Abstract
Sex differences in the nervous system come in many forms. Although a majority of sexually dimorphic characteristics in brain have been described in older animals, mechanisms that determine sexually differentiated brain characteristics often operate during critical perinatal periods. Both genetic and hormonal factors likely contribute to physiological mechanisms in development to generate the ontogeny of sexual dimorphisms in brain. Relevant mechanisms may include neurogenesis, cell migration, cell differentiation, cell death, axon guidance and synaptogenesis. On a molecular level, there are several ways to categorize factors that drive brain development. These range from the actions of transcription factors in cell nuclei that regulate the expression of genes that control cell development and differentiation, to effector molecules that directly contribute to signaling from one cell to another. In addition, several peptides or proteins in these and other categories might be referred to as “biomarkers” of sexual differentiation with undetermined functions in development or adulthood. While a majority of sex differences are revealed as a direct consequence of hormone actions, some may only be revealed following genetic or environmental disruption. Sex differences in cell positions in the developing hypothalamus, and steroid hormone influences on cell movements in vitro, suggest that cell migration may be one target for early molecular actions that impact brain development and sexual differentiation.
Keywords: sexual differentiation, GABA, brain derived neurotrophic factor, estradiol, nitric oxide, migration
The ontogeny of sex differences in the developing brain occurs through genetic and hormonal influences. Steroid hormone signaling events are involved for many of the sex differences seen in development [1,2], however, it is also evident that genetic contributions provide another important factor in determining brain sexual differentiation [3]. Some processes through which sex differences may emerge include neurogenesis, cell migration, cell differentiation, cell death, axon guidance and synaptogenesis [4]. Molecular mechanisms underlying these cell biological processes are varied, but also sometimes converge to particular signaling pathways. This review presents several molecular players that, from the perspective of cell migration, might provide key signals for hypothalamic development and sexual differentiation and at some points may do so interactively.
Estrogens as direct modulators of cell behaviors in development
The original dogma of steroid hormone dependent brain ‘organization’ or sexual differentiation assumed that the activation of nuclear steroid receptors initiated genomic signaling events. Steroid hormones would presumably bind to nuclear steroid receptor superfamily members (for example, estrogen receptor alpha (ERα) or beta (ERβ) that would act via hormone response elements at the promoters of select genes to activate or inhibit new protein transcription and subsequent translation. While this is likely the predominant mechanism of steroid influences, other molecular mechanisms may also contribute to steroid activated sexual differentiation. For example, there are many recently identified proteins that are not members of the steroid receptor superfamily that are thought to bind steroid hormones [5,6]. Many of these steroid hormone-binding proteins are not located in cell nuclei and have been shown to promote cell signaling through classical second messenger signal transduction cascades involving either protein phosphorylation or calcium entry into the cell [7]. Additionally, some events modulated by steroid hormones take place either too quickly to be regulated by changes in transcription or cannot be blocked by transcriptional inhibitors [6].
There is increasing evidence that sexually dimorphic brain characteristics may derive, in part, from genetic sex and/or hormone influences on neuronal migration. In our studies, we have found several lines of evidence that suggest that gonadal steroid hormones influence the movements and positions of identified cells in the embryonic brain. Early indirect evidence came from the discovery of sex differences and hormone dependence of antigen expression in radial cell fibers within the preoptic area/anterior hypothalamus (POA/AH) of rats [8]. As these cells are likely important for neuronal migration, we hypothesized that sex differences in the molecular characteristics of these cells would translate into differences in migration. The first direct demonstration of sex differences in cell migration within the developing mouse POA/AH came from an examination of migratory characteristics of randomly labeled cells using the carbocyanine dye DiI [9]. At the same time, in an avian model, estradiol was also shown to influence cell movements in brain [10]. More recently, we examined the direct effect of estradiol on neuron migration in vitro by tracking the movement of fluorescent cells expressing yellow fluorescent protein (YFP) under the control of a Thy1 promoter in organotypic brain slices [11]. Estradiol administration caused rapid changes in cell movement characteristics in a region specific manner, whereas exposure of these cells to dihydrotestosterone did not affect cell motions or interfere with the subsequent influence of estradiol.
