Abstract
Background
The role of magnesium in maintaining muscle integrity and function in older adults is largely unknown.
Objective
We aimed to investigate the relation between serum magnesium concentrations and muscle performance in older subjects.
Design
Data are from the baseline examination conducted between September 1998 and March 2000 of the InCHIANTI (aging in the Chianti area) study, a prospective epidemiologic survey of risk factors for late-life disability. From among 1453 randomly selected community residents completing a home interview, 1138 men (46%) and women (aged 66.7 ± 15.2 y; x̄ ± SD) with complete data on muscle performance and serum magnesium who were not severely cognitively compromised and had no evidence of kidney disease or hypercalcemia were included in the analysis. Muscle performance was evaluated by grip strength, lower-leg muscle power, knee extension torque, and ankle extension isometric strength and was normalized for age and body mass index (BMI) within each sex.
Results
After adjustment for age, sex, BMI, laboratory variables, presence of chronic diseases, muscle area, muscle density, and physical activity level, serum magnesium concentrations were significantly associated with indexes of muscle performance, including grip strength (β = 2.0 ± 0.5, P = 0.0002), lower-leg muscle power (β = 8.8 ± 2.7, P = 0.001), knee extension torque (β = 31.2 ± 7.9, P < 0.0001), and ankle extension strength (β = 3.8 ± 0.5, P < 0.0001).
Conclusions
The serum magnesium concentration is an independent correlate of muscle performance in older persons. Whether magnesium supplementation improves muscle function remains to be shown.
Keywords: Magnesium, aging, sarcopenia, muscle performance, muscle strength
INTRODUCTION
Magnesium, the second most abundant intracellular cation after potassium (1), is essential in a wide variety of fundamental cellular activities and metabolic pathways, such as peptide hormone receptor signal transduction, cellular glucose metabolism, stimulus-contraction coupling, stimulus-secretion coupling, and ion channel translocation (1, 2). Magnesium is implicated in this pleiomorphic activity because of its role as part of the activated MgATP complex required for the activity of all rate-limiting glycolytic enzymes, protein kinases, and more generally, all ATP- and phosphate transfer–associated enzymes. Magnesium may also bind the enzymes directly (eg, RNA and DNA polymerases) and alter their structure (1–4). Therefore, the availability of adequate quantities of magnesium may be considered a critical factor for normal human function.
In athletes, magnesium depletion is associated with structural damage to muscle cells, probably as a result of increased production of reactive oxygen species, lipid and protein damage, and impaired intracellular calcium homeostasis (5). Furthermore, adequate magnesium concentrations seem necessary for maintaining optimal muscle performance and exercise tolerance (6), and magnesium supplementation has been shown to significantly increase muscle strength in young subjects (7).
Older age is frequently characterized by sarcopenia, a loss of skeletal muscle mass and function (8) that is a strong independent risk factor for disability and mortality (9–14). Recent epidemiologic data (15–20) show that inadequate magnesium intake is common, especially in older persons (18–20). Because magnesium status is strictly related to muscle ATP, and because both magnesium deficiency and sarcopenia tend to be more prevalent at older ages, we hypothesized that poor magnesium status contributes to late-life sarcopenia.
This hypothesis is consistent with clinical and epidemiologic evidence supporting the importance of the magnesium ion as a determinant of muscle performance in young subjects. However, to the best of our knowledge, no one has explored the association of magnesium status and muscle performance in a representative cohort of older subjects. Thus, the present study aimed to investigate whether, independent of disease and metabolic disturbances, serum magnesium concentration is related to muscle performance in community-resident persons aged 65 y and older.
SUBJECTS AND METHODS
We analyzed data from the Invecchiare in Chianti (InCHIANTI, Aging in the Chianti area) study, a prospective population-based study of older persons that was designed by the Laboratory of Clinical Epidemiology of the Italian National Research Council of Aging (Florence, Italy) with the aim of identifying risk factors for mobility disability in older persons. Details of the design, sampling procedures, and data collection methods of the InCHIANTI study were published previously (21). At baseline, data were collected from 1453 participants, who were randomly selected from residents in 2 towns of the Chianti geographic area (Greve in Chianti and Bagno a Ripoli in Tuscany, Italy), who participated in an in-home interview between September 1998 and March 2000. The Italian National Research Council on Aging Ethical Committee approved the study protocol, which conformed to the principles of the Declaration of Helsinki for human research.
The present analyses were performed on 1138 (76.5% older than 65 y) of the initial 1453 subjects, excluding those in whom indexes of muscle performance or laboratory data on serum magnesium were not collected and those with evidence of chronic kidney disease (creatinine clearance < 30 mL/min), cognitive impairment (Mini-Mental State Examination score ≤ 18) (22), or hypercalcemia (serum calcium ≥ 5 mmol/dL).
Muscle performance
After a home interview, the participants received a comprehensive standardized medical and functional evaluation by a geriatrician and a physical therapist, respectively, both of whom had received specialized training on the assessment tools used in this study. Objective assessment of physical function was performed within 4 wk of the interview in a dedicated laboratory by use of a standard protocol (23). Strength was measured with isometric dynamometry to permit comparable strength assessment in the study clinic and in the participants’ homes for those unable to come to the study clinic. The dynamometers used were regularly calibrated.
Handgrip strength was measured in kilograms by using a hand-held dynamometer (hydraulic hand “baseline”; Smith & Nephew, Agrate Brianza, Milan, Italy). During testing, the participant was strongly encouraged to exhibit the best possible force. For the analysis, the best of 2 attempts with each hand were averaged.
Knee extension strength was measured with a hand-held dynamometer (Nicholas Muscle Tester; Sammon Preston Inc, Chicago, IL) according to a standard protocol (23). The participants lay down in lateral decubitus (opposite to the examined limb) with the hip and knee in 45° and 60° flexed positions, respectively, and were asked to perform the task twice with each leg. The subjects were instructed to exert maximal effort against the dynamometer, avoiding explosive movements. Strength was measured as the peak force that the examiner had to apply to break the isometric contraction, indicated by a slight movement of the subject’s leg in the direction opposite to the voluntary movement. The subject was also instructed to maintain a fixed posture during the entire testing procedure and to grasp the edge of the bed with the right hand when lying on the left side and vice versa, or with both hands when lying on the back, for stabilization. Measurements obtained when the subject was in an incorrect position or in case of explosive movements were considered invalid and were repeated until performed correctly. The average of the best results obtained for each leg was used for the present analyses. Knee extension torque was calculated according to the formula
| (1) |
where knee extension is the mean of repeated right and left measurements.
