Abstract
Neuronal nicotinic acetylcholine receptors (nAChRs) can regulate the activity of many neurotransmitter pathways throughout the central nervous system and are considered important modulators of cognition and emotion. nAChRs also are the primary site of action in the brain for nicotine, the major addictive component of tobacco smoke. nAChRs consist of five membrane-spanning subunits (α and β isoforms) that can associate in various combinations to form functional nAChR ion channels. Because of a dearth of nAChR subtype-selective ligands, the precise subunit composition of the nAChRs that regulate the rewarding effects of nicotine and the development of nicotine dependence are unknown. However, the advent of mice with genetic nAChR subunit modifications has provided a useful experimental approach to assess the contribution of individual subunits in vivo. Here we review data generated from nAChR subunit knockout and genetically modified mice supporting a role for discrete nAChR subunits in nicotine reinforcement and dependence processes. Importantly, the rates of tobacco dependence are far higher in patients suffering from comorbid psychiatric illnesses compared with the general population, which may at least partly reflect disease-associated alterations in nAChR signaling. An understanding of the role of nAChRs in psychiatric disorders associated with high rates of tobacco addiction, therefore, may reveal novel insights into mechanisms of nicotine dependence. Thus, we also briefly review data generated from genetically modified mice to support a role for discrete nAChR subunits in anxiety disorders, depression, and schizophrenia.
Keywords: Nicotine, nicotinic acetylcholine receptor, knockout, acetylcholine, addiction, anxiety, depression, schizophrenia
Cigarette smoking is one of the largest preventable causes of death and disease in developed countries. Nevertheless, approximately 24% of adults were current smokers in the United States in 1997-1998 (Centers for Disease Control and Prevention, 2002). Tobacco-related disease is responsible for approximately 440,000 deaths annually and results in approximately $160 billion in health-related costs in the United States (Centers for Disease Control and Prevention, 2002). Moreover, by 2020, tobacco-related disease is projected to become the largest single health problem worldwide, resulting in approximately 8.4 million deaths each year (Murray and Lopez, 1997). Despite the well-known negative health consequences associated with the tobacco smoking habit, only about 10% of smokers who attempt to quit annually remain abstinent after 1 year.
Nicotine contained in tobacco smoke is one of the most widely consumed psychotropic agents in the world. Nicotine is considered the primary reinforcing component of tobacco responsible for addiction in human smokers (Stolerman and Jarvis, 1995). Nevertheless, many other components of tobacco smoke also may contribute to tobacco addiction, perhaps by increasing the reinforcing effects of nicotine (Fowler et al., 1996; Agatsuma et al., 2006; Guillem et al., 2006; Rose, 2006; Villegier et al., 2007; Guillem et al., 2008). Consistent with a role for nicotine in the tobacco smoking habit, nicotine elicits a positive affective state in both human smokers and non-smokers (Barr et al., 2007; Sofuoglu et al., 2008) and is self-administered by humans (Henningfield et al., 1983; Rose et al., 2003; Sofuoglu et al., 2008), non-human primates (Goldberg et al., 1981; Le Foll et al., 2007), and rodents (Corrigall and Coen, 1989; Picciotto et al., 1998; Watkins et al., 1999).
Importantly, the tobacco smoking habit may depend not only on the positive reinforcing actions of nicotine, but also on escape from the aversive consequences of nicotine withdrawal (i.e., negative reinforcement) (Doherty et al., 1995; Kenny and Markou, 2001; George et al., 2007). Prolonged tobacco consumption can result in the development of nicotine dependence, and smoking cessation can elicit an aversive withdrawal syndrome in nicotine-dependent human smokers (Shiffman and Jarvik, 1976; Hughes et al., 1991). The duration and severity of nicotine withdrawal may predict relapse in abstinent human smokers (Piasecki et al., 1998; Piasecki et al., 2000; Piasecki et al., 2003). Furthermore, the efficacy of nicotine replacement therapy for smoking cessation may be related to the reduction of nicotine withdrawal in abstinent smokers, at least in certain individuals (Fagerstrom, 1988; Sachs and Leischow, 1991).
Neuronal nicotinic acetylcholine receptors (nAChRs) are the primary site of action for nicotine in the brain. As such, nAChRs are considered important targets for the development of therapeutic agents that may facilitate smoking cessation efforts. For example, varenicline (Chantix), a partial agonist at nAChRs, was approved recently by the U.S. Food and Drug Administration (FDA) as a pharmacological aid for smoking cessation and is effective in preventing relapse to tobacco smoking during abstinence (Gonzales et al., 2006; Jorenby et al., 2006; West et al., 2007). Thus, identification of the nAChRs at which nicotine acts in the brain to elicit its reinforcing effects, and also the nAChRs at which nicotine acts to induce the development of dependence and expression of withdrawal, may provide valuable insights into the neurobiology of the nicotine habit in human tobacco smokers and facilitate the development of novel therapeutic strategies for the treatment of tobacco addiction (for review, see Domino, 2000). However, little is known regarding the nAChR subtypes that regulate the reinforcing actions of nicotine in vivo. This lack of knowledge reflects an absence of small-molecule ligands that are selective for specific nAChR subtypes. Although many nAChR ligands may be more selective for certain nAChR subtypes, they nonetheless exhibit binding affinities for other nAChR subtypes. Therefore, attributing any particular behavioral effect of nicotine to an action at a discrete nAChR subtype has been difficult. The construction of genetically modified mice with null mutations in the genes that encode individual nAChR subunits offers a promising approach to identifying the nAChR subtypes that regulate the actions of nicotine in vivo and the contribution of specific nAChR subtypes to physiology and disease. Indeed, as summarized in Table 1, nAChR mutant mice have been employed extensively over recent years to examine the roles of nAChR subunits in a broad range of physiological processes. Although the use of knockout mice is a promising strategy, certain limitations should be taken into account with interpretation of the data. First of all, the animal has been without the gene for the duration of development, and physiological compensation may have occurred. For example, the α7 knockout exhibits increased expression of both the α3 and α4 subunits (Yu et al., 2007), whereas the β3 knockout displays decreased α6 subunit expression (Gotti et al., 2005). Secondly, primary effects on behavior may be noted in secondary measures. For instance, decreased open field activity may be indicative of increased anxiety, rather than decreased locomotion. In this respect, it is important to employ varying techniques and behavioral measures to fully address the research questions. Until the development of more sophisticated methods that afford precise control over anatomical and temporal factors, as well as the pharmacological development of specific ligands, knockout mice remain an essential tool in defining the mechanisms underlying addiction processes. In this review, we will outline the progress that has been made in identifying nAChR subunit components of the nicotinic receptors that regulate the rewarding properties of nicotine, as well as those that contribute to nicotine dependence and withdrawal. In particular, we will highlight those studies that have utilized mice with genetic modifications of nAChR subunits.
Table 1.
Behavioral and pharmacological effects of nicotinic acetylcholine receptor subunit knockout in mice (in vivo only; no binding studies included).
| Knockout | Baseline phenotype | Effects of nicotine in knockout vs. wildtype | References |
|---|---|---|---|
| α3 | impaired growth dilated pupils do not constrict to light enlarged bladder, dribbling urination, urinary tract infection, bladder stones lethal postnatal (1 day to 3 months) |
lack of nicotine-induce bladder contractility | (Xu et al., 1999a) |
| α4 | ↑ locomotor activity ↑ sniffing ↑ rearing ↑ striatal dopamine levels ↑ CD40-stimulated B lymphocytes ↑ dopamine neuron terminal arbors ↓ exploration in elevated plus maze ↓ serum IgG ↓ IgG-producing cells in spleen |
↑ recovery from nicotine-induced depression of locomotor activity ↓ antinociception ↓ raphe magnus neuronal response ↓ thalamic neuronal response ↓ tolerance to hypothermia ↓ locomotor activity no nicotine-induced ↑ in dopamine release — dopamine neuron terminal arbor size — dopamine transporter immunoreactivity in dorsal striatum |
(Marubio et al., 1999; Ross et al., 2000; Marubio et al., 2003; Parish et al., 2005; Skok et al., 2005; Tapper et al., 2007) |
| α5 | ↓ α3 receptor expression ↑ experimental colitis normal hearing normal spiral ganglion neurons |
↑ seizure activity ↓ seizure activity no effect in open field ameliorates colitis |
(Salas et al., 2003a; Kedmi et al., 2004; Bao et al., 2005; Orr-Urtreger et al., 2005) |
| α6 | not tested | not tested | (Champtiaux et al., 2002) |
| α7 | ↑— y-maze activity ↑ open-field activity ↑ sympathetic response (↑ heart rate) to sodium nitroprusside-induced vasodilation ↑ CD40-stimulated B lymphocytes ↑ α4 receptors ↑ α3 receptors ↓ appetitive learning ↓ serum IgG ↓ delayed-matching-to-place performance ↓ IgG-producing cells in spleen ↓ 5-choice serial reaction time task performance (i.e., ↑ impulsivity) ↓ limbic c-fos expression induced by α7 agonist ↓ β-amyloid1-41-induced dopamine release in prefrontal cortex — sperm number, morphology, viability — dopamine neuron firing rate/bursts — brain injury-induced tissue loss or brain inflammation — object recognition task no facilitatory effect of α7 agonist in object recognition task |
— somatic signs of withdrawal — operant responding for nicotine — 5-choice serial reaction time task performance — antinociception in tail flick test — increase in spinal calcium/calmodulin-dependent protein kinase II ↓ hyperalgesia during withdrawal ↓ sperm motility ↓ B lymphocytes in spleen ↓ dopamine neuron firing rate/bursts exhibit conditioned place preference — contextual cued fear conditioning — trace cued fear conditioning — nicotine withdrawal-induced deficits in contextual fear conditioning |
(Franceschini et al., 2000; Tritto et al., 2004; Bowers et al., 2005; Bray et al., 2005; Grabus et al., 2005; Keller et al., 2005; Naylor et al., 2005; Skok et al., 2005; Fernandes et al., 2006; Hoyle et al., 2006; Kelso et al., 2006; Mameli-Engvall et al., 2006; Walters et al., 2006; Damaj, 2007; Hansen et al., 2007; Pichat et al., 2007; Wu et al., 2007; Yu et al., 2007; Portugal et al., 2008) |
| α9 | ↓ number of outer hair cells in cochlea ↓ olivocochlear function abnormal cochlear terminal morphology |
not tested | (Vetter et al., 1999) |
| β2 | ↑ passive avoidance latency ↑ corticosterone levels ↑ visual response latency ↑ preference for higher temporal frequencies ↑ contrast sensitivity ↑ CD40-stimulated B lymphocytes ↑ B lymphocytes in bone marrow ↓ freezing in tone-conditioned fear (aged mice) ↓ visual acuity at cortical level ↓ locomotor activity in familiar environment ↓ hippocampal pyramidal neurons ↓ spatial learning ↓ serum IgG ↓ IgG-producing cells in spleen ↓ spiral ganglion neurons ↓ dopamine neuron firing rate/bursts ↓ antidepressant-like effect of mecamylamine in forced swim test abnormal cholinergic transmission neural tissue atrophy neocortical hypotrophy astrogliosis microgliosis hearing loss do not self-administer intra-VTA nicotine self-administer intra-VTA morphine normal nicotine withdrawal syndrome normal β-amyloid1-41-induced dopamine release in prefrontal cortex |
↑ locomotor activity (initial low dose) — locomotor activity (repeated high dose) ↑ body temperature (initial low dose) — body temperature (repeated high dose) ↑ passive avoidance latency ↓ startle ↓— nicotine self-administration ↓ dopamine response in substantia nigra ↓ dopamine response in ventral tegmental area ↓ thalamic neuronal response ↓ raphe magnus neuronal response ↓ antinociception ↓ CD40-stimulated B lymphocytes ↓ B lymphocytes in bone marrow ↓ antinociception in tail flick test ↓ increase in spinal calcium/calmodulin-dependent protein kinase II ↓ contextual cued fear conditioning ↓ trace cued fear conditioning ↓ nicotine withdrawal-induced deficits in contextual fear conditioning — B lymphocytes in spleen — dopamine neuron firing rate/bursts no conditioned place preference |
(Picciotto et al., 1995; Marubio et al., 1999; Zoli et al., 1999; Caldarone et al., 2000; Rossi et al., 2001; Owens et al., 2003; Grubb and Thompson, 2004; King et al., 2004; Tritto et al., 2004; Bao et al., 2005; Skok et al., 2005; Besson et al., 2006; Mameli-Engvall et al., 2006; McCallum et al., 2006; Rabenstein et al., 2006; Skok et al., 2006; Walters et al., 2006; Damaj, 2007; Davis and Gould, 2007; Wu et al., 2007; Portugal et al., 2008) |
| β3 | ↑ open field activity ↓ prepulse inhibition of startle ↓ nigrostriatal dopamine release ↓ α6 receptor expression |
↑ striatal dopaminergic transmission | (Cui et al., 2003; Gotti et al., 2005) |
| β4 | ↑ heart rate ↑ exploration in elevated plus maze ↑ activity in stair case test ↓ α3 receptor expression impaired bladder contractility ↓ core body temperature |
↓ seizure activity ↓ somatic withdrawal signs ↓ hyperalgesia ↓ hypothermic response |
(Xu et al., 1999b; Salas et al., 2003b; Kedmi et al., 2004; Sack et al., 2005) |
| α5β4 | ↓ α3 receptor expression | ↓ seizure activity | (Kedmi et al., 2004) |
| α7β2 | ↑ rotarod performance ↓ passive avoidance latency |
(Marubio and Paylor, 2004) | |
| β2β4 | dilated pupils do not contract to light impaired bladder contractility, enlarged bladder, dribbling urination, urinary tract infection, bladder stones lethal at 1-3 weeks postnatal |
not tested | (Xu et al., 1999b) |
↓, decreased measure; ↑, increased measure; —, no change; parts of the table are reproduced with permission from (Koob and Le Moal, 2006), and modified and updated from (Cordero-Erausquin et al., 2000).
Strong epidemiological evidence suggests that the rate of tobacco dependence is far higher in individuals who suffer from psychiatric illnesses, such as anxiety disorders, depression, or schizophrenia, compared with the rate of tobacco dependence observed in the general population (Dani and Harris, 2005). Thus, the increased susceptibility to tobacco addiction in patients with comorbid psychiatric illness may reflect disease-associated deficits in nAChR signaling. An alternative, but not mutually exclusive, explanation is that smoking represents an attempt at self-medication of disease-associated symptoms through consumption of nicotine (see below for more detailed discussion of these possibilities). Based on these observations, the role of nAChRs in psychiatric disorders associated with high levels of tobacco addiction is an important consideration because such knowledge may reveal important clues regarding the role and identity of nAChR subtypes in nicotine dependence and disease states. We also will briefly discuss recent advances in our knowledge related to the role of nAChRs in anxiety disorders, depression, and schizophrenia, again focusing on data generated from mice with genetic modifications of nAChR subunits. Throughout this paper, the asterisk (*) annotation beside a specific subunit denotes a nAChR that contains the indicated subunit but for which the complete subunit composition is unknown.
Structural architecture and brain distribution of neuronal nicotinic acetylcholine receptors
nAChRs are composed of five distinct membrane-spanning subunits (α and β subunits) that combine to form a functional receptor (Albuquerque et al., 1995; Lena and Changeux, 1998) (Fig. 1). Each subunit comprises four membrane-spanning helices (M1-M4 domains). These membrane-spanning domains are arranged in concentric layers around a central aqueous pore with the M2 domain lining the pore (Unwin, 1995; Grosman and Auerbach, 2000; Miyazawa et al., 2003; Taly et al., 2005; Unwin, 2005; Cymes and Grosman, 2008). Domains M1 and M3 shield M2 from the surrounding lipid bilayer, and M4 is the outermost and most lipid-exposed segment (Unwin, 1995; Grosman and Auerbach, 2000; Miyazawa et al., 2003; Taly et al., 2005; Unwin, 2005; Cymes and Grosman, 2008). Upon nAChR activation by an endogenous agonist (e.g., acetylcholine) or exogenous agonist (e.g., nicotine), the domains of each of the five nAChR subunits rearrange such that the central pore opens and permits cationic trafficking. The nAChR α subunit exists in nine isoforms (α2-α10), whereas the neuronal β subunit exists in three isoforms (β2-β4) (Chini et al., 1994; Elgoyhen et al., 2001; Le Novere et al., 2002; Lips et al., 2002). The receptor subunits arrange in various combinations to form functionally distinct pentameric nAChR subtypes, with α and β subunits combining with a putative stoichiometry of 2α:3β (Deneris et al., 1991; Sargent, 1993) (Fig. 1). However, α7, α8, and α9 subunits usually form homopentameric complexes composed of five α subunits and no β subunits, with only the α7 pentameric nAChR present in the central nervous system (CNS).
