Abstract
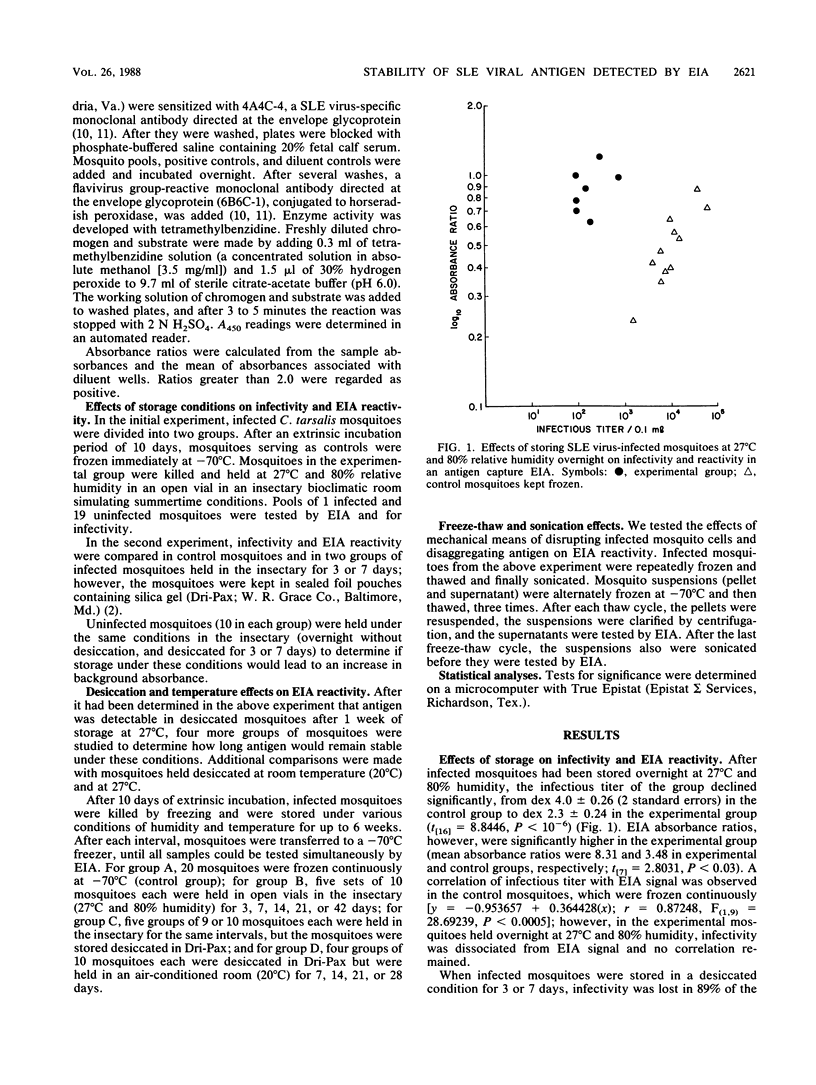
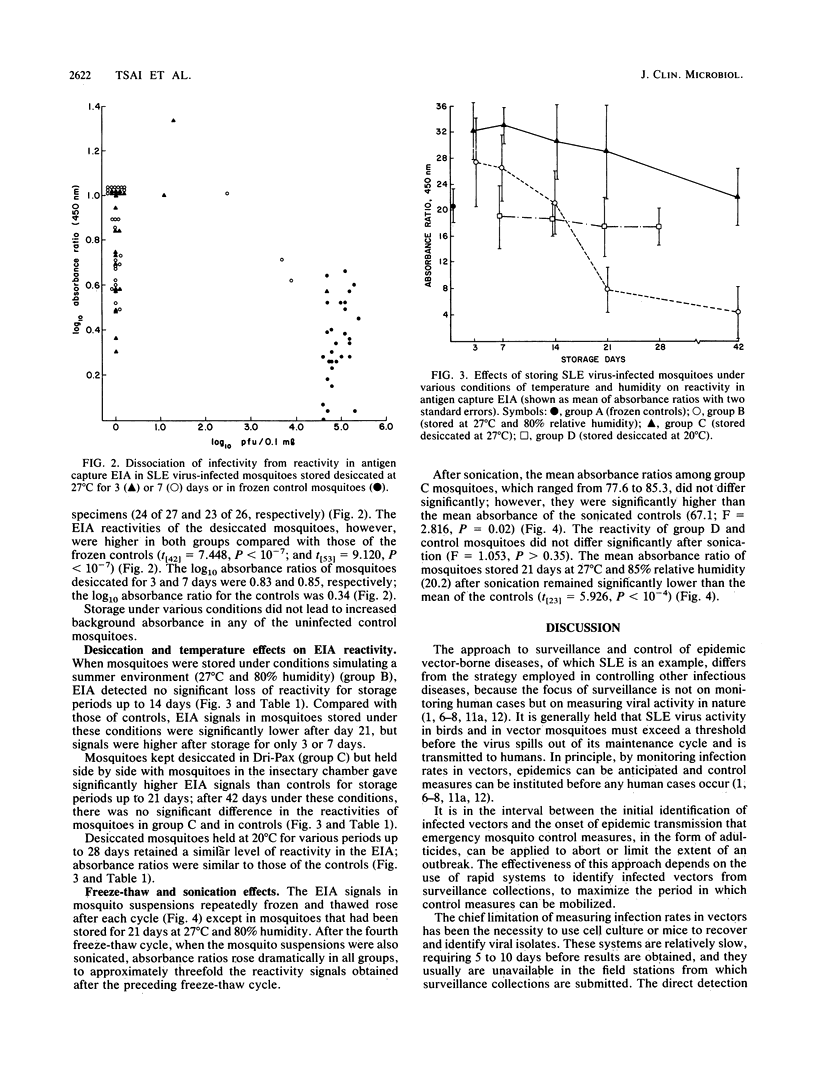
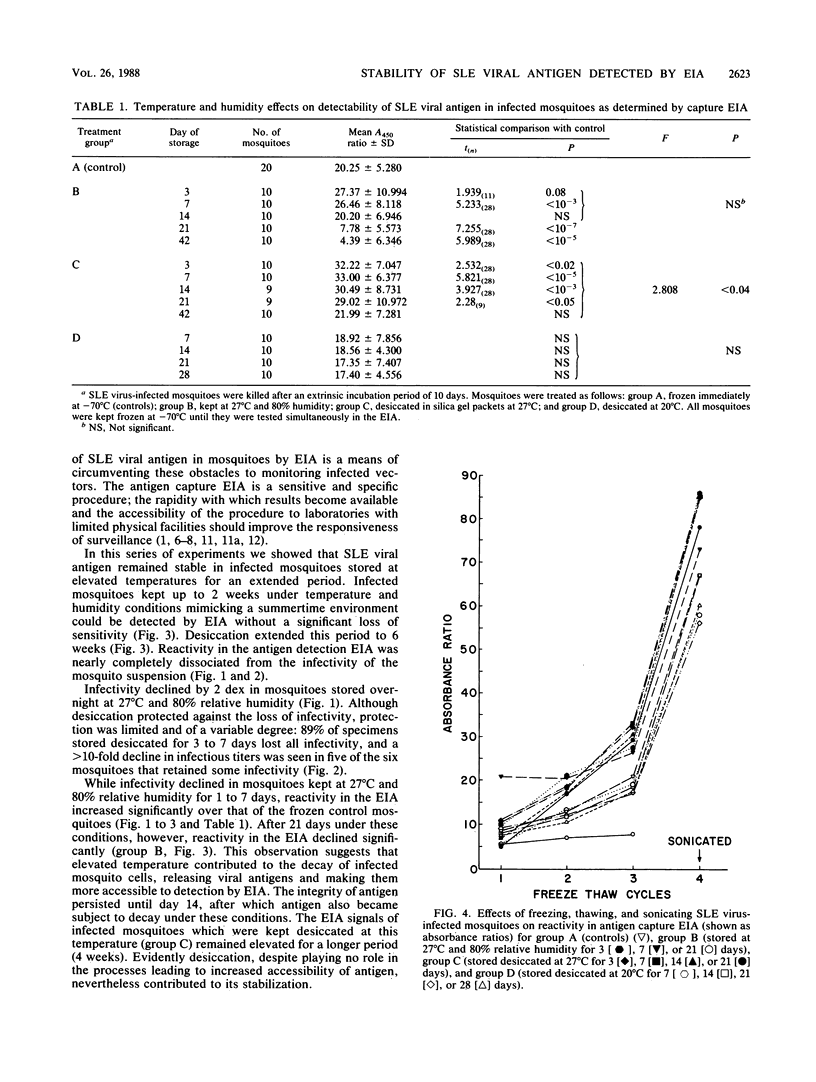
The use of enzyme immunoassay to detect St. Louis encephalitis (SLE) viral antigen in vector mosquitoes enhances the effectiveness of surveillance because infected mosquitoes can be identified more rapidly than with conventional virus isolation systems and because it is a simple and accessible procedure. Infectivity among mosquitoes experimentally infected with SLE virus was lost within 24 h after the mosquitoes were stored at 27 degrees C and 80% relative humidity; however, viral antigen remained stable under these conditions and could be detected by enzyme immunoassay 2 weeks later. Desiccation further extended the period during which antigen could be detected to 6 weeks. Absorbances were higher in infected mosquitoes stored at 27 degrees C than in mosquitoes frozen continuously. Absorbances in infected mosquitoes also increased after repeated freezing and thawing and sonication. Both phenomena may be related to the release of antigen from decaying or disrupted cells. The relative stability of SLE viral antigen at ambient temperatures lends flexibility to schemes