Finding an influence of a sex steroids on cell movements begs the question of the mechanisms through which they act, as well as which receptor(s) might be activated. There are a number of candidate proteins that bind estradiol [5,6], although it has been argued that many, if not all, known non-genomic actions of estradiol can be attributed to the actions of ERα or ERβ acting at extra-nuclear sites [12]. In early work we localized immunoreactive ERα to cell nuclei in the same region that we have seen sex differences or hormone influence on cell movements [9]. To date, however, we have been unable to co-localize ERα specifically to cells whose movement characteristics are influenced by hormone administration in vitro [11]. The question, therefore, still remains as to whether expression of ERα or ERβ, below the sensitivity of current detection methods, exist on neuronal cell membranes during development, or whether other mediators are involved in transducing estrogen-dependent changes in neuron migration.
For hormones to impact neuronal migration early in development, all necessary signaling components must be present at appropriate times. The primary steroid effector in the development of sexually dimorphic features in the rodent brain is hypothesized to be estradiol [13]. Alpha-fetoprotein (AFP), a steroid binding protein present in both embryonic male and female rodents, prevents direct access of estradiol to cells in the brain. AFP does not bind testosterone that is free to enter the brain, where it can be converted to estradiol by cytochrome p450-aromatase (AROM). For estradiol signaling to impact neuron migration in the developing brain, both aromatase activity and proper expression of the downstream signal transduction components must occur in temporally and spatially specific patterns. For example, sex differences in basal movement of neurons in vitro were evident in slices derived from brains at embryonic day (E) 14 [11] and E15 [9], but not E13 [11]. This suggests that alterations of migratory characteristics may be one of the first influences of gonadal steroids for brain sexual differentiation. Because administration of estradiol was able to influence cell movements in slices created on E13, it further suggests that the hormone-responsive machinery might be in place prior to the initial exposure to steroid hormones that occurs after the formation of the gonads (around E12.5 in mice).
If sex differences and hormone influences alter cell motions (and thereby migration), then there should be an increasing number of sexual dimorphisms identified based on the positions of cells. One example is a sex difference in the location of cells expressing either ERβ or the R1 subunit (GABABR1) of the γ-aminobutyric acid (GABA)B receptor in the POA/AH of mice at E17 [14]. The location of each subset of cells containing immunoreactive ERβ or GABABR1 at E17 was sex dependent, even though the total number of cells did not differ by sex. Immunoreactive ERβ cells were located more ventrally and medially in males than in females and immunoreactive GABABR1 neurons were located more ventrally in males than in females. The area in which these differences were noted corresponds to the location where estradiol was found to decrease the rate and frequency of movement of YFP-positive cells [11]. In adult mice, the location of cells containing immunoreactive neuronal nitric oxide synthase (nNOS) appeared to be spread more laterally in the POA/AH of ERα gene disrupted males compared to wild type [15]. In rats, a sex difference was detected in the location of immunoreactive ERβ neurons in the anteroventral periventricular nucleus [AVPV; 16]. In this case, immunoreactive ERβ neurons were located more laterally in males than in females. Interestingly, this difference was reversed by neonatal orchidectomy of males or estradiol treatment of females, providing further evidence of steroid hormones directly affecting the position of specific neurons.