Figure 1.
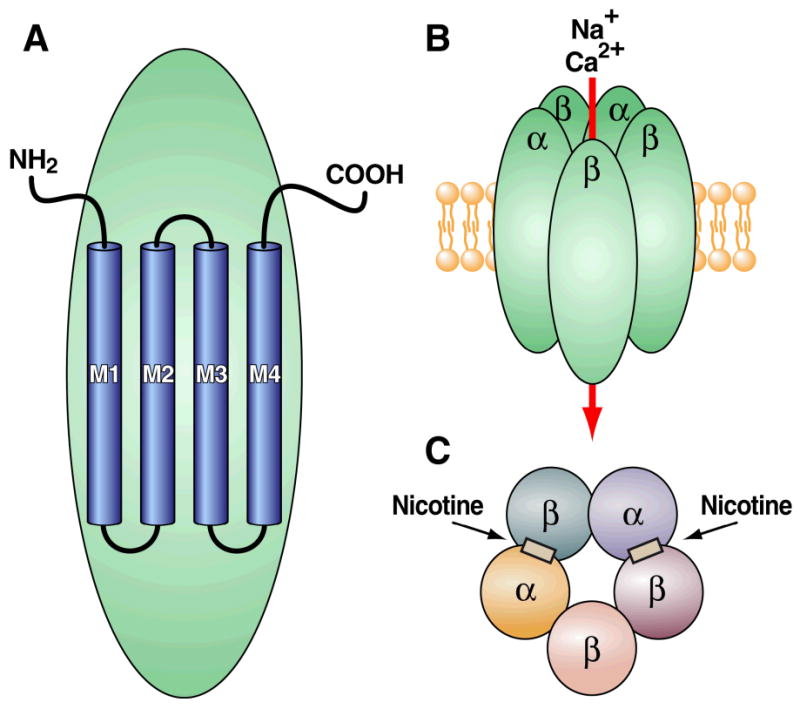
Because of the large number of subunits, potentially many nAChR subtypes can exist, each composed of various combinations of α and β subunits. However, the assembly of nAChRs is a tightly regulated process that requires appropriate subunit interactions to produce functional nAChRs. As a result, the number of functional nAChR subtypes appears to be far less than that which could be generated theoretically (Table 2). As noted above, because of a lack of receptor agonists and antagonists with selectivity for individual nAChR subunits, the precise combinations of nAChR subunits that constitute functional neuronal nAChR subtypes in vivo are unknown. However, recent studies have begun to identify the nAChR subunits expressed by individual neurons in the brain. For example, single-cell reverse transcription polymerase chain reaction (RT-PCR) coupled with patch-clamp recordings suggests that approximately 90-100% of neurons located in the medial habenula (MH) express the α3, α4, α5, β2, and β4 nAChR subunits (Sheffield et al., 2000). In contrast, only about 40% of MH neurons express the α6, α7, and β3 subunits, whereas the α2 subunit was not found (Sheffield et al., 2000). Similar techniques revealed a significant co-expression of α3 and α5, α4 and β2, α4 and β3, α7 and β2, β2 and β3, and β3 and β4 within individual neurons in the rat hippocampus (Sudweeks and Yakel, 2000). Such investigations eventually may reveal the subunit composition of nAChR subtypes that are expressed by distinct cell populations.
Table 2.
Likely combinations of α and β subunits that produce functional neuronal nAChR subtypes in the central nervous system. Evidence for the existence of particular subunit combinations is summarized from electrophysiological and immunoprecipitation data obtained from oocyte and mammalian cell line expression systems.
| α Subunit | Combines with β subunits | Reference |
|---|---|---|
| α2 | β2 or β4 | (Duvoisin et al., 1989; Papke et al., 1989) |
| α3 | β2 or β4; β3 and 4 | (Duvoisin et al., 1989; Papke et al., 1989; Groot-Kormelink et al., 1998) |
| α4 | β2 or β4; β2 and β3 and β4 | (Duvoisin et al., 1989; Papke et al., 1989) |
| α6 | β2 or β4; β3 and β4 | (Gerzanich et al., 1997; Fucile et al., 1998; Kuryatov et al., 2000) |
| α7 | None (pentamer) | (Couturier et al., 1990) |
| α2 and α5 | β2 | (Balestra et al., 2000) |
| α3 and α5 | β2 or β4; β2and β4 | (Conroy and Berg, 1995; Wang et al., 1996) |
| α3 and α6 | β2 or β4; β3 and β4 | (Vailati et al., 1999; Kuryatov et al., 2000) |
| α4 and α5 | β2 | (Ramirez-Latorre et al., 1996) |
| α6 and α5 | β2 | (Kuryatov et al., 2000) |
| α4 and α6 and α5 | β2 | (Klink et al., 2001) |
In situ hybridization has been utilized to examine the distribution of messenger RNAs (mRNAs) for each nAChR subunit throughout the CNS of humans, non-human primates, rats, and mice (Table 3) (for review, see Drago et al., 2003). These experiments demonstrate marked differences in the distribution and density of the various subunits throughout the mammalian brain. Overall, α4, β2, and α7 subunits are the most widely expressed, whereas α2, α5, α6, β3, and β4 subunits demonstrate a more restricted expression profile (Table 2). In addition, although their relative densities may vary, considerable overlap exists in the expression profiles of the α4 and β2 subunits, suggesting that α4β2 nAChRs represent a major subtype of nAChR in the brain. Importantly, α2, α3, α4, α5, α6, α7, β2, and β3 subunits are expressed in brain regions that are known to play a role in the reinforcing effects of addictive drugs and in the cognitive and emotional deficits associated with various neuropsychiatric disorders. Indeed, these nAChR subunits are abundantly expressed in the amygdala, nucleus accumbens (NAc), ventral tegmental area (VTA), hippocampus, and throughout cortical areas (Table 3).
Table 3.
Summary of the brain regions that demonstrate a significant density of each nAChR subunit throughout the mammalian brain.
| Subunit | Brain region | Reference |
|---|---|---|
| α2 | interpeduncular nucleus hippocampus subiculum |
(Wada et al., 1989) |
| α3 | medial habenula entorhinal cortex thalamus hypothalamus ventral tegmental area |
(Wada et al., 1989; Champtiaux et al., 2002) |
| α4 | amygdala hippocampus hypothalamus medial habenula substantia nigra pars compacta |
(Wada et al., 1989) |
| α5 | interpeduncular nucleus ventral tegmental area substantia nigra pars compacta |
(Wada et al., 1990) |
| α6 | substantia nigra pars compacta ventral tegmental area locus coeruleus medial habenula interpeduncular nucleus |
(Le Novere et al., 1996) |
| α7 | amygdala hypothalamus hippocampus |
(Seguela et al., 1993) |
| α8 | not expressed in mammalian brain? | (Gotti et al., 1997) |
| α9 | not expressed in mammalian brain? | (Park et al., 1997) |
| α10 | not expressed in mammalian brain? | (Elgoyhen et al., 2001) |
| α2 | Thalamus substantia nigra pars compacta ventral tegmental area entorhinal cortex |
(Wada et al., 1989) |
| α3 | ventral tegmental area substantia nigra pars compacta thalamus medial habenula |
(Deneris et al., 1989) |
| α4 | interpeduncular nucleus medial habenula |
(Wada et al., 1989) |
Nicotinic receptors within the CNS are situated mainly on presynaptic terminals (Wonnacott, 1997) but also are found at somatodendritic, axonal, and postsynaptic locations (for review, see Sargent, 1993). One of the predominant roles of nAChRs in the CNS has been proposed to be the modulation of transmitter release as heteroreceptors (Wonnacott, 1997). Accordingly, nicotine has been shown to stimulate the release of most neurotransmitters in the brain, including dopamine, glutamate, γ-aminobutyric acid (GABA), norepinephrine, and serotonin (Carboni et al., 2000; Kenny et al., 2000b; Mansvelder and McGehee, 2000; Fu et al., 2003). Importantly, although not discussed in detail in the present review, the stimulatory action of nicotine on neurotransmitter release and on cell excitability is hypothesized to regulate many of the behavioral actions of nicotine in human tobacco smokers (for review, see Benowitz, 2008).
Neurobiological mechanisms of nicotine reinforcement
In order to identify the subunit composition of the nAChRs that regulate the reinforcing effects of nicotine, it is important to have an understanding of the neurobiological mechanisms by which nicotine acts in the brain to elicit its effects. Attention can thereby be focused on identifying those nAChRs located within reinforcement-related neural circuitries. It is important to note that dynamic rearrangement of the subunit content of constitutively expressed nAChRs in reinforcement circuitries may occur upon repeated exposure to rewarding doses of nicotine. Chronic exposure to nicotine in vitro robustly upregulates α4* and β2* nAChRs, whereas the α7, α3*, and β4* nAChRs are more resistant to upregulation (for review see Gentry and Lukas, 2002). Consistent with this in vitro observation, increased expression of α4β2* receptors is found in postmortem brain tissues of human smokers (Benwell et al., 1988; Breese et al., 1997). The α5 nAChR subunit appears to plays an important modulatory role within nAChRs. In Xenopus oocytes, inserting the α5 subunit into α4β2, α3β2 or α3β4 nAChRs increases receptor desensitization in response to nicotine (Wang et al., 1996; Ramirez-Latorre et al., 1996). Interestingly, the α3α5β2 subunit combination has increased affinity for nicotine compared with the α3β2 subtype (Wang et al., 1996), an alteration in vivo which could result in altered susceptibility to nicotine reward and vulnerability to tobacco addiction. Thus, nicotine-induced alterations in the expression of nAChR subunits may have a profound impact on the function of nAChRs in brain reward circuitries that may contribute to tobacco addiction. However, because current knowledge regarding nicotine-induced alterations in the subunit content of nAChRs in vivo is very limited, the contribution of this process to tobacco addiction is not reviewed in detail here.
Similar to other major drugs of abuse, nicotine enhances mesocorticolimbic dopamine transmission, and this action is hypothesized to play an important role in the positive reinforcing effects of nicotine (Merlo-Pich et al., 1997; Pidoplichko et al., 1997; Balfour et al., 2000; Koob and Le Moal, 2006). In vivo microdialysis studies have demonstrated that subcutaneous injections of nicotine (0.1 mg/kg) or cocaine (3 mg/kg) increases accumbal dopamine release by a similar magnitude (Zernig et al., 1997). The powerful impact of nicotine on the brain reward pathways is further evidenced by the significant lowering of intracranial self-stimulation (ICSS) thresholds (see below) observed in rats in response to intravenously self-administered nicotine. Indeed, although rats self-administer relatively low numbers of nicotine infusions compared with the amouts of cocaine consumed under similar conditions, rats nevertheless titrate their nicotine intake at a level that significantly lowers ICSS thresholds, with a magnitude of effect comparable with that induced by self-administered heroin or cocaine (Kenny et al., 2003; Kenny, 2006; Kenny and Markou, 2006; Kenny et al., 2006). Taken together, these observations suggest that nicotine can impact brain reward circuitries and facilitate their activity in a manner similar to other major drugs of abuse.
Whole-cell electrophysiological and in vivo microdialysis studies have demonstrated that systemic or direct VTA administration of nicotine increases the firing rate of mesoaccumbens dopamine neurons and increases dopamine release in the NAc (Andersson et al., 1981; Di Chiara and Imperato, 1988; Benwell and Balfour, 1992), particularly the shell region of the NAc (Pontieri et al., 1996), but also in other mesolimbic terminal regions, such as the bed nucleus of the stria terminalis (BNST) (Carboni et al., 2000). Systemic nicotine-induced dopamine release in the NAc can be blocked by infusion of the nAChR antagonist mecamylamine into the VTA but not into the NAc; however, site-specific infusion of nicotine into the VTA or NAc can increase extracellular accumbal dopamine levels (Nisell et al., 1994). Thus, nicotine likely acts preferentially in the VTA to increase mesoaccumbens dopamine transmission but also may act directly in the striatum to modulate dopamine transmission. Furthermore, systemic administration of dopamine receptor antagonists (Corrigall and Coen, 1991) or lesions of the NAc (Corrigall et al., 1992) attenuate the reinforcing effects of nicotine, reflected in decreased intravenous nicotine self-administration in rats. Infusion of the nAChR antagonist dihydro-β-erythroidine (DHβE) directly into the VTA decreases nicotine self-administration in rats (Corrigall et al., 1994). Accumulating evidence suggests that, in addition to the direct stimulatory action of nicotine at postsynaptic nAChRs located on dopamine-containing VTA neurons, nicotine may increase mesoaccumbens dopamine transmission by indirect actions involving glutamate and GABA transmission in the VTA. Indeed, nicotine activates nAChRs located on VTA glutamate terminals (Grillner and Svensson, 2000; Mansvelder and McGehee, 2000; Jones and Wonnacott, 2004), and thereby induces a persistent increase in glutamate transmission in this brain site (Fu et al., 2000; Grillner and Svensson, 2000; Schilstrom et al., 2000). In turn, this enhanced glutamate transmission stimulates postsynaptic glutamate receptors located on dopamine-containing neurons, thereby increasing their burst firing (Kalivas, 1993; Hu and White, 1996; Schilstrom et al., 1998; Fu et al., 2000; Mansvelder and McGehee, 2000; Kosowski et al., 2004). In addition, nicotine activates nAChRs located on GABAergic neurons in the VTA (Mansvelder et al., 2002), resulting in a transient increase in inhibitory GABAergic transmission. However, nAChRs located on GABAergic neurons in the VTA appear to be very sensitive to nicotine-induced desensitization, and following nicotine-induced activation may become nonfunctional for prolonged periods of time (Mansvelder et al., 2002). Additionally, nAChRs located presynaptically on cholinergic fibers into the VTA also may regulate mesoaccumbens dopamine transmission (Maskos, 2008). Identification of the subunit composition of nAChRs in the VTA, especially those located on dopamine-, glutamate-, and GABA-containing neurons but also located on cholinergic neurons, is likely to provide important insights to the identity of the nAChR subtypes that regulate nicotine reinforcement. Similarly, identification of the nAChR subunits expressed in terminals brain regions of VTA dopamine neurons, such as the NAc, also may provide important clues to the nAChR subtypes that regulate nicotine reinforcement. The data outlined below describe recent advances in our understanding of the nAChR subunits that are expressed in the VTA and NAc and highlight the utility of nAChR genetically modified mice in this regard. Importantly, although not reviewed in detail here, nicotine exerts actions in reward-relevant brain regions other than the VTA and NAc, and extra-mesolimbic nAChRs also play a central role in nicotine reinforcement (Kenny et al., 2008).
