Abstract
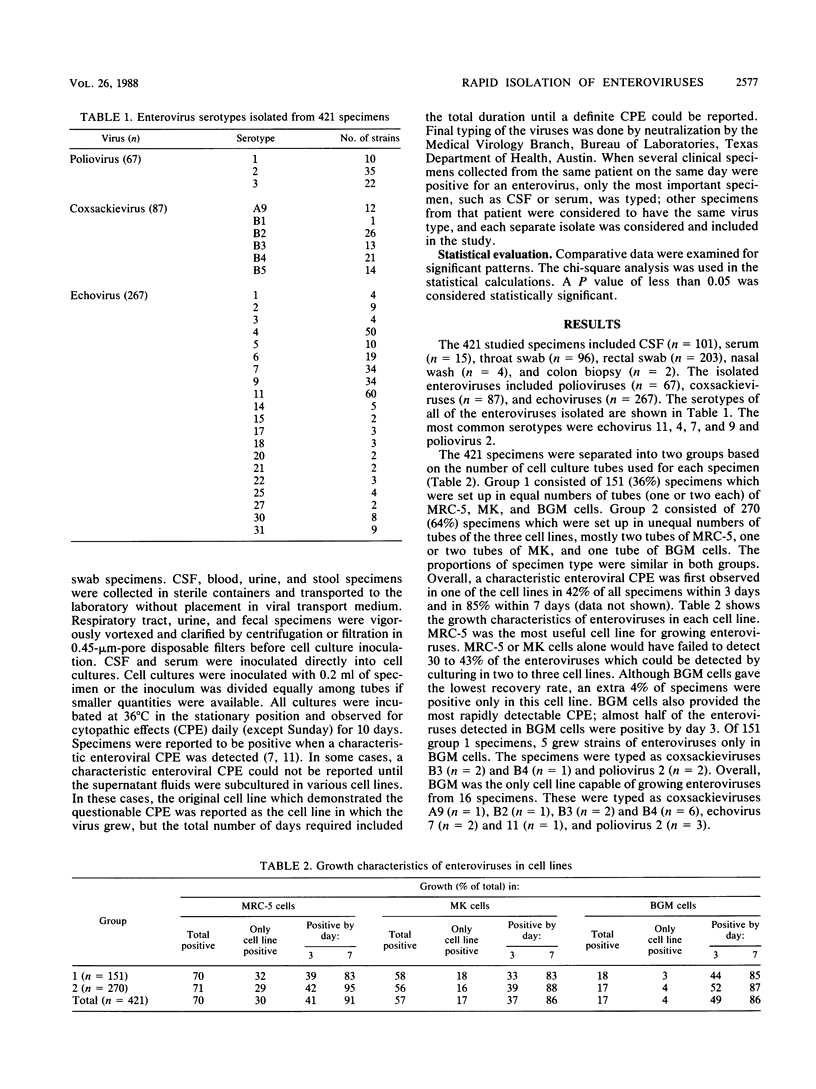
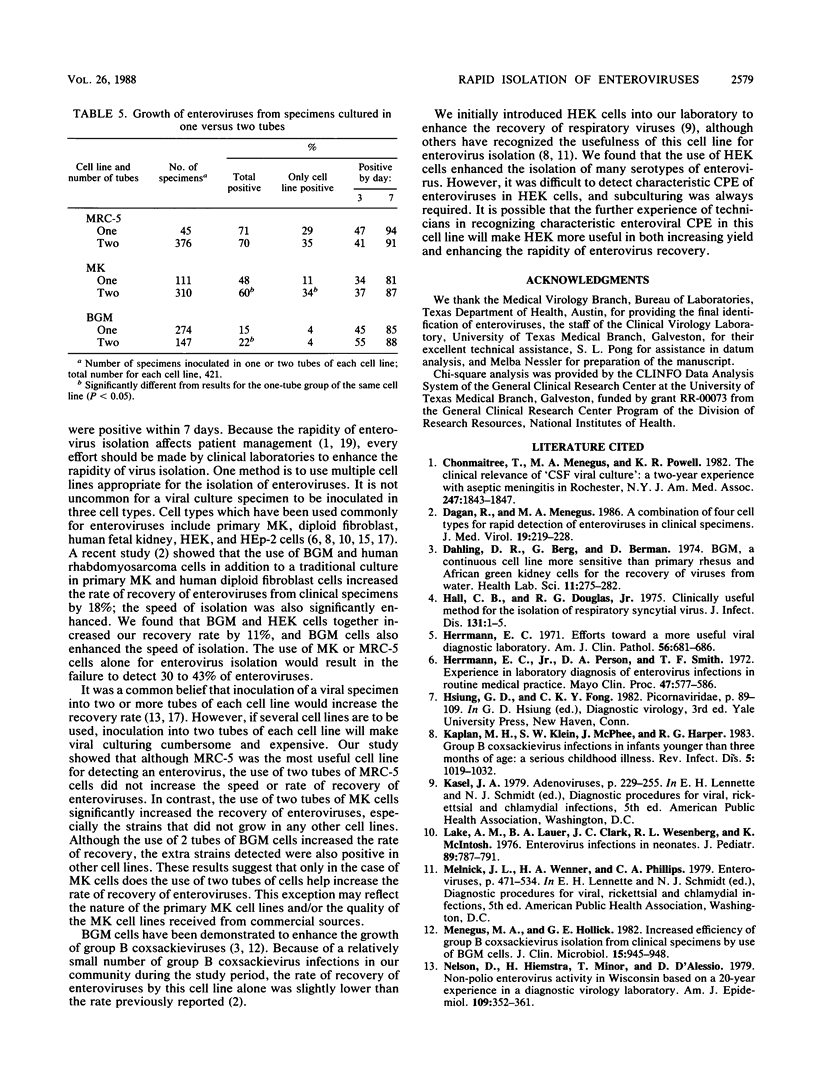
Cell culture isolation is still the most reliable method for the detection of enteroviruses from clinical specimens. Rapid diagnosis of enterovirus infection affects patient management. To increase yield and enhance the rapidity of enterovirus isolation in cell cultures, we used Buffalo green monkey kidney (BGM) cells and subpassages of primary human embryonic kidney (HEK) cells in addition to the human diploid fibroblast (MRC-5) cells and primary cynomolgus or rhesus monkey kidney (MK) cells routinely used for enterovirus culturing. Growth characteristics of enteroviruses from 421 specimens were studied. All specimens were cultured in MRC-5, MK, and BGM cells, and 204 of these specimens were also cultured in HEK cells. Forty-two percent of the enteroviruses became positive within 3 days, and 85% did so within 7 days. MRC-5 cells provided the highest yield of enteroviruses overall and were the best cell type for the recovery of poliovirus and echovirus. MK cells provided the second best yield but were more useful than MRC-5 cells for coxsackievirus. BGM cells supported the growth of additional isolates of coxsackievirus and enhanced the speed of isolation. HEK cells supported the growth of additional isolates of both coxsackievirus and echovirus, but subculturing was always required for definite enterovirus cytopathic effects. The recovery rate increased 11% when two additional cell lines were used. The use of two tubes of MK cells significantly increased the yield of all enterovirus types. We conclude that the use of multiple appropriate cell lines increases yield and enhances the rapidity of enterovirus isolation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chonmaitree T., Menegus M. A., Powell K. R. The clinical relevance of 'CSF viral culture'. A two-year experience with aseptic meningitis in Rochester, NY. JAMA. 1982 Apr 2;247(13):1843–1847. [PubMed] [Google Scholar]
- Dagan R., Menegus M. A. A combination of four cell types for rapid detection of enteroviruses in clinical specimens. J Med Virol. 1986 Jul;19(3):219–228. doi: 10.1002/jmv.1890190304. [DOI] [PubMed] [Google Scholar]
- Dahling D. R., Berg G., Berman D. BGM, a continuous cell line more sensitive than primary rhesus and African green kidney cells for the recovery of viruses from water. Health Lab Sci. 1974 Oct;11(4):275–282. [PubMed] [Google Scholar]
