Abstract
The solution phase parallel synthesis of a 121 member library of multi-substituted benzo[b]furans is described. 2,3,5-Trisubstituted benzo[b]furans have been prepared by the palladium-catalyzed substitution of 3-iodobenzofurans by Suzuki-Miyaura, carbonylative Suzuki, Sonogashira, Heck, and carboalkoxylation chemistry. The 3-iodobenzofurans are readily prepared in good to excellent yields by the palladium/copper-catalyzed cross-coupling of various o-iodoanisoles and terminal alkynes, followed by electrophilic cyclization with IC1.
Introduction
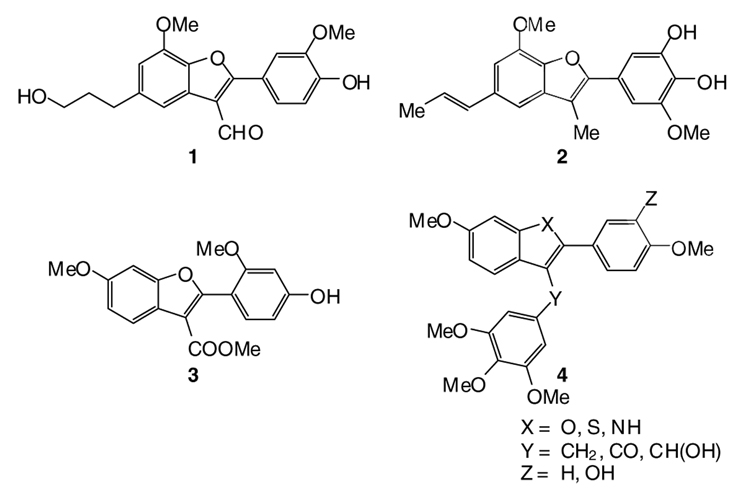
Benzo[b]furan derivatives are of considerable interest because of their widespread occurrence among natural products and their physiological properties.1–6 For instance, 2,3-disubstituted benzo[b]furans and derivatives exhibit a broad range of biological activities. A small selection of biologically and pharmacologically active benzo[b]furan compounds is shown in Figure 1. Adenosine antagonist XH-14 (1) was isolated from the plant Salvia miltiorrhiza, which has been widely used in China for the treatment of coronary heart diseases, such as myocardial infarction and angina pectoris.7–9 Obovaten (2) is known as a very important antitumor agent.10,11 The methyl ester 3 was isolated from exudates released by the roots of iron-deficient alfalfa (Medicago sativa).12,13 2-Aryl-3-aroylbenzo[b]furans serve as core structures of many naturally occurring products and pharmaceutical drug candidates.14,15 Recently, Flynn et al. prepared 2,3-disubstituted benzo[b]furan analogues (4) of some benzo[b]thiophenes identified as inhibitors of tubulin polymerization and their biological activity was assessed.16–18 Furopyridine derivatives are also highly biologically active.19–22
Figure 1.
Some biologically active benzo[b]furans
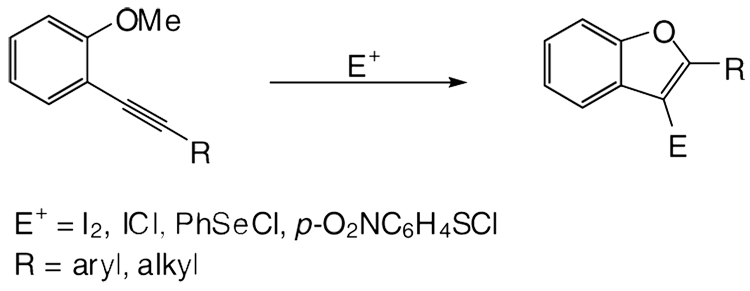
For these reasons, various routes to substituted benzo[b]furans have been the subject of extensive experimental studies. Major synthetic strategies for the construction of furan rings from various arene derivatives,23,24 alkyne-based palladium-catalyzed reactions,25–34 and C-O bond formation35,36 have been reported. Recently, we developed a general synthesis of 2,3-disubstituted benzo[b]furans by the palladium/copper-catalyzed cross-coupling of various o-iodoanisoles and terminal alkynes, followed by electrophilic cyclization with I2, PhSeCl or p-O2NC6H4SC1 under very mild reaction conditions (Scheme 1).37
Scheme 1.