Nicotinic acetylcholine receptor subunits that regulate the positive reinforcing effects of nicotine: In vitro evidence
Based on accumulating evidence that the VTA is a core brain site regulating behavioral actions of nicotine with relevance to addiction, much effort has been directed toward elucidating the nicotinic subunits expressed in this site. Charpantier and colleagues (1998) used RT-PCR to analyze mRNA content of individual nAChR subunits in the VTA following unilateral lesions of the mesoaccumbens system in rats. In contrast to the unlesioned side, mRNA signals for α2, α3, α5, α6, α7, and β4 subunits were absent in the VTA of the lesioned hemisphere (Charpantier et al., 1998). These nAChR subunits, therefore, may be expressed preferentially in dopamine-containing neurons that originate in the VTA. Interestingly, mRNA for α4, β2, and β3 subunits was detected after the dopamine lesion (Charpantier et al., 1998), suggesting that these subunits may be expressed in both dopamine-containing, as well as GABAergic, neurons in the VTA. Klink and colleagues (2001) utilized RT-PCR and single-cell electrophysiological recordings in wildtype and nAChR knockout mice to investigate subunit composition of nAChR subtypes expressed in the VTA. Almost 100% of cells in the VTA (i.e., dopamine- and GABA-containing) expressed mRNA for the α4 and β2 subunit (Klink et al., 2001), suggesting that these subunits may be incorporated into many nAChR subtypes in this area. Approximately 90% of dopamine-containing cells and 20% of GABA-containing cells in the VTA contained β3 nAChR subunits (Klink et al., 2001). Similarly, approximately 70% of dopamine-containing neurons in the VTA expressed α5 nAChR mRNA, with a much lower proportion of GABAergic cells (<20%) expressing this subunit (Klink et al., 2001). Approximately 75% of dopamine-containing cells in the VTA expressed α6 nAChR subunits, compared with about 10% of GABAergic cells. Around 40% of both dopamine and GABAergic cells expressed mRNA for the α7 nAChR subunit (Klink et al., 2001). A relatively low concentration of β4 mRNA was observed in VTA cells, consistent with the high concentrations of this subunit that are restricted to the MH and IPN. Therefore, the majority of dopamine neurons in the VTA expressed α4, α5, α6, β2, and β3 mRNA, whereas the majority of GABA neurons expressed α4 and β2 subunits, with α7 subunits demonstrating a similar incidence in both cell types. Interestingly, in the VTA, electrophysiological analyses suggested that the only currents elicited by acetylcholine in β2 knockout mice were regulated by α7 pentameric nAChRs subunit (Klink et al., 2001). In α4 knockout mice, acetylcholine-evoked currents were qualitatively similar but quantitatively smaller than those observed in wildtype mice (Klink et al., 2001). Finally, in α7 knockout mice, acetylcholine-evoked currents were qualitatively and quantitatively similar to those observed in wildtype mice, with the exception that putative α7-mediated currents were absent. Based on the above, three subtypes of pentameric nAChRs were proposed to be putatively functional on VTA dopamine-containing neurons: α4α6α5(β2)2, (α4)2α5(β2)2, and (α7)5. On VTA GABAergic neurons, two nAChR subtypes predominated: (α4)3(β4)2 and (α7)5.
Interestingly, although mRNA for the β3 nAChR is abundant in the VTA, this subunit does not appear to contribute to functional nAChRs in this brain site. Instead, the β3 subunit may be targeted to terminal domains in the NAc where it contributes to the stimulatory effects of nicotine on accumbal dopamine transmission. Immunocytochemical analyses demonstrated that β3 subunits are transported to the projection areas of VTA neurons (Forsayeth and Kobrin, 1997) and that the striatum has high β3 protein concentrations (Forsayeth and Kobrin, 1997; Reuben et al., 2000). Using experimental approaches similar to those described above, much progress has been made in elucidating putative nAChR subtypes in the striatum that regulate the stimulatory effects of nicotine on dopamine transmission in this brain region. This work has been facilitated by combining data generated from nAChR mutant mice with classical pharmacological approaches. In particular, the discovery that the novel nAChR antagonist α-conotoxin MII (α-CtxMII) can attenuate, but not fully block, the stimulatory effects of nicotine on striatal dopamine release suggested that at least two sub-populations of nAChRs regulate the actions of nicotine, a finding that has proven to be very useful in characterizing putative nAChR subtypes in the striatum. Zoli and colleagues (2002) used α-CtxMII combined with immunoprecipitation and targeted brain lesion to provide evidence that four species of nAChRs may be expressed on the terminals of mesoaccumbens dopamine neurons in the striatum that contain the following subunits: α4β2*, α4α5β2*, α4α6β2β3*, or α6β2β3* (Zoli et al., 2002). Interestingly, by using α-CtxMII and various nAChR ligands in combination with nAChR knockout mice, Salminen and colleagues similarly concluded that α4β2*, α4α5β2*, α4α6β2β3*, and α6β2β3* nAChRs may be located on dopamine terminals in the striatum, where they can regulate the stimulatory effects of nicotine on mesoaccumbens dopamine transmission (Salminen et al., 2004). In addition, two further putative nAChR subtypes were identified in these studies: α6β2β3* and α6β2* (for recent review, see Grady et al., 2007). The effects of acetylcholine-evoked dopamine release from striatal synaptosomes and nicotine-evoked striatal dopamine release measured by in vivo microdialysis have been assessed in α4, α6, α4α6, and β2 knockout mice (Champtiaux et al., 2003). α4β2*, α6β2*, and α4α6β2* nAChRs were shown to be expressed on dopamine terminal fields in the striatum. Additionally, α6β2* nAChRs were found to be functional and sensitive to α-CtxMII inhibition but do not contribute to dopamine release evoked by systemic nicotine administration, and somatodendritic (non-α6)α4β2* nAChRs were posited to be the most likely contribution to nicotine reinforcement (Champtiaux et al., 2003). Taken together, the above data suggest that α4, α5, α6, β2, and β3 may be important components of functionally expressed nAChR subtypes in the VTA and striatum. These nAChR subunits likely play an important role in regulating nicotine reinforcement processes.
Electrophysiological investigations also have provided important insights into the nAChR subtypes likely to be expressed in the VTA and striatum that may regulate nicotine reinforcement. Initially, Picciotto and colleagues (1998) found that nicotine increased the discharge frequency of mesencephalic dopaminergic neurons from wildtype but not β2 knockout mice. Mameli-Engvall and colleagues (2006) extended these findings specifically within VTA dopamine-containing neurons; basal levels of firing were decreased and burst firing was absent in VTA dopaminergic neurons from β2 knockout mice. Furthermore, the stimulatory effect of intravenous nicotine on VTA dopamine cells was abolished in β2 knockout mice (Mameli-Engvall et al., 2006). The role of β2* nAChRs in regulating the effects of endogenous cholinergic transmission on dopamine release from mouse striatal slices also has been assessed (Zhou et al., 2001). β2 knockout mice were shown to have reduced levels of electrically evoked dopamine release compared with wildtype controls (Zhou et al., 2001). These electrophysiological data from nAChR knockout mice support the hypothesis that β2* nAChRs play an important role in regulating the effects of nicotine on the neuroanatomical substrates that are implicated in nicotine reinforcement.
Nicotinic acetylcholine receptor subunits that regulate the positive reinforcing effects of nicotine: In vivo evidence
In addition to the in vitro approaches described above, in vivo studies are beginning to shed more direct light on nAChR subunits involved in nicotine reinforcement and dependence processes. Many of the published studies assessing the role of nAChR subtypes in nicotine reinforcement have utilized the intravenous nicotine self-administration procedure. This procedure is considered the most direct and reliable measure of the reinforcing properties of drugs of abuse. Indeed, the majority of drugs that are abused by humans are also self-administered by animals (Weeks, 1962; Wilson and Schuster, 1972; Risner and Jones, 1975; Griffiths and Balster, 1979; Griffiths et al., 1981; Criswell and Ridings, 1983; Donny et al., 1995). Consistent with a role for nicotine in initiating and maintaining the tobacco smoking habit in humans, nicotine acts as a reinforcer and is self-administered by humans (Harvey et al., 2004), non-human primates (Goldberg et al., 1981), dogs (Risner and Goldberg, 1983) and rats (Corrigall and Coen, 1989; Watkins et al., 1999). Nicotine self-administration produces an inverted U-shaped dose-response curve in all cases, similar to that obtained for other reinforcing drugs such as opiates and cocaine. It is likely that the shape of the nicotine dose-response curve is the result of the competing positive and negative effects of nicotine at different doses. Thus, the increased responding for nicotine over the ascending limb of the curve may reflect the increasing reinforcing effects of nicotine as the unit dose per infusion increases. In contrast, the decreased responding over the descending limb of the curve may reflect increasing aversive properties of nicotine. Consistent with the hypothesis that nicotine may elicit both positive and negative effects, doses of nicotine self-administered by non-human primates can also serve as the substrate for negative reinforcement to occur, and monkeys will work to avoid such IV nicotine infusions (Spealman and Goldberg, 1982). Further, anxiety-like behavior was increased in rats following volitional intravenous self-administration of nicotine (Irvine et al., 2001a), and benzodiazepine treatment, which counters the aversive properties of nicotine, increased nicotine self-administration in rats (Hanson et al., 1979). Finally, it is important to note that the nicotine dose-response curve is ‘shallow’ and ‘narrow’ compared with that obtained for opiates or cocaine, suggesting that responding for nicotine is relatively insensitive to alterations in the dose available for infusion, and that nicotine is reinforcing over a narrow range of doses only.
Consistent with a role for neuronal nAChRs in regulating nicotine reinforcement, administration of antagonists at neuronal nAChRs decreases nicotine self-administration in non-human primates and rats (see below). This decrease in nicotine intake is in contrast to human tobacco smokers, in which there was a transient increase in smoking behavior (Stolerman et al., 1979) or intravenous nicotine self-administration (Rose et al., 1989), after antagonist blockade of nAChRs. This finding in rats is also in contrast to findings with other drugs of abuse such as opiates or cocaine, where administration of opioid or dopamine receptor antagonists increased opiate or cocaine intake, respectively. Such compensatory increases in drug intake likely occur to counter antagonist-induced reductions in the reward value of unit injections of drugs of abuse. Thus, the net effect of antagonist treatment is usually a right-ward shift in the dose-response curve. One possible explanation for the absence of such antagonist-induced compensatory increases in nicotine intake in laboratory animals may be that different subtypes of nAChRs regulate nicotine's reinforcing properties and its aversive properties, and nAChR antagonists in these animals may selectively block the reinforcing effects of nicotine but not its aversive effects. Such an action of nAChR antagonists would prevent laboratory animals from self-administering compensatory nicotine injections. Whatever the explanation, the net effect of decreasing the reinforcing properties of nicotine in rodents appears to be a shift the nicotine self-administration dose-response curve down-ward instead of right-ward, resulting in an overall decrease in responding for nicotine at each unit dose of nicotine available. Thus, pharmacological or genetic manipulations that decrease nicotine reinforcement may be expected to result in a decrease in responsing for nicotine self-administration.
Systemic or intra-VTA administration of the neuronal nAChR antagonist DHβE decreases nicotine self-administration in rats (Corrigall and Coen, 1989; Watkins et al., 1999). In addition, DHβE reduces the stimulatory effects of nicotine on brain reward systems, demonstrated by attenuated nicotine-induced lowering of ICSS thresholds in rats (Ivanova and Greenshaw, 1997; Harrison et al., 2002). DHβE is relatively selective for α4* and β2* nAChRs compared with other classes of nAChRs (Harvey and Luetje, 1996; Harvey et al., 1996). The novel nAChR ligand SSR591813, considered a partial agonist at nAChRs containing α4 and β2 subunits, decreases nicotine self-administration in rats (Cohen et al., 2003). Similarly, the novel nAChR ligand UCI-30002, a partial agonist at α4β2* nAChRs (but also with actions at α7 and α3β4-containing nAChRs), also decreases nicotine self-administration in rats (Yoshimura et al., 2007). Varenicline, a partial agonist at α4β2* nAChRs (but a full agonist at α7* nAChRs) (Mihalak et al., 2006), dose-dependently decreases nicotine self-administration in rats (Rollema et al., 2007). Liu and colleagues have shown that 5-iodo-A-85380, a putative agonist at β2* nAChRs, is actively self-administered by rats (Liu et al., 2003). Full dose-response analyses of these novel drugs should be determined in further studies. Nevertheless, these pharmacological data suggest that α4* and β2* nAChRs play an important role in nicotine reinforcement.
Recent observations in nAChR mutant mice also support the role of these receptor subunits in nicotine addiction. To date, the reinforcing effects of intravenously self-administered nicotine have been assessed only in β2 knockout mice (Picciotto et al., 1998; Epping-Jordan et al., 1999). In these studies, β2 knockout mice and their wildtype counterparts were trained to nose-poke for cocaine under a fixed-ratio 2, time-out 20 s schedule of reinforcement, in which two nose-pokes into an “active” nose-poke hole resulted in an intravenous infusion of cocaine (0.8 mg/kg/infusion) (Picciotto et al., 1998). Under this schedule of reinforcement, β2 knockout and wildtype mice demonstrated similar responding for cocaine (Picciotto et al., 1998; Epping-Jordan et al., 1999). However, when cocaine was substituted with nicotine infusions (0.03 mg/kg/infusion), wildtype mice persisted in responding for nicotine, whereas β2 knockout mice rapidly extinguished operant responding in a manner similar to that observed in wildtype mice when cocaine was substituted with saline (Picciotto et al., 1998; Epping-Jordan et al., 1999). Most recently, Maskos and colleagues (2005) utilized lentivirus-mediated gene transfer to re-express β2 nAChR subunits in the VTA of β2 knockout mice. In these “rescued” β2 knockout mice, systemic nicotine administration significantly increased dopamine transmission in the NAc similar to wildtype mice, an effect absent in non-rescued β2 knockout mice (Maskos et al., 2005). Moreover, wildtype and rescued β2 knockout mice demonstrated preference for the arm of a Y-maze paired with the delivery of intra-VTA nicotine infusions upon arm entry. In contrast, non-rescued β2 knockout mice did not display preference for either the nicotine-paired or non-paired arm of the Y-maze (Maskos et al., 2005). Additionally, Walters and colleagues (2006) found that β2 knockout mice do not demonstrate a conditioned place preference for an environment previously paired with nicotine injections. Furthermore, using β2 knockout mice, Shoaib and colleagues (2002) showed that β2* nAChRs regulate the discriminative stimulus properties of nicotine. The above observations suggest that β2 nAChR subunits play a central role in nicotine reinforcement, consistent with the high density of these subunits in the VTA and other reward-relevant brain regions.
In addition to gene knockout technology, Tapper and colleagues (2004) utilized a complementary approach to investigate the role of α4 nAChR subunits in regulating the reinforcing effects of nicotine. Specifically, an endogenous exon of the α4 nAChR gene was replaced in mice with one containing a single point mutation (Leu 9′ П Ala 9′). This point mutation renders mutant “knock-in” α4-containing receptors hypersensitive to nicotine. Using these α4 knock-in mice, nicotine was shown to induce a significant conditioned place preference, a measure of the conditioned rewarding effects of drugs of abuse, at doses 50-fold lower than those necessary to induce a place preference in wildtype mice (Tapper et al., 2004). These data suggest that, similar to β2 nAChR subunits, α4 subunits also likely are important regulators of nicotine reinforcement processes. Importantly, however, the role of α4 subunits in nicotine reinforcement has not yet been directly tested by intravenous nicotine self-administration behavior in α4 nAChR knockout mice.
In contrast to most other nAChR subunits expressed in the CNS, the α5 nAChR subunit does not form functional receptors when expressed alone or when co-expressed with β subunits in Xenopus oocytes (Ramirez-Latorre et al., 1996; Yu and Role, 1998). Rather, the function of the α5 nAChR subunit is restricted to a modulatory role when incorporated into functional nAChRs, regulating the activation and desensitization kinetics and other characteristics of nAChRs (Girod et al., 1999; Nelson and Lindstrom, 1999). Unfortunately, compounds selective for α5* nAChRs are not available. Therefore, in vivo pharmacological data supporting a role for α5 subunits in nicotine self-administration has not been generated. Nevertheless, accumulating circumstantial evidence suggests that α5* nAChRs may play a key role in nicotine reward and dependence processes. As stated above, the α5 nAChR subunit is expressed at high concentrations in the VTA (Klink et al., 2001), an important neuroanatomical substrate that regulates nicotine reward. Indeed, the α5 subunit is considered an important component of two putative functional nAChR subtypes expressed on VTA dopamine neurons (Klink et al., 2001): α4α6α5(β2)2 and (α4)2α5(β2)2. Furthermore, α5 knockout mice have decreased behavioral sensitivity to the effects of nicotine, measured by nicotine-induced seizures and hypolocomotion (Salas et al., 2003a). The most convincing evidence supporting a role for α5* nAChRs in nicotine dependence has been generated from genetic analyses of human smokers. A non-synonymous single nucleotide polymorphism (SNP) in the α5 nAChR gene was associated with a two-fold increase in the risk of developing nicotine dependence in humans after they were exposed to cigarette smoking (Saccone et al., 2007). Similarly, Schlaepfer and colleagues (2007) found that two linked SNPs on the gene cluster that incorporates the α3, α5, and β4 nAChR subunits predicted early-onset tobacco use in young adults. Berrettini and colleagues (2008) showed in three independent populations of human samples, totaling approximately 15000 individuals, that a common genetic haplotype in the α3/α5/β4 gene cluster predisposes individuals to nicotine dependence. Recently, polymorphism in the α3/α5/β4 gene cluster, most notably in the α5 nAChR subunit gene, predisposed smokers to increased risk of developing lung cancer (Hung et al., 2008), perhaps by increasing the reinforcing effects of tobacco smoke, resulting in greater tobacco intake and associated carcinogens, and increased risk of developing cancer (Thorgeirsson et al., 2008). Interestingly, polymorphism in the α5 nAChR subunit gene was found to be protective against cocaine dependence (Grucza et al., 2008), a finding suggesting that nicotine and cocaine reinforcement may be dissociated at the level of α5* nAChRs. Taken together, these data suggest that the α5 nAChR subunit likely plays a critical role in nicotine dependence processes. Nevertheless, direct assessment of the reinforcing effects of nicotine in α5 knockout mice will be necessary to support this conclusion.
