Abstract
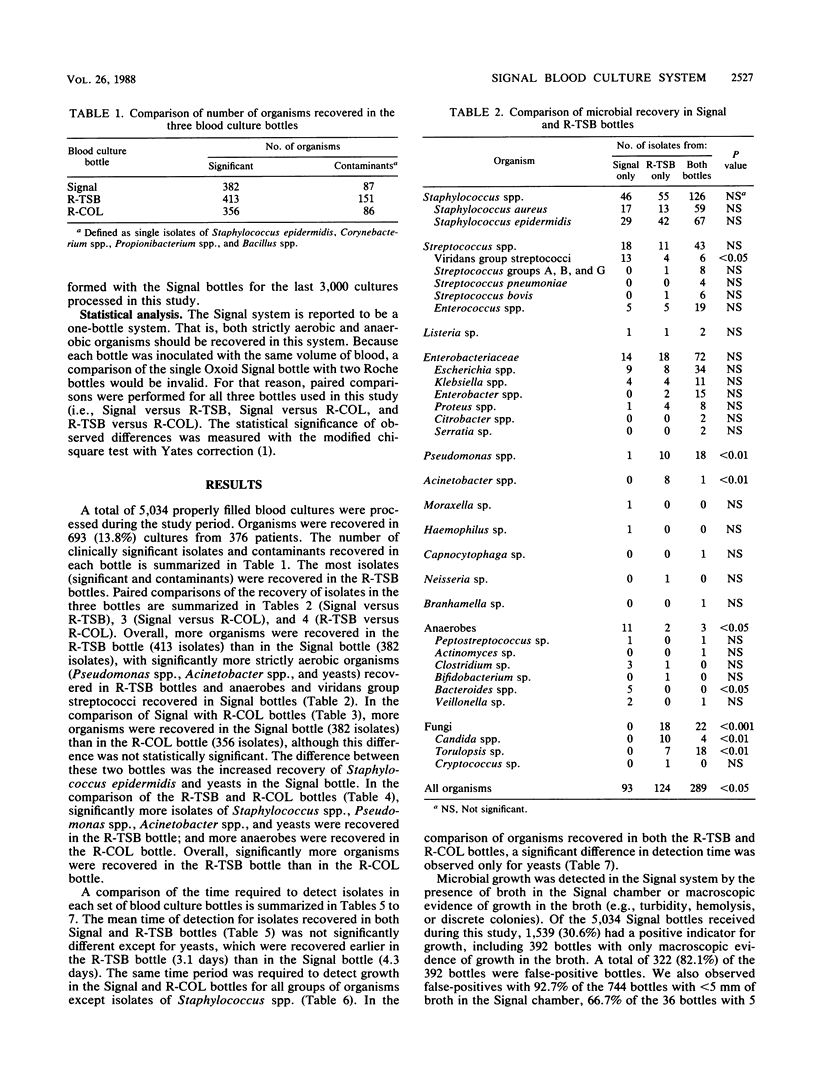
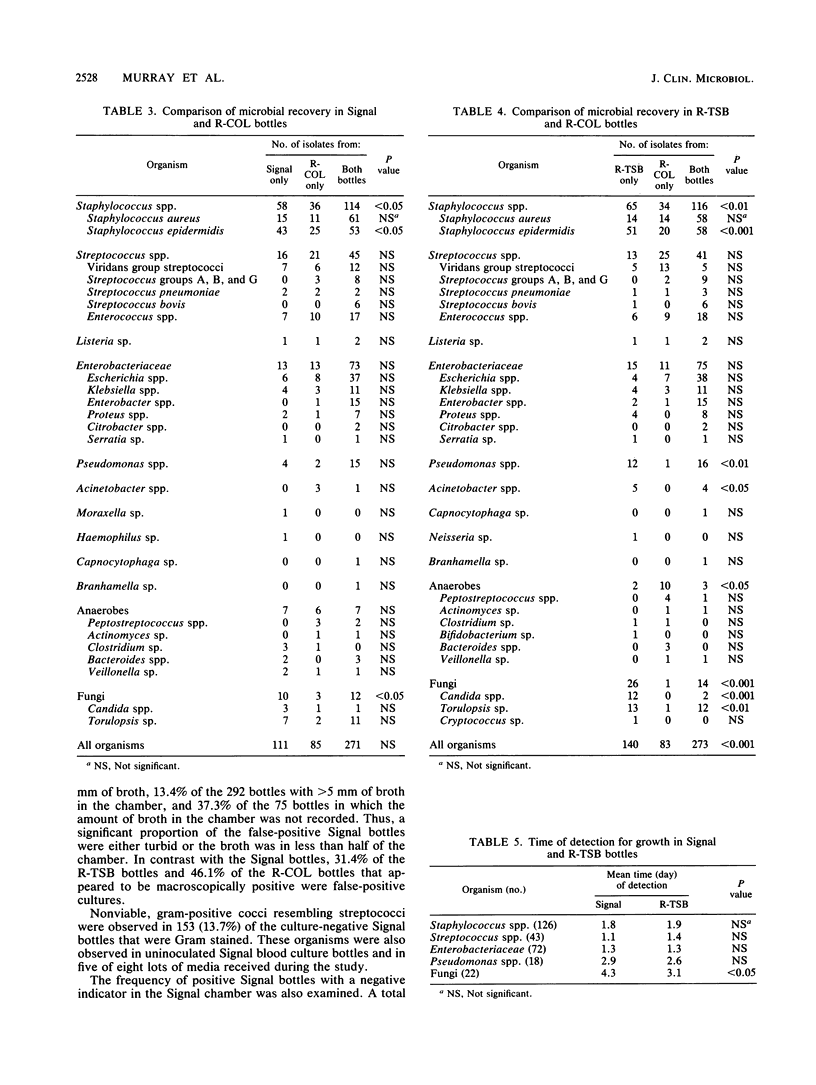
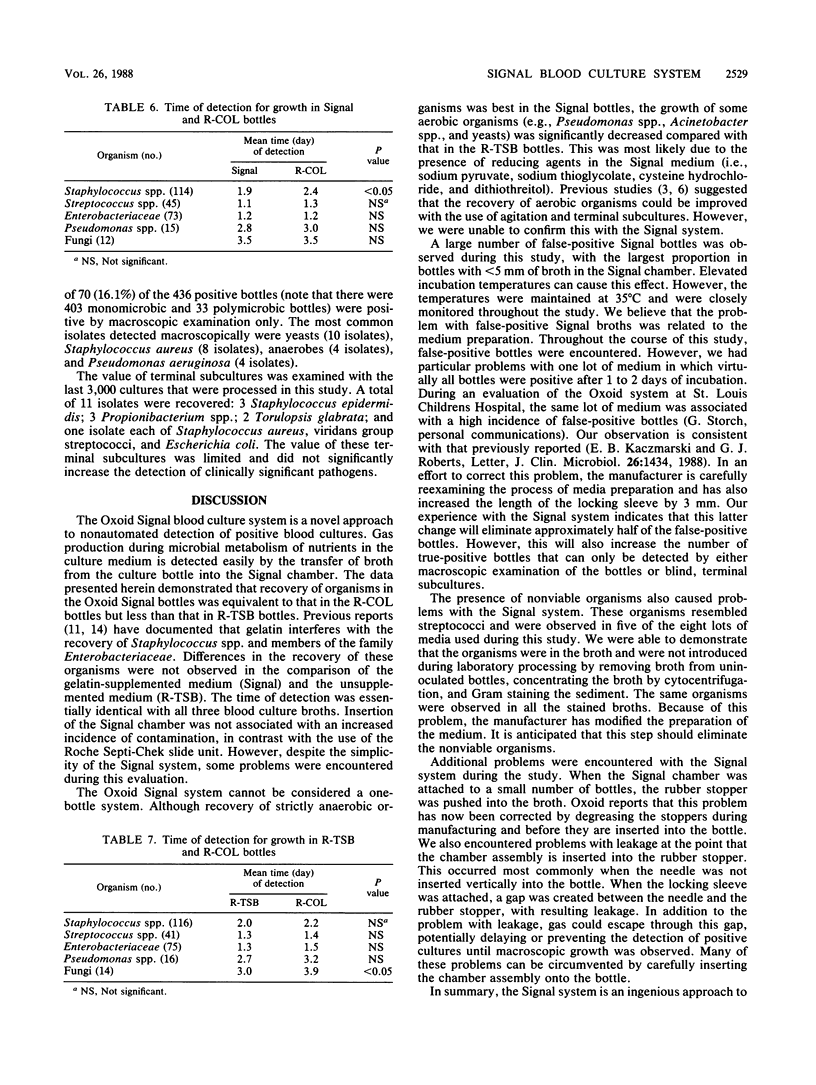
The Oxoid Signal blood culture system (Oxoid USA, Inc., Columbia, Md.) was compared with the Roche Septi-Chek system (Roche Diagnostics, Div. Hoffmann-La Roche Inc., Nutley, N.J.), with the latter consisting of a tryptic soy broth (R-TSB) bottle with an attached agar slide unit and a Columbia broth bottle. A total of 5,034 cultures with equal volumes of blood in each bottle were processed. Overall, more organisms were recovered in the R-TSB bottle than in the Signal bottle, with significantly more aerobic organisms (Pseudomonas spp., Acinetobacter spp., and yeasts) recovered in the R-TSB bottles and anaerobes and viridans group streptococci recovered in Signal bottles. Approximately equivalent numbers of organisms were recovered in the Signal and Columbia broth bottles. The times of detection were essentially identical with the three blood culture broth systems. During the study, 30.6% of the Signal bottles had a positive indicator of growth, of which 1,103 (71.7%) were false-positive cultures. Additionally, nonviable organisms resembling streptococci were observed in 13.7% of the Signal bottles that were Gram stained and in unioculated blood culture bottles. With appropriate modifications of the preparation of the media, the latter problem can be eliminated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeLand F. H., Wagner H. N., Jr Early detection of bacterial growth, with carbon-14-labeled glucose. Radiology. 1969 Jan;92(1):154–155. doi: 10.1148/92.1.154. [DOI] [PubMed] [Google Scholar]