Synthesis of 2,3-Disubstituted Benzo[b]furans by Electrophilic Cyclization
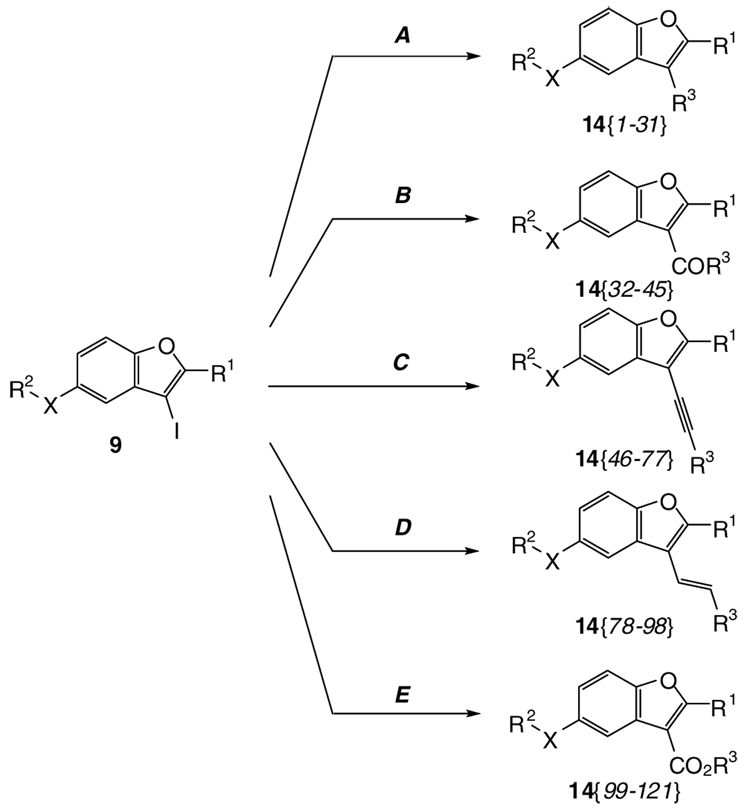
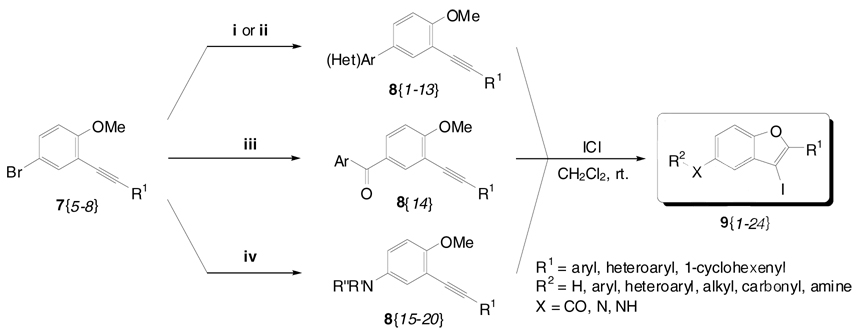
In our continuing research efforts to adapt heterocyclization chemistry to a high-throughput synthesis format, we herein report the first solution phase library synthesis of benzofurans by our electrophilic cyclization chemistry. We demonstrate the significance of this methodology by elaborating the resulting 3-iodobenzofurans via various palladium-catalyzed couplings, such as Suzuki-Miyaura, carbonylative Suzuki, Sonogashira, Heck, and carboalkoxylation chemistry, to a 121 member library of 2,3,5-trisubstituted benzo[b]furans 14 (see Scheme 4) in modest yields.
Scheme 4.
Synthesis of Benzo[b]furans 14 using Various Palladium-Catalyzed Reactions
Method A (Suzuki-Miyaura coupling): 10% Pd(PPh3)4, KOH (3.0 equiv), 10 (1.5 equiv), toluene/EtOH/H2O (20/5/1), reflux; Method B (carbonylative Suzuki coupling): CO (1 atm), 3 mol % PdCl2(PPh3)2, K2CO3 (3.0 equiv), NaI (3.0 equiv), 10 (1.1 equiv), anisole, 80 °C; Method C (Sonogashira coupling): 3% PdCl2(PPh3)2, 3% CuI, Et2NH, 6 (1.2 equiv), DMF, 50 °C; Method D (Heck coupling): 5 mol % Pd(OAc)2, n-Bu4NI (1.0 equiv), Na2CO3 (2.5 equiv), R3CH=CH2 12 (1.2 equiv), DMF, 80 °C; Method E (carboalkoxylation): CO (1 atm), 3 mol % Pd(OAc)2, 5 mol % dppf, TEA (2.0 equiv), R3OH 13 (1.5 equiv), DMF, 70 °C.
Results and Discussion
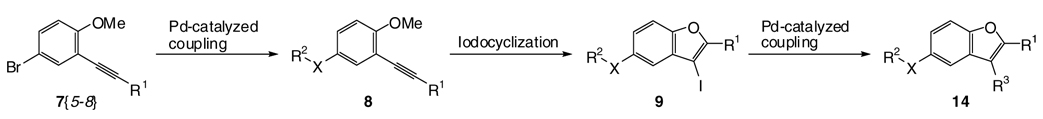
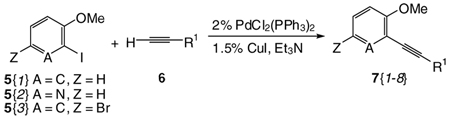
The strategy for library production is shown in Scheme 2. We hypothesized that our previously described iodocyclization process should readily afford 2,3,5-trisubstituted benzo[b]furans 14 as key intermediates to compounds of biological interest. Also, numerous analogues of 14 should be readily prepared through various coupling reactions and subsequent functionalization at the C-3 and C-5 positions. The starting material, 4-bromo-2-iodoanisole 5{3} (see Table 1), can be easily prepared through regioselective iodination of 4-bromoanisole.38 2-Iodoanisole [5{1}] and 2-iodo-3-methoxypyridine [5{2}] were obtained commercially. The alkynes 7 required for cyclization are readily prepared by the palladium/copper-catalyzed Sonogashira cross-coupling39 of the methoxy-substituted aryl and/or heteroaryl iodides 5 with terminal alkynes 6 at room temperature. The results are summarized in Table 1. As shown in the table, the requisite alkynes 7{1–8} are readily obtained by this straightforward approach.
Scheme 2.
Library Design for 2,3,5-Trisubstituted Benzo[b]furans 14
Table 1.
Library Data for Compounds 7{1–8}
 | ||||
|---|---|---|---|---|
| compd | A | Z | R1 | yield (%) |
| 7{1} | C | H | 4-MeOC6H4 | 93 |
| 7{2} | C | H | 3-MeC6H4 | 95 |
| 7{3} | N | H | 4-MeOC6H4 | 98 |
| 7{4} | N | H | 3,5-(MeO)2C6H3 | 93 |
| 7{5} | C | Br | 1-cyclohexenyl | 84 |
| 7{6} | C | Br | 4-MeOC6H4 | 90 |
| 7{7} | C | Br | 3-thiophenyl | 94 |
| 7{8} | C | Br | 3-MeC6H4 | 93 |
As outlined in Scheme 3, the bromoalkynes 7{5–8} are readily elaborated to the more highly substituted alkynes 8 via standard palladium-based methodology. As summarized in Table 2, the palladium-catalyzed Suzuki-Miyaura coupling of bromoalkynes 7{5–8} with boronic acids 10 proceeds in the presence of a base to provide alkynes 8{1–13}.40,41 Bromoalkyne 7{6} readily reacts with 4-methoxyphenylboronic acid [10{1}] under one atmosphere of carbon monoxide in the presence of PdCl2(dppf) as a catalyst to give the corresponding ketone 8{14}.42 Unfortunately, this process affords only a low yield of 8{14} (38%) due to the formation of the direct coupling product 8{1} (14%), as well as recovery of the starting material 7{6}. Work is currently underway to improve this process. The corresponding amines 8{15–20} were obtained by palladium-catalyzed amination of several bromoalkynes 7 with amines 11.43 The secondary amines morpholine [11{1}] and pyrrolidine [11{2}] gave the corresponding products 8{15–18} in moderate yields after 12 h reaction time. As expected, compounds 8{19} and 8{20} were obtained in excellent yields within 3 h when employing n-butylamine [11{3}] and 4-methylaniline [11{4}] (see the Supporting Information for the experimental details).