Maximum lower-extremity muscle power was measured in a single leg extension movement, according to the method described by Bassey and Short (24). The value of the best performance obtained over 8 repetitions each on the right and left sides were used in the analysis. Using this method, Bassey and Short (24) reported that the CV for retests obtained after 1 wk is 9.4%. Crude values of muscle power were divided by body weights, with the resulting value multiplied by the sex-specific average body weight for the study population, to obtain body-size-adjusted values.
Ankle extension isometric strength was measured with a hand-held dynamometer (Nicholas Muscle Tester, Sammon Preston, Chicago, IL). Participants lay down in lateral decubitus (opposite to the examined limb) with the hip and the knee extended and the ankle in a neutral position and were asked to perform the task twice with the right foot. The average of the results obtained was used for the analyses.
Muscle area, muscle density, and degree of physical activity
A lower-leg peripheral quantitative computerized tomography (pQCT) scan was performed in all study participants by using the Stratec XCT 2000 (Pforzheim, Germany). A detailed description of the pQCT exams has been published elsewhere (25). Standard 2.5-mm thick transverse scans at 66% of the tibial length, proximal to the terminal tip of the tibia, were obtained. Previous studies have shown that this is the region with the largest outer calf diameter, with little variability across individuals (26). Muscle density was calculated from X-ray attenuation, and is expressed in mg/cm3. Images were analyzed by using BONALYSE software (BonAlyse Oy, Jyvaskyla, Finland), as previously described in detail (8).
Levels of physical activity in the year before the interview were coded into an ordinal scale on the basis of responses to a standard questionnaire as follows: 1, hardly any physical activity; 2, mostly sitting (occasional walks, easy gardening); 3, light exercise (no sweating) 2–4 h/wk; 4, moderate exercise (sweat) 1–2 h/wk (level 4); 5, moderate exercise >3 h/wk; 6, intense exercise (at the limits) >3 times/wk. According to this classification, we grouped the participants as follows: 1–3, inactive or light physical activity; 4–5, moderate physical activity; 6, intense physical activity.
Analytic methods
Blood samples were obtained from participants after they had fasted for 12 h and after they had been in a sitting or supine position for ≥15 min. Serum albumin (as a percentage) was detected by electrophoresis (Hydragel 7 Protein, Sebia, France; mean interassay coefficient, 0.8%), and its concentration was calculated from serum total proteins (Roche Diagnostics, inter-assay CV <1%). Serum creatinine was detected by a standard creatinine Jaffe method (Roche Diagnostics, Mannheim, Germany); the interassay CV was <2.5%. Serum calcium and magnesium concentrations were measured by use of a colorimetric assay with endpoint determination and sample blank (27). The measure unit was expressed in mg/dL. For calcium, the analytic sensitivity was 0.2 mg/dL, the intraassay CV was 0.9%, and the interassay CV was 1.5%. For magnesium, the analytic sensitivity was 0.07 mg/dL, the intraassay CV was 1.2%, and the interassay CV was 1.4%. Serum concentrations of 25-hydroxyvitamin D were measured by radioimmunoassay in samples frozen at −80 °C (DiaSorin, Stillwater, MN) after extraction of the samples with acetonitrile. Intraassay and interassay CVs were 8.1% and 10.2%, respectively.
Covariates
Covariates included sociodemographic characteristics (age, sex), body mass index (BMI) calculated as weight in kilograms divided by the square of height in meters, biological indexes [serum calcium concentration, serum albumin, hemoglobin, plasma glucose, creatinine, and 25-hydroxyvitamin D], the presence of self-reported or diagnosed diseases, calf muscle area and density, and levels of physical activity in the year before the recruitment. The diagnosis of major medical conditions (hypertension, angina pectoris, myocardial infarction, stroke, cancer, diabetes mellitus, congestive heart failure, and chronic obstructive pulmonary disease) was established by using an adjudication process that considered information obtained from self-reported history, the medical examination, and medical records. Potential chronic kidney disease was established for serum creatinine clearance <30 mL/min.
Statistical analyses
The analyses were performed considering serum magnesium as a continuous variable and as a categorical variable with sample-based tertiles of magnesium concentration values. Linear regression models were used to examine the relations of serum magnesium to each index of muscle performance (handgrip strength, maximum power of lower-leg strength, knee extension torque, and ankle extension strength). Sex interactions were assessed by including an interaction term in the adjusted models as a covariate. Differences in muscle performance across magnesium concentration tertiles were tested by using analysis of covariance. Differences in proportions and means of covariates according to magnesium concentration tertiles were assessed with chi-square and ANOVA statistics, respectively, with post-hoc testing (Tukey’s multiple comparisons test) for significance. Statistical analyses were performed with EPI INFO version 3.3.2 software (Centers for Disease Control and Prevention, Atlanta, GA) and GRAPH PAD version 4.0 software (GraphPad Software Inc, San Diego, CA). P values <0.05 were considered to be statistically significant.
RESULTS
The anthropometric characteristics of the participants by sex are shown in Table 1. The participants’ mean (±SD) age was 66.7 ±15.2 y and 54.0% were women. Mean (±SD) concentrations of serum magnesium were 2.0 ± 0.5 mg/dL and 2.0 ± 0.6 mg/dL for women and men, respectively. Mean serum magnesium concentrations did not differ significantly between participants aged >65 y, who accounted for 76.5% of the study sample (2.0 ± 0.53 mg/dL), and participants aged <65 y (2.0 ± 0.44 mg/dL). Overall, 345 (30.3%) participants had a serum magnesium concentration <1.8 mg/dL, with no significant differences according to age and sex group.
TABLE 1.