Much interest has centered on the potential role of α6 nAChR subunits in regulating nicotine reinforcement processes. This interest has arisen, in large part, because of the high concentrations and relatively restricted patterns of expression of mRNA transcripts for α6 subunits in the VTA (Quik et al., 2000; Azam et al., 2002; Champtiaux et al., 2002). The expression of α6 nAChR subunits was shown to undergo robust upregulation in rats following repeated exposure to intravenous nicotine self-administration (Parker et al., 2004). More recently, pharmacological data have emerged that further support a role for this subunit in nicotine reinforcement. Neugebauer and colleagues demonstrated that the novel nAChR antagonist bPiDDB, which may selectively antagonize α6* and/or α3* nAChRs (Dwoskin et al., 2004; Neugebauer et al., 2006), dose-dependently decreases nicotine self-administration in rats and the hyperactivity induced by acute and repeated nicotine administration (Neugebauer et al., 2006). Furthermore, Le Novere and colleagues (1999) demonstrated that knockdown of α6 mRNA through the use of antisense oligonucleotides attenuates the locomotor stimulatory effects of nicotine in mice. These data provide circumstantial evidence implicating α6-containing nAChRs in nicotine reinforcement processes, but the role for this subunit has not yet been directly tested.
The nAChR antagonist methyllycaconitine (MLA) is considered relatively selective for α7 pentameric nAChRs (Ward et al., 1990), and a high concentration of α7 nAChRs exists in the VTA and other reward-related brain sites, including the amygdala (Seguela et al., 1993). MLA was shown to attenuate the conditioned rewarding effects of nicotine following direct intra-VTA administration in rats (Laviolette and van der Kooy, 2003). Furthermore, intra-VTA administration of MLA attenuated the ICSS threshold-lowering effects of nicotine in rats (Panagis et al., 2000). More importantly, Markou and Paterson (2001) demonstrated that MLA decreased intravenous nicotine self-administration in rats. These observations suggest that α7 nAChRs play a role in nicotine reinforcement. In contrast, Grottick and co-workers (2000) demonstrated that MLA did not alter nicotine self-administration or nicotine-stimulated locomotor activity in rats. Based on these findings, α7 nAChRs were speculated to play no role in regulating nicotine reinforcement (Grottick et al., 2000). Consistent with this conclusion, nicotine was shown to elicit a conditioned place preference in α7 mice that was very similar to that observed in wildtype mice (Walters et al., 2006). Thus, the precise role of α7 nAChRs in nicotine reinforcement remains unclear, and testing of α7 knockout mice in the intravenous nicotine self-administration procedure may provide important insights into the role of this receptor subunit in nicotine reinforcement. Overall, the above data support a role for α4 and β2, and perhaps α5, α6, and α7, nAChR subunits in regulating the positive reinforcing effects of nicotine. In addition, these data demonstrate the utility of nAChR knockout mice for investigating the nAChR subtypes that regulate nicotine reinforcement.
Nicotinic acetylcholine receptor subunits regulate the positive reinforcing effects of other drugs of abuse
Accumulating evidence suggests that nAChRs may play a role in the reinforcing effects of drugs of abuse other than nicotine, such as cocaine and alcohol. Cue-induced cocaine craving is potentiated by nicotine pretreatment in human cocaine users whom are also cigarette smokers (Reid et al., 1998). In rats, the development of escalated levels of cocaine intake that is associated with extended daily access to the drug was abolished by co-administration of the nAChR antagonist mecamylamine (Hansen and Mark, 2007). This observation suggests that nAChRs may regulate the development of compulsive cocaine-seeking. Perfusion of mecamylamine or co-perfusion of the nAChR antagonists DHβE and MLA (see above) into the NAc blocked the stimulatory effects of cocaine on NAc dopamine release (Zanetti et al., 2007). More importantly, β2 knockout mice appear to be less sensitive to the conditioned rewarding effects of cocaine (Zachariou et al., 2001). Furthermore, induction of chronic Fos-related antigens by cocaine, which may contribute to cocaine-associated neuroplasticity, was abolished in β2 knockout mice compared with wildtype siblings, implying that the actions of cocaine downstream of membrane-expressed receptors may be regulated by β2* nAChRs (Zachariou et al., 2001).
In addition to cocaine, nAChRs play a role in alcohol dependence. In human populations, polymorphisms in the α3/α5/β4 nAChR gene cluster were associated with increased vulnerability to alcoholism (Schlaepfer et al., 2007; Wang et al., 2008), as well as with altered steady-state levels of α5 nAChR mRNA in the brain (Wang et al., 2008). Mecamylamine decreased the desire to consume alcohol in humans (Young et al., 2005) and blocked the self-reported euphoric effects of alcohol (Chi and de Wit, 2003). In rats, mecamylamine decreased ethanol intake (Blomqvist et al., 1996; Ericson et al., 1998; Le et al., 2000) and attenuated the stimulatory effects of ethanol on dopamine release in the NAc when administered either systemically (Blomqvist et al., 1993) or directly into the anterior, but not posterior, VTA (Ericson et al., 2008). Systemic or direct intra-NAc administration of mecamylamine decreased ethanol consumption by rats (Nadal et al., 1998; Le et al., 2000). Similarly, the α4β2* nAChR partial agonist varenicline was shown recently to decrease ethanol consumption by rats (Steensland et al., 2007). Interestingly, chronic ethanol consumption decreased the expression of α7 and α4β2* nAChRs in the hippocampus (Robles and Sabria, 2008). Patch-clamp recordings from human embryonic kidney (HEK) cells that heterologously express human nAChR subunits have shown that ethanol can act directly at nAChRs to potentiate the activity α4* nAChRs (Zuo et al., 2001; Zuo et al., 2002). These data suggest that nAChRs play a role in the neurochemical actions of alcohol that participate in the regulation of alcohol intake. Interestingly, α7 knockout mice are more sensitive than wildtype mice to some of the behavioral actions of ethanol (Bowers et al., 2005), including ethanol-induced locomotor stimulation and loss of righting reflex (Bowers et al., 2005). However, little is known about the reinforcing effects of alcohol in α7* knockout and other strains of nAChR knockout mice. Taken together, the above data support an important role for nAChRs, particularly those containing β2 subunits but perhaps also α5* and α7* subunits, in the reinforcing actions of cocaine and alcohol, as well as in the neuroplasticity induced by these drugs that may contribute to compulsive drug-seeking.
Nicotinic acetylcholine receptor subunits in nicotine dependence and withdrawal
Addiction to tobacco smoking depends not only on the positive reinforcing and hedonic actions of nicotine, but also on escape from the aversive consequences of nicotine withdrawal (Doherty et al., 1995; Kenny and Markou, 2001). Indeed, prolonged nicotine exposure results in the development of nicotine dependence, and smoking cessation commonly elicits an aversive nicotine withdrawal syndrome in human smokers (Shiffman and Jarvik, 1976; Hughes et al., 1991). This syndrome can be directly attributed to a reduction of nicotine intake in nicotine-dependent individuals (West et al., 1984). Importantly, withdrawal duration and severity can predict relapse in abstinent human smokers (Piasecki et al., 1998; Piasecki et al., 2000; Piasecki et al., 2003). Furthermore, the efficacy of nicotine replacement therapy, at least in certain individuals (Fagerstrom, 1988; Sachs and Leischow, 1991), is related to its ability to prevent the onset and reduce the duration of nicotine withdrawal.
Isola and colleagues have shown that spontaneous or mecamylamine-precipitated withdrawal from chronic nicotine injections (2 mg/kg salt, four times daily for 14 days) resulted in increased expression of physical or “somatic” withdrawal signs (e.g., rearing, jumping, shakes, abdominal constrictions, chewing, scratching, facial tremor) in mice (Isola et al., 1999). Similarly, spontaneous or antagonist-precipitated withdrawal from nicotine delivered via osmotic minipump (24-48 mg/kg/day free-base, 7-60 day continuous treatment) also increased somatic withdrawal signs, and these signs were increased by a greater magnitude in C57BL/6 than in 129/SvEv strains of mice (Damaj et al., 2003). This strain difference is important to note because most nAChR knockout mice are bred on a C57BL/6 background. Besson and colleagues have shown that somatic withdrawal signs were increased by a similar magnitude in wildtype and β2 knockout mice during mecamylamine-precipitated withdrawal from nicotine delivered via osmotic minipump (2.4 mg/kg/day free-base, 28 day continuous treatment) (Besson et al., 2006). The α7-selective nAChR antagonist MLA increased somatic withdrawal signs in wildtype and β2 knockout mice following chronic nicotine treatment (24-36 mg/kg/day free-base via osmotic minipump, 13-28 day continuous treatment) (Salas et al., 2004; Jackson et al., 2008). However, under similar treatment conditions, somatic withdrawal signs were diminished in α5, α7, and β4 knockout mice (Salas et al., 2004; Salas et al., 2007; Jackson et al., 2008). Based on these observations, α5, α7, and β4, but not β2, subunits likely are components of the nAChRs that regulate the development of physical dependence on nicotine and the expression of somatic withdrawal signs.
Affective components of nicotine and tobacco withdrawal include depressed mood, dysphoria, anxiety, irritability, difficulty concentrating, and craving (Parrott, 1993; Doherty et al., 1995; Kenny and Markou, 2001). Accumulating evidence suggests that affective components of withdrawal may play a more important role than somatic aspects in the maintenance of dependence to drugs of abuse, including nicotine (Markou et al., 1998; Kenny and Markou, 2001; George et al., 2007). In mice, withdrawal from nicotine delivered via osmotic minipump (Damaj et al., 2003; Jonkman et al., 2005) or repeated daily injections (Costall et al., 1989) increased the expression of anxiety-like behaviors in mice. Similarly, the time spent immobile in the forced swim test, considered an index of depression-like behavior, was increased in mice undergoing withdrawal from nicotine (2 mg/kg, four injections daily for 15 days) compared with saline-treated controls (Mannucci et al., 2006). Recent findings in nAChR knockout mice have provided insights into the nAChR subunits that may regulate affective aspects of nicotine withdrawal. Affective signs of spontaneous or mecamylamine-precipitated nicotine withdrawal (36 mg/kg/day via osmotic minipump for 14 or 28 days), reflected in increased anxiety-related behavior and induction of a conditioned place aversion, were absent in β2 knockout mice but were readily observed in α5 knockout and α7 knockout mice (Jackson et al., 2008). Furthermore, the deficits in fear conditioning typically observed in mice undergoing withdrawal from nicotine (6.3 mg/kg/day free-base via osmotic minipump, 12 day treatment) were intact in α7 knockout mice but were greatly diminished in β2 knockout mice (Portugal et al., 2008). Therefore, β2*, but not α5* or α7*, nAChRs may contribute to the affective components of the nicotine withdrawal syndrome.
Withdrawal from nicotine and other major drugs of abuse decreases brain reward function, reflected in elevated ICSS thresholds in rats (Epping-Jordan et al., 1998; Watkins et al., 2000; Kenny and Markou, 2005). Such reward deficits are considered a particularly important affective component of withdrawal that maintains drug-taking behavior (Koob and Le Moal, 2005; Kenny, 2006). Systemic administration of the nAChR antagonist DHβE, but not MLA, precipitated withdrawal-associated elevations of ICSS thresholds in nicotine-dependent rats (3.16 mg/kg/day for 7-14 days) (Ivanova and Greenshaw, 1997; Markou and Paterson, 2001; Harrison et al., 2002). Infusion of DHβE into the VTA also precipitated elevations of ICSS thresholds in nicotine-dependent, but not control, rats (Bruijnzeel and Markou, 2004). As stated above, DHβE is relatively selective for α4* and β2* nAChRs (Harvey and Luetje, 1996; Harvey et al., 1996). Therefore, these data provide pharmacological support for a role of α4* and β2* nAChR subtypes, particularly those located in the VTA, in regulating the development of nicotine dependence and the expression of nicotine withdrawal. Nevertheless, the role of these or other nAChR subunits in regulating the reward deficits associated with nicotine withdrawal has not been directly tested in nAChR knockout mice. However, recent data from our laboratory has shown that nicotine withdrawal does indeed elevate ICSS thresholds in mice similar to rats (Johnson et al., 2008), highlighting the potential utility of the ICSS procedure for assessing nicotine withdrawal-associated reward deficits in nAChR knockout mouse strains (Johnson et al., 2008).
Finally, in a series of interesting studies, Nashmi and colleagues (2007) generated genetically modified mice in which the α4 nAChR subunit was altered to express a fluorescent tag (yellow fluorescent protein [YFP]), and these mice were used to assess the effects of chronic nicotine administration on the expression of α4* nAChRs in cell populations located in the VTA (Nashmi et al., 2007). The α4*-YFP mice were treated continuously for 10 days with nicotine (48 mg/kg/day) via osmotic minipump. Levels of α4-YFP in VTA dopamine-containing cells were not significantly altered by chronic nicotine exposure. In contrast, α4-YFP levels were dramatically increased in GABAergic cells in the VTA. A similar increase in α4-YFP expression in VTA GABAergic cells also was observed in mice chronically treated with a lower dose of nicotine (9.6 mg/kg/day). These data demonstrate that VTA cell populations are differentially sensitive to nicotine-induced plasticity in α4* nAChR expression and suggest that α4* nAChRs located in VTA GABAergic cells may be a particularly important target for the induction of nicotine dependence (Nashmi et al., 2007).
Anxiety disorders and subtypes of nicotinic acetylcholine receptors
Anxiety may be defined as an emotional condition characterized by an unpleasant and diffuse sense of apprehension usually accompanied by autonomic symptoms such as headache or palpitations (Millet et al., 1998). Anxiety is distinguished from fear by the fact that in anxiety a threat generated “internally” results in an emotional response, whereas in the case of fear, the threat is external and known. In humans, anxiety disorders encompass a number of related conditions, including generalized anxiety disorder (GAD), panic disorder, phobia, obsessive-compulsive disorder (OCD), and posttraumatic stress disorder (PTSD) (American Psychiatric Association, 1994). Many pharmacological agents used to treat anxiety disorders, including fluoxetine (Prozac) or buspirone (Buspar), have direct actions on nAChRs (Dilsaver and Davidson, 1987; Fryer and Lukas, 1999; Hennings et al., 1999; Lopez-Valdes and Garcia-Colunga, 2001; Miller et al., 2002; Garwood and Potts, 2007), suggesting a role of nAChR modulation in the therapeutic actions of these compounds. A high prevalence of tobacco addiction exists among individuals who suffer from anxiety disorders (Koenen et al., 2006; Morissette et al., 2007; Goodwin et al., 2008). Indeed, Hughes and colleagues reported that the incidence of smoking was approximately 47% in patients suffering from an anxiety disorder compared with 30% in control patients (Hughes et al., 1986). However, patients suffering from OCD may have reduced rates of tobacco dependence compared with the general population (Bejerot and Humble, 1999; Lopes et al., 2002). Many smokers report that cigarettes have an anxiety-reducing influence (Speilberger, 1986) and that mood control is a core reason for maintaining their smoking habit (Parrott, 1994). Conversely, smoking abstinence and associated nicotine withdrawal is known to increase subjective levels of anxiety in smokers (Hughes et al., 1991). Thus, nicotine in tobacco smoke appears to have subjective anxiolytic effects that are important in maintaining the tobacco habit and may be particularly important in individuals with comorbid anxiety disorders. Nevertheless, the precise relationship between nicotine consumption and anxiety state is unclear. Although accumulating evidence suggests that nicotine in tobacco smoke may have subjective anxiety-reducing properties, nicotine itself actually may contribute to the development of anxiety disorders (West and Hajek, 1997; Isensee et al., 2003). Taken together, the above observations are consistent with the notion that deficits may exist in nAChR signaling pathways in the brains of smokers who suffer from comorbid anxiety disorders, and these deficits may enhance the reinforcing effects of nicotine and result in higher rates of tobacco dependence in such individuals.