Scheme 3.
Suzuki-Miyaura Coupling [8{1–13}], Carbonylative Suzuki Coupling [8{14}], Amination [8{15–20}], and Subsequent Iodocyclization [9{1–24}]
i. 5 mol % Pd(PPh3)4, K2CO3 (3.0 equiv), and ArB(OH)2 10{1,4,7–8} (1.5 equiv) in toluene/EtOH, 80 °C
ii. 5 mol % Pd(dba)2, 8 mol % PPh3, KOH (3.0 equiv), and (Het)ArB(OH)2 10{6} (1.5 equiv) in toluence/EtOH, 80 °C
iii. 5 mol % PdCl2(dppf), K2CO3 (3.0 equiv), NaI (3.0 equiv), CO (1.0 atm), and ArB(OH)2 10{1} (1.1 equiv) in toluene/EtOH, 80 °C
iv. 5 mol % Pd2(dba)3, DavePhos (0.1 equiv), NaOtBu (1.4 equiv), and amines 11 (1.5 equiv) in toluene, 60 °C
Table 2.
Library Data for Compounds 8{1–20} and 9{1–24}
| alkyne 7/8 | R1 | R2 | t (h) | yield (%)b | 3-iodobenzofurana9 | yield (%)b |
|---|---|---|---|---|---|---|
| 7{1} | 4-MeOC6H4 | - | - | - | 9{1} | 97 |
| 7{2} | 3-MeC6H4 | - | - | - | 9{2} | 92 |
| 7{3} | 4-MeOC6H4 | - | - | - | 9{3} | 76 |
| 7{4} | 3,5-(MeO)2C6H3 | - | - | - | 9{4} | 58 |
| 8{1} | 4-MeOC6H4 | 10{1} | 8 | 69 | 9{5} | 93 |
| 8{2} | 4-MeOC6H4 | 10{4} | 8 | 63 | 9{6} | 89 |
| 8{3} | 4-MeOC6H4 | 10{6} | 8 | 57 | 9{7} | 87 |
| 8{4} | 4-MeOC6H4 | 10{7} | 8 | 63 | 9{8} | 90 |
| 8{5} | 4-MeOC6H4 | 10{8} | 8 | 72 | 9{9} | 83 |
| 8{6} | 3-thiophenyl | 10{1} | 8 | 78 | 9{10} | 91 |
| 8{7} | 3-thiophenyl | 10{4} | 8 | 81 | 9 {11} | 83 |
| 8{8} | 3-thiophenyl | 10{6} | 8 | 62 | 9{12} | 78 |
| 8{9} | 3-thiophenyl | 10{7} | 8 | 83 | 9{13} | 88 |
| 8{10} | 1-cyclohexenyl | 10{4} | 8 | 71 | 9{14} | 91 |
| 8{11} | 1-cyclohexenyl | 10{6} | 8 | 68 | 9{15} | 88 |
| 8{12} | 1-cyclohexenyl | 10{7} | 8 | 68 | 9{16} | 92 |
| 8{13} | 3-MeC6H4 | 10{4} | 8 | 67 | 9{17} | 87 |
| 8{14} | 4-MeOC6H4 | 10{1} | 24 | 38(14)c | 9{18} | 82 |
| 8{15} | 4-MeOC6H4 | 11{1} | 12 | 78 | 9{19} | 84 |
| 8{16} | 3-MeC6H4 | 11{1} | 12 | 73 | 9{20} | 82 |
| 8{17} | 3-thiophenyl | 11{1} | 12 | 71 | 9{21} | 81 |
| 8{18} | 3-MeC6H4 | 11{2} | 12 | 59 | 9{22} | 77 |
| 8{19} | 4-MeOC6H4 | 11{3} | 3 | 87 | 9{23} | 51d |
| 8{20} | 4-MeOC6H4 | 11{4} | 3 | 93 | 9{24} | 28d,e |
All reactions were carried out using alkynes 7{1–4} or 8{1–20} and 1.5 equiv of IC1 in CH2C12 at room temperature within 2–3 h, unless otherwise indicated.
Isolated yields after column chromatography.
Some starting material remained. In parentheses is the yield of the Suzuki-Miyaura direct coupling product.
IC1 (2.0 + 1.0 equiv) was used.
An inseparable mixture was obtained.