Characteristics of the men and women in the study population1
| Women (n = 615) | Men (n = 523) | P | |
|---|---|---|---|
| Age (y) | 67.4 ± 14.92 | 65.9 ± 15.4 | NS |
| Sex (%) | 54.0 | 45.9 | <0.05 |
| BMI (kg/m2) | 27.3 ± 4.6 | 27.0 ± 3.4 | NS |
| <20 [n(%)] | 21 (3.4) | 7 (1.3) | |
| 20–24.9 [n(%)] | 178 (28.9) | 142 (27.2) | |
| 25–30 [n(%)] | 245 (39.8) | 257 (49.1) | |
| >30 [n(%)] | 161 (26.2) | 107 (20.5) | |
| Serum magnesium (mg/dL) | 2.0 ± 0.5 | 2.0 ± 0.6 | NS |
| Serum calcium (mg/dL) | 9.5 ± 0.4 | 9.4 ± 0.4 | NS |
| Albumin (g/dL) | 4.2 ± 0.3 | 4.3 ± 0.3 | <0.001 |
| Hemoglobin (g/dL) | 13.3 ± 1.0 | 14.6 ± 1.2 | <0.001 |
| Plasma glucose (mg/dL) | 92.7 ± 25.0 | 95.8 ± 25.7 | <0.05 |
| Creatinine (mg/dL) | 0.83 ± 0.13 | 0.99 ± 0.16 | <0.001 |
| 25-Hydroxyvitamin D (ng/mL) | 19.7 ± 14.0 | 24.6 ± 14.2 | <0.001 |
| Angina pectoris [n (%)] | 12 (2.0) | 30 (5.7) | <0.001 |
| COPD [n (%)] | 5 (0.8) | 60 (11.5) | <0.001 |
| Cancer [n (%)] | 39 (6.3) | 18 (3.4) | <0.05 |
| CHF [n (%)] | 16 (2.6) | 16 (3.1) | NS |
| DM2 [n (%)] | 45 (7.3) | 54 (10.3) | NS |
| Hypertension, by BP measurements [n (%)] | 263 (42.8) | 221 (42.3) | NS |
| Hypertension, self-reported or drug use [n (%)] | 203 (33.0) | 128 (24.5) | <0.001 |
| MI [n (%)] | 19 (3.1) | 28 (5.4) | NS |
| Stroke [n (%)] | 13 (2.1) | 22 (4.2) | NS |
| Maximum handgrip strength (kg) | 25.3 ± 10.5 | 43.6 ± 14.1 | <0.001 |
| Maximum LE muscle power (W) | 92.4 ± 57.5 | 186.3 ± 85.7 | <0.001 |
| Knee extension torque (N/dm) | 363.6 ± 140.5 | 582.5 ± 197.0 | <0.001 |
| Maximum ankle plantar flexion (kg) | 30.5 ± 11.4 | 39.6 ± 11.1 | <0.001 |
| MMSE score | 25.9 ± 3.0 | 26.7 ± 2.6 | <0.001 |
BP, blood pressure; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; DM2, type 2 diabetes; MI, myocardial infarction; LE, lower extremity; MMSE, Mini-Mental State Examination. Differences between men and women were assessed by t tests. To convert mg/dL to mmol/L, multiply by 0.411 for serum magnesium, by 0.25 for serum calcium, by 10 for albumin, by 0.155 for hemoglobin, by 0.055 for plasma glucose, by 88.4 for creatinine, and by 2.496 for 25-hydroxyvitamin D.
x̄ ± SD (all such values).
The relations of serum magnesium with maximum grip strength, lower-leg muscle power, knee extension torque, and ankle extension isometric strength in men and women are shown in Figure 1. The slopes of the regression lines were not significantly different for men and women. Adjusted regression coefficients for the association of serum magnesium concentration with all indexes of muscle performance studied are shown in Table 2 and Table 3. Model 1 shows the regression coefficient for the association of magnesium concentration and muscle performance adjusted for age, sex, and BMI. Even after adjustment for other factors that may influence muscle function or magnesium metabolism, such as serum calcium, albumin, hemoglobin, plasma glucose, creatinine, and vitamin D (model 2), the relation remained highly significant. To further investigate whether this relation may have been affected by the presence of chronic diseases that may influence either muscle indexes or serum magnesium concentrations, or by muscle area, muscle density, or degree of physical activity, we added the presence of these diseases and muscle variables as covariates in the model (model 3). Even after this further adjustment, serum magnesium remained strongly correlated with muscle performance (grip strength: β= 2.0 ± 0.5, P = 0.0002; lower-leg muscle power: β = 8.8 ± 2.7, P = 0.001; knee extension torque: β = 31.2 ± 7.9, P < 0.0001; ankle extension strength: β = 3.8 ± 0.5, P < 0.0001). Indeed, even though several parameters included in the model were significantly related to muscle measurements (age, sex, BMI, hemoglobin, creatinine, albumin, vitamin D, muscle area, and physical activity level), the relation between magnesium and muscle function was still strong and statistically significant. There was no interaction of sex by serum magnesium in the linear regression.
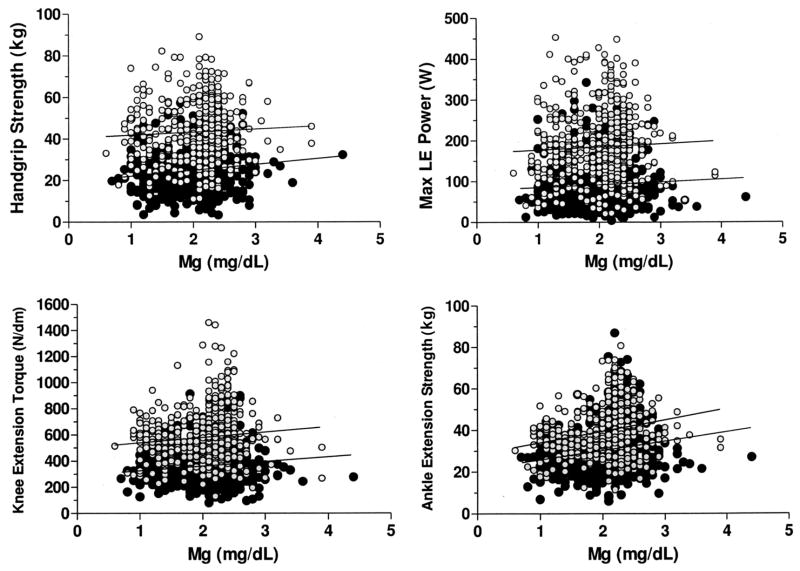
Figure 1.