In rodents, acute administration of nicotine or nAChR agonists has complex actions on anxiety-related behaviors (Brioni et al., 1993; Kenny et al., 2000a). Nicotine can increase or decrease anxiety-like behaviors in rats, depending on the dose of nicotine and the baseline state of anxiety-like behavior at testing (File et al., 1998b). Similarly, nAChR antagonists also have complex actions on anxiety-like behavior (File et al., 1998a; Newman et al., 2001; Newman et al., 2002). This complexity likely reflects populations of nAChRs located in different neurotransmitter systems in the brain that have opposing actions on anxiety behaviors (for reviews, see File et al., 2000a; File et al., 2000b). Consistent with this interpretation, administration of nicotine into the dorsal hippocampus increased anxiety-like behaviors in rats, and this effect was blocked by co-administration of the α7* nAChR-selective antagonist MLA (Tucci et al., 2003). Conversely, nicotine administered into the dorsal raphe nucleus decreased anxiety-like behaviors in rats, and this effect was abolished by DHβE (Cheeta et al., 2001a; Cheeta et al., 2001b). Interestingly, α7 knockout mice were shown to spend significantly more time in the center of an open-field apparatus than wildtype controls, suggesting that α7 knockout mice may have reduced levels of anxiety (Paylor et al., 1998). Importantly, however, Salas and colleagues did not observe any difference in anxiety-like behaviors between α7 knockout mice and wildtype controls (Salas et al., 2007). Recent data suggest that β3 nAChR knockout mice may display reduced anxiety-like behaviors compared with wildtype mice, reflected in more time spent on the open arm of an elevated plus-maze compared to wildtype littermates (Booker et al., 2007). β4 knockout mice also displayed decreased anxiety-like behaviors on an elevated plus-maze and staircase maze compared with wildtype mice (Salas et al., 2003b). The reduction in anxiety-like behaviors in β4 knockout mice compared with wildtype mice on the elevated plus maze was supported by telemetry measures of heart rate; the β4 knockout exhibited lower increases in heart rate than controls (Salas et al., 2003b). Therefore, these data suggest a role for the β3* and β4* nAChRs in anxiety-like behaviors in mice. In contrast to β3 and β4 knockout mice, α4 knockout mice were shown to spend significantly less time on the open arm of an elevated plus-maze apparatus than wildtypes (Ross et al., 2000), suggesting that α4 knockout mice may have an anxiogenic-like phenotype. Paradoxically, mice with a point mutation in the α4 nAChR subunit gene, which renders α4* nAChRs hypersensitive, also exhibited increased anxiety-like behaviors compared with wildtype controls (Labarca et al., 2001). Finally, anxiety-like behaviors were unaltered relative to wildtype controls in mice simultaneously lacking both the α7 and β2 nAChR subunits, although these mice exhibited deficits in a test of fear-based learning (Marubio and Paylor, 2004). These behavioral genetics data suggest that β3 nAChR subunits, and perhaps α7 and α4 subunits, may regulate anxiogenic-like actions of endogenous cholinergic transmission in the brains of mice. Furthermore, these observations highlight the complex and sometimes contradictory nature of nAChR modulation of anxiety states.
In common with abstinent human tobacco smokers, nicotine withdrawal elicits an anxiogenic-like state in rodents (Cheeta et al., 2001a; Irvine et al., 2001a; Irvine et al., 2001b; Biala and Weglinska, 2005; Jonkman et al., 2005). The nAChR antagonist DHβE increases anxiety-like behaviors in nicotine-dependent mice (Damaj et al., 2003). β2 knockout mice do not shown the decreased time spent on the open arm of an elevated plus-maze (i.e., increased anxiety-like behavior) usually observed in wildtype mice during mecamylamine-precipitated nicotine withdrawal (Jackson et al., 2008). Importantly, nicotine withdrawal elicits an increase in anxiety-like behavior in α5 and α7 knockout mice that is similar to the magnitude observed in wildtype mice (Jackson et al., 2008), suggesting that these nAChR subunits are not involved in the expression of nicotine withdrawal-associated increases in anxiety-like behaviors. Thus, α4* and β2*, but not α5* nor α7*, nAChRs likely regulate the anxiogenic-like state associated with nicotine withdrawal.
Finally, increased activity of the hypothalamic-pituitary-adrenal axis (HPA) may play an important role in the etiology of anxiety disorders, as well as depression and schizophrenia (see below). Accumulating evidence indicates that nAChRs regulate HPA function, possibly by modulating corticotropin-releasing factor (CRF) and corticosterone levels. Axon terminals in the median eminence appear to contain CRF-like immunoreactive synaptic vesicles and membrane-bound nAChRs (Okuda et al., 1993). In rats, nicotine administered systemically or by self-administration acutely increased plasma levels of corticosterone and adrenocorticotropic hormone (Cam and Bassett, 1983; Chen et al., 2008), whereas chronic self-administration decreased CRF mRNA expression in the paraventricular nucleus (Yu et al., 2008). Mecamylamine-precipitated nicotine withdrawal enhanced anxiety-associated behaviors and CRF-like immunoreactivity in the amygdala, likely via CRF1 receptors (George et al., 2007), and CRF antagonism prevented, but could not reverse, the elevation of ICSS brain reward thresholds found during mecamylamine-precipitated nicotine withdrawal (Bruijnzeel et al., 2007). The nAChR subtypes that participate in the regulation of these effects on the HPA are unclear. Interestingly, however, β3 knockout mice had increased basal and stress-induced corticosterone levels compared with wildtype controls (Cui et al., 2003; Booker et al., 2007), suggesting increased stress responsively. Previously, systemic corticosterone administration was shown to reduce anxiety-like behavior in rats (File et al., 1979), possibly through feedback inhibition of stress cascades in the brain. Thus, the lower levels of anxiety-like behavior observed in β3 knockout mice may be secondary increased anxiety-reducing corticosterone. Further, these data implicate the β3* nAChR or non-β3* nAChRs that have undergone adaptation in the β3 knockout mice, in regulation of the HPA system.
Depression and subtypes of nicotinic acetylcholine receptors
In humans, major depression often is comorbidly expressed with tobacco addiction, similar to the relationship described above between tobacco smoking and anxiety disorders (Breslau, 1995; Diwan et al., 1998). Human tobacco smokers are more likely than the general population to display symptoms of depression and to be diagnosed with major depression (Covey et al., 1997; Laje et al., 2001). Nicotine is reported to have mood-enhancing effects in smokers (Foulds et al., 1997), whereas cessation of the tobacco habit can precipitate depression-like systems in smokers (Glassman et al., 1990). Many antidepressant agents that are used to treat major depression can potently antagonize nAChRs (Fryer and Lukas, 1999; Miller et al., 2002; Shytle et al., 2002), and this action may contribute to their therapeutic effects. Thus, cigarette smoking may represent a form of self-medication behavior in depressed individuals in which the nicotine contained in tobacco smoke may induce antidepressant or other beneficial effects (Rabenstein et al., 2006).
Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants can increase limbic serotonin (5-hydroxytryptamine, 5-HT) transmission, and this action is hypothesized to contribute to their antidepressant actions. Additionally, antidepressants also increase the expression of brain-derived neurotrophic factor (BDNF) in limbic brain regions, and this action also may contribute to the therapeutic utility of these agents (Nibuya et al., 1996; Duman et al., 1997). Consistent with an antidepressant-like action of nicotine, previous studies have shown that nicotine increases the release of serotonin from superfused hippocampal slices (Kenny et al., 2000b). Furthermore, chronic nicotine exposure increased BDNF mRNA in the hippocampus of rats (Kenny et al., 2000c), whereas nicotine withdrawal decreased hippocampal BDNF mRNA expression (Kenny et al., 2000c). These effects of nicotine could potentially contribute to the subjective mood-enhancing effects of the drug in human smokers, as well as the negative affect associated with nicotine withdrawal. Intriguingly, mecamylamine also increases hippocampal serotonin release similar to the stimulatory effects of nicotine (Kenny et al., 2000b), and also has antidepressant-like effects in animals (Caldarone et al., 2004; Rabenstein et al., 2006). Indeed, both nicotine and nAChR antagonists can potentiate the antidepressant-like effects of tricyclic antidepressant drugs in mice (Popik et al., 2003). These observations likely reflect preferential actions of nicotine and mecamylamine at different populations of nAChRs that have opposing actions, similar to the scenario described above for nAChR modulation of anxiety-like behaviors. Such an explanation would account for the apparently contradictory findings in which both an agonist (nicotine) and antagonist (mecamylamine) at nAChRs can elicit similar neurochemical and behavioral effects.
Neurogenesis is the process by which new cells are born and mature into functional neurons and occurs in the adult mammalian brain (Fowler et al., 2008). Neurogenesis is potentiated by antidepressant treatment, likely through a mechanism involving hippocampal serotonin and BDNF transmission, and is hypothesized to play a role in the therapeutic efficacy of antidepressant drugs (Brezun and Daszuta, 1999; Gould, 1999; Brezun and Daszuta, 2000; Lee et al., 2000; Duman et al., 2001; Pencea et al., 2001; Katoh-Semba et al., 2002; Santarelli et al., 2003; Mattson et al., 2004; Sairanen et al., 2005). In vitro, nicotine appears to be cytotoxic to newly born neurons by inducing apoptosis in hippocampal progenitor cells (Berger et al., 1998). Similarly, in vivo, intravenous nicotine self-administration decreases neurogenesis in the hippocampus of adult rats (Abrous et al., 2002). It is worth noting that in this study rats were euthanized 48 h after the last self-administration session. Thus, these observations may be more indicative of nicotine withdrawal rather than the primary actions of nicotine. More recently, the proliferation of newly born cells were shown to be significantly reduced in the hippocampus of β2 knockout mice compared with wildtype controls (Harrist et al., 2004), suggesting that constitutive cholinergic transmission at β2* nAChRs promotes hippocampal neurogenesis. Thus, nAChRs appear to regulate hippocampal neurogenesis in a complex manner, and this regulation may contribute to the multifaceted effects of nicotine on mood.
Emerging evidence from nAChR knockout mice support the hypothesis that nAChRs play a central role in mood regulation and in gating the beneficial actions of antidepressant drugs. In the learned helplessness test, wildtype mice were shown to respond to the antidepressant-like effects of the tricyclic antidepressant amitriptyline (Caldarone et al., 2004). In contrast, these beneficial effects of amitriptyline were absent in β2 knockout mice (Caldarone et al., 2004). Similarly, the antidepressant-like effects of amitriptyline observed in wildtype mice tested in the tail suspension and forced swim tests were absent in β2 knockout mice (Caldarone et al., 2004). In addition to the key role noted above for β2* nAChRs in regulating the stimulatory effects of endogenous cholinergic transmission on hippocampal neurogenesis (Harrist et al., 2004), β2* nAChRs also appear to regulate the stimulatory effects of antidepressants on this process. Indeed, the stimulatory effects of amitriptyline on cell proliferation observed in the adult hippocampus of wildtype mice were absent in β2 knockout mice (Caldarone et al., 2004). Most recently, Rabenstein and colleagues demonstrated that the antidepressant-like effects of mecamylamine in wildtype mice were absent in β2 and α7 nAChR knockout mice, measured in the tail suspension test (Rabenstein et al., 2006). These data provide further support for a role of nAChRs in the beneficial effects of antidepressants.
Taken together, the above data demonstrate that nAChRs may play a key role in regulating baseline affective state and in regulating the beneficial effects of antidepressant drugs. The observation that hippocampal neurogenesis is impaired in β2 knockout mice supports the hypothesis that constitutive deficits in nAChR signaling cascades may contribute to vulnerability to depression and other mood disorders and also may contribute to the increased motivation to consume nicotine in tobacco smoke in humans.
Schizophrenia and subtypes of nicotinic acetylcholine receptors
Approximately 1% of the population of the United States, around 2 million people, suffer from schizophrenia (Rupp and Keith, 1993). Similarly to anxiety disorders and depression, the rate of tobacco smoking is dramatically higher in schizophrenia patients compared with the general population (Masterson and O'Shea, 1984; Hughes et al., 1986; Goff et al., 1992; Dalack et al., 1998). Estimates indicate that the prevalence of tobacco smoking in schizophrenia patients is between 60-90%. Several explanations have been proposed to account for the high incidence of tobacco use in schizophrenia patients. First, this high rate may reflect an attempt by schizophrenia patients to reduce neuroleptic-induced extrapyramidal side-effects (Jarvik, 1991). Second, the nicotine obtained from tobacco smoke may ameliorate the generalized cognitive or sensory gating deficits associated with schizophrenia (Curzon et al., 1994; Acri et al., 1995; Kumari et al., 1996; Kumari and Gray, 1999). Third, the rewarding properties of nicotine may be increased in schizophrenia patients, resulting in greater abuse potential (Chambers et al., 2001; Spring et al., 2003). Finally, nicotine obtained through tobacco smoking may ameliorate the negative symptoms of schizophrenia (Markou and Kenny, 2002; Cook et al., 2007). A combination of the aforementioned factors likely contributes to the increased motivation to consume nicotine in tobacco smoke in human schizophrenia patients and to the beneficial effects that nicotine has on various symptoms of schizophrenia (Glynn and Sussman, 1990; Dalack and Meador-Woodruff, 1996).
Accumulating evidence from human patient populations suggests that a genetic linkage may exist between symptoms of schizophrenia and polymorphisms in nAChR subunits (Freedman et al., 2001; Leonard et al., 2002; De Luca et al., 2006), particularly the α7 nAChR subunit. Indeed, abnormal sensory gating in schizophrenia has been associated with polymorphisms in chromosome 15q13-14, which encompasses the α7 nAChR gene locus (Freedman et al., 1997). Nicotine has been reported to improve sensory gating and attention in patients with schizophrenia (Depatie et al., 2002). α7 nAChR knockout mice display deficits in working memory (Fernandes et al., 2006) and attention (Hoyle et al., 2006; Young et al., 2007). α7 nAChR knockout mice also have marked deficits in the acquisition of an operant response to obtain food rewards, suggesting that associative learning processes may be disrupted in α7 nAChR knockout mice (Keller et al., 2005). Similar cognitive deficits also are observed in rats following antisense-mediated knockdown of α7 nAChR subunits (Curzon et al., 2006). Consistent with a role for α7* nAChRs in cognition, the nAChR partial agonist SSR180711 enhances episodic memory in wildtype but not in α7 knockout mice (Pichat et al., 2007). Furthermore, viral-mediated overexpression of α7 nAChR subunits in the mouse hippocampus improves spatial memory (Ren et al., 2007). Because α7 receptors are highly expressed in the hippocampus (Seguela et al., 1993), and decreased nicotinic receptor density is found in the hippocampus in schizophrenia patients (Freedman et al., 1995), the above data suggest that the cognitive deficits associated with schizophrenia may at least partly reflect decreased α7* nAChR signaling. The high rates of smoking in schizophrenia patients may reflect high motivation to obtain the stimulatory effects of nicotine on α7* nAChRs, thereby improving compromised cognitive performance.
In addition to α7* nAChRs, accumulating evidence generated from knockout mice supports a role for other nAChR subunits in the neuropathology associated with schizophrenia. β3 nAChR knockout mice have deficits in prepulse inhibition (PPI) compared with wildtype mice. PPI is the process by which a weaker prepulse stimulus can attenuate the reaction of an organism to a subsequent stronger startling stimulus. Deficits in PPI likely reflect abnormalities in sensorimotor gating and are manifested in individuals who suffer from schizophrenia (Braff et al., 2001). Interestingly, β3 knockout mice have higher constitutive levels of striatal dopamine transmission in response to cholinergic (nicotinic receptor) stimulation (Cui et al., 2003), possibly reflecting compensatory increases in the function of non-β3* nAChRs in response to β3 nAChR subunit null mutation (Cui et al., 2003). Importantly, enhanced striatal dopamine-mediated transmission may contribute to the sensorimotor gating deficits observed in schizophrenia patients (Braff and Geyer, 1990). Thus, an interesting possibility is that deficits in β3* nAChR signaling cascades or compensatory alterations in non-β3* nAChRs may contribute to the sensorimotor gating deficits in schizophrenia. nAChRs also are found in glutamate-containing terminals throughout the brain, including the prefrontal cortex (Jones and Wonnacott, 2004). Nicotine or acetylcholine increases glutamate-mediated transmission in prefrontal cortical slices, and this action is absent in β2 knockout mice (Lambe et al., 2003). Based on the fact that the prefrontal cortex is an important brain region in schizophrenia-associated neuropathology, deficits in β2* nAChRs in the prefrontal cortex have been hypothesized to contribute to schizophrenia-associated cognitive and behavioral deficits (Lambe et al., 2003).