Steroid signaling for cell movements in non-neural cells
There is a long history of rapid steroid hormone signaling and localization of steroid hormone receptors to compartments other than nuclei [12]. There is also strong evidence of sex steroid influences on cell movements in non-neural circumstances including cancer metastasis, angiogenesis, vascular cell remodeling, wound healing and the control of inflammation [17]. For its role in metastasis, estrogen receptor signaling (via ERα) may utilize the actin binding protein moesin as a key partner for modifying the cytoskeleton to promote migration [18]. Moesin is part of the ezrin-radixin-moesin family of actin binding proteins that has potential functions in brain development [19], but has not been investigated for hormone interactions in brain. Estrogenic signaling may not require either ERα or ERβ if estradiol is metabolized to 2-methoxy-estradiol. This metabolite of estradiol has been implicated in altering cell motility, migration and adhesion by interfering with cytoskeletal function through other mechanisms [20,21]. Another migratory factor that has sexually differentiated expression is macrophage migration inhibitory factor (MIF). MIF is a mediator of delayed healing in several species. Surprisingly, the effectiveness of MIF is sexually dimorphic, and is regulated by steroid hormones in adulthood [22].
Other signaling molecules that might mediate hormonal modulation of cell movements
GABA
While direct effects of steroid hormones such as estradiol on cell motions might be considered ‘atypical’, the effect of steroid hormones may occur through other molecules that are more frequently thought to influence cell migration (Figure 1). Several neurotransmitters (including, GABA, serotonin, dopamine, and endogenous opiates) have been suggested to act as neurotrophic factors or morphogens in various brain regions [23,24]. Of those molecules that might be considered major effectors of hormone action, the neurotransmitter GABA might be particularly important in hypothalamic development [25,26]. The focus of several of our studies has examined the effects of GABA on the development of hypothalamic nuclear groups, specifically the ventromedial (VMN) and paraventricular (PVN) nuclei of the hypothalamus. The distribution of GABAergic elements surrounding these developing nuclei may be essential for the arrangement of the cytoarchitecture in these regions [VMN, 26; and PVN, 27]. Several lines of evidence suggest that GABA plays a role in the embryonic differentiation of the VMN. First, GABA is synthesized in positions that could potentially provide boundary information for the embryonic VMN [28]. Second, disrupting the steroidogenic factor-1 (SF-1) gene disrupts the distribution of GABAergic elements that normally surrounds the VMN prior to its organization [29]. This may partially contribute to the altered distribution of numerous phenotypically identified cells in the VMN of SF-1 knockout mice [29,30]. Third, activation of GABAA receptors affected cell movements and the distribution of identified cells in the region of the VMN [31]. Activation of GABAB receptors also decreased cell movements in the region of the VMN [32]. Finally, antagonism of GABAB action, either pharmacologically in vitro or by genetic disruption of the GABAB R1 subunit of the receptor, also affected cell movement and the distribution of immunoreactive ERα containing cells in the ventrolateral VMN [33]. Although the specifics of GABAA and GABAB receptor actions may differ, likely due to differences in their molecular mechanisms, strong indications of morphogenetic roles for GABA have been characterized in the developing cerebral cortex [34-37].
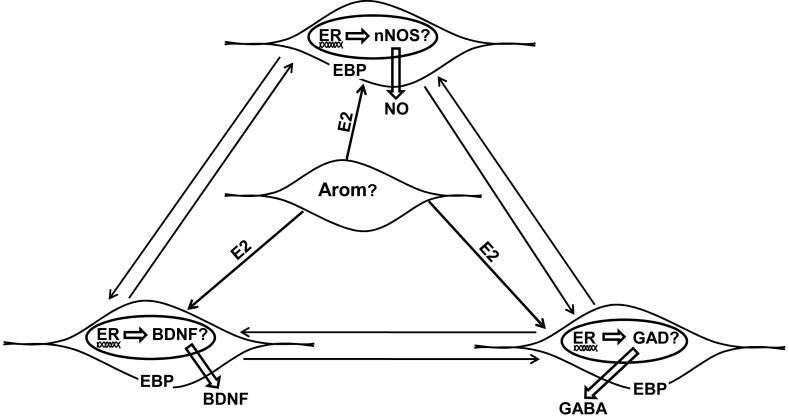
Figure 1.
