Abstract
The costimulatory receptors CD28 and cytotoxic T-lymphocyte antigen (CTLA)-4 and their ligands, CD80 and CD86, are expressed on T lymphocytes; however, their functional roles during T cell–T cell interactions are not well known. The consequences of blocking CTLA-4–CD80/CD86 interactions on purified mouse CD4+ T cells were studied in the context of the strength of signal (SOS). CD4+ T cells were activated with phorbol 12-myristate 13-acetate (PMA) and different concentrations of a Ca2+ ionophore, Ionomycin (I), or a sarcoplasmic Ca2+ ATPase inhibitor, Thapsigargin (TG). Increasing concentrations of I or TG increased the amount of interleukin (IL)-2, reflecting the conversion of a low to a high SOS. During activation with PMA and low amounts of I, intracellular concentrations of calcium ([Ca2+]i) were greatly reduced upon CTLA-4–CD80/CD86 blockade. Further experiments demonstrated that CTLA-4–CD80/CD86 interactions reduced cell cycling upon activation with PMA and high amounts of I or TG (high SOS) but the opposite occurred with PMA and low amounts of I or TG (low SOS). These results were confirmed by surface T-cell receptor (TCR)–CD3 signalling using a low SOS, for example soluble anti-CD3, or a high SOS, for example plate-bound anti-CD3. Also, CTLA-4–CD80/CD86 interactions enhanced the generation of reactive oxygen species (ROS). Studies with catalase revealed that H2O2 was required for IL-2 production and cell cycle progression during activation with a low SOS. However, the high amounts of ROS produced during activation with a high SOS reduced cell cycle progression. Taken together, these results indicate that [Ca2+]i and ROS play important roles in the modulation of T-cell responses by CTLA-4–CD80/CD86 interactions.
Keywords: costimulation, intracellular Ca2+, reactive oxygen species, strength of signal, T-cell activation and cycling
Introduction
Optimal CD4+ T-cell activation, in addition to the first signal mediated by T-cell receptor (TCR) major histocompatibility compex (MHC) class II/peptide binding, requires another costimulatory signal which is delivered by the binding of CD28 on T cells to CD80/CD86 on antigen-presenting cells (APCs). This is known as the ‘two signal hypothesis’.1 The two important costimulatory receptors are CD28 and cytotoxic T-lymphocyte antigen (CTLA)-4 (CD152), which bind to the identical ligands CD80 and CD86. CD28–CD80/CD86 interactions, together with TCR signalling, enhances the production of interleukin (IL)-2 and survival factors leading to increased cell cycle progression.2,3 In fact, Cd28−/− T cells can initiate but cannot sustain T-cell proliferation.4 CTLA-4, unlike CD28, is translocated from intracellular stores and is expressed on the immunological synapse only after activation. It also binds to CD80 and CD86, but with a greater affinity than CD28, thereby sequestering these ligands from binding to CD28. In addition, crosslinking of CTLA-4 greatly reduces activation of mitogen-activated protein kinases and the transcription factor nuclear factor of activated T cells, leading to low amounts of IL-2 and reduced cell cycle progression.5–7 The mechanisms by which CTLA-4 inhibits T-cell activation and proliferation are complex and are not completely understood. Notably, there is marked expansion of CD4+ T cells in Ctla-4−/− mice, which die at or before 4–6 weeks of age.8,9 Interestingly, reducing Ctla-4 expression using small interfering RNA causes rapid onset of diabetes in mice.10 This finding is important as mutations in CTLA-4 are associated with several autoimmune disorders in humans.11