Conclusions and Future Directions
Based on the data reviewed above, mice with genetic modifications in discrete nAChR subunits serve as powerful tools that can increase our understanding of the subunit composition of the nAChRs that regulate the rewarding properties of nicotine and also that regulate the development of nicotine dependence and expression of nicotine withdrawal. nAChR knockout mice have provided strong evidence that α4* and β2* nAChR subunits are critically involved in the rewarding effects of nicotine. Furthermore, based on data from nAChR knockout mice, β2*, but not α5* or α7*, nAChRs may contribute to the affective components of nicotine withdrawal. Conversely, α5*, α7*, and β4*, but not β2*, nAChRs may contribute to the somatic aspects of nicotine withdrawal.
In addition to their utility in delineating the mechanisms of nicotine reward and dependence, nAChR mutant mice are providing important insights into the contribution of nAChRs to psychiatric illnesses, as well as to the mechanisms that may contribute to the increased incidence of tobacco dependence associated with many psychiatric disorders. nAChR knockout mice have provided evidence that α4*, α7*, and β3* nAChRs may play an important role in anxiety-related disorders. β2* and α7* nAChRs may regulate the mood-enhancing effects of antidepressant drugs and also may regulate baseline affective state. Studies in nAChR knockout mice suggest that α7* and perhaps β2* and β3* nAChRs may regulate expression of various symptoms of schizophrenia.
Many important aspects of the mechanisms by which nAChRs regulate nicotine addiction processes and contribute to psychiatric illness remain to be resolved. For example, what is the precise subunit composition of the nAChR subtypes in vivo that regulate tobacco addiction? If addiction-related sub-populations of nAChRs can be identified, will selective modulation of their activity be possible, independent of other populations of nAChRs that are involved in essential neurophysiological processes? Finally, what are the signaling mechanisms through which addiction-associated nAChRs regulate behavior? Considering the progress that has been made to date using genetically modified mice, continuing advances in this field will provide answers to these important questions.
Acknowledgments
Supported by the National Institute on Drug Abuse (DA020686 to PJK), and The James and Esther King Biomedical Research Program, Florida Department of Health (07KN-06 to PJK). We are very grateful to Dr. George F. Koob for helpful comments on the manuscript and Janet Hightower from the Department of Biomedical Graphics at The Scripps Research Institute for assistance with graphics. This is manuscript 19512 from The Scripps Research Institute.
Cited Literature
- Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le Moal M, et al. Nicotine self-administration impairs hippocampal plasticity. J Neurosci. 2002;22:3656–3662. doi: 10.1523/JNEUROSCI.22-09-03656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acri JB, Brown KJ, Saah MI, Grunberg NE. Strain and age differences in acoustic startle responses and effects of nicotine in rats. Pharmacol Biochem Behav. 1995;50:191–198. doi: 10.1016/0091-3057(94)00285-q. [DOI] [PubMed] [Google Scholar]
- Agatsuma S, Lee M, Zhu H, Chen K, Shih JC, Seif I, et al. Monoamine oxidase A knockout mice exhibit impaired nicotine preference but normal responses to novel stimuli. Hum Mol Genet. 2006;15:2721–2731. doi: 10.1093/hmg/ddl206. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Castro NG, Alkondon M, Reinhardt S, Schroder H, et al. Nicotinic receptor function in the mammalian central nervous system. Ann N Y Acad Sci. 1995;757:48–72. doi: 10.1111/j.1749-6632.1995.tb17464.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Book Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Andersson K, Fuxe K, Agnati LF, Eneroth P. Effects of acute central and peripheral administration of nicotine on ascending dopamine pathways in the male rat brain: evidence for nicotine induced increases of dopamine turnover in various telencephalic dopamine nerve terminal systems. Med Biol. 1981;59:170–176. [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Balestra B, Vailati S, Moretti M, Hanke W, Clementi F, Gotti C. Chick optic lobe contains a developmentally regulated α2α5β2 nicotinic receptor subtype. Mol Pharmacol. 2000;58:300–311. doi: 10.1124/mol.58.2.300. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Wright AE, Benwell ME, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- Bao J, Lei D, Du Y, Ohlemiller KK, Beaudet AL, Role LW. Requirement of nicotinic acetylcholine receptor subunit β2 in the maintenance of spiral ganglion neurons during aging. J Neurosci. 2005;25:3041–3045. doi: 10.1523/JNEUROSCI.5277-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2007.09.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejerot S, Humble M. Low prevalence of smoking among patients with obsessive-compulsive disorder. Compr Psychiatry. 1999;40:268–272. doi: 10.1016/s0010-440x(99)90126-8. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, et al. α-5/α-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, et al. Genetic dissociation of two behaviors associated with nicotine addiction: β-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology (Berl) 2006;187:189–199. doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- Biala G, Weglinska B. Blockade of the expression of mecamylamine-precipitated nicotine withdrawal by calcium channel antagonists. Pharmacol Res. 2005;51:483–488. doi: 10.1016/j.phrs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Booker TK, Butt CM, Wehner JM, Heinemann SF, Collins AC. Decreased anxiety-like behavior in beta3 nicotinic receptor subunit knockout mice. Pharmacol Biochem Behav. 2007;87:146–157. doi: 10.1016/j.pbb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM. Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcohol Clin Exp Res. 2005;29:295–302. doi: 10.1097/01.alc.0000156116.40817.a2. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bray C, Son JH, Meizel S. Acetylcholine causes an increase of intracellular calcium in human sperm. Mol Hum Reprod. 2005;11:881–889. doi: 10.1093/molehr/gah245. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, et al. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus. 2000;10:37–46. doi: 10.1002/(SICI)1098-1063(2000)10:1<37::AID-HIPO4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Brioni JD, O'Neill AB, Kim DJ, Decker MW. Nicotinic receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. Eur J Pharmacol. 1993;238:1–8. doi: 10.1016/0014-2999(93)90498-7. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in rats. Neuropharmacology. 2004;47:572–579. doi: 10.1016/j.neuropharm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Duman CH, Picciotto MR. Fear conditioning and latent inhibition in mice lacking the high affinity subclass of nicotinic acetylcholine receptors in the brain. Neuropharmacology. 2000;39:2779–2784. doi: 10.1016/s0028-3908(00)00137-4. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56:657–664. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Cam GR, Bassett JR. The effect of acute nicotine administration on plasma levels of the thyroid hormones and corticosterone in the rat. Pharmacol Biochem Behav. 1983;19:559–561. doi: 10.1016/0091-3057(83)90135-1. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Rolando MT, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci. 2000;20:RC102. doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Annual smoking - attributable mortality, years of potential life lost, and economic costs - United States, 1995-1999. MMWR. 2002;51:300–303. [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, et al. Distribution and pharmacology of α6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpantier E, Barneoud P, Moser P, Besnard F, Sgard F. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport. 1998;9:3097–3101. doi: 10.1097/00001756-199809140-00033. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Irvine EE, Kenny PJ, File SE. The dorsal raphe nucleus is a crucial structure mediating nicotine's anxiolytic effects and the development of tolerance and withdrawal responses. Psychopharmacology (Berl) 2001a;155:78–85. doi: 10.1007/s002130100681. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Tucci S, File SE. Antagonism of the anxiolytic effect of nicotine in the dorsal raphe nucleus by dihydro-β-erythroidine. Pharmacol Biochem Behav. 2001b;70:491–496. doi: 10.1016/s0091-3057(01)00641-4. [DOI] [PubMed] [Google Scholar]
- Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic-pituitary-adrenal responses to mild acute stress. Neuropsychopharmacology. 2008;33:721–730. doi: 10.1038/sj.npp.1301466. [DOI] [PubMed] [Google Scholar]
- Chi H, de Wit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- Chini B, Raimond E, Elgoyhen AB, Moralli D, Balzaretti M, Heinemann S. Molecular cloning and chromosomal localization of the human α7-nicotinic receptor subunit gene (CHRNA7) Genomics. 1994;19:379–381. doi: 10.1006/geno.1994.1075. [DOI] [PubMed] [Google Scholar]
- Cohen C, Bergis OE, Galli F, Lochead AW, Jegham S, Biton B, et al. SSR591813, a novel selective and partial α4β2 nicotinic receptor agonist with potential as an aid to smoking cessation. J Pharmacol Exp Ther. 2003;306:407–420. doi: 10.1124/jpet.103.049262. [DOI] [PubMed] [Google Scholar]
- Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology (Berl) 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Marubio LM, Klink R, Changeux JP. Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol Sci. 2000;21:211–217. doi: 10.1016/s0165-6147(00)01489-9. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 1991;104:171–176. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Costall B, Kelly ME, Naylor RJ, Onaivi ES. The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol Biochem Behav. 1989;33:197–203. doi: 10.1016/0091-3057(89)90450-4. [DOI] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, et al. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. Am J Psychiatry. 1997;154:263–265. doi: 10.1176/ajp.154.2.263. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ridings A. Intravenous self-administration of morphine by naïve mice. Pharmacol Biochem Behav. 1983;18:467–470. doi: 10.1016/0091-3057(83)90471-9. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, et al. The β3 nicotinic receptor subunit: a component of α-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon P, Anderson DJ, Nikkel AL, Fox GB, Gopalakrishnan M, Decker MW, et al. Antisense knockdown of the rat α7 nicotinic acetylcholine receptor produces spatial memory impairment. Neurosci Lett. 2006;410:15–19. doi: 10.1016/j.neulet.2006.09.061. [DOI] [PubMed] [Google Scholar]
- Curzon P, Kim DJ, Decker MW. Effect of nicotine, lobeline, and mecamylamine on sensory gating in the rat. Pharmacol Biochem Behav. 1994;49:877–882. doi: 10.1016/0091-3057(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Cymes GD, Grosman C. Pore-opening mechanism of the nicotinic acetylcholine receptor evinced by proton transfer. Nat Struct Mol Biol. 2008;15:389–396. doi: 10.1038/nsmb.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155:1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Meador-Woodruff JH. Smoking, smoking withdrawal and schizophrenia: case reports and a review of the literature. Schizophr Res. 1996;22:133–141. doi: 10.1016/s0920-9964(96)80441-5. [DOI] [PubMed] [Google Scholar]
- Damaj MI. Nicotinic regulation of calcium/calmodulin-dependent protein kinase II activation in the spinal cord. J Pharmacol Exp Ther. 2007;320:244–249. doi: 10.1124/jpet.106.111336. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. β2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V, Voineskos S, Wong G, Kennedy JL. Genetic interaction between α4 and β2 subunits of high affinity nicotinic receptor: analysis in schizophrenia. Exp Brain Res. 2006;174:292–296. doi: 10.1007/s00221-006-0458-y. [DOI] [PubMed] [Google Scholar]
- Deneris ES, Boulter J, Swanson LW, Patrick J, Heinemann S. β3: a new member of nicotinic acetylcholine receptor gene family is expressed in brain. J Biol Chem. 1989;264:6268–6272. [PubMed] [Google Scholar]
- Deneris ES, Connolly J, Rogers SW, Duvoisin R. Pharmacological and functional diversity of neuronal nicotinic acetylcholine receptors. Trends Pharmacol Sci. 1991;12:34–40. doi: 10.1016/0165-6147(91)90486-c. [DOI] [PubMed] [Google Scholar]
- Depatie L, O'Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, et al. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilsaver SC, Davidson RK. Fluoxetine subsensitizes a nicotinic mechanism involved in the regulation of core temperature. Life Sci. 1987;41:1165–1169. doi: 10.1016/0024-3205(87)90636-9. [DOI] [PubMed] [Google Scholar]
- Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW. Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr Res. 1998;33:113–118. doi: 10.1016/s0920-9964(98)00045-0. [DOI] [PubMed] [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Domino EF. Round table on nicotinic receptors in addiction: summary report. Eur J Pharmacol. 2000;393:315–320. doi: 10.1016/s0014-2999(99)00890-0. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Drago J, McColl CD, Horne MK, Finkelstein DI, Ross SA. Neuronal nicotinic receptors: insights gained from gene knockout and knockin mutant mice. Cell Mol Life Sci. 2003;60:1267–1280. doi: 10.1007/s00018-003-2259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 2001;25:836–844. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron. 1989;3:487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Sumithran SP, Zhu J, Deaciuc AG, Ayers JT, Crooks PA. Subtype-selective nicotinic receptor antagonists: potential as tobacco use cessation agents. Bioorg Med Chem Lett. 2004;14:1863–1867. doi: 10.1016/j.bmcl.2003.10.073. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. α10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Picciotto MR, Changeux JP, Merlo-Pich EM. Assessment of nicotinic acetylcholine receptor subunit contributions to nicotine self-administration in mutant mice. Psychopharmacology (Berl) 1999;147:25–26. doi: 10.1007/s002130051135. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Ericson M, Lof E, Stomberg R, Chau P, Soderpalm B. Nicotinic acetylcholine receptors in the anterior, but not posterior, VTA mediate ethanol induced elevation of accumbal dopamine levels. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.108.137489. advance online publication. [DOI] [PubMed] [Google Scholar]
- Exley R, Moroni M, Sasdelli F, Houlihan LM, Lukas RJ, Sher E, et al. Chaperone protein 14-3-3 and protein kinase A increase the relative abundance of low agonist sensitivity human alpha 4 beta 2 nicotinic acetylcholine receptors in Xenopus oocytes. J Neurochem. 2006;98:876–885. doi: 10.1111/j.1471-4159.2006.03915.x. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Efficacy of nicotine chewing gum: a review. Prog Clin Biol Res. 1988;261:109–128. [PubMed] [Google Scholar]
- Fernandes C, Hoyle E, Dempster E, Schalkwyk LC, Collier DA. Performance deficit of α7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Genes Brain Behav. 2006;5:433–440. doi: 10.1111/j.1601-183X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- File SE, Cheeta S, Kenny PJ. Neurobiological mechanisms by which nicotine mediates different types of anxiety. Eur J Pharmacol. 2000a;393:231–236. doi: 10.1016/s0014-2999(99)00889-4. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE, Andrews N. Endogenous acetylcholine in the dorsal hippocampus reduces anxiety through actions on nicotinic and muscarinic1 receptors. Behav Neurosci. 1998a;112:352–359. doi: 10.1037//0735-7044.112.2.352. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav. 2000b;66:65–72. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Ouagazzal AM. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav Neurosci. 1998b;112:1423–1429. doi: 10.1037//0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- File SE, Vellucci SV, Wendlandt S. Corticosterone: an anxiogenic or an anxiolytic agent? J Pharm Pharmacol. 1979;31:300–305. doi: 10.1111/j.2042-7158.1979.tb13505.x. [DOI] [PubMed] [Google Scholar]
- Forsayeth JR, Kobrin E. Formation of oligomers containing the β3 and β4 subunits of the rat nicotinic receptor. J Neurosci. 1997;17:1531–1538. doi: 10.1523/JNEUROSCI.17-05-01531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Stapleton JA, Bell N, Swettenham J, Jarvis MJ, Russell MA. Mood and physiological effects of subcutaneous nicotine in smokers and never-smokers. Drug Alcohol Depend. 1997;44:105–115. doi: 10.1016/s0376-8716(96)01327-0. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res Rev. 2008;57:342–351. doi: 10.1016/j.brainresrev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Franceschini D, Orr-Urtreger A, Yu W, Mackey LY, Bond RA, Armstrong D, et al. Altered baroreflex responses in α7 deficient mice. Behav Brain Res. 2000;113:3–10. doi: 10.1016/s0166-4328(00)00195-9. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Freedman R, Leonard S, Gault JM, Hopkins J, Cloninger CR, Kaufmann CA, et al. Linkage disequilibrium for schizophrenia at the chromosome 15q13-14 locus of the α7-nicotinic acetylcholine receptor subunit gene (CHRNA7) Am J Med Genet. 2001;105:20–22. [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Antidepressants noncompetitively inhibit nicotinic acetylcholine receptor function. J Neurochem. 1999;72:1117–1124. doi: 10.1046/j.1471-4159.1999.0721117.x. [DOI] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Gao W, Brower VG, Sharp BM. Systemic nicotine stimulates dopamine release in nucleus accumbens: re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. J Pharmacol Exp Ther. 2000;294:458–465. [PubMed] [Google Scholar]