This schematic diagram depicts several signaling systems that may contribute to sexual differentiation of the vertebrate diencephalon. Estradiol (E2) synthesized via the aromatase (Arom) enzyme may bind estrogen receptors (ER’s) located in cell nuclei or estrogen binding proteins (EBP; that may or may not be identified ER’s) found in cell membranes. E2 signaling through ER’s may influence the transcription of genes involved in nitric oxide (nitric oxide synthase; nNOS) or GABA synthesis (glutamic acid decarboxylase; GAD). In turn, the end products, NO and GABA, may diffuse locally to influence neighboring cells or form molecular gradients to impact cells at greater distances. E2 signaling through ER’s may also influence brain derived neurotrophic factor (BDNF). The arrows between cells indicates the hypothesis that cells which synthesize nNOS, BDNF, and GAD may also interact with each other to influence the migration and cell positions of neurons in the developing preoptic area (except BDNF) and hypothalamus (see text for specific evidence). Aromatase may be localized in a separate cell type as depicted in the diagram, or be co-localized in one of the other cell types.
GABAergic neurons are likely to be direct targets of steroid hormone actions since co-localization has been noted with both steroid binding [38] and immunoreactive receptors [39,40]. Perinatal administration of the GABAA receptor agonists muscimol and diazepam have resulted in sex-related morphological changes in several brain regions [41,42]. In locations where GABAergic inhibitory input to cells of the PVN has been characterized in adults [43], there is a large population of cells containing immunoreactive ERα at early ages in development. These GABAergic cells are likely targets of steroid hormone action, and may have an important role in the development of the PVN [27].
Nitric Oxide
Another potential molecular effector of differentiation is nitric oxide (NO), which is a product of the enzymatic conversion of L-arginine to citrulline. NO plays many roles in development as well as in adulthood. NO has been suggested to help direct cell migration, cell proliferation, and survival [44-46], which are all important factors for sexual differentiation. Since NO is a highly reactive molecule that is difficult to isolate in vivo, many studies evaluate nitric oxide synthase (NOS) expression as an indicator of the location of NO production. NO is produced by three forms of NOS: neuronal (nNOS), inducible (iNOS) and endothelial (eNOS) [47]. Sex differences or hormone influences have been found in adults for nNOS mRNA-positive cells in rats [48,49] and immunoreactive nNOS cells in mice [15,50,51]. We recently examined the distribution of immunoreactive nNOS containing cells in developing mice and found sex differences in locations that may contribute to the development of sex differences in hypothalamic development [52].
Brain Derived Neurotrophic Factor
Another potential mediator of hormone actions on cell movements during development is brain derived neurotrophic factor (BDNF). Data from brain regions other than hypothalamus suggested a role for BDNF in cell migration [53]. It is highly expressed in restricted regions of the developing hypothalamus [54,55]. The BDNF gene promoter contains a consensus regulatory region for estrogen receptors [56] and estradiol has been shown to increase the release of BDNF from specific cell types [57]. BDNF expression specifically in the VMN has been shown to depend upon circulating gonadal steroids [see figure 1 in 58]. However, examination of brains from BDNF knockout mice showed no alteration of positions of identified cells in the region of the VMN [McClellan and Tobet, unpublished observations; 59]. On the other hand, in the same dataset we noted possible changes in immunoreactive ERα cells in the region of the PVN (McClellan and Tobet, unpublished observations).