- Hawkins B. L., Peterson E. M., de la Maza L. M. Improvement of positive blood culture detection by agitation. Diagn Microbiol Infect Dis. 1986 Sep;5(3):207–213. doi: 10.1016/0732-8893(86)90003-9. [DOI] [PubMed] [Google Scholar]
- Henry N. K., McLimans C. A., Wright A. J., Thompson R. L., Wilson W. R., Washington J. A., 2nd Microbiological and clinical evaluation of the isolator lysis-centrifugation blood culture tube. J Clin Microbiol. 1983 May;17(5):864–869. doi: 10.1128/jcm.17.5.864-869.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungkind D., Millan J., Allen S., Dyke J., Hill E. Clinical comparison of a new automated infrared blood culture system with the BACTEC 460 system. J Clin Microbiol. 1986 Feb;23(2):262–266. doi: 10.1128/jcm.23.2.262-266.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. J., Gottschall R. L., Schwabe L. D., Randall E. L. Effect of agitation and frequent subculturing on recovery of aerobic and facultative pathogens by Roche Septi-Chek and BACTEC blood culture systems. J Clin Microbiol. 1987 Feb;25(2):312–315. doi: 10.1128/jcm.25.2.312-315.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Sibley T. K., Westfall L. M., Hoppe-Bauer J. E., Keating M. A., Murray P. R. Clinical laboratory comparison of a slide blood culture system with a conventional broth system. J Clin Microbiol. 1982 Sep;16(3):525–530. doi: 10.1128/jcm.16.3.525-530.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney D., Hinder S., Swaine D., Bridson E. Y. Novel method for detecting micro-organisms in blood cultures. J Clin Pathol. 1986 Nov;39(11):1259–1263. doi: 10.1136/jcp.39.11.