Scatterplots describing the relations of serum magnesium and maximum handgrip strength (r = 0.08, P < 0.05), lower-extremity (LE) muscle power (r = 0.06, p = NS), knee extension torque (r = 0.10, P = 0.01), and ankle extension isometric strength (r = 0.19, P < 0.0001) in men ( ; n = 523) and women (●; n = 615) who participated in the InCHIANTI study. To convert mg/dL to mmol/L, multiply by 0.411. Pearson’s correlation coefficients were used to analyze the linear correlations between muscle function and serum magnesium.
; n = 523) and women (●; n = 615) who participated in the InCHIANTI study. To convert mg/dL to mmol/L, multiply by 0.411. Pearson’s correlation coefficients were used to analyze the linear correlations between muscle function and serum magnesium.
TABLE 2.
Linear regression models testing the relation of serum magnesium with handgrip strength and knee extension torque, independent of confounders1
| Handgrip strength |
Knee extension torque |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1: R2 = 0.71 |
Model 2: R2 = 0.71 |
Model 3: R2 =0.73 |
Model 1: R2 = 0.57 |
Model 2: R2 = 0.59 |
Model 3: R2 = 0.62 |
|||||||
| β ±SE | P | β ±SE | P | β ±SE | P | β ±SE | P | β ±SE | P | β ±SE | P | |
| Age (y) | −0.5 ± 0.0 | <0.0001 | −0.5 ± 0.0 | <0.0001 | −0.4 ± 0.0 | <0.0001 | −5.7 ± 0.3 | <0.0001 | −5.9 ± 0.3 | <0.0001 | −5.0 ± 0.3 | <0.0001 |
| Sex | 16.9 ± 0.5 | <0.0001 | 15.4 ± 0.7 | <0.0001 | 13.2 ± 0.8 | <0.0001 | 207.0 ± 8.0 | <0.0001 | 169.1 ± 10.6 | <0.0001 | 137.3 ± 12.2 | <0.0001 |
| BMI (kg/m2) | −1.0 ± 0.1 | <0.0001 | −1.0 ± 0.1 | <0.0001 | −1.2 ± 0.1 | <0.0001 | −10.7 ± 1.0 | <0.0001 | −11.5 ± 1.0 | <0.0001 | −15.0 ± 1.3 | <0.0001 |
| Magnesium (mg/dL) | 2.0 ± 0.5 | 0.0001 | 2.3 ± 0.6 | <0.0001 | 2.0 ± 0.5 | 0.0002 | 33.0 ± 7.8 | <0.0001 | 33.7 ± 8.3 | <0.0001 | 31.2 ± 7.9 | <0.0001 |
| Calcium (mg/dL) | — | — | −0.7 ± 0.8 | 0.42 | −0.9 ± 0.8 | 0.27 | — | — | −1.1 ± 12.0 | 0.93 | −2.0 ± 12.0 | 0.87 |
| Albumin (g/dL) | — | — | −0.3 ± 1.1 | 0.79 | −0.7 ± 1.1 | 0.53 | — | — | −28.7 ± 16.5 | 0.08 | −35.0 ± 16.3 | 0.03 |
| Hemoglobin (g/dL) | — | — | 0.6 ± 0.3 | 0.02 | 0.7 ± 0.3 | 0.01 | — | — | 11.8 ± 3.9 | 0.002 | 11.7 ± 3.9 | 0.003 |
| Plasma glucose (mg/dL) | — | — | −0.0 ± 0.0 | 0.24 | −0.0 ± 0.0 | 0.36 | — | — | −0.0 ± 0.2 | 0.88 | 0.1 ± 0.2 | 0.59 |
| Creatinine (mg/dL) | — | — | 3.4 ± 1.9 | 0.07 | 3.6 ± 1.9 | 0.06 | — | — | 164.1 ± 28.0 | <0.0001 | 163.5 ± 28.6 | <0.0001 |
| 25-Hydroxyvitamin D (ng/mL) | — | — | 0.0 ± 0.0 | 0.03 | 0.0 ± 0.0 | 0.19 | — | — | 0.1 ± 0.1 | 0.24 | −0.0 ± 0.1 | 0.99 |
| Angina pectoris | — | — | — | — | 0.4 ± 0.8 | 0.61 | — | — | — | — | 10.4 ± 11.7 | 0.37 |
| COPD | — | — | — | — | −0.2 ± 0.7 | 0.78 | — | — | — | — | 3.1 ± 10.3 | 0.77 |
| Cancer | — | — | — | — | 0.7 ± 1.2 | 0.58 | — | — | — | — | 8.8 ± 18.0 | 0.62 |
| CHF | — | — | — | — | 0.5 ± 0.4 | 0.18 | — | — | — | — | −6.2 ± 5.8 | 0.28 |
| DM2 | — | — | — | — | 0.2 ± 1.0 | 0.87 | — | — | — | — | −9.9 ± 14.4 | 0.49 |
| HTN 1 | — | — | — | — | −0.6 ± 0.6 | 0.33 | — | — | — | — | −14.9 ± 8.7 | 0.09 |
| HTN 2 | — | — | — | — | −0.2 ± 0.4 | 0.62 | — | — | — | — | −3.0 ± 6.2 | 0.62 |
| MI | — | — | — | — | −2.9 ± 1.2 | 0.01 | — | — | — | — | −38.8 ± 18.0 | 0.03 |
| Stroke | — | — | — | — | −0.4 ± 0.7 | 0.56 | — | — | — | — | −28.9 ± 10.5 | 0.005 |
| Muscle area (mm2) | — | — | — | — | 0.0 ± 0.0 | <0.0001 | — | — | — | — | 0.0 ± 0.0 | <0.0001 |
| Muscle density (mg/cm3) | — | — | — | — | 0.0 ± 0.1 | 0.96 | — | — | — | — | −1.7 ± 1.3 | 0.17 |
| Physical activity level | — | — | — | — | 4.2 ± 1.3 | <0.001 | — | — | — | — | 35.6 ± 19.2 | 0.06 |
Linear regression models. n = 1138; 615 women; 523 men. Men equals 1 and women equals 0. Model 1 was adjusted for age, sex, and BMI. Model 2 was adjusted for age, sex, BMI, calcium, albumin, hemoglobin, plasma glucose, creatinine, and 25-hydroxyvitamin D. Model 3 was adjusted for age; sex; BMI; calcium; albumin; hemoglobin; plasma glucose; creatinine; 25-hydroxyvitamin D; the presence of angina pectoris, chronic abstructive pulmonary disease (COPD), cancer, congestive heart failure (CHF), type 2 diabetes (DM2), hypertension detected via blood pressure reading (HTN 1), hypertension detected via self-report or drug use (HTN 2), myocardial infarction (MI), and stroke; muscle area; muscle density; and physical activity level.