Cyclic AMP Response Element Binding Protein
It is interesting to note that several signaling systems already highlighted to be important for sexual differentiation may converge on the phosphorylation of the cyclic AMP response element binding protein (CREB). CREB phosphorylation in hypothalamic cells has been noted for sex differences [60,61] and hormone influences [62]. The site of CREB phosphorylation may play an important role in the activity of CREB binding protein and its ability to influence gene transcription. Historically, phospho-CREB (pCREB) represents phosphorylation at serine 133; however, calcium influx can induce phosphorylation of CREB at two additional locations (Ser142 and Ser143), along with serine 133, which then inhibit pCREB interaction with CREB-binding protein [63]. During development, GABA may influence calcium influx and therefore downstream phosphorylation of CREB and pCREB activity. Treatment with the GABAA agonist, muscimol, induced a sex difference in pCREB, which was blocked by an L-type voltage gated calcium channel blocker nimodipine [64]. The identification of this effect highlights calcium influx as an interesting target for sexual differentiation via GABA signaling [65]. GABA signaling also affects expression of BDNF and nNOS via CREB dependent mechanisms [65,66]. In turn, BDNF may drive CREB dependent gene expression in a NO dependent manner [67]. This complex interaction of signal transduction pathways is impacted by gonadal steroid hormones at all levels, supporting the hypothesis that these pathways may play an important role in the modulation of sex specific differences in neuron migration during development.
Sexual Dimorphisms Following Disruption of Normal Development
While a majority of sex differences are a direct consequence of hormone actions, some may only be revealed following either genetic or environmental disruptions. Identification of these sexual dimorphisms allows both insight into the mechanisms behind normal brain development, and potential side effects of disruption on adult phenotypes. Although there are a number of indications of sex differences in PVN regulation and function [68,69], there are relatively few findings of sexual dimorphism (based on cytoarchitecture or anatomy) in the perinatal or adult PVN. One of the few demonstrations of sexual dimorphism in the PVN is a difference in the number of corticotropin releasing-hormone (CRH) containing neurons in the brains of human subjects. Men had more immunoreactive CRH neurons than women in the PVN, and men, but not women, had a significant increase in the number of immunoreactive CRH neurons with age [70]. We recently noted an additional sex difference in the PVN of mice in regards to the positioning of cells containing immunoreactive ERα that was only revealed when GABAB receptor signaling was impaired [27]. Some sex differences in the PVN may only become apparent during specific physiological states or under circumstances of altered development.
CONCLUSION
Sex differences in the positions of cells in the developing hypothalamus and steroid hormone influences on cell movements in vitro suggest that cell migration may be one target for early molecular actions that impact brain development and sexual differentiation. A potential relationship exists between steroid hormone signaling and various other signaling molecules described above (NO, GABA, BDNF, and CREB). Future experiments will be important for directly testing the interactions of these signaling molecules as they relate to hypothalamic development.
Acknowledgments
This works is supported by NIH grants RO1-HD61376 and PO1-MH082679 (SAT).
References