- Hall C. B., Douglas R. G., Jr Clinically useful method for the isolation of respiratory syncytial virus. J Infect Dis. 1975 Jan;131(1):1–5. doi: 10.1093/infdis/131.1.1. [DOI] [PubMed] [Google Scholar]
- Herrmann E. C., Jr Efforts toward a more useful viral diagnostic laboratory. Am J Clin Pathol. 1971 Dec;56(6):681–686. doi: 10.1093/ajcp/56.6.681. [DOI] [PubMed] [Google Scholar]
- Herrmann E. C., Jr, Person D. A., Smith T. F. Experience in laboratory diagnosis of enterovirus infections in routine medical practice. Mayo Clin Proc. 1972 Aug;47(8):577–586. [PubMed] [Google Scholar]
- Kaplan M. H., Klein S. W., McPhee J., Harper R. G. Group B coxsackievirus infections in infants younger than three months of age: a serious childhood illness. Rev Infect Dis. 1983 Nov-Dec;5(6):1019–1032. doi: 10.1093/clinids/5.6.1019. [DOI] [PubMed] [Google Scholar]
- Lake A. M., Lauer B. A., Clark J. C., Wesenberg R. L., McIntosh K. Enterovirus infections in neonates. J Pediatr. 1976 Nov;89(5):787–791. doi: 10.1016/s0022-3476(76)80808-6. [DOI] [PubMed] [Google Scholar]
- Menegus M. A., Hollick G. E. Increased Efficiency of Group B Coxsackievirus Isolation from Clinical Specimens by Use of BGM Cells. J Clin Microbiol. 1982 May;15(5):945–948. doi: 10.1128/jcm.15.5.945-948.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D., Hiemstra H., Minor T., D'Alessio D. Non-polio enterovirus activity in Wisconsin based on a 20-year experience in a diagnostic virology laboratory. Am J Epidemiol. 1979 Mar;109(3):352–361. doi: 10.1093/oxfordjournals.aje.a112688. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Ho H. H., Riggs J. L., Lennette E. H. Comparative sensitivity of various cell culture systems for isolation of viruses from wastewater and fecal samples. Appl Environ Microbiol. 1978 Sep;36(3):480–486. doi: 10.1128/aem.36.3.480-486.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Ho H. H. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis. 1974 Mar;129(3):304–309. doi: 10.1093/infdis/129.3.304. [DOI] [PubMed] [Google Scholar]
- Singer J. I., Maur P. R., Riley J. P., Smith P. B. Management of central nervous system infections during an epidemic of enteroviral aseptic meningitis. J Pediatr. 1980 Mar;96(3 Pt 2):559–563. doi: 10.1016/s0022-3476(80)80866-3. [DOI] [PubMed] [Google Scholar]
- Wildin S., Chonmaitree T. The importance of the virology laboratory in the diagnosis and management of viral meningitis. Am J Dis Child. 1987 Apr;141(4):454–457. doi: 10.1001/archpedi.1987.04460040112030. [DOI] [PubMed] [Google Scholar]