TABLE 3.
Linear regression models testing the relation of serum magnesium with lower-extremity muscle power and ankle extension isometric strength, independent of confounders1
| Maximum lower-extremity muscle power |
Ankle extension isometric strength |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1: R2 = 0.72 |
Model 2: R2 = 0.73 |
Model 3: R2 = 0.74 |
Model 1: R2 = 0.51 |
Model 2: R2 = 0.53 |
Model 3: R2 = 0.54 |
|||||||
| β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P | |
| Age (y) | −3.5 ± 0.1 | <0.0001 | −3.5 ± 0.1 | <0.0001 | −3.2 ± 0.1 | <0.0001 | −0.3 ± 0.0 | <0.0001 | −0.37 ± 0.0 | <0.0001 | −0.3 ± 0.0 | <0.0001 |
| Sex | 87.5 ± 2.7 | <0.0001 | 75.7 ± 3.7 | <0.0001 | 63.7 ± 4.3 | <0.0001 | 8.4 ± 0.5 | <0.0001 | 6.6 ± 0.7 | <0.0001 | 5.5 ± 0.8 | <0.0001 |
| BMI (kg/m2) | −1.8 ± 0.3 | <0.0001 | −2.1 ± 0.4 | <0.0001 | −3.4 ± 0.4 | <0.0001 | −1.0 ± 0.1 | <0.0001 | −1.1 ± 0.1 | <0.0001 | −1.1 ± 0.1 | <0.0001 |
| Magnesium (mg/dL) | 8.9 ± 2.7 | 0.0008 | 8.8 ± 2.7 | 0.001 | 8.8 ± 2.7 | 0.001 | 3.7 ± 0.5 | <0.0001 | 4.2 ± 0.5 | <0.0001 | 3.8 ± 0.5 | <0.0001 |
| Calcium (mg/dL) | — | — | −0.9 ± 4.0 | 0.82 | 1.1 ± 4.2 | 0.79 | — | — | −0.9 ± 0.8 | 0.26 | 0.7 ± 0.8 | 0.35 |
| Albumin (g/dL) | — | — | −3.3 ± 3.6 | 0.36 | −10.5 ± 5.8 | 0.07 | — | — | −3.4 ± 1.1 | 0.001 | −3.7 ± 1.1 | 0.0006 |
| Hemoglobin (g/dL) | — | — | 3.5 ± 1.4 | 0.01 | 3.6 ± 1.4 | 0.008 | — | — | 0.7 ± 0.3 | 0.004 | 0.8 ± 0.3 | 0.003 |
| Plasma glucose (mg/dL) | — | — | −0.1 ± 0.1 | 0.28 | 0.0 ± 0.1 | 0.66 | — | — | 0.0 ± 0.0 | 0.27 | 0.0 ± 0.0 | 0.12 |
| Creatinine (mg/dL) | — | — | 51.6 ± 9.8 | <0.0001 | 49.7 ± 10.0 | <0.0001 | — | — | 7.3 ± 1.9 | <0.0001 | 7.8 ± 1.9 | <0.0001 |
| 25-Hydroxyvitamin D(nmol/L) | — | — | 0.0 ± 0.0 | 0.57 | −0.0 ± 0.0 | 0.65 | — | — | 0.0 ± 0.0 | 0.59 | 0.0 ± 0.0 | 0.99 |
| Angina pectoris | — | — | — | — | 3.7 ± 4.1 | 0.37 | — | — | — | — | −0.3 ± 0.8 | 0.74 |
| COPD | — | — | — | — | −2.6 ± 3.6 | 0.47 | — | — | — | — | −0.1 ± 0.7 | 0.93 |
| Cancer | — | — | — | — | 1.1 ± 6.3 | 0.86 | — | — | — | — | 2.2 ± 1.2 | 0.06 |
| CHF | — | — | — | — | −1.1 ± 2.0 | 0.60 | — | — | — | — | 0.3 ± 0.4 | 0.51 |
| DM2 | — | — | — | — | −4.3 ± 5.0 | 0.39 | — | — | — | — | −1.1 ± 0.9 | 0.25 |
| HTN 1 | — | — | — | — | 0.7 ± 3.1 | 0.82 | — | — | — | — | −1.3 ± 0.6 | 0.03 |
| HTN 2 | — | — | — | — | −4.6 ± 2.2 | 0.03 | — | — | — | — | −0.5 ± 0.4 | 0.24 |
| MI | — | — | — | — | −11.5 ± 6.1 | 0.06 | — | — | — | — | −2.2 ± 1.2 | 0.07 |
| Stroke | — | — | — | — | −1.4 ± 3.6 | 0.70 | — | — | — | — | −0.2 ± 0.7 | 0.77 |
| Muscle area (mm2) | — | — | — | — | 0.0 ± 0.0 | <0.0001 | — | — | — | — | 0.0 ± 0.0 | 0.02 |
| Muscle density (mg/cm3) | — | — | — | — | −0.4 ± 0.4 | 0.32 | — | — | — | — | 0.0 ± 0.1 | 0.95 |
| Physical activity level | — | — | — | — | 15.0 ± 6.8 | 0.03 | — | — | — | — | 1.1 ± 1.3 | 0.39 |
Linear regression models. n = 1138; 615 women; 523 men. Men equals 1 and women equals 0. Model 1 was adjusted for age, sex, and BMI. Model 2 was adjusted for age, sex, BMI, calcium, albumin, hemoglobin, plasma glucose, creatinine, and 25-hydroxyvitamin D. Model 3 was adjusted for age; sex; BMI; calcium; albumin; hemoglobin; plasma glucose; creatinine, 25-hydroxyvitamin D; the presence of angina pectoris, chronic obstructive pulmonary disease (COPD), cancer, congestive heart failure (CHF), type 2 diabetes, (DM2), hypertension detected via blood pressure reading (HTN 1), hypertension detected via self-report or drug use (HTN 2), myocardial infarction (MI), and stroke; muscle area; muscle density; and physical activity level.