The functional consequences of CTLA-4–CD80/CD86 interactions are not autonomous but are dependent on several factors. The extent of CTLA-4 inhibition is greater during T-cell responses to tolerogenic proteins12 or antigens presented as tissue antigens compared with antigens injected along with adjuvants.13 Also, CTLA-4 on differentiated T cells plays a major role in inhibiting secondary as opposed to primary responses.14 Several studies have also implicated the strength of signal (SOS) in modulating CTLA-4 function. First, CTLA-4 accumulation (but not accumulation of CD28 or protein kinase C θ) at the immunological synapse was enhanced with increased SOS, resulting in inhibition of T-cell activation.15 Secondly, activation of T cells at high, but not low, antigen concentrations, together with CTLA-4 blockade, favoured a T helper type 2 (Th2) response.16 Thirdly, in an experimental autoimmune encephalitis model, immunization with a disease antagonistic peptide, but not with a disease agonist peptide, together with CTLA-4 blockade inhibited the generation of cross-reactive T-cell clones.17 It is possible that antagonistic peptides generate low primary signals and CTLA-4 interactions enhance some T-cell responses under these conditions. In fact, CTLA-4 blockade inhibited or enhanced the generation of T cells expressing distinct TCRs of identical specificities obtained from a patient with multiple sclerosis, on activation with a myelin basic protein (MBP) peptide.16 CTLA-4 ligation may enhance activation in some cell types or conditions. CTLA-4 signalling enhanced activation and reduced survival of double-positive thymocytes, whereas the opposite effect was observed with single-positive thymocytes.18 Surprisingly, a bispecific tandem single-chain Fv reagent to CTLA-4 alone enhanced the association between CTLA-4 and protein phosphatase 2A, leading to increased IL-2 production and T-cell proliferation.19 Also, the transfer of specific residues from the cytoplasmic tail of CD28 to CTLA-4 resulted in positive signalling via CTLA-4.20 Finally, CTLA-4 lacking the cytoplasmic domain costimulated the production of IL-2 in a T-cell hybridoma.21 These studies clearly demonstrate that the extent of CTLA-4 inhibition or activation of T-cell responses, in some cases, depends on multiple factors.
Our laboratory has been investigating the functional roles of CD28 and CTLA-4 on purified primary CD4+ T cells. This work has revealed that the SOS together with CTLA-4–CD80/CD86 interactions modulates T-cell proliferation.22–24 Importantly, differences in the outcome of CTLA-4–CD80/CD86 interactions were observed using signalling with anti-CD3, which is more physiologically relevant compared to pharmacological compounds. CTLA-4–CD80/CD86 interactions inhibited T-cell activation in cells treated with plate-bound anti-CD3 (pb anti-CD3), whereas enhancement was found in cells treated with soluble anti-CD3 (sol anti-CD3) and suboptimal amounts of anti-CD28.24 However, the intracellular mediators involved in the differential roles of CTLA-4–CD80/CD86 interactions and SOS were not identified. In this study, we show that the intracellular calcium concentration ([Ca2+]i) plays a key role in the conversion of a low-intensity signal into a high-intensity signal. The roles of CTLA-4–CD80/CD86 interactions were studied in the context of these varying intensities of signal strength to demonstrate roles of [Ca2+]i and reactive oxygen species (ROS) during this process.
Materials and methods
Mice
C57BL/6 mice of either sex, aged 6–8 weeks, were used for the isolation of CD4+ T cells. Mice were obtained from the Central Animal Facility of the Indian Institute of Science, and housed in our departmental facility, according to institutional guidelines.