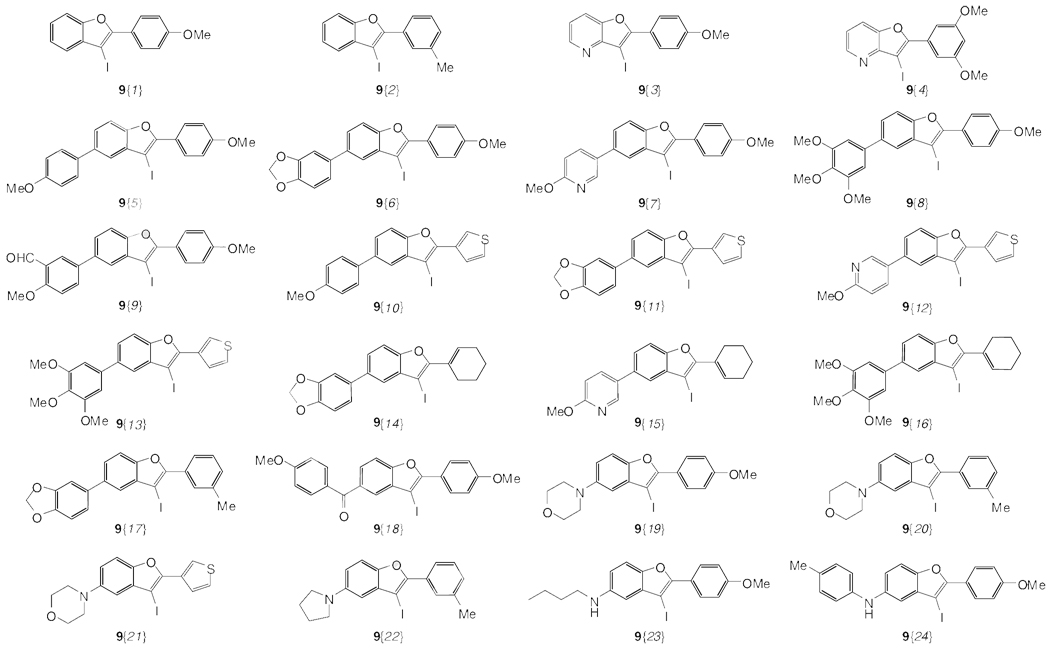
As the key step in our library synthesis, variously substituted iodobenzofuran derivatives 9 were efficiently prepared within 1–2 h by electrophile cyclization of the corresponding methoxy-containing alkynes 7{1–4} and 8{1–20} using ICl in CH2Cl2 at ambient temperature (Table 2 and Figure 2). All of the reactions were monitored by thin layer chromatography and the products purified by column chromatography. We did not systematically investigate the effects of various substituents in the C-5 position of the aromatic ring system. However, it is noteworthy that pyridine derivatives were treated with ICl under our standard electrophilic cyclization conditions, affording the desired furopyridines 9{3–4} in moderate yields. The presence of an electron-donating OMe group in the para position of the phenyl ring of the R1 group gave excellent yields of the desired iodobenzofuran products 9{5–9} and 9{19}. Also noteworthy is the fact that substituting the alkynyl unit with a thiophene heterocycle gave 3-iodobenzofurans 9{10–13} in good to excellent yields. Alkynes 8{10–12}, having a 1-cyclohexenyl group, also reacted by electrophilic cyclization to give the desired products 9{14–16}. Alkynes with an m-tolyl substituent gave the desired products 9{17}, 9{20} and 9{22}. The iodobenzofuran 9{18} having a carbonyl group in the C-5 position was produced in a good yield. The reaction of C-5 amine-substituted aryl alkynes generally afforded the desired benzofuran products in good yields under ordinary reaction conditions. However, the secondary amine-substituted alkyne 8{19} produced the desired product 9{23} in a slightly lower yield. As expected, an aniline-substituted alkyne 8{20} also generated the desired product 9{24}, but in a lower yield. The low yield here is probably due to electrophilic substitution of the aniline ring of the product 9{24}. This iodobenzofuran synthesis tolerates a wide variety of substituents, including halides, ethers, acetals, aldehydes, ketones, amines, aryl, heteroaryl, and alkyl groups, and proceeds under mild reaction conditions. The 3-iodobenzofurans 9{1–24} produced by this chemistry should be very useful for the synthesis of a wide variety of substituted benzo[b]furans 14.
Figure 2.
Synthesis of 3-iodobenzofurans 9{1–24}
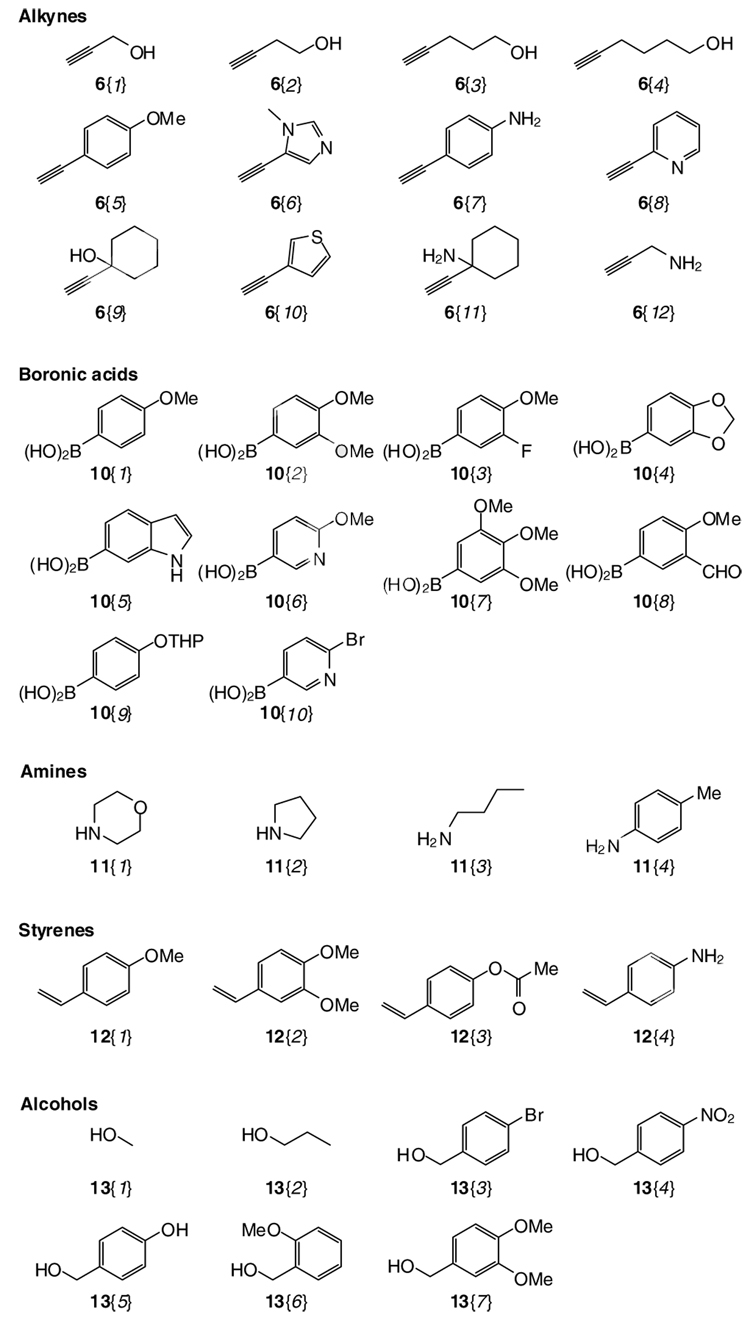
The 3-iodobenzofurans 9 can be further elaborated by using a variety of palladium-mediated processes, such as Suzuki-Miyaura coupling,40,41 carbonylative Suzuki coupling,42 Sonogashira coupling,39 Heck coupling,44 and carboalkoxylation45 (Scheme 4). The crude products 14 were purified by either column chromatography or preparative HPLC. The results of this parallel library synthesis are summarized in Table 3, which indicates that the products 14 can be obtained in moderate to good yields with high purities. The reagents used for substitution of the iodine-containing products 9 were chosen on the basis of their commercial availability (e.g. boronic acids 10, terminal alkynes 6, styrenes 12, and alcohols 13) (Figure 3) and potential drug-like properties, resulting in a virtual library of all theoretically possible products of approximately 5,000 potential compounds. This number was arrived at by determining all possible combinations of the R group variation with available starting materials. However, only a small subset of 121 compounds out of these 5,000 virtual structures was actually made in the laboratory. Most of the selected 121 benzo[b]furan library members were Lipinski46,47 compliant. The molecular weight, clog P, number of hydrogen bond donors and acceptors, and the number of rotatable bonds were either specified or calculated for each of the library members using the SYBYL48 program.