To determine whether the observed relations were driven by persons with magnesium deficiency or hypermagnesemia, we examined these associations excluding subjects with serum magnesium concentrations <1.8 mg/dL (n = 345, or 30.3%) and >3 mg/dL (n = 13, or 1.1%) (below and above the laboratory reference value, respectively). In persons with magnesium concentrations within the normal range, the relations were still highly significant for all the indexes of muscle performance adjusted by serum calcium, albumin, hemoglobin, plasma glucose, creatinine, vitamin D, the presence of chronic diseases, muscle area, muscle density, and degree of physical activity (grip strength: β = 2.8 ± 1.1, P = 0.01; lower-leg muscle power: β = 13.2 ± 6.1, P = 0.03; knee extension torque: β = 59.1 ± 18.8, P = 0.001; ankle extension strength: β = 5.0 3 1.2, P < 0.0001), which suggests that this association is continuous, in the full range of serum magnesium concentrations.
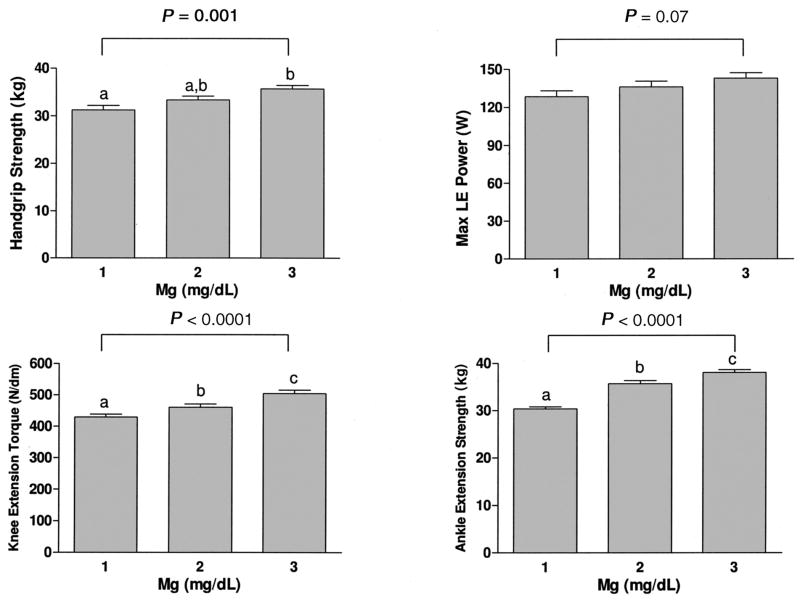
Adjusted values of handgrip strength, lower-extremity muscle power, knee extension torque, and ankle extension strength are shown in Figure 2, according to tertiles of serum magnesium concentration. Handgrip strength, knee extension torque, and ankle extension were significantly higher with higher serum magnesium. Lower-extremity muscle power tended to be higher with higher serum magnesium; however, the relation was not significant.
Figure 2.
Mean (±SE) isometric muscle strength measurements [handgrip strength, lower-extremity (LE) muscle power, knee extension torque, and ankle extension isometric strength] according to serum magnesium concentrations. Tertile 1 is the lowest tertile, tertile 2 is the intermediate, and tertile 3 is the highest. To convert mg/dL to mmol/L, multiply by 0.411. Bars with different letters are significantly different, P < 0.05 (ANOVA followed by Tukey’s multiple-comparisons test). P values in the figure correspond to P for trend.
DISCUSSION
Using data from a well-characterized, representative sample of older men and women, we found a significant, independent, and strong relation between circulating magnesium and muscle performance, which was consistent across several muscle variables for both men and women. At least 3 mechanisms may explain these findings: 1) the role of magnesium in energetic metabolism, 2) the increased reactive oxygen species production in magnesium deficiency, and 3) the proinflammatory effect of magnesium depletion.
Previous studies conducted in young volunteers found that magnesium status strongly affects muscle performance, probably due to magnesium’s key role in energetic metabolism, trans-membrane transport, and muscle contraction and relaxation (28). Magnesium depletion causes structural damage to muscle cells through increased oxidative stress and impaired intracellular calcium homeostasis (5). In one study, magnesium supplementation (up to 8 mg/kg daily) enhanced muscle strength (+20% of peak knee-extension torque) in young untrained individuals (7). Similarly, physically active young subjects experienced improved endurance performance and decreased oxygen use during sub-maximal exercise after magnesium supplementation (29). Conversely, postmenopausal women fed 180 versus 320 mg Mg/d had higher heart rate and oxygen consumption during submaximal exercise, probably related to decreased erythrocyte and skeletal muscle magnesium concentrations (6).
A large portion of the energy used for physiologic functions in humans is produced by mitochondria through the movement of electrons over the respiratory chain (30). Magnesium is critical for basic mitochondrial functions, including ATP synthesis, electron transport chain complex subunits, and oxygen detoxification (1). Inadequate availability of magnesium may lead to reduced mitochondrial efficiency and increased production of reactive oxygen species with consequent structural and functional impairment to proteins (31), DNA (30), and other essential molecules. Magnesium in the mitochondria accounts for one-third of total cellular magnesium and is present as a complex with ATP and as a component of membranes and nucleic acids (1). Studies of magnesium-deficient cultured human cells and animals show evidence of decreased antioxidant capacity (32, 33), and one study showed mitochondrial swelling and altered ultrastructure in muscle taken from magnesium-deficient animals (5). Hence, magnesium seems fundamental for the control of oxidative stress and to maintain the normal function of muscle mitochondria.