Media, antibodies and cell lines
CD4+ T cells were cultured in RPMI-1640 supplemented with 25 mm HEPES (Sigma Chemical Co., St Louis, MO), 2 mm l-glutamine (HiMedia Labs, Mumbai, India), 5 μmβ-mercaptoethanol (Merck, Darmstadt, Germany), 100 μg/ml penicillin, 250 μg/ml streptomycin, 50 μg/ml gentamycin (HiMedia Labs) and 5% heat-inactivated fetal bovine serum (FBS; Sigma). Anti-CD3 (145-2C11), anti-CD28 (37·51), and hamster control antibodies were obtained from eBioScience (San Diego, CA). Ascites containing anti-CTLA-4 and mouse CTLA-4 human immunoglobulin G (mCTLA-4hIgG1) were used for blocking studies, as previously described.22–24 For flow cytometry, culture supernatants containing the appropriate antibody were used and the respective fluorescein isothiocyanate (FITC)-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PE). Anti-CD8 (3·155) and heat-stable antigen (J11D) culture supernatants were used to purify lymph node CD4+ T cells.22–24
Isolation of CD4+ T cells and activation
CD4+ T cells were purified by complement-mediated depletion of J11D+ and CD8+ cells. Live cells were collected by Histopaque 1083 (Sigma) gradient centrifugation, and subjected to panning over a T25 flask coated with 100 μg/ml goat anti-mouse antibody. CD4+ T-cell preparations were typically ∼95% pure as measured by flow cytometry. Purified T cells were plated at ∼6–7 × 104 cells/well in 96-well U-bottom plates (Becton Dickenson Labware, Franklin Lakes, NJ) in a final volume of 100 μl/well. All wells were precoated with RPMI-1640 containing 5% FBS to minimize non-specific adhesion of monoclonal antibodies (mAbs) to the plate. T cells were activated with 10 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma) and 0·1 or 0·5 μm Ionomycin (I; Sigma), unless otherwise stated. Thapsigargin (TG; Sigma) was also used along with PMA for activation. The concentrations used in most experiments were 0·001 and 0·01 μm. Cells were also activated with anti-CD3 (0·1 μg/ml), either plate-immobilized or added in soluble form. To cells activated with sol aCD3, suboptimal amounts of anti-CD28 (0·1 μg/ml) for triggering were added. In all other experiments, anti-CD28 was used at a concentration of 0·5 μg/ml and anti-CTLA-4 and mCTLA-4hIgG ascites were used at a final concentration of 1 : 100. For proliferation assays, cultures were pulsed 36 hr after activation with ∼0·8 μCi/well of [3H]thymidine (BRIT, Mumbai, India) and harvested 12 hr later. Incorporated radioactivity was measured using a liquid scintillation counter (Beckman LS6500; Beckman Coulter, Fullerton, CA) to assess levels of proliferation. Details of the data collection and presentation are given in the figure legends.
Cytokine assays
Supernatants from CD4+ T-cell assays were collected 24 or 36 hr after activation and IL-2 was quantified using an enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions (eBioscience).
Flow cytometric analysis
For surface staining, ∼2 × 105 cells were washed in cold Hanks’ balanced salt solution (HBSS; Sigma) containing 0·5% FBS, stained with pretitred amounts of culture supernatants, washed and incubated with the appropriate FITC-conjugated pre-adsorbed secondary antibodies. Flow cytometry was performed on FACScan (Becton Dickinson, San Jose, CA), using CellQuest (Becton Dickinson) software for acquisition and WinList (Verity, Topsham, ME) software for analysis. Debris and cellular fragments were excluded from the analysis by electronic logical gates based on forward- and side-scatter profiles. Cell cycle analysis was performed using propidium iodide (Sigma) as previously reported.22–24 Production of ROS was assessed using the oxidation sensitive fluorescent probe 2′, 7′-dichlorofluorescein diacetate (DCFDA). Cells treated under the different conditions were incubated with 5 μm DCFDA for 20 min and acquired on a FACScan. Cytoplasmic free Ca2+ levels were measured by loading the cells with 1 μm fluro-3 acetoxymethyl ester (Fluo-3AM; Calbiochem, San Diego, CA). The acetoxymethyl ester component of the dye renders it cell-permeable, and after hydrolytic cleavage within the cell, the fluorophore is caged within cells. The increase in fluorescence intensity on binding Ca2+ was measured on a FACScan. Pluronic F127 (Calbiochem) was used as a dispersant, and ethyleneglycoltetraacetic acid (EGTA; Sigma) was included in the wash buffer to greatly reduce any fluorescence caused by dye leakage from cells.
Statistical analysis
One-tailed paired Student’s t-tests were performed to determine P-values. Unless otherwise stated, the significance of the difference between control cells and cells subjected to CTLA-4 blockade was calculated. P-values are given in the respective figure legends.