- 1.Simerly RB. Wired on hormones: endocrine regulation of hypothalamic development. Curr Opin Neurobiol. 2005;15:81–85. doi: 10.1016/j.conb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- 4.Tobet SA, Hanna IK. Ontogeny of sex differences in the mammalian hypothalamus and preoptic area. Cell Mol Neurobiol. 1997;17:565–601. doi: 10.1023/A:1022529918810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 6.Kelly MJ, Rønnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasudevan N, Pfaff DW. Membrane-intiated actions of estrogens in neuroendocrinology: emerging principles. Endocrinology Review. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 8.Tobet SA, Fox TO. Sex- and hormone-dependent antigen immunoreactivity in developing rat hypothalamus. Proc Natl Acad Sci USA. 1989;86:382–386. doi: 10.1073/pnas.86.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson RG, Brown AE, Tobet SA. Sex differences in cell migration in the preoptic area/anterior hypothalamus of mice. J Neurobiol. 1999;41:252–266. doi: 10.1002/(sici)1097-4695(19991105)41:2<252::aid-neu8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Williams S, Leventhal C, Lemmon V, Nedergaard M, Goldman SA. Estrogen promotes the initial migration and inception of NgCAM-dependent calcium-signaling by new neurons of the adult songbird brain. Mol Cell Neurosci. 1999;13:41–55. doi: 10.1006/mcne.1998.0729. [DOI] [PubMed] [Google Scholar]
- 11.Knoll JG, Wolfe CA, Tobet SA. Estrogen modulates neuronal movements within the developing preoptic area-anterior hypothalamus. Eur J Neurosci. 2007;26:1091–1099. doi: 10.1111/j.1460-9568.2007.05751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 13.Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Frontiers in Neuroendocrinology. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Dev Brain Res. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol. 2002;453:336–44. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- 16.Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci USA. 2002;99:3306–3311. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giretti MS, Simoncini T. Rapid regulatory actions of sex steroids on cell movement through the actin cytoskeleton. Steroids. 2008;73:895–900. doi: 10.1016/j.steroids.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Giretti MS, Fu XD, De Rosa G, Sarotto I, Baldacci C, Garibaldi S, Mannella P, Biglia N, Sismondi P, Genazzani AR, Simoncini T. Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS ONE. 2008;3:e2238. doi: 10.1371/journal.pone.0002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas MA, Vickers JC, Dickson TC. Rho kinase activates ezrin-radixin-moesin (ERM) proteins and mediates their function in cortical neuron growth, morphology and motility in vitro. J Neurosci Res. 2007;85:34–46. doi: 10.1002/jnr.21102. [DOI] [PubMed] [Google Scholar]
- 20.D’Amato RJ, Lin CM, Flynn E, Folkman J, Hamel E. 2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site. Proc Natl Acad Sci USA. 1994;91:3964–8. doi: 10.1073/pnas.91.9.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattler M, Quinnan LR, Pride YB, Gramlich JL, Chu SC, Even GC, Kraeft SK, Chen LB, Salgia R. 2-methoxyestradiol alters cell motility, migration, and adhesion. Blood. 2003;102:289–96. doi: 10.1182/blood-2002-03-0729. [DOI] [PubMed] [Google Scholar]
- 22.Gilliver SC, Ruckshanthi JP, Hardman MJ, Nakayama T, Ashcroft GS. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology. 2008 Jul 24; doi: 10.1210/en.2008-0355. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tiss Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy MM, Auger AP, Perrot-Sinal TS. Getting excited about GABA and sex differences in the brain. Trends Neurosci. 2002;25:307–12. doi: 10.1016/s0166-2236(02)02182-3. [DOI] [PubMed] [Google Scholar]
- 26.McClellan KM, Parker KL, Tobet S. Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol. 2006;27:193–209. doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.McClellan KM, Tobet SA. A relationship between GABA, nNOS and BDNF in the development of the paraventricular and ventromedial hypothalamic nuclei. Soc Neurosci Abstr. 2008 2008. 278.20. [Google Scholar]
- 28.Tobet SA, Henderson RG, Whiting PJ, Sieghart W. Special relationship of gamma-aminobutyric acid to the ventromedial nucleus of the hypothalamus during embryonic development. J Comp Neurol. 1999;405:88–98. doi: 10.1002/(sici)1096-9861(19990301)405:1<88::aid-cne7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker K, Tobet SA. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Davis AM, Seney ML, Stallings NR, Zhao L, Parker KL, Tobet SA. Loss of steroidogenic factor 1 alters cellular topography in the mouse ventromedial nucleus of the hypothalamus. J Neurobiol. 2004;60:424–436. doi: 10.1002/neu.20030. [DOI] [PubMed] [Google Scholar]