A state of chronic inflammation has been proposed as one of the main causes of frailty in older persons (34). Poor magnesium status may trigger the development of a proinflammatory state both by causing excessive production and release of interleukin 1β and tumor necrosis factor α (35) and by elevating circulating concentrations of proinflammatory neuropeptides that trigger activation of low-grade chronic inflammation (36). On the other hand, it is possible that oxidative mitochondrial decay linked to aging may itself favor hypomagnesemia. A recent study showed that a mutation in a mitochondrial gene results in low circulating magnesium, which became apparent as the affected subjects aged, possibly because magnesium reabsorption at the distal convoluted tubule requires a high amount of ATP (37).
Poor muscle strength is a major cause of disability in the elderly (9–14). If the findings of the present study are confirmed in other cross-sectional and longitudinal studies, the potential implications are two-fold. First, because measurement of serum magnesium is relatively inexpensive, it should be incorporated as part of a routine physical work-up. Second, the role of magnesium supplementation as a possible intervention for delaying or preventing disability in older adults deserves some consideration.
Defining magnesium deficiency is complex, in part because of the lack of available clinical tests for assessing total-body magnesium content. Currently, the serum magnesium concentration, which normally ranges between 1.8 and 3.0 mg/dL and is tightly maintained within this range (2, 3), is the most clinically available test for assessing magnesium status. For practical reasons, magnesium deficiency is defined as a serum concentration below the reference interval for the laboratory, which is not necessarily related to a pathophysiologic state of deficiency. Serum concentrations <1.8 mg/dL usually indicate some degree of magnesium depletion (2, 3), but low intracellular magnesium has been documented even in patients with serum concentrations >1.8 mg/dL (38). Our results showing a continuous relation between serum magnesium and indexes of muscle performance, not simply driven by subjects with the lowest or highest magnesium concentration, lead us to propose that it is crucial not only to avoid deficiency according to the normal laboratory reference value, but also to obtain optimal magnesium concentrations to attain the best possible muscle performance.
Despite the physiologic importance of magnesium, the multiple problems associated with its deficiency, and the ease of supplementation, inadequate magnesium intake remains highly prevalent in various populations (15–20). The typical Western diet high in processed foods and low in whole grains and green vegetables is often deficient in magnesium. Data from the National Health and Nutrition Examination Survey found that daily magnesium intake decreases with age, and older persons are well below the recommended minimal quantity (average of 225 and 166 mg/d compared with the recommended 420 and 320 mg/d for men and women, respectively) (16). Among US adults, 68% consume less than the recommended daily allowance (RDA) of magnesium, 45% consume <75% of the RDA, and 19% consume <50% of the RDA (15). The risk of inadequate magnesium intake is particularly high in those affected by chronic conditions and receiving chronic drug treatment (18–20). Magnesium supplementation has been shown to be beneficial in several conditions, such as neuropsychiatric disorders, ischemic heart disease and cardiac arrhythmias, asthma, diabetes, and chronic fatigue (39, 40). Because magnesium supplementation is inexpensive and in general well tolerated, it should be a key consideration in older subjects at particular risk of magnesium deficiency.
Some caveats for the present study should be considered. This analysis used cross-sectional data; longitudinal data are needed to verify whether magnesium deficiency predicts accelerated decline of muscle performance with age. Because serum magnesium may not accurately reflect intracellular magnesium, it is possible that a greater proportion of older persons than those detected with use of circulating concentrations may have intracellular magnesium deficiency.
In conclusion, the results of the present study show an independent concurrent relation between serum magnesium and muscle performance in a large cohort of community-resident older persons. A possible mechanism of this association is the effect of magnesium concentrations on mitochondrial function in muscle, which may be particularly critical in aging muscle.
The limited attention given to suboptimal magnesium status in older populations at higher risk raises several questions that should be the focus of future studies. Some of these are as follows: What mechanisms underlie inadequate magnesium concentrations? What dietary magnesium intake is required in older persons to maintain adequate muscle performance? What is the role of inflammatory cytokines in mediating the adverse effect of magnesium deficiency on muscle? Is low magnesium a component of the frailty syndrome? Can magnesium supplementation influence muscle strength or performance and cytokine concentrations in older persons? The importance of elucidating the role of low magnesium status on the development of sarcopenia cannot be overlooked, because the aging population at risk of related disability continues to grow and contribute to extensive health care costs.
Footnotes
The InCHIANTI study was supported as a targeted project (ICS 110.1\RS97.71) by the Italian Ministry of Health and in part by the US National Institute on Aging (contracts N01-AG-916413, N01-AG-821336, 263 MD 9164 13, and 263 MD 821336).
Author contributions were as follows: study concept and design, LJD, MB, and LF; data acquisition, LF, SB, FL, and AMC; analysis and interpretation of data, LJD, LF, MB, AB, and EMS; drafting of the manuscript, LJD, LF, MB, and EMS; critical revision of the manuscript for important intellectual content, LJD, MB, FL, SB, AMC, AB, EMS, and LF; statistical analysis: LJD, AB, and LF; obtaining funding: LJD and LF; administrative, technical, or material support: LF, SB, and FL; and study supervision: LF, SB, and FL. The funding sources had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, and approval of the manuscript. None of the authors had a conflict of interest to report.