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliva H. S., Washington J. A., 2nd Optimal time for routine early subculture of blood cultures. J Clin Microbiol. 1980 Sep;12(3):445–446. doi: 10.1128/jcm.12.3.445-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W., Weinstein M. P., Mirrett S., Paisley J., Lauer B. A., Reller L. B. Controlled evaluation of blood culture medium containing gelatin and V-factor-analog for detection of septicemia in children. J Clin Microbiol. 1988 Apr;26(4):747–749. doi: 10.1128/jcm.26.4.747-749.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley C., Anderson J. D. SIGNAL blood culture system for detection of bacteremia in neonates. J Clin Microbiol. 1987 Nov;25(11):2098–2101. doi: 10.1128/jcm.25.11.2098-2101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. P., Mirrett S., Reller L. B. Comparative evaluation of Oxoid Signal and BACTEC radiometric blood culture systems for the detection of bacteremia and fungemia. J Clin Microbiol. 1988 May;26(5):962–964. doi: 10.1128/jcm.26.5.962-964.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. P., Reller L. B., Mirrett S., Reimer L. G., Wang W. L., Stratton C. W. Controlled evaluation of modified radiometric blood culture medium supplemented with gelatin for detection of bacteremia and fungemia. J Clin Microbiol. 1987 Aug;25(8):1373–1375. doi: 10.1128/jcm.25.8.1373-1375.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. P., Reller L. B., Mirrett S., Stratton C. W., Reimer L. G., Wang W. L. Controlled evaluation of the agar-slide and radiometric blood culture systems for the detection of bacteremia and fungemia. J Clin Microbiol. 1986 Feb;23(2):221–225. doi: 10.1128/jcm.23.2.221-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