- 31.Dellovade TL, Davis AM, Ferguson C, Sieghart W, Homanics GE, Tobet SA. GABA influences the development of the ventromedial nucleus of hypothalamus. J Neurobiol. 2001;49:264–276. doi: 10.1002/neu.10011. [DOI] [PubMed] [Google Scholar]
- 32.Davis AM, Henion TR, Tobet SA. Gamma-aminobutyric acidB receptors and the development of the ventromedial nucleus of the hypothalamus. J Comp Neurol. 2002;449:270–280. doi: 10.1002/cne.10293. [DOI] [PubMed] [Google Scholar]
- 33.McClellan KM, Calver AR, Tobet SA. GABAB receptors role in cell migration and positioning within the ventromedial nucleus of the hypothalamus. Neuroscience. 2008;151:1119–1131. doi: 10.1016/j.neuroscience.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behar TN, Li YX, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behar TN, Schaffner AE, Scott CA, O’Connell C, Barker JL. Differential response of cortical plate and ventricular zone cells to GABA as a migratory stimulus. J Neurosci. 1998;18:6378–6387. doi: 10.1523/JNEUROSCI.18-16-06378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- 37.Behar TN, Smith SV, Kennedy RT, McKenzie JM, Maric I, Barker JL. GABA(B) receptors mediate motility signals for migrating embryonic cortical cells. Cereb Cortex. 2001;11:744–753. doi: 10.1093/cercor/11.8.744. [DOI] [PubMed] [Google Scholar]
- 38.Flugge G, Oertel WH, Wuttke W. Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology. 1986;43:1–5. doi: 10.1159/000124500. [DOI] [PubMed] [Google Scholar]
- 39.Herbison AE. Immunocytochemical evidence for oestrogen receptors within GABA neurones located in the perinuclear zone of the supraoptic nucleus and GABAA receptor beta 2/beta 3 subunits on supraoptic oxytocin neurones. J Neuroendocrinol. 1994;6:5–11. doi: 10.1111/j.1365-2826.1994.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 40.Herbison AE, Fenelon VS. Estrogen regulation of GABAA receptor subunit mRNA expression in preoptic area and bed nucleus of the stria terminalis of female rat brain. J Neurosci. 1995;15:2328–2337. doi: 10.1523/JNEUROSCI.15-03-02328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bach F, Flügge G, Wuttke W. GABAergic influence on the development of the sexually dimorphic nucleus of male and female rats. Brain Res. 1992;573:341–344. doi: 10.1016/0006-8993(92)90785-8. [DOI] [PubMed] [Google Scholar]
- 42.Segovia S, del Cerro MC, Ortega E, Pérez-Laso C, Rodriguez-Zafra C, Izquierdo MA, Guillamón A. Role of GABAA receptors in the organization of brain and behavioural sex differences. Neuroreport. 1996;7:2553–2557. doi: 10.1097/00001756-199611040-00030. [DOI] [PubMed] [Google Scholar]
- 43.Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213:63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- 44.Bicker G. STOP and GO with NO: nitric oxide as a regulator of cell motility in simple brains. Bioessays. 2005;27:495–505. doi: 10.1002/bies.20221. [DOI] [PubMed] [Google Scholar]
- 45.Bulotta S, Perrotta C, Cerullo A, De Palma C, Clementi E, Borgese N. A cellular system to study the role of nitric oxide in cell death, survival, and migration. Neurotoxicology. 2005;26:841–845. doi: 10.1016/j.neuro.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Contestabile A, Ciani E. Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem Int. 2004;b:903–914. doi: 10.1016/j.neuint.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 47.Mungrue IN, Bredt DS, Stewart DJ, Husain M. From molecules to mammals: what’s NOS got to do with it? Acta Physiol Scand. 2003;179:123–135. doi: 10.1046/j.1365-201X.2003.01182.x. [DOI] [PubMed] [Google Scholar]