Table 3.
Library Data for Compounds 14{1–121}
| product | 9 | R3 | method | yield (%)b | purity (%)e | product | 9 | R3 | method | yield (%)b | purity (%)e |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 14{1} | 9{1} | 10{5} | A | 39 | 84 | 14{65} | 9{8} | 6{10} | C | 77 | >99 |
| 14{2} | 9{2} | 10{9} | Aa | 47 | >99 | 14{66} | 9{12} | 6{9} | C | 76 | >99 |
| 14{3} | 9{3} | 10{1} | A | 51 | >99 | 14{67} | 9{13} | 6{5} | C | 82c | 99 |
| 14{4} | 9{3} | 10{6} | A | 38 | 99 | 14{68} | 9{13} | 6{9} | C | 67 | >99 |
| 14{5} | 9{3} | 10{7} | A | 55 | 99 | 14{69} | 9{17} | 6{6} | C | 52 | 98 |
| 14{6} | 9{5} | 10{1} | A | 88c | >99 | 14{70} | 9{18} | 6{2} | C | 85 | >99 |
| 14{7} | 9{5} | 10{6} | A | 78 | >99 | 14{71} | 9{19} | 6{1} | C | 78 | >99 |
| 14{8} | 9{5} | 10{7} | A | 83 | >99 | 14{72} | 9{19} | 6{7} | C | 57 | >99 |
| 14{9} | 9{8} | 10{10} | A | 71c | 99 | 14{73} | 9{19} | 6{9} | C | 78 | >99 |
| 14{10} | 9{9} | 10{1} | A | 57 | >99 | 14{74} | 9{19} | 6{12} | C | 34 | 99 |
| 14{11} | 9{10} | 10{10} | A | 73c | 98 | 14{75} | 9{21} | 6{5} | C | 83c | >99 |
| 14{12} | 9{11} | 10{1} | A | 62 | >99 | 14{76} | 9{21} | 6{7} | C | 61 | >99 |
| 14{13} | 9{11} | 10{6} | A | 53 | 99 | 14{77} | 9{21} | 6{9} | C | 86c | >99 |
| 14{14} | 9{14} | 10{1} | A | 47 | >99 | 14{78} | 9{1} | 12{1} | D | 66c | 96 |
| 14{15} | 9{14} | 10{2} | A | 28 | >99 | 14{79} | 9{1} | 12{3} | D | 61c | 99 |
| 14{16} | 9{14} | 10{6} | A | 51 | >99 | 14{80} | 9{3} | 12{1} | D | 47c | 95 |
| 14{17} | 9{14} | 10{7} | A | 69 | >99 | 14{81} | 9{3} | 12{2} | D | 72c | 98 |
| 14{18} | 9{14} | 10{9} | A | 53 | 99 | 14{82} | 9{3} | 12{3} | D | 58 | 97 |
| 14{19} | 9{15} | 10{3} | A | 32 | 98 | 14{83} | 9{5} | 12{1} | D | 69c | 99 |
| 14{20} | 9{17} | 10{1} | A | 72c | >99 | 14{84} | 9{5} | 12{3} | D | 71c | >99 |
| 14{21} | 9{17} | 10{6} | A | 67 | >99 | 14{85} | 9{6} | 12{1} | D | 76c | 98 |
| 14{22} | 9{17} | 10{7} | A | 73c | >99 | 14{86} | 9{9} | 12{3} | D | 71 | 99 |
| 14{23} | 9{17} | 10{9} | A | 85c | >99 | 14{87} | 9{11} | 12{2} | D | 71 | >99 |
| 14{24} | 9{18} | 10{9} | Aa | 69c | >99 | 14{88} | 9{12} | 12{1} | D | 78 | 99 |
| 14{25} | 9{19} | 10{1} | A | 81c | >99 | 14{89} | 9{13} | 12{1} | D | 69 | 93 |
| 14{26} | 9{19} | 10{6} | A | 73 | >99 | 14{90} | 9{14} | 12{1} | D | 61 | 98 |
| 14{27} | 9{21} | 10{1} | A | 83c | >99 | 14{91} | 9{14} | 12{4} | D | 57 | 98 |
| 14{28} | 9{21} | 10{6} | A | 71c | >99 | 14{92} | 9{17} | 12{1} | D | 76c | 99 |
| 14{29} | 9{22} | 10{1} | A | 67 | >99 | 14{93} | 9{17} | 12{4} | D | 68 | 96 |
| 14{30} | 9{23} | 10{3} | A | 57c | 98 | 14{94} | 9{19} | 12{2} | D | 58 | 99 |
| 14{31} | 9{23} | 10{9} | A | 68c | 99 | 14{95} | 9{19} | 12{3} | D | 64c | >99 |
| 14{32,(33)} | 9{5} | 10{4} | B | 34(29)c,d | >99 | 14{96} | 9{20} | 12{1} | D | 81c | >99 |
| 14{34,(35)} | 9{6} | 10{6} | B | 44(33)c,d | >99 | 14{97} | 9{21} | 12{3} | D | 53c | >99 |
| 14{36,(37)} | 9{7} | 10{1} | B | 46(27)c,d | >99 | 14{98} | 9{22} | 12{3} | D | 57 | >99 |
| 14{38,(39)} | 9{8} | 10{1} | B | 41(31)c,d | >99 | 14{99} | 9{1} | 13{1} | E | 58c | 98 |
| 14{40,(41)} | 9{8} | 10{6} | B | 43(35)c,d | >99 | 14{100} | 9{1} | 13{3} | E | 29 | >99 |
| 14{42,(43)} | 9{10} | 10{1} | B | 49(32)c,d | >99 | 14{101} | 9{1} | 13{5} | E | 56c | >99 |
| 14{44,(45)} | 9{20} | 10{6} | B | 37(28)c,d | >99 | 14{102} | 9{1} | 13{6} | E | 26 | >99 |
| 14{46} | 9{1} | 6{6} | C | 43 | 96 | 14{103} | 9{1} | 13{7} | E | 27 | 96 |
| 14{47} | 9{2} | 6{2} | C | 81c | 99 | 14{104} | 9{3} | 13{1} | E | 59 | 98 |
| 14{48} | 9{2} | 6{4} | C | 82c | 99 | 14{105} | 9{3} | 13{5} | E | 37 | 97 |
| 14{49} | 9{3} | 6{1} | C | 77c | >99 | 14{106} | 9{5} | 13{1} | E | 71c | 99 |
| 14{50} | 9{3} | 6{8} | C | 41 | 96 | 14{107} | 9{5} | 13{5} | E | 73 | >99 |
| 14{51} | 9{3} | 6{9} | C | 48 | 98 | 14{108} | 9{6} | 13{6} | E | 37 | >99 |
| 14{52} | 9{4} | 6{2} | C | 21 | 97 | 14{109} | 9{7} | 13{1} | E | 76c | >99 |