Results
Reduction in CD4+ T-cell proliferation and survival observed on blockade of CTLA-4–CD80/CD86 interactions is dependent on [Ca2+]i
For studying the roles of costimulatory receptors and SOS in modulating T-cell activation, our cell culture system consisted of highly purified CD4+ T cells. This system has also been used by other groups3,25 and is advantageous as all effects observed are attributable to blockade of cell surface receptor–ligand interactions and interpretations are not complicated by signalling events, i.e. binding of antibodies to Fc receptors on APCs and crosslinking cell surface molecules. Also, all effects are attributable to T cells and not to effects of pharmacological agents on APCs, which could affect T-cell responses. It is important to point out that CD80 and CD86, apart from being present on APCs, are also expressed on T cells upon activation. In fact, biochemical and functional differences have been reported between CD80/CD86 molecules on APCs and T cells: CD80 and CD86 on T cells are hypoglycosylated and can bind to CTLA-4 but not to CD28.26 Therefore, CTLA-4–CD80/CD86, not CD28–CD80/CD86, interactions play the dominant role here. This property may be advantageous as it enables the study of CTLA-4 function on T cells without any interference from CD28. CD80 and CD86 molecules on T cells are functional as mouse T cells expressing high levels of these costimulatory ligands proliferate, in the absence of costimulation, in response to low concentrations of anti-CD3.27 IL-4 production is enhanced by CD80 on APCs but inhibited by CD80 on T cells.28 The expression of CD80/CD86 on T cells is immunologically relevant as CTLA-4 binding to CD80 and CD86 on T cells lowers alloresponses in a mouse model for graft versus host disease.29
Previously, we had shown that blockade of CTLA-4–CD80/CD86 interactions in primary CD4+ T cells activated with 10 ng/ml PMA and 0·1 μm I led to a substantial decrease in proliferation and survival.22 The roles of Fas and caspases in mediating increases in cell death were investigated in this system. Blockade of Fas–FasL interactions using a blocking mAb did not show any effect in terms of proliferation (Supplementary Fig. S1a). Also, use of the caspase inhibitor Boc-d-fluoromethyl ketone (BDfmk) had no effect [N-benzyloxycarbonyl phe-ala-fluoromethane (ZFAfmk) was used as a control]. We confirmed that Fas–FasL interactions were not involved in our system by the use of lpr mice (CD95-deficient), which contain a point mutation in Fas, rendering it non-functional. No difference in terms of [3H]thymidine incorporation was observed in CD4+ T cells from lpr and wild-type mice (Supplementary Fig. S1b). These data suggested that the reduction in proliferation with a concomitant increase in hypodiploidy observed on blockade of CTLA-4–CD80/CD86 interactions in this system was Fas- and caspase-independent.