- Fu Y, Matta SG, Kane VB, Sharp BM. Norepinephrine release in amygdala of rats during chronic nicotine self-administration: an in vivo microdialysis study. Neuropharmacology. 2003;45:514–523. doi: 10.1016/s0028-3908(03)00201-6. [DOI] [PubMed] [Google Scholar]
- Fucile S, Matter JM, Erkman L, Ragozzino D, Barabino B, Grassi F, et al. The neuronal α6 subunit forms functional heteromeric acetylcholine receptors in human transfected cells. Eur J Neurosci. 1998;10:172–178. doi: 10.1046/j.1460-9568.1998.00001.x. [DOI] [PubMed] [Google Scholar]
- Garwood CL, Potts LA. Emerging pharmacotherapies for smoking cessation. Am J Health Syst Pharm. 2007;64:1693–1698. doi: 10.2146/ajhp060427. [DOI] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Kuryatov A, Anand R, Lindstrom J. “Orphan” α6 nicotinic AChR subunit can form a functional heteromeric acetylcholine receptor. Mol Pharmacol. 1997;51:320–327. [PubMed] [Google Scholar]
- Girod R, Crabtree G, Ernstrom G, Ramirez-Latorre J, McGehee D, Turner J, et al. Heteromeric complexes of α5 and/or α7 subunits: effects of calcium and potential role in nicotine-induced presynaptic facilitation. Ann N Y Acad Sci. 1999;868:578–590. doi: 10.1111/j.1749-6632.1999.tb11331.x. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, et al. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Glynn SM, Sussman S. Why patients smoke. Hosp Community Psychiatry. 1990;41:1027–1028. doi: 10.1176/ps.41.9.1027. [DOI] [PubMed] [Google Scholar]
- Goff DC, Henderson DC, Amico E. Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry. 1992;149:1189–1194. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Zvolensky MJ, Keyes KM. Nicotine dependence and mental disorders among adults in the USA: evaluating the role of the mode of administration. Psychol Med. 2008 doi: 10.1017/S0033291708003012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, et al. Expression of nigrostriatal α6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by β3 subunit gene deletion. Mol Pharmacol. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Maggi R, Longhi R, Hanke W, Klinke N, et al. α7 and α8 nicotinic receptor subtypes immunopurified from chick retina have different immunological, pharmacological and functional properties. Eur J Neurosci. 1997;9:1201–1211. doi: 10.1111/j.1460-9568.1997.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21(2 suppl):46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Damaj MI. Nicotine physical dependence in the mouse: involvement of the α7 nicotinic receptor subtype. Eur J Pharmacol. 2005;515:90–93. doi: 10.1016/j.ejphar.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Balster RL. Opioids: similarity between evaluations of subjective effects and animal self-administration results. Clin Pharmacol Ther. 1979;25:611–617. doi: 10.1002/cpt1979255part1611. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Bigelow GE. Predicting the dependence liability of stimulant drugs. NIDA Res Monogr. 1981;37:182–196. [PubMed] [Google Scholar]
- Grillner P, Svensson TH. Nicotine-induced excitation of midbrain dopamine neurons in vitro involves ionotropic glutamate receptor activation. Synapse. 2000;38:1–9. doi: 10.1002/1098-2396(200010)38:1<1::AID-SYN1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Luyten WH, Colquhoun D, Sivilotti LG. A reporter mutation approach shows incorporation of the “orphan” subunit beta3 into a functional nicotinic receptor. J Biol Chem. 1998;273:15317–15320. doi: 10.1074/jbc.273.25.15317. [DOI] [PubMed] [Google Scholar]
- Grosman C, Auerbach A. Kinetic, mechanistic, and structural aspects of unliganded gating of acetylcholine receptor channels: a single-channel study of second transmembrane segment 12′ mutants. J Gen Physiol. 2000;115:621–635. doi: 10.1085/jgp.115.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, et al. Evidence that nicotinic α7 receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–1119. [PubMed] [Google Scholar]
- Grubb MS, Thompson ID. Visual response properties in the dorsal lateral geniculate nucleus of mice lacking the β2 subunit of the nicotinic acetylcholine receptor. J Neurosci. 2004;24:8459–8469. doi: 10.1523/JNEUROSCI.1527-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruza RA, Wang JC, Stitzel JA, Hinrichs AL, Saccone SF, Saccone NL, et al. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.04.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, et al. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur J Neurosci. 2006;24:3532–3540. doi: 10.1111/j.1460-9568.2006.05217.x. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically prolongs the duration of nicotine withdrawal-induced place aversion. Biol Psychiatry. 2008;63:158–163. doi: 10.1016/j.biopsych.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Timmermann DB, Peters D, Walters C, Damaj MI, Mikkelsen JD. Alpha-7 nicotinic acetylcholine receptor agonists selectively activate limbic regions of the rat forebrain: an effect similar to antipsychotics. J Neurosci Res. 2007;85:1810–1818. doi: 10.1002/jnr.21293. [DOI] [PubMed] [Google Scholar]
- Hansen ST, Mark GP. The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology (Berl) 2007;194:53–61. doi: 10.1007/s00213-007-0822-z. [DOI] [PubMed] [Google Scholar]
- Hanson HM, Ivester CA, Morton BR. Nicotine self-administration in rats. NIDA Res Monogr. 1979;23:70–90. [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DHβE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Harrist A, Beech RD, King SL, Zanardi A, Cleary MA, Caldarone BJ, et al. Alteration of hippocampal cell proliferation in mice lacking the β2 subunit of the neuronal nicotinic acetylcholine receptor. Synapse. 2004;54:200–206. doi: 10.1002/syn.20081. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Luetje CW. Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor β subunits. J Neurosci. 1996;16:3798–3806. doi: 10.1523/JNEUROSCI.16-12-03798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-β-erythroidine sensitivity on rat neuronal nicotinic receptor α subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004;175:134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Miyasato K, Jasinski DR. Cigarette smokers self-administer intravenous nicotine. Pharmacol Biochem Behav. 1983;19:887–890. doi: 10.1016/0091-3057(83)90099-0. [DOI] [PubMed] [Google Scholar]
- Hennings EC, Kiss JP, De Oliveira K, Toth PT, Vizi ES. Nicotinic acetylcholine receptor antagonistic activity of monoamine uptake blockers in rat hippocampal slices. J Neurochem. 1999;73:1043–1050. doi: 10.1046/j.1471-4159.1999.0731043.x. [DOI] [PubMed] [Google Scholar]
- Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl) 2006;189:211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YN, Amin J, Weiss DS, Wecker L. Sustained nicotine exposure differentially affects alpha 3 beta 2 and alpha 4 beta 2 neuronal nicotinic receptors expressed in Xenopus oocytes. J Neurochem. 1996;66:667–697. doi: 10.1046/j.1471-4159.1996.66020667.x. [DOI] [PubMed] [Google Scholar]
- Hu XT, White FJ. Glutamate receptor regulation of rat nucleus accumbens neurons in vivo. Synapse. 1996;23:208–218. doi: 10.1002/(SICI)1098-2396(199607)23:3<208::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: a replication and extension. Arch Gen Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Bagnalasta M, Marcon C, Motta C, Tessari M, File SE, et al. Nicotine self-administration and withdrawal: modulation of anxiety in the social interaction test in rats. Psychopharmacology (Berl) 2001a;153:315–320. doi: 10.1007/s002130000586. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Tolerance to nicotine's effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol Biochem Behav. 2001b;68:319–325. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Isensee B, Wittchen HU, Stein MB, Hofler M, Lieb R. Smoking increases the risk of panic: findings from a prospective community study. Arch Gen Psychiatry. 2003;60:692–700. doi: 10.1001/archpsyc.60.7.692. [DOI] [PubMed] [Google Scholar]
- Isola R, Vogelsberg V, Wemlinger TA, Neff NH, Hadjiconstantinou M. Nicotine abstinence in the mouse. Brain Res. 1999;850:189–196. doi: 10.1016/s0006-8993(99)02131-9. [DOI] [PubMed] [Google Scholar]
- Ivanova S, Greenshaw AJ. Nicotine-induced decreases in VTA electrical self-stimulation thresholds: blockade by haloperidol and mecamylamine but not scopolamine or ondansetron. Psychopharmacology (Berl) 1997;134:187–192. doi: 10.1007/s002130050441. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik ME. Beneficial effects of nicotine. Br J Addict. 1991;86:571–575. doi: 10.1111/j.1360-0443.1991.tb01810.x. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Hollander JA, Kenny PJ. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: Evidence from intracranial self-stimulation (ICSS) studies. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.03.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IW, Wonnacott S. Precise localization of α7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Henry B, Semenova S, Markou A. Mild anxiogenic effects of nicotine withdrawal in mice. Eur J Pharmacol. 2005;516:40–45. doi: 10.1016/j.ejphar.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Asano T, Ueda H, Morishita R, Takeuchi IK, Inaguma Y, et al. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 2002;16:1328–1330. doi: 10.1096/fj.02-0143fje. [DOI] [PubMed] [Google Scholar]
- Kedmi M, Beaudet AL, Orr-Urtreger A. Mice lacking neuronal nicotinic acetylcholine receptor β4-subunit and mice lacking both α5- and β4-subunits are highly resistant to nicotine-induced seizures. Physiol Genomics. 2004;17:221–229. doi: 10.1152/physiolgenomics.00202.2003. [DOI] [PubMed] [Google Scholar]
- Keller JJ, Keller AB, Bowers BJ, Wehner JM. Performance of α7 nicotinic receptor null mutants is impaired in appetitive learning measured in a signaled nose poke task. Behav Brain Res. 2005;162:143–152. doi: 10.1016/j.bbr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kelso ML, Wehner JM, Collins AC, Scheff SW, Pauly JR. The pathophysiology of traumatic brain injury in α7 nicotinic cholinergic receptor knockout mice. Brain Res. 2006;1083:204–210. doi: 10.1016/j.brainres.2006.01.127. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Brain reward systems and compulsive drug use. Trends Pharmacol Sci. 2006;28:135–141. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff EH, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.58. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Cheeta S, File SE. Anxiogenic effects of nicotine in the dorsal hippocampus are mediated by 5-HT1A and not by muscarinic M1 receptors. Neuropharmacology. 2000a;39:300–307. doi: 10.1016/s0028-3908(99)00114-8. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, File SE, Neal MJ. Evidence for a complex influence of nicotinic acetylcholine receptors on hippocampal serotonin release. J Neurochem. 2000b;75:2409–2414. doi: 10.1046/j.1471-4159.2000.0752409.x. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, File SE, Rattray M. Acute nicotine decreases, and chronic nicotine increases the expression of brain-derived neurotrophic factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 2000c;85:234–238. doi: 10.1016/s0169-328x(00)00246-1. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Polis I, Koob GF, Markou A. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur J Neurosci. 2003;17:191–195. doi: 10.1046/j.1460-9568.2003.02443.x. [DOI] [PubMed] [Google Scholar]
- King SL, Caldarone BJ, Picciotto MR. β2-subunit-containing nicotinic acetylcholine receptors are critical for dopamine-dependent locomotor activation following repeated nicotine administration. Neuropharmacology. 2004;47 1:132–139. doi: 10.1016/j.neuropharm.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, Stroud L, Niaura R, McCaffery J, et al. Posttraumatic stress disorder and late-onset smoking in the Vietnam era twin registry. J Consult Clin Psychol. 2006;74:186–190. doi: 10.1037/0022-006X.74.1.186. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. London: Elsevier; 2006. [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kosowski AR, Cebers G, Cebere A, Swanhagen AC, Liljequist S. Nicotine-induced dopamine release in the nucleus accumbens is inhibited by the novel AMPA antagonist ZK200775 and the NMDA antagonist CGP39551. Psychopharmacology (Berl) 2004;175:114–123. doi: 10.1007/s00213-004-1797-7. [DOI] [PubMed] [Google Scholar]
- Kumari V, Checkley SA, Gray JA. Effect of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacology (Berl) 1996;128:54–60. doi: 10.1007/s002130050109. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA. Smoking withdrawal, nicotine dependence and prepulse inhibition of the acoustic startle reflex. Psychopharmacology (Berl) 1999;141:11–15. doi: 10.1007/s002130050800. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J. Human α6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology. 2000;39:2570–2590. doi: 10.1016/s0028-3908(00)00144-1. [DOI] [PubMed] [Google Scholar]
- Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, et al. Point mutant mice with hypersensitive α4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci U S A. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laje RP, Berman JA, Glassman AH. Depression and nicotine: preclinical and clinical evidence for common mechanisms. Curr Psychiatry Rep. 2001;3:470–474. doi: 10.1007/s11920-001-0040-z. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The motivational valence of nicotine in the rat ventral tegmental area is switched from rewarding to aversive following blockade of the α7-subunit-containing nicotinic acetylcholine receptor. Psychopharmacology (Berl) 2003;166:306–313. doi: 10.1007/s00213-002-1317-6. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS ONE. 2007;2:e230. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Zoli M, Changeux JP. Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci. 1996;8:2428–2439. doi: 10.1111/j.1460-9568.1996.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Zoli M, Lena C, Ferrari R, Picciotto MR, Merlo-Pich E, et al. Involvement of α6 nicotinic receptor subunit in nicotine-elicited locomotion, demonstrated by in vivo antisense oligonucleotide infusion. Neuroreport. 1999;10:2497–2501. doi: 10.1097/00001756-199908200-00012. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Lena C, Changeux JP. Allosteric nicotinic receptors, human pathologies. J Physiol Paris. 1998;92:63–74. doi: 10.1016/S0928-4257(98)80140-X. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, et al. Association of promoter variants in the α7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Lips KS, Pfeil U, Kummer W. Coexpression of α9 and α10 nicotinic acetylcholine receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;115:1–5. doi: 10.1016/s0306-4522(02)00274-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Koren AO, Yee SK, Pechnick RN, Poland RE, London ED. Self-administration of 5-iodo-A-85380, a β2-selective nicotinic receptor ligand, by operantly trained rats. Neuroreport. 2003;14:1503–1505. doi: 10.1097/00001756-200308060-00020. [DOI] [PubMed] [Google Scholar]
- Lopes FL, Nascimento I, Zin WA, Valenca AM, Mezzasalma MA, Figueira I, et al. Smoking and psychiatric disorders: a comorbidity survey. Braz J Med Biol Res. 2002;35:961–967. doi: 10.1590/s0100-879x2002000800013. [DOI] [PubMed] [Google Scholar]
- Lopez-Valdes HE, Garcia-Colunga J. Antagonism of nicotinic acetylcholine receptors by inhibitors of monoamine uptake. Mol Psychiatry. 2001;6:511–519. doi: 10.1038/sj.mp.4000885. [DOI] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Mannucci C, Tedesco M, Bellomo M, Caputi AP, Calapai G. Long-term effects of nicotine on the forced swimming test in mice: an experimental model for the study of depression caused by smoke. Neurochem Int. 2006;49:481–486. doi: 10.1016/j.neuint.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Markou A, Kenny PJ. Neuroadaptations to chronic exposure to drugs of abuse: relevance to depressive symptomatology seen across psychiatric diagnostic categories. Neurotox Res. 2002;4:297–313. doi: 10.1080/10298420290023963. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res. 2001;3:361–373. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d'Exaerde A, et al. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, et al. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Marubio LM, Paylor R. Impaired passive avoidance learning in mice lacking central neuronal nicotinic acetylcholine receptors. Neuroscience. 2004;129:575–582. doi: 10.1016/j.neuroscience.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153(Suppl 1):S438–S445. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Masterson E, O'Shea B. Smoking and malignancy in schizophrenia. Br J Psychiatry. 1984;145:429–432. doi: 10.1192/bjp.145.4.429. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Collins AC, Paylor R, Marks MJ. Deletion of the beta 2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology (Berl) 2006;184:314–327. doi: 10.1007/s00213-005-0076-6. [DOI] [PubMed] [Google Scholar]
- Merlo-Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Miller DK, Wong EH, Chesnut MD, Dwoskin LP. Reboxetine: functional inhibition of monoamine transporters and nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2002;302:687–695. doi: 10.1124/jpet.302.2.687. [DOI] [PubMed] [Google Scholar]
