Abstract
Chronic respiratory tract infections caused by Staphylococcus aureus are common in patients with cystic fibrosis (CF). Recently, it was shown in a few CF patients that S. aureus isolates produce capsular polysaccharides (CPs). However, it is not known whether this is a common feature and whether an immune response to CPs in CF is detectable. Therefore, we examined 170 S. aureus isolates from CF patients and healthy individuals for production of CP types 5 and 8 by using monoclonal antibodies. We found that CP-producing staphylococcal isolates were randomly distributed among CF patients and healthy carriers. Eighty-five percent of all isolates produced CPs, 77% of which were type 8. Examination of one sputum sample by an immunofluorescence technique suggested that production of CPs is not an in vitro phenomenon. S. aureus isolates from various sites of a single person often yielded more than one CP type. A random distribution of S. aureus strains with CP type 5 or 8 from the skin and respiratory tracts of patients and from the skin of healthy individuals was found. Antibody response to CP types 5 and 8, measured by enzyme-linked immunosorbent assay, was not elevated in CF patients with chronic S. aureus lung infection in comparison with healthy carriers. On the contrary, in CF patients the ratios of antibodies to CP 8 were significantly lower (P less than 0.005; alpha = 0.025). The ratios of antibodies to CP types did not change when monitored longitudinally over several months. This study suggests that the production of CPs is a universal property of S. aureus and that infected CF patients do not have elevated ratios of antibodies to these antigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit R. D., Karakawa W. W., Vann W. F., Robbins J. B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984 Apr;2(2):85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- Arbeit R. D., Nelles M. J. Capsular polysaccharide antigenemia in rats with experimental endocarditis due to Staphylococcus aureus. J Infect Dis. 1987 Feb;155(2):242–246. doi: 10.1093/infdis/155.2.242. [DOI] [PubMed] [Google Scholar]
- Berger M. Immunoglobulin G subclass determination in diagnosis and management of antibody deficiency syndromes. J Pediatr. 1987 Feb;110(2):325–328. doi: 10.1016/s0022-3476(87)80182-8. [DOI] [PubMed] [Google Scholar]
- Buggy B. P., Schaberg D. R., Swartz R. D. Intraleukocytic sequestration as a cause of persistent Staphylococcus aureus peritonitis in continuous ambulatory peritoneal dialysis. Am J Med. 1984 Jun;76(6):1035–1040. doi: 10.1016/0002-9343(84)90854-4. [DOI] [PubMed] [Google Scholar]
- Ericsson A., Granström M., Möllby R., Strandvik B. Antibodies to staphylococcal teichoic acid and alpha toxin in patients with cystic fibrosis. Acta Paediatr Scand. 1986 Jan;75(1):139–144. doi: 10.1111/j.1651-2227.1986.tb10170.x. [DOI] [PubMed] [Google Scholar]
- Falcieri E., Vaudaux P., Huggler E., Lew D., Waldvogel F. Role of bacterial exopolymers and host factors on adherence and phagocytosis of Staphylococcus aureus in foreign body infection. J Infect Dis. 1987 Mar;155(3):524–531. doi: 10.1093/infdis/155.3.524. [DOI] [PubMed] [Google Scholar]
- Fournier J. M., Hannon K., Moreau M., Karakawa W. W., Vann W. F. Isolation of type 5 capsular polysaccharide from Staphylococcus aureus. Ann Inst Pasteur Microbiol. 1987 Sep-Oct;138(5):561–567. doi: 10.1016/0769-2609(87)90041-x. [DOI] [PubMed] [Google Scholar]
- Fournier J. M., Vann W. F., Karakawa W. W. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect Immun. 1984 Jul;45(1):87–93. doi: 10.1128/iai.45.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Lepow M. L., Gotschlich E. C., Mauck F. T., Bachl F., Randolph M. Immunogenicity of group A and group C meningococcal polysaccharides in human infants. J Infect Dis. 1973 Dec;128(6):769–776. doi: 10.1093/infdis/128.6.769. [DOI] [PubMed] [Google Scholar]