| 14{53} | 9{4} | 6{3} | C | 18 | 98 | 14{110} | 9{7} | 13{2} | E | 69c | >99 |
| 14{54} | 9{5} | 6{1} | C | 83 | >99 | 14{111} | 9{7} | 13{4} | E | 48 | >99 |
| 14{55} | 9{5} | 6{2} | C | 84 | >99 | 14{112} | 9{7} | 13{5} | E | 55 | >99 |
| 14{56} | 9{5} | 6{5} | C | 73 | 98 | 14{113} | 9{8} | 13{1} | E | 76c | >99 |
| 14{57} | 9{5} | 6{9} | C | 81 | 98 | 14{114} | 9{8} | 13{3} | E | 59 | >99 |
| 14{58} | 9{5} | 6{11} | C | 69 | >99 | 14{115} | 9{13} | 13{1} | E | 80c | 99 |
| 14{59} | 9{5} | 6{12} | C | 31 | 95 | 14{116} | 9{15} | 13{1} | E | 53 | >99 |
| 14{60} | 9{6} | 6{5} | C | 69 | >99 | 14{117} | 9{16} | 13{1} | E | 58 | 98 |
| 14{61} | 9{6} | 6{9} | C | 60 | >99 | 14{118} | 9{17} | 13{5} | E | 71c | >99 |
| 14{62} | 9{7} | 6{2} | C | 79c | 99 | 14{119} | 9{18} | 13{1} | E | 74c | >99 |
| 14{63} | 9{7} | 6{9} | C | 57 | >99 | 14{120} | 9{20} | 13{5} | E | 59 | >99 |
| 14{64} | 9{8} | 6{8} | C | 72 | >99 | 14{121} | 9{21} | 13{5} | E | 54c | 99 |
The THP ether-protected boronic acid 10{9} was deprotected in situ using aq. HCl in THF at room temperature.
Isolated yield after preparative HPLC.
Isolated yield after column chromatography.
The number in parentheses is the yield of Suzuki-Miyaura direct coupling product.
UV purity determined at 214 nm after preparative HPLC.
Figure 3.
Diverse terminal alkynes 6{1–12}, boronic acids 10{1–10}, amines 11{1–4}, styrenes 12{1–4}, and alcohols 13{1–7} used for library synthesis
The Suzuki-Miyaura coupling of the 3-iodobenzofurans 9 with various arylboronic acids 10 proceeded smoothly to give the desired products 14{1–31} in modest yields. Most reactions were complete within 6 h in refluxing toluene (Method A).40,41 Product 14{24}, containing a tetrahydropyranyl (THP) ether protecting group, was deprotected in situ in 69% yield using aqueous hydrochloric acid in THF at room temperature for a few minutes.49 The carbonylative Suzuki coupling of 3-iodobenzofurans 9 with arylboronic acids 10 has been carried out in anisole in the presence of PdCl2(PPh3)2 under carbon monoxide at atmospheric pressure (Method B).42 These reactions produced both the corresponding CO inserted carbonyl products and the direct Suzuki-Miyaura coupling products. The products were separated by column chromatography to give 14{32,34,36,38,40,42} and 14{33,35,37,39,41,43}, respectively. Sonogashira coupling of the 3-iodobenzofurans 9 with various terminal acetylenes 6 nicely provides the corresponding alkynyl products 14{46–77} (Method C).39 Additionally, we have been able to perform Heck coupling on the 3-iodobenzofurans 9. By allowing the compound to react under Heck reaction conditions in the presence of the styrenes 12, the substituted olefin-containing benzo[b]furan products 14{78–98} were obtained (Method D).44 Carboalkoxylation of the 3-iodobenzofurans 9 using one atmosphere of carbon monoxide and various alcohols 13 in the presence of catalytic amounts of Pd(OAc)2 and dppf ligand afforded the ester-containing benzo[b]furans 14{99–121} (Method E).45
In conclusion, the 3-iodobenzofurans 9 have proven to be very useful templates for further diversification by a variety of C-C, C-N, and C-O bond forming reactions,50–53 and are thus valuable building blocks for combinatorial chemistry.54,55 We have demonstrated that 3-iodobenzofurans 9 readily react with various building blocks, e.g. boronic acids 10, terminal alkynes 6, styrenes 12, and carbon monoxide plus alcohols 13, to efficiently construct a 121 member library of highly substituted benzo[b]furans 14. The desired 3-iodobenzofurans 9 are readily prepared by our previously published electrophilic cyclization chemistry. Of the 121 compounds produced, 74 compounds were obtained in >99% purity. The benzo[b]furan library members 14 will be evaluated against various biological screens by the National Institutes of Health Molecular Library Screening Center Network.