- Millet B, Bayle FJ, Olie JP. Prospects for an anxiolytic therapy: a reflection from different viewpoints. Drug Development Today. 1998;3:471–479. [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: a critical review of interrelationships. Psychol Bull. 2007;133:245–272. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Nadal R, Chappell AM, Samson HH. Effects of nicotine and mecamylamine microinjections into the nucleus accumbens on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 1998;22:1190–1198. [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, et al. Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor C, Quarta D, Fernandes C, Stolerman IP. Tolerance to nicotine in mice lacking α7 nicotinic receptors. Psychopharmacology (Berl) 2005;180:558–563. doi: 10.1007/s00213-005-2187-5. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Lindstrom J. Single channel properties of human α3 AChRs: impact of β2, β4 and α5 subunits. J Physiol. 1999;516:657–678. doi: 10.1111/j.1469-7793.1999.0657u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Effect of a novel nicotinic receptor antagonist, N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology (Berl) 2006;184:426–434. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- Newman MB, Manresa JJ, Sanberg PR, Shytle RD. Anxiolytic effects of mecamylamine in two animal models of anxiety. Exp Clin Psychopharmacol. 2002;10:18–25. doi: 10.1037//1064-1297.10.1.18. [DOI] [PubMed] [Google Scholar]
- Newman MB, Nazian SJ, Sanberg PR, Diamond DM, Shytle RD. Corticosterone-attenuating and anxiolytic properties of mecamylamine in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:609–620. doi: 10.1016/s0278-5846(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Okuda H, Shioda S, Nakai Y, Nakayama H, Okamoto M, Nakashima T. The presence of corticotropin-releasing factor-like immunoreactive synaptic vesicles in axon terminals with nicotinic acetylcholine receptor-like immunoreactivity in the median eminence of the rat. Neurosci Lett. 1993;161:183–186. doi: 10.1016/0304-3940(93)90289-w. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Kedmi M, Rosner S, Karmeli F, Rachmilewitz D. Increased severity of experimental colitis in alpha 5 nicotinic acetylcholine receptor subunit-deficient mice. Neuroreport. 2005;16:1123–1127. doi: 10.1097/00001756-200507130-00018. [DOI] [PubMed] [Google Scholar]
- Owens JC, Balogh SA, McClure-Begley TD, Butt CM, Labarca C, Lester HA, et al. α4β2* nicotinic acetylcholine receptors modulate the effects of ethanol and nicotine on the acoustic startle response. Alcohol Clin Exp Res. 2003;27:1867–1875. doi: 10.1097/01.ALC.0000102700.72447.0F. [DOI] [PubMed] [Google Scholar]
- Palma E, Eusebi F, Miledi R. Co-expression of the neuronal alpha7 and L247T alpha7 mutant subunits yields hybrid nicotinic receptors with properties of both wild-type alpha7 and alpha7 mutant homeric receptors. Proc Natl Acad Sci USA. 1997;94:1539–1543. doi: 10.1073/pnas.94.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagis G, Kastellakis A, Spyraki C, Nomikos G. Effects of methyllycaconitine (MLA), an α7 nicotinic receptor antagonist, on nicotine- and cocaine-induced potentiation of brain stimulation reward. Psychopharmacology (Berl) 2000;149:388–396. doi: 10.1007/s002130000384. [DOI] [PubMed] [Google Scholar]
- Papke RL, Boulter J, Patrick J, Heinemann S. Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron. 1989;3:589–596. doi: 10.1016/0896-6273(89)90269-9. [DOI] [PubMed] [Google Scholar]
- Parish CL, Nunan J, Finkelstein DI, McNamara FN, Wong JY, Waddington JL, et al. Mice lacking the α4 nicotinic receptor subunit fail to modulate dopaminergic neuronal arbors and possess impaired dopamine transporter function. Mol Pharmacol. 2005;68:1376–1386. doi: 10.1124/mol.104.004820. [DOI] [PubMed] [Google Scholar]
- Park HJ, Niedzielski AS, Wenthold RJ. Expression of the nicotinic acetylcholine receptor subunit, α9, in the guinea pig cochlea. Hear Res. 1997;112:95–105. doi: 10.1016/s0378-5955(97)00111-1. [DOI] [PubMed] [Google Scholar]
- Parker SL, Fu Y, McAllen K, Luo J, McIntosh JM, Lindstrom JM, et al. Up-regulation of brain nicotinic acetylcholine receptors in the rat during long-term self-administration of nicotine: disproportionate increase of the α6 subunit. Mol Pharmacol. 2004;65:611–622. doi: 10.1124/mol.65.3.611. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Cigarette smoking: effects upon self-rated stress and arousal over the day. Addict Behav. 1993;18:389–395. doi: 10.1016/0306-4603(93)90055-e. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Individual differences in stress and arousal during cigarette smoking. Psychopharmacology (Berl) 1994;115:389–396. doi: 10.1007/BF02245082. [DOI] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. α7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol. 1998;107:238–251. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003;112:14–27. [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, et al. Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pichat P, Bergis OE, Terranova JP, Urani A, Duarte C, Santucci V, et al. SSR180711, a novel selective α7 nicotinic receptor partial agonist: II. Efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2007;32:17–34. doi: 10.1038/sj.npp.1301188. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Popik P, Kozela E, Krawczyk M. Nicotine and nicotinic receptor antagonists potentiate the antidepressant-like effects of imipramine and citalopram. Br J Pharmacol. 2003;139:1196–1202. doi: 10.1038/sj.bjp.0705359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. β2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Polonskaya Y, Gillespie A, Jakowec M, Lloyd GK, Langston JW. Localization of nicotinic receptor subunit mRNAs in monkey brain by in situ hybridization. J Comp Neurol. 2000;425:58–69. doi: 10.1002/1096-9861(20000911)425:1<58::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not β2- or α7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology (Berl) 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Reid MS, Ho LB, Berger SP. Behavioral and neurochemical components of nicotine sensitization following 15-day pretreatment: studies on contextual conditioning. Behav Pharmacol. 1998;9:137–148. [PubMed] [Google Scholar]
- Ren K, Thinschmidt J, Liu J, Ai L, Papke RL, King MA, et al. α7 Nicotinic receptor gene delivery into mouse hippocampal neurons leads to functional receptor expression, improved spatial memory-related performance, and tau hyperphosphorylation. Neuroscience. 2007;145:314–322. doi: 10.1016/j.neuroscience.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Reuben M, Boye S, Clarke PBS. Nicotinic receptors modulating somatodendritic and terminal dopamine release differ pharmacologically. Eur J Pharmacol. 2000;393:39–49. doi: 10.1016/s0014-2999(00)00004-2. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983;224:319–326. [PubMed] [Google Scholar]
- Risner ME, Jones BE. Self-administration of CNS stimulants by dog. Psychopharmacologia. 1975;43:207–213. doi: 10.1007/BF00429252. [DOI] [PubMed] [Google Scholar]
- Robles N, Sabria J. Effects of moderate chronic ethanol consumption on hippocampal nicotinic receptors and associative learning. Neurobiol Learn Mem. 2008;89:497–503. doi: 10.1016/j.nlm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Sampson A, Levin ED, Henningfield JE. Mecamylamine increases nicotine preference and attenuates nicotine discrimination. Pharmacol Biochem Behav. 1989;32:933–938. doi: 10.1016/0091-3057(89)90061-0. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE. Mecamylamine acutely increases human intravenous nicotine self-administration. Pharmacol Biochem Behav. 2003;76:307–313. doi: 10.1016/j.pbb.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, et al. Phenotypic characterization of an α4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor β2 subunit for the anatomical and functional development of the visual system. Proc Natl Acad Sci U S A. 2001;98:6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp A, Keith SJ. The costs of schizophrenia: assessing the burden. Psychiatr Clin North Am. 1993;16:413–423. [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs DP, Leischow SJ. Pharmacologic approaches to smoking cessation. Clin Chest Med. 1991;12:769–791. [PubMed] [Google Scholar]
- Sack R, Gochberg-Sarver A, Rozovsky U, Kedmi M, Rosner S, Orr-Urtreger A. Lower core body temperature and attenuated nicotine-induced hypothermic response in mice lacking the β4 neuronal nicotinic acetylcholine receptor subunit. Brain Res Bull. 2005;66:30–36. doi: 10.1016/j.brainresbull.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the α7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit α5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003a;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the β4 subunit of the nicotinic receptor. J Neurosci. 2003b;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Ann Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Fagerquist MV, Zhang X, Hertel P, Panagis G, Nomikos GG, et al. Putative role of presynaptic α7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse. 2000;38:375–383. doi: 10.1002/1098-2396(20001215)38:4<375::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Nomikos GG, Nisell M, Hertel P, Svensson TH. N-methyl-d-aspartate receptor antagonism in the ventral tegmental area diminishes the systemic nicotine-induced dopamine release in the nucleus accumbens. Neuroscience. 1998;82:781–789. doi: 10.1016/s0306-4522(97)00243-1. [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, et al. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.10.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield EB, Quick MW, Lester RA. Nicotinic acetylcholine receptor subunit mRNA expression and channel function in medial habenula neurons. Neuropharmacology. 2000;39:2591–2603. doi: 10.1016/s0028-3908(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 1976;50:35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- Skok M, Grailhe R, Agenes F, Changeux JP. The role of nicotinic acetylcholine receptors in lymphocyte development. J Neuroimmunol. 2006;171:86–98. doi: 10.1016/j.jneuroim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Skok M, Grailhe R, Changeux JP. Nicotinic receptors regulate B lymphocyte activation and immune response. Eur J Pharmacol. 2005;517:246–251. doi: 10.1016/j.ejphar.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Yoo S, Hill KP, Mooney M. Self-administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacology. 2008;33:715–720. doi: 10.1038/sj.npp.1301460. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Maintenance of schedule-controlled behavior by intravenous injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1982;223:402–408. [PubMed] [Google Scholar]
- Spring B, Pingitore R, McChargue DE. Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. Am J Psychiatry. 2003;160:316–322. doi: 10.1176/appi.ajp.160.2.316. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Stolerman I, Goldfarb T, Fink R, Jarvik M. Influencing cigarette smoking with nicotine antagonists. Psychopharmacology (Berl) 1979;28:247–59. doi: 10.1007/BF00429305. [DOI] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527:515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Delarue M, Grutter T, Nilges M, Le Novere N, Corringer PJ, et al. Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophys J. 2005;88:3954–3965. doi: 10.1529/biophysj.104.050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Marks MJ, Lester HA. Nicotine responses in hypersensitive and knockout α4 mice account for tolerance to both hypothermia and locomotor suppression in wild-type mice. Physiol Genomics. 2007;31:422–428. doi: 10.1152/physiolgenomics.00063.2007. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, et al. Null mutant analysis of responses to nicotine: deletion of β2 nicotinic acetylcholine receptor subunit but not α7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob Res. 2004;6:145–158. doi: 10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- Tucci SA, Genn RF, File SE. Methyllycaconitine (MLA) blocks the nicotine evoked anxiogenic effect and 5-HT release in the dorsal hippocampus: possible role of α7 receptors. Neuropharmacology. 2003;44:367–373. doi: 10.1016/s0028-3908(02)00391-x. [DOI] [PubMed] [Google Scholar]
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Vailati S, Hanke W, Bejan A, Barabino B, Longhi R, Balestra B, et al. Functional α6-containing nicotinic receptors are present in chick retina. Mol Pharmacol. 1999;56:11–19. doi: 10.1124/mol.56.1.11. [DOI] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, et al. Role of α9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron. 1999;23:93–103. doi: 10.1016/s0896-6273(00)80756-4. [DOI] [PubMed] [Google Scholar]
- Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007;52:1415–1425. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW. The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (α5) in the rat central nervous system. Brain Res. 1990;526:45–53. doi: 10.1016/0006-8993(90)90248-a. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, et al. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The β2 but not α7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Wanamaker CP, Christianson JC, Green WN. Regulation of nicotinic acetylcholine receptor assembly. Ann NY Acad Sci. 2003;998:66–80. doi: 10.1196/annals.1254.009. [DOI] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, et al. Assembly of human neuronal nicotinic receptor alpha5 subunits with α3, β2, and β4 subunits. J Biol Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.42. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Cockcroft VB, Lunt GG, Smillie FS, Wonnacott S. Methyllycaconitine: a selective probe for neuronal α-bungarotoxin binding sites. FEBS Lett. 1990;270:45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl) 2007;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P. What happens to anxiety levels on giving up smoking? Am J Psychiatry. 1997;154:1589–1592. doi: 10.1176/ajp.154.11.1589. [DOI] [PubMed] [Google Scholar]
- West RJ, Russell MA, Jarvis MJ, Feyerabend C. Does switching to an ultra-low nicotine cigarette induce nicotine withdrawal effects? Psychopharmacology (Berl) 1984;84:120–123. doi: 10.1007/BF00432039. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Schuster CR. The effects of chlorpromazine on psychomotor stimulant self-administration in the rhesus monkey. Psychopharmacologica. 1972;26:115–126. doi: 10.1007/BF00422098. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Wu J, Khan GM, Nichols RA. Dopamine release in prefrontal cortex in response to β-amyloid activation of α7 * nicotinic receptors. Brain Res. 2007;1182:82–89. doi: 10.1016/j.brainres.2007.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou CN, et al. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1999a;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, et al. Multiorgan autonomic dysfunction in mice lacking the β2 and the β4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999b;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita T, Ando K, Kato S, Takada K. Psychopharmacological studies on nicotine and tobacco smoking in rhesus monkeys. Psychopharmacology Bull. 1983;19:409–412. [PubMed] [Google Scholar]
- Yoshimura RF, Hogenkamp DJ, Li WY, Tran MB, Belluzzi JD, Whittemore ER, et al. Negative allosteric modulation of nicotinic acetylcholine receptors blocks nicotine self-administration in rats. J Pharmacol Exp Ther. 2007;323:907–915. doi: 10.1124/jpet.107.128751. [DOI] [PubMed] [Google Scholar]
- Young EM, Mahler S, Chi H, de Wit H. Mecamylamine and ethanol preference in healthy volunteers. Alcohol Clin Exp Res. 2005;29:58–65. doi: 10.1097/01.alc.0000150007.34702.16. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, et al. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Yu CR, Role LW. Functional contribution of the α5 subunit to neuronal nicotinic channels expressed by chick sympathetic ganglion neurones. J Physiol. 1998;509:667–681. doi: 10.1111/j.1469-7793.1998.667bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Chen H, Zhao W, Matta SG, Sharp BM. Nicotine self-administration differentially regulates hypothalamic corticotropin-releasing factor and arginine vasopressin mRNAs and facilitates stress-induced neuronal activation. J Neurosci. 2008;28:2773–2782. doi: 10.1523/JNEUROSCI.3837-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WF, Guan ZZ, Nordberg A. Postnatal upregulation of α4 and α3 nicotinic receptor subunits in the brain of α7 nicotinic receptor-deficient mice. Neuroscience. 2007;146:1618–1628. doi: 10.1016/j.neuroscience.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, et al. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Zanetti L, Picciotto MR, Zoli M. Differential effects of nicotinic antagonists perfused into the nucleus accumbens or the ventral tegmental area on cocaine-induced dopamine release in the nucleus accumbens of mice. Psychopharmacology (Berl) 2007;190:189–199. doi: 10.1007/s00213-006-0598-6. [DOI] [PubMed] [Google Scholar]
- Zernig G, O'Laughlin IA, Fibiger HC. Nicotine and heroin augment cocaine-induced dopamine overflow in nucleus accumbens. Eur J Pharmacol. 1997;337:1–10. doi: 10.1016/s0014-2999(97)01184-9. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Picciotto MR, Ferrari R, Cocchi D, Changeux JP. Increased neurodegeneration during ageing in mice lacking high-affinity nicotine receptors. EMBO J. 1999;18:1235–1244. doi: 10.1093/emboj/18.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Aistrup GL, Marszalec W, Gillespie A, Chavez-Noriega LE, Yeh JZ, et al. Dual action of n-alcohols on neuronal nicotinic acetylcholine receptors. Mol Pharmacol. 2001;60:700–711. [PubMed] [Google Scholar]
- Zuo Y, Kuryatov A, Lindstrom JM, Yeh JZ, Narahashi T. Alcohol modulation of neuronal nicotinic acetylcholine receptors is α subunit dependent. Alcohol Clin Exp Res. 2002;26:779–784. [PubMed] [Google Scholar]