- Hasche K. D., Brückler J., Schaeg W., Blobel H. Einfluss des "Clumping Factors" von Staphylococcus aureus auf die Phagozytose. Zentralbl Bakteriol Orig A. 1975 Aug;232(4):446–453. [PubMed] [Google Scholar]
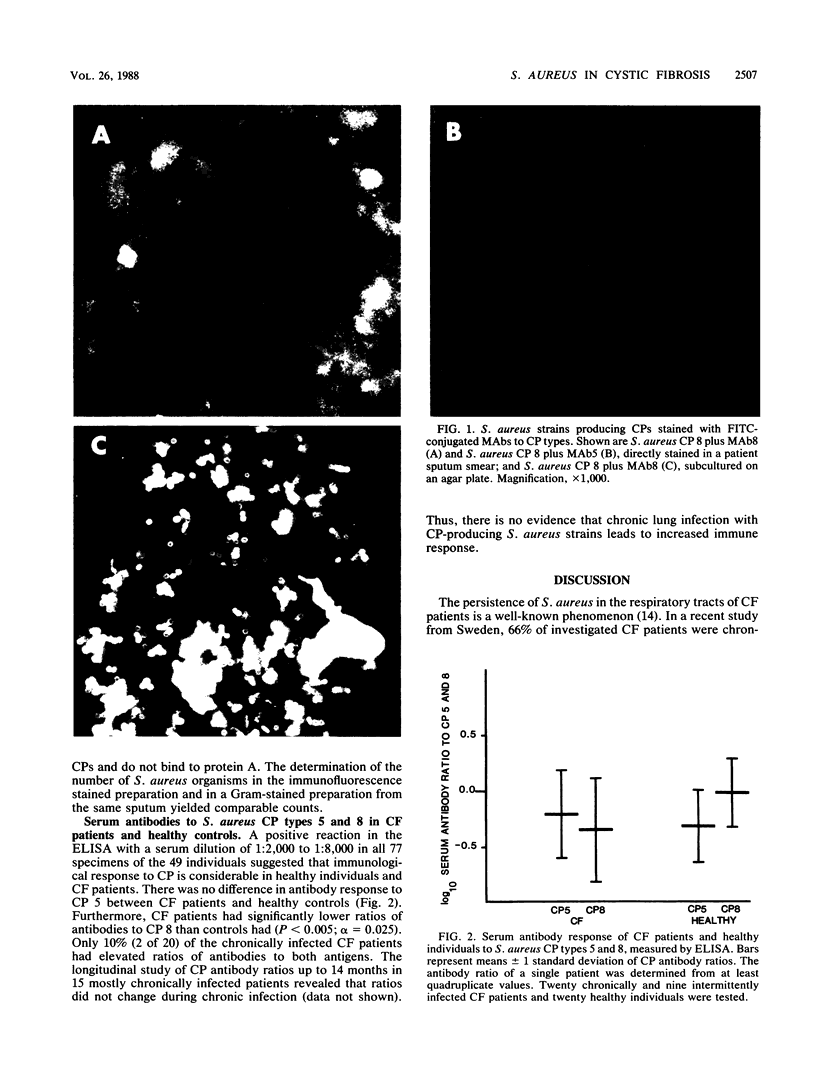
- Hochkeppel H. K., Braun D. G., Vischer W., Imm A., Sutter S., Staeubli U., Guggenheim R., Kaplan E. L., Boutonnier A., Fournier J. M. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol. 1987 Mar;25(3):526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoidal J. R., Schmeling D., Peterson P. K. Phagocytosis, bacterial killing, and metabolism by purified human lung phagocytes. J Infect Dis. 1981 Jul;144(1):61–71. doi: 10.1093/infdis/144.1.61. [DOI] [PubMed] [Google Scholar]
- Hollsing A. E., Granström M., Strandvik B. Prospective study of serum staphylococcal antibodies in cystic fibrosis. Arch Dis Child. 1987 Sep;62(9):905–911. doi: 10.1136/adc.62.9.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N. N., Schidlow D. V., Szatrowski T. H., Palmer J., Laraya-Cuasay L. R., Yeung W., Hardy K., Quitell L., Fiel S. Clinical features, survival rate, and prognostic factors in young adults with cystic fibrosis. Am J Med. 1987 May;82(5):871–879. doi: 10.1016/0002-9343(87)90147-1. [DOI] [PubMed] [Google Scholar]
- Jarløv J. O., Espersen F., Christensson B., Jensen B. A., Hedström S. A., Hertz J. B. Antibody response to whole cells and teichoic acid of Staphylococcus aureus strain E 1369 in human sera. Acta Pathol Microbiol Immunol Scand B. 1987 Apr;95(2):115–120. doi: 10.1111/j.1699-0463.1987.tb03097.x. [DOI] [PubMed] [Google Scholar]
- Julander I. G., Granström M., Hedström S. A., Möllby R. The role of antibodies against alpha-toxin and teichoic acid in the diagnosis of staphylococcal infections. Infection. 1983 Mar-Apr;11(2):77–83. doi: 10.1007/BF01641071. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langone J. J. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- Lee J. C., Betley M. J., Hopkins C. A., Perez N. E., Pier G. B. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J Infect Dis. 1987 Nov;156(5):741–750. doi: 10.1093/infdis/156.5.741. [DOI] [PubMed] [Google Scholar]
- Moss R. B., Hsu Y. P., Sullivan M. M., Lewiston N. J. Altered antibody isotype in cystic fibrosis: possible role in opsonic deficiency. Pediatr Res. 1986 May;20(5):453–459. doi: 10.1203/00006450-198605000-00015. [DOI] [PubMed] [Google Scholar]
- Poutrel B., Boutonnier A., Sutra L., Fournier J. M. Prevalence of capsular polysaccharide types 5 and 8 among Staphylococcus aureus isolates from cow, goat, and ewe milk. J Clin Microbiol. 1988 Jan;26(1):38–40. doi: 10.1128/jcm.26.1.38-40.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUIE P., WANNAMAKER L. W. SERUM ANTIBODIES IN STAPHYLOCOCCAL DISEASE. Pediatrics. 1964 Jan;33:63–70. [PubMed] [Google Scholar]
- Sompolinsky D., Samra Z., Karakawa W. W., Vann W. F., Schneerson R., Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985 Nov;22(5):828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Rydén C., Rubin K., Ljungh A., Hök M., Wadström T. Binding of fibronectin to Staphylococcus strains. Infect Immun. 1983 Nov;42(2):628–633. doi: 10.1128/iai.42.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Peters R., Rozenberg-Arska M., Peterson P. K., Verhoef J. Antibodies to cell wall peptidoglycan of Staphylococcus aureus in patients with serious staphylococcal infections. J Infect Dis. 1981 Jul;144(1):1–9. doi: 10.1093/infdis/144.1.1. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Nguyen B. Y., Sisson S. P., Kim Y. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol. 1982 Oct;129(4):1681–1687. [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]