Supplementary Material
Synthetic methods, spectral assignments and copies of 1H and 13C NMR spectra for all previously unreported starting materials and products. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the National Institute of General Medical Sciences (GM070620 and GM079593) and the National Institutes of Health Kansas University Chemical Methodologies and Library Development Center of Excellence (GM069663) for support of this research; Johnson Matthey, Inc. and Kawaken Fine Chemicals Co., Ltd. for donations of palladium catalysts; and Frontier Scientific and Synthonix for donations of boronic acids.
REFERENCES
- 1.Crenshaw RR, Jeffries AT, Luke GM, Cheney LC, Bialy G. J. Med. Chem. 1971;14:1185–1190. doi: 10.1021/jm00294a011. [DOI] [PubMed] [Google Scholar]
- 2.Teo CC, Kon OL, Sim KY, Ng SC. J. Med. Chem. 1992;35:1330–1339. doi: 10.1021/jm00086a002. [DOI] [PubMed] [Google Scholar]
- 3.Halabalaki M, Aligiannis N, Papoutsi Z, Mitakou S, Moutsatsou P, Sekeris C, Skaltsounis AL. J. Nat. Prod. 2000;63:1672–1674. doi: 10.1021/np000071b. [DOI] [PubMed] [Google Scholar]
- 4.Hocke C, Prante O, Lober S, Hubner H, Gmeiner P, Kuwert T. Bioorg. Med. Chem. Lett. 2004;14:3963–3966. doi: 10.1016/j.bmcl.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 5.Gfesser GA, Faghih R, Bennani YL, Curtis MP, Esbenshade TA, Hancock AA, Cowart MD. Bioorg. Med. Chem. Lett. 2005;15:2559–2563. doi: 10.1016/j.bmcl.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Xiang JS, DiGrandi MJ, Du X, Ipek M, Laakso LM, Li J, Li W, Rush TS, Schmid J, Skotnicki JS, Tam S, Thomason JR, Wang Q, Levin JI. Bioorg. Med. Chem. 2005;13:6629–6644. doi: 10.1016/j.bmc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 7.Chang HM, Cheng KP, Choang TF, Chow HF, Chui KY, Hon PM, Tan FWL, Yang Y, Zhong ZP, Lee CM, Sham HL, Chan CF, Cui YX, Wong HNC. J. Org. Chem. 1990;55:3537–3543. [Google Scholar]
- 8.Yang Z, Liu HB, Lee CM, Chang HM, Wong HNC. J. Org. Chem. 1992;57:7248–7257. [Google Scholar]
- 9.Hutchinson SA, Luetjens H, Scammells PJ. Bioorg. Med. Chem. Lett. 1997;7:3081–3084. [Google Scholar]
- 10.Tsai I-L, Hsieh C-F, Duh C-Y. Phytochemistry. 1998;48:1371–1375. doi: 10.1016/s0031-9422(97)00948-5. [DOI] [PubMed] [Google Scholar]
- 11.Kao CL, Chern JW. J. Org. Chem. 2002;67:6772–6787. doi: 10.1021/jo0258960. [DOI] [PubMed] [Google Scholar]
- 12.Koshino H, Masaoka Y, Ichihara A. Phytochemistry. 1993;33:1075–1077. [Google Scholar]
- 13.Hiroya K, Suzuki N, Yasuhara A, Egawa Y, Kasano A, Sakamoto T. J. Chem. Soc., Perkin Trans. 1. 2000:4339–4346. [Google Scholar]
- 14.Twyman LJ, Allsop D. Tetrahedron Lett. 1999;40:9383–9384. [Google Scholar]
- 15.Allsop D, Gibson G, Martin IK, Moore S, Turnbull S, Twyman LJ. Bioorg. Med. Chem. Lett. 2001;11:255–257. doi: 10.1016/s0960-894x(00)00645-4. [DOI] [PubMed] [Google Scholar]
- 16.Flynn BL, Hamel E, Jung MK. J. Med. Chem. 2002;45:2670–2673. doi: 10.1021/jm020077t. [DOI] [PubMed] [Google Scholar]
- 17.Kerr DJ, Hamel E, Jung MK, Flynn BL. Bioorg. Med. Chem. 2007;15:3290–3298. doi: 10.1016/j.bmc.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Flynn BL, Verdier-Pinard P, Hamel E. Org. Lett. 2001;3:651–654. doi: 10.1021/ol0067179. [DOI] [PubMed] [Google Scholar]
- 19.Elliott RL, Ryther KB, Anderson DJ, Piattoni-Kaplan M, Kuntzweiler TA, Donnelly-Roberts D, Arneric SP, Holladay MW. Bioorg. Med. Chem. Lett. 1997;7:2703–2708. [Google Scholar]
- 20.Wishka DG, Graber DR, Kopta LA, Olmsted RA, Friis JM, Hosley JD, Adams WJ, Seest EP, Castle TM, Dolak LA, Keiser BJ, Yagi Y, Jeganathan A, Schlachter ST, Murphy MJ, Cleek GJ, Nugent RA, Poppe SM, Swaney SM, Han F, Watt W, White WL, Poel TJ, Thomas RC, Voorman RL, Stefanski KJ, Stehle RG, Tarpley WG, Morris J. J. Med. Chem. 1998;41:1357–1360. doi: 10.1021/jm9801049. [DOI] [PubMed] [Google Scholar]
