structural basis for domain-domain communication in a protein tyrosine kinase, the C-terminal Src kinase. J Mol Biol. 2006;357:1263–1273. doi: 10.1016/j.jmb.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 113.Mikkola ET, Bergman M. Conserved hydrophobicity in the SH2-kinase linker is required for catalytic activity of Csk and CHK. FEBS Lett. 2003;544:11–14. doi: 10.1016/s0014-5793(03)00405-8. [DOI] [PubMed] [Google Scholar]
- 114.Sondhi D, Cole PA. Domain interactions in protein tyrosine kinase Csk. Biochemistry. 1999;38:11147–11155. doi: 10.1021/bi990827+. [DOI] [PubMed] [Google Scholar]
- 115.Wong L, et al. Dynamic coupling between the SH2 domain and active site of the COOH terminal Src kinase, Csk. J Mol Biol. 2004;341:93–106. doi: 10.1016/j.jmb.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 116.Bose R, Holbert MA, Pickin KA, Cole PA. Protein tyrosine kinase-substrate interactions. Curr Opin Struct Biol. 2006;16:668–675. doi: 10.1016/j.sbi.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 117.Johnson SA, Hunter T. Kinomics: methods for deciphering the kinome. Nat Methods. 2005;2:17–25. doi: 10.1038/nmeth731. [DOI] [PubMed] [Google Scholar]
- 118.Remenyi A, Good MC, Lim WA. Docking interactions in protein kinase and phosphatase networks. Curr Opin Struct Biol. 2006;16:676–685. doi: 10.1016/j.sbi.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 119.Goldsmith EJ, Akella R, Min X, Zhou T, Humphreys JM. Substrate and docking interactions in serine/threonine protein kinases. Chem Rev. 2007;107:5065–5081. doi: 10.1021/cr068221w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 121.Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shoelson SE, Chatterjee S, Chaudhuri M, White MF. YMXM motifs of IRS-1 define substrate specificity of the insulin receptor kinase. Proc Natl Acad Sci USA. 1992;89:2027–2031. doi: 10.1073/pnas.89.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee S, Ayrapetov MK, Kemble DJ, Parang K, Sun G. Docking-based substrate recognition by the catalytic domain of a protein tyrosine kinase, C-terminal Src kinase (Csk) J Biol Chem. 2006;281:8183–8189. doi: 10.1074/jbc.M508120200. [DOI] [PubMed] [Google Scholar]
- 124.Lee S, Lin X, Nam NH, Parang K, Sun G. Determination of the substrate-docking site of protein tyrosine kinase C-terminal Src kinase. Proc Natl Acad Sci USA. 2003;100:14707–14712. doi: 10.1073/pnas.2534493100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Levinson NM, Seeliger MA, Cole PA, Kuriyan J. Structural basis for the recognition of c-Src by its inactivator Csk. Cell. 2008;134:124–134. doi: 10.1016/j.cell.2008.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Joseph RE, Min L, Xu R, Musselman ED, Andreotti AH. A remote substrate docking mechanism for the tec family tyrosine kinases. Biochemistry. 2007;46:5595–5603. doi: 10.1021/bi700127c. [DOI] [PubMed] [Google Scholar]
- 127.Qi Q, Sahu N, August A. Tec kinase Itk forms membrane clusters specifically in the vicinity of recruiting receptors. J Biol Chem. 2006;281:38529–38534. doi: 10.1074/jbc.M609180200. [DOI] [PubMed] [Google Scholar]
- 128.Marquez JA, et al. Conformation of full-length Bruton tyrosine kinase (Btk) from synchrotron X-ray solution scattering. EMBO J. 2003;22:4616–4624. doi: 10.1093/emboj/cdg448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu W, et al. Direct inhibition of Bruton's tyrosine kinase by IBtk, a Btk-binding protein. Nat Immunol. 2001;2:939–946. doi: 10.1038/ni1001-939. [DOI] [PubMed] [Google Scholar]
- 130.Yamadori T, et al. Bruton's tyrosine kinase activity is negatively regulated by Sab, the Btk-SH3 domain-binding protein. Proc Natl Acad Sci USA. 1999;96:6341–6346. doi: 10.1073/pnas.96.11.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang P, Heitman J. The cyclophilins. Genome Biol. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gothel SF, Marahiel MA. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol Life Sci. 1999;55:423–436. doi: 10.1007/s000180050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brazin KN, Mallis RJ, Fulton DB, Andreotti AH. Regulation of the tyrosine kinase Itk by the peptidyl-prolyl isomerase cyclophilin A. Proc Natl Acad Sci USA. 2002;99:1899–1904. doi: 10.1073/pnas.042529199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DeLano WL. The PyMOL Molecular Graphics System. 2002. [Google Scholar]