References
- 1.Wolf FI, Cittadini A. Chemistry and biochemistry of magnesium. Mol Aspects Med. 2003;24:3–9. doi: 10.1016/s0098-2997(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 2.Reinhart RA. Magnesium metabolism. Arch Intern Med. 1988;148:2415–20. doi: 10.1001/archinte.148.11.2415. [DOI] [PubMed] [Google Scholar]
- 3.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium: an update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 4.Barbagallo M, Dominguez LJ, Galioto A, et al. Role of magnesium in insulin action, diabetes and cardiometabolic syndrome X. Mol Aspects Med. 2003;24:39–52. doi: 10.1016/s0098-2997(02)00090-0. [DOI] [PubMed] [Google Scholar]
- 5.Rock E, Astier C, Lab X, et al. Dietary magnesium deficiency in rats enhances free radical production in skeletal muscle. J Nutr. 1995;125:1205–10. doi: 10.1093/jn/125.5.1205. [DOI] [PubMed] [Google Scholar]
- 6.Lukaski HC, Nielsen FH. Dietary magnesium depletion affects metabolic responses during submaximal exercise in postmenopausal women. J Nutr. 2003;132:930–5. doi: 10.1093/jn/132.5.930. [DOI] [PubMed] [Google Scholar]
- 7.Brilla LR, Haley TF. Effect of magnesium supplementation on strength training in humans. J Am Coll Nutr. 1992;11:326–9. doi: 10.1080/07315724.1992.10718233. [DOI] [PubMed] [Google Scholar]
- 8.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallance RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried L, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–41. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 11.Onder G, Penninx BWJH, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women’s Health and Aging Study. J Gerontol Med Sci. 2005;60:74–9. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 12.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–60. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 13.Ploutz-Snyder LL, Manini T, Ploutz-Snyder RJ, Wolf DA. Functionally relevant thresholds of quadriceps femoris strength. J Gerontol A Biol Sci Med Sci. 2002;57:B144–52. doi: 10.1093/gerona/57.4.b144. [DOI] [PubMed] [Google Scholar]
- 14.Al Snih S, Markides KS, Ray L, Ostir GV, Goodwin JS. Handgrip strength and mortality in older Mexican Americans. J Am Geriatr Soc. 2002;50:1250–6. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 15.King DE, Mainous AG, Geesey ME, Woolson RF. Dietary magnesium and C-reactive protein levels. J Am Coll Nutr. 2005;24:166–71. doi: 10.1080/07315724.2005.10719461. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of U.S. adults. J Nutr. 2003;133:2879–82. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 17.Galan P, Preziosi P, Durlach V, et al. Dietary magnesium intake in a French adult population. Magnes Res. 1997;10:321–8. [PubMed] [Google Scholar]
- 18.Vaquero MP. Magnesium and trace elements in the elderly: intake, status and recommendations. J Nutr Health Aging. 2002;6:147–53. [PubMed] [Google Scholar]
- 19.Dror Y, Berner YN, Stern F, Polyak Z. Dietary intake analysis in institutionalized elderly: a focus on nutrient density. J Nutr Health Aging. 2002;6:237–42. [PubMed] [Google Scholar]
- 20.Padro L, Benacer R, Foix S, et al. Assessment of dietary adequacy for an elderly population based on a Mediterranean model. J Nutr Health Aging. 2002;6:31–3. [PubMed] [Google Scholar]
- 21.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 22.Measso G, Cavarzeran F, Zappalà G, et al. The Mini-Mental State Examination: normative study of an Italian random sample. Dev Neuropsychol. 1993;9:77–85. [Google Scholar]
- 23.Bandinelli S, Benvenuti E, Del Lungo I, et al. Measuring muscular strength of the lower limbs by hand-held dynamometer: a standard protocol. Aging Clin Exp Res. 1999;11:287–93. doi: 10.1007/BF03339802. [DOI] [PubMed] [Google Scholar]
- 24.Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol. 1990;60:385–90. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- 25.Russo CR, Lauretani F, Bandinelli S, et al. Aging bone in men and women: beyond changes in bone mineral density. Osteoporos Int. 2003;14:531–8. doi: 10.1007/s00198-002-1322-y. [DOI] [PubMed] [Google Scholar]
- 26.Simonsick EM, Maffeo CE, Rogers SK, et al. Methodology and feasibility of a home-based examination in disabled older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52:M264–74. doi: 10.1093/gerona/52a.5.m264. [DOI] [PubMed] [Google Scholar]
- 27.Elin RJ. Determination of serum magnesium concentration by clinical laboratories. Magnes Trace Elem. 1991;10:60–6. [PubMed] [Google Scholar]
- 28.Lukaski HC. Vitamin and mineral status: effects on physical performance. Nutrition. 2004;20:632–44. doi: 10.1016/j.nut.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Brilla LR, Gunther KB. Effect of magnesium supplementation on exercise time to exhaustion. Med Exerc Nutr Health. 1995;4:230–3. [Google Scholar]
- 30.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–23. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Head E, Gharib AM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-α-lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–61. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickens BF, Weglicki WB, Li YS, Mak IT. Magnesium deficiency in vitro enhances free radical induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett. 1992;311:187–91. doi: 10.1016/0014-5793(92)81098-7. [DOI] [PubMed] [Google Scholar]
- 33.Freedman AM, Mak IT, Stafford RE, et al. Erythrocytes from magnesium-deficient hamsters display an enhanced susceptibility to oxidative stress. Am J Physiol. 1992;262:C1371–5. doi: 10.1152/ajpcell.1992.262.6.C1371. [DOI] [PubMed] [Google Scholar]
- 34.Ferrucci L, Guralnik JM. Inflammation, hormones, and body composition at a crossroad. Am J Med. 2003;115:501–2. doi: 10.1016/j.amjmed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Weglicki WB, Dickens BF, Wagner TL, Chemielinska JJ, Phillips TM. Immunoregulation by neuropeptides in magnesium deficiency: ex vivo effect of enhanced substance P production on circulation T lymphocytes from magnesium-deficient mice. Magnes Res. 1996;9:3–11. [PubMed] [Google Scholar]
- 36.Kramer JH, Mak IT, Phillips TM, Weglicki WB. Dietary magnesium intake influences circulating pro-inflammatory neuropeptide levels and loss of myocardial tolerance to postischemic stress. Exp Biol Med. 2003;228:665–73. doi: 10.1177/153537020322800604. [DOI] [PubMed] [Google Scholar]
- 37.Wilson FH, Hariri A, Farhi A, et al. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004;306:1190–4. doi: 10.1126/science.1102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rude RK, Stephen A, Nadler J. Determination of red blood cell intracellular free magnesium by nuclear magnetic resonance as an assessment of magnesium depletion. Magnes Trace Elem. 1991;10:117–21. [PubMed] [Google Scholar]
- 39.McLean RM. Magnesium and its therapeutic uses: a review. Am J Med. 1994;96:63–76. doi: 10.1016/0002-9343(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 40.Manuel y Keenoy B, Moorkens G, Vertommen J, Noe M, Neve J, De Leeuw I. Magnesium status and parameters of the oxidant-antioxidant balance in patients with chronic fatigue: effects of supplementation with magnesium. J Am Coll Nutr. 2000;19:374–82. doi: 10.1080/07315724.2000.10718934. [DOI] [PubMed] [Google Scholar]