which use direct antigen detection to identify vectors. Surveillance systems can be designed without regard to collecting living mosquitoes, and a cold chain in unnecessary to preserve specimens, thus reducing the cost of surveillance and expanding the geographic areas to which it is accessible.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hildreth S. W., Beaty B. J. Economic comparison of enzyme immunoassay and virus isolation procedures for surveillance of arboviruses in mosquito populations. J Clin Microbiol. 1987 Jun;25(6):976–981. doi: 10.1128/jcm.25.6.976-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth S. W., Beaty B. J., Maxfield H. K., Gilfillan R. F., Rosenau B. J. Detection of eastern equine encephalomyelitis virus and Highlands J virus antigens within mosquito pools by enzyme immunoassay (EIA). II. Retrospective field test of the EIA. Am J Trop Med Hyg. 1984 Sep;33(5):973–980. doi: 10.4269/ajtmh.1984.33.973. [DOI] [PubMed] [Google Scholar]
- Hildreth S. W., Beaty B. J., Meegan J. M., Frazier C. L., Shope R. E. Detection of La Crosse arbovirus antigen in mosquito pools: application of chromogenic and fluorogenic enzyme immunoassay systems. J Clin Microbiol. 1982 May;15(5):879–884. doi: 10.1128/jcm.15.5.879-884.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklasson B. S., Gargan T. P., 2nd Enzyme-linked immunosorbent assay for detection of Rift Valley fever virus antigen in mosquitoes. Am J Trop Med Hyg. 1985 Mar;34(2):400–405. doi: 10.4269/ajtmh.1985.34.400. [DOI] [PubMed] [Google Scholar]
- Roehrig J. T., Mathews J. H., Trent D. W. Identification of epitopes on the E glycoprotein of Saint Louis encephalitis virus using monoclonal antibodies. Virology. 1983 Jul 15;128(1):118–126. doi: 10.1016/0042-6822(83)90323-9. [DOI] [PubMed] [Google Scholar]
- Tasi T. F., Smith G. C., Ndukwu M., Jakob W. L., Happ C. M., Kirk L. J., Francy D. B., Lampert K. J. Entomologic studies after a St. Louis encephalitis epidemic in Grand Junction, Colorado. Am J Epidemiol. 1988 Aug;128(2):285–297. doi: 10.1093/oxfordjournals.aje.a114969. [DOI] [PubMed] [Google Scholar]
- Tsai T. F., Bolin R. A., Montoya M., Bailey R. E., Francy D. B., Jozan M., Roehrig J. T. Detection of St. Louis encephalitis virus antigen in mosquitoes by capture enzyme immunoassay. J Clin Microbiol. 1987 Feb;25(2):370–376. doi: 10.1128/jcm.25.2.370-376.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