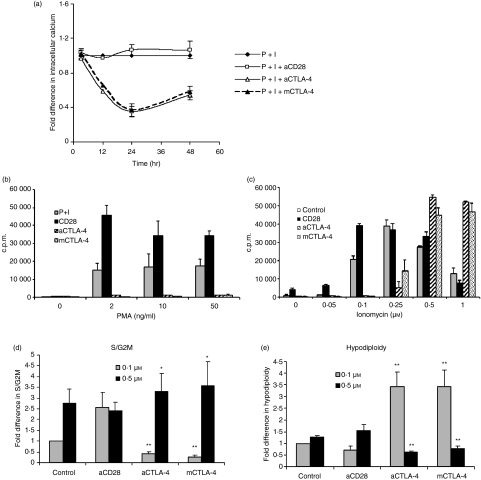
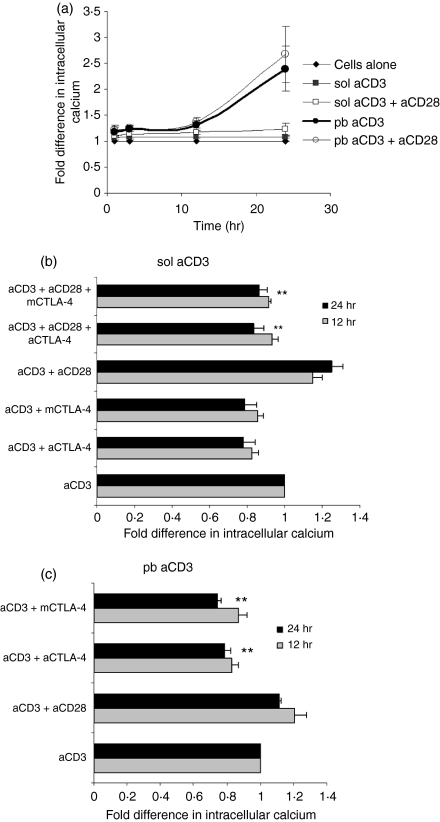
An increase in cytosolic [Ca2+]i is one of the first processes initiated after TCR signalling, and Ca2+ mobilization occurs in two stages. The first involves the phospholipase C γ1 and inositol trisphosphate (IP3) pathway, which is triggered seconds after TCR signalling, to bring about a rapid but rather transient release of Ca2+ from endoplasmic reticulum stores. Depletion of [Ca2+]i leads to the activation of Ca2+ release-activated Ca2+ channels across the plasma membrane, resulting in a sustained Ca2+ influx from the extracellular space by a process termed ‘capacitative Ca2+ entry’.30 There were two reasons to investigate the role of [Ca2+]i in this system. First, a sustained decrease in IL-2 levels in the presence of CTLA-4 blockade is observed.22 Secondly, modulation of Ca2+ is an important trigger for caspase-independent cell death.31,32 Therefore, free [Ca2+]i was studied, using Fluo3-AM fluorescence, in the presence or absence of blockade of CTLA-4–CD80/CD86 interactions. A slight increase was observed in cells activated with CD28; however, blockade of CTLA-4–CD80/CD86 interactions decreased [Ca2+]i. There was no difference in [Ca2+]i at 3 hr, while maximum differences were observed 24 hr post activation (Fig. 1a).
Figure 1.
The intracellular Ca2+ concentration ([Ca2+]i) is modulated by cytotoxic T-lymphocyte antigen (CTLA)-4–CD80/CD86 interactions in CD4+ T cells activated with phorbol 12-myristate 13-acetate (PMA) + Ionomycin (I). (a) CD4+ T cells were activated with 10 ng/ml PMA + 0·1 μm I in the presence or absence of anti-CD28, anti-CTLA-4 or mouse CTLA-4. At different times after activation, [Ca2+]i was measured using fluro-3 acetoxymethyl ester (Fluo-3AM). The mean fluorescence intensities (MFIs) obtained for different conditions were normalized to the MFI of cells activated with just PMA + I, which was taken as unity. Data shown are the mean ± standard error (SE) for three independent experiments. (b–e) Next, CD4+ T cells were activated with I (0·1 μm) plus the indicated concentrations of PMA (b) or 10 ng/ml PMA plus the indicated concentrations of I (c) in the presence or absence of anti-CD28, anti-CTLA-4 or mCTLA-4, and [3H]thymidine incorporation was measured. Cells activated with 10 ng/ml PMA plus 0·1 or 0·5 μm I in the presence of the indicated reagents were permeabilized and stained with propidium iodide after 48 hr of culture. The percentage of cells in the S/G2M and hypodiploid phases was determined by flow cytometry. Fold differences in percentages of S/G2M (d) and hypodiploid cells (e) under different activation conditions were normalized to that of cells activated with PMA + I (0·1 μm) alone, which was taken as unity. Data shown are the mean ± SE for three independent experiments. *P < 0·1; **P < 0·05. c.p.m., counts per minute.