- 21.Mathes BM, Hudziak KJ, Schaus JM, Xu Y-C, Nelson DL, Wainscott DB, Nutter SE, Gough WH, Branchek TA, Zgombick JM, Filla SA. Bioorg. Med. Chem. Lett. 2004;14:167–170. doi: 10.1016/j.bmcl.2003.09.091. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki Y, Nakano M, Sato H, Truesdale AT, Stuart JD, Nartey EN, Hightower KE, Kane-Carson L. Bioorg. Med. Chem. Lett. 2007;17:250–254. doi: 10.1016/j.bmcl.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita A, Toy A, Scahill TA. J. Org. Chem. 1989;54:3625–3634. [Google Scholar]
- 24.Sakamoto T, Kondo Y, Yasuhara A, Yamanaka H. Tetrahedron. 1991;47:1877–1886. [Google Scholar]
- 25.Kondo Y, Shiga F, Murata N, Sakamoto T, Yamanaka H. Tetrahedron. 1994;50:11803–11812. [Google Scholar]
- 26.Arcadi A, Cacchi S, Del Rosario M, Fabrizi G, Marinelli F. J. Org. Chem. 1996;61:9280–9288. [Google Scholar]
- 27.Hu Y, Zhang Y, Yang Z, Fathi R. J. Org. Chem. 2002;67:2365–2368. doi: 10.1021/jo010839c. [DOI] [PubMed] [Google Scholar]
- 28.Colobert F, Castanet A-S, Abillard O. Eur. J. Org. Chem. 2005:3334–3341. [Google Scholar]
- 29.Liao Y, Smith J, Fathi R, Yang Z. Org. Lett. 2005;7:2707–2709. doi: 10.1021/ol050876g. [DOI] [PubMed] [Google Scholar]
- 30.Bernini R, Cacchi S, De Salve I, Fabrizi G. Synthesis. 2007:873–882. [Google Scholar]
- 31.Cacchi S, Fabrizi G, Moro L. Tetrahedron Lett. 1998;39:5101–5104. [Google Scholar]
- 32.Nan Y, Miao H, Yang Z. Org. Lett. 2000;2:297–299. doi: 10.1021/ol991327b. [DOI] [PubMed] [Google Scholar]
- 33.Arcadi A, Cacchi S, Di Giuseppe S, Fabrizi G, Marinelli F. Org. Lett. 2002;4:2409–2412. doi: 10.1021/ol0261581. [DOI] [PubMed] [Google Scholar]
- 34.Arcadi A, Cacchi S, Fabrizi G, Marinelli F, Moro L. Synlett. 1999:1432–1434. [Google Scholar]
- 35.Willis MC, Taylor D, Gillmore AT. Org. Lett. 2004;6:4755–4757. doi: 10.1021/ol047993g. [DOI] [PubMed] [Google Scholar]
- 36.Anderson KW, Ikawa T, Tundel RE, Buchwald SL. J. Am. Chem. Soc. 2006;128:10694–10695. doi: 10.1021/ja0639719. [DOI] [PubMed] [Google Scholar]
- 37.Yue D, Yao T, Larock RC. J. Org. Chem. 2005;70:10292–10296. doi: 10.1021/jo051299c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muraki T, Togo H, Yokoyama M. J. Org. Chem. 1999;64:2883–2889. doi: 10.1021/jo9825207. [DOI] [PubMed] [Google Scholar]
- 39.Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975;16:4467–4470. [Google Scholar]
- 40.Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457–2483. [Google Scholar]
- 41.Suzuki A. J. Organomet. Chem. 1999;576:147–168. [Google Scholar]
- 42.Ishiyama T, Kizaki H, Hayashi T, Suzuki A, Miyaura N. J. Org. Chem. 1998;63:4726–4731. [Google Scholar]
- 43.Ali MH, Buchwald SL. J. Org. Chem. 2001;66:2560–2565. doi: 10.1021/jo0008486. [DOI] [PubMed] [Google Scholar]
- 44.Heck RF. Org. React. 1982;27:345–390. [Google Scholar]
- 45.Chambers RJ, Marfat A. Synth. Commun. 1997;27:515–520. [Google Scholar]
- 46.Lipinski CA, Lombardo F, Dominay BW, Feeney PJ. Adv. Drug Del. Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 47.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv. Drug Del. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 48.SYBYL, version 8.0. St. Louis, MO: The Tripos Associate; 2007. [Google Scholar]
- 49.Pez D, Leal I, Zuccotto F, Boussard C, Brun R, Croft SL, Yardley V, Ruiz Perez LM, Gonzalez Pacanowska D, Gilbert IH. Bioorg. Med. Chem. 2003;11:4693–4711. doi: 10.1016/j.bmc.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki A. Chem. Commun. 2005:4759–4763. doi: 10.1039/b507375h. [DOI] [PubMed] [Google Scholar]
- 51.Hartwig JF. Angew. Chem., Int. Ed. Engl. 1998;37:2046–2067. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 52.Buchwald SL, Mauger C, Mignani G, Scholz U. Adv. Synth. Catal. 2006;348:23–39. [Google Scholar]
- 53.Zhang H, Cao W, Ma D. Synth. Commun. 2007;37:25–35. [Google Scholar]
- 54.Liao Y, Reitman M, Zhang Y, Fathi R, Yang Z. Org. Lett. 2002;4:2607–2609. doi: 10.1021/ol020111y. [DOI] [PubMed] [Google Scholar]
- 55.Bui CT, Flynn BL. J. Comb. Chem. 2006;8:163–167. doi: 10.1021/cc050066w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthetic methods, spectral assignments and copies of 1H and 13C NMR spectra for all previously unreported starting materials and products. This material is available free of charge via the Internet at http://pubs.acs.org.