- 48.Ishihara T, Orikasa C, Araki T, Sakuma Y. Sex difference in the expression and regulation of nitric oxide synthase gene in the rat preoptic area. Neurosci Res. 2002;43:147–154. doi: 10.1016/s0168-0102(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 49.Carrillo B, Pinos H, Guillamón A, Panzica G, Collado P. Morphometrical and neurochemical changes in the anteroventral subdivision of the rat medial amygdala during estrous cycle. Brain Res. 2007;1150:83–93. doi: 10.1016/j.brainres.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 50.Panzica GC, Viglietti-Panzica C, Sica M, Gotti S, Martini M, Pinos H, Carrillo B, Collado P. Effects of gonadal hormones on central nitric oxide producing systems. Neuroscience. 2006;138:987–995. doi: 10.1016/j.neuroscience.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 51.Büdefeld T, Grgurevic N, Tobet SA, Majdic G. Sex differences in brain developing in the presence or absence of gonads. Dev Neurobiol. 2008;68:981–995. doi: 10.1002/dneu.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev Neurobiol. 2007;67:1371–1381. doi: 10.1002/dneu.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borghesani PR, Peyrin JM, Klein R, Rubin J, Carter AR, Schwartz PM, Luster A, Corfas G, Segal RA. BDNF stimulates migration of cerebellar granule cells. Development. 2002;129:1435–1442. doi: 10.1242/dev.129.6.1435. [DOI] [PubMed] [Google Scholar]
- 54.Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem. 1997;69:34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- 55.Sugiyamaa N, Kanbaa S, Arita J. Temporal changes in the expression of brain-derived neurotrophic factor mRNA in the ventromedial nucleus of the hypothalamus of the developing rat brain. Mol Brain Res. 2003;115:69–77. doi: 10.1016/s0169-328x(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 56.Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. beta-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–120. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 58.Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 59.Holm PC, Duckworth JK, Ringstedt T, Harkany T, Hermanson O. Premature expression of Leptin receptor in prenatal ventromedial hypothalamus of Bdnf-deficient mice. Soc Neurosci Abstr. 2007 671.8. [Google Scholar]
- 60.Auger AP. Sex differences in the developing brain: crossroads in the phosphorylation of cAMP response element binding protein. J Neuroendocrinol. 2003;15:622–7. doi: 10.1046/j.1365-2826.2003.01041.x. [DOI] [PubMed] [Google Scholar]
- 61.Abrahám IM, Herbison AE. Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience. 2005;131:945–51. doi: 10.1016/j.neuroscience.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 62.Abrahám IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 63.Kornhauser JM, Cowan CW, Shaywitz AJ, Dolmetsch RE, Griffith EC, Hu LS, Haddad C, Xia Z, Greenberg ME. CREB transcriptional activity in neurons is regulated by multiple, calcium-specific phosphorylation events. Neuron. 2002;34:221–233. doi: 10.1016/s0896-6273(02)00655-4. [DOI] [PubMed] [Google Scholar]
- 64.Perrot-Sinal TS, Auger AP, McCarthy MM. Excitatory actions of GABA in developing brain are mediated by l-type Ca2+ channels and dependent on age, sex, and brain region. Neuroscience. 2003;116:995–1003. doi: 10.1016/s0306-4522(02)00794-7. [DOI] [PubMed] [Google Scholar]
- 65.Obrietan K, Gao XB, Van Den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism--a positive feedback circuit in developing neurons. J Neurophysiol. 2002;88:1005–1015. doi: 10.1152/jn.2002.88.2.1005. [DOI] [PubMed] [Google Scholar]
- 66.Mantelas A, Stamatakis A, Fameli M, Stylianopoulou F. Sex differences in the control of neuronal nitric oxide synthase by GABA-A receptors in the developing rat diencephalon. Brain Res. 2007;1149:38–49. doi: 10.1016/j.brainres.2007.02.075. [DOI] [PubMed] [Google Scholar]
- 67.Riccio A, Alvania RS, Lonze BE, Ramanan N, Kim T, Huang Y, Dawson TM, Snyder SH, Ginty DD. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol Cell. 2006;21:283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 69.Rhodes ME, Rubin RT. Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Res Rev. 1999;30:135–152. doi: 10.1016/s0165-0173(99)00011-9. [DOI] [PubMed] [Google Scholar]
- 70.Bao AM, Swaab DF. Gender difference in age-related number of corticotropin-releasing hormone-expressing neurons in the human hypothalamic paraventricular nucleus and the role of sex hormones. Neuroendocrinology. 2007;85:27–36. doi: 10.1159/000099832. [DOI] [PubMed] [Google Scholar]