Increasing doses of I greatly enhances cell cycling and survival of CD4+ T cells activated with PMA + I together with CTLA-4–CD80/CD86 blockade
Next, we attempted to determine whether increasing [Ca2+]i, using increasing amounts of I, could affect the functional outcome of this blockade. The concentration of I was increased while PMA (a protein kinase C activator) was kept constant at 10 ng/ml (Fig. 1c). Proliferation initially increased with increase in I concentration but decreased considerably at higher concentrations. The addition of anti-CD28 enhanced proliferation at lower concentrations but had no effect at higher concentrations of I. The addition of anti-CTLA-4 or mCTLA-4 decreased proliferation, compared with control, at lower concentrations. However, an increase in the concentration of I increased proliferation, and the difference between control and CTLA-4–CD80/CD86 blockade was reduced. Most interestingly, at the two highest concentrations of I, blockade of CTLA-4–CD80/CD86 interactions resulted in more proliferation compared with the control. This suggested that these interactions stimulated T-cell proliferation in cells activated with low doses of I but were inhibitory in cells activated with higher concentrations of I. In other words, the outcomes of these interactions changed with the intensity of the primary activating signal which was varied by changing the concentration of I. Notably, this effect was not observed upon increasing PMA (Fig. 1b), which demonstrated the specific role of [Ca2+]i. Also, the [3H]thymidine incorporation data (Fig. 1c) were consistent with cell cycle analysis (Fig. 1d and e). Increasing the I concentration from 0·1 to 0·5 μm increased cell cycling by ∼3-fold and only marginally increased hypodiploidy (Fig. 1d and e). Increasing I in cells subjected to blockade of CTLA-4–CD80/CD86 interactions not only increased cycling but also considerably decreased hypodiploidy (Fig. 1d and e).
Cell surface expression of costimulatory receptors and their ligands on T cells
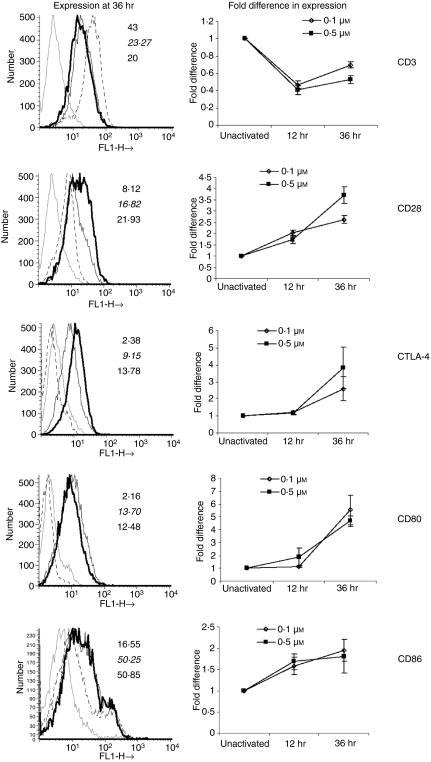
Next, the cell surface expression of CD3 and costimulatory receptors and ligands was studied upon activation with low and high amounts of I (Fig. 2). CD3 expression was reduced upon activation at 12 and 36 hr. Expression of both CD28 and CTLA-4 was enhanced upon activation, although with different kinetics, and CTLA-4 expression was only detected at 36 hr. CD86 was expressed on unactivated cells but no CD80 expression was detected. The levels of both CD80 and CD86 were substantially increased upon activation. However, for both CD80 and CD86, no difference was observed between expression on cells activated with 0·1 μm I and that on cells activated with 0·5 μm I. Overall, the surface expression of both costimulatory receptors and ligands increased with T-cell activation but no significant differences in expression were observed with increased amounts of I.
Figure 2.
Cell surface expression of CD3, costimulatory receptors and ligands is modulated upon T-cell activation. CD4+ T cells were activated with 10 ng/ml phorbol 12-myristate 13-acetate (PMA) plus 0·1 or 0·5 μm Ionomycin (I). Cell surface expression of CD3, CD28, CD80, CD86 and cytotoxic T-lymphocyte antigen (CTLA)-4 was studied 12 and 36 hr post activation by flow cytometry using appropriate antibodies. The broken lines are for expression on unactivated cells, thin black lines are for expression on cells activated with PMA + 0·1 μm I, and thick black lines are for cells activated with PMA + 0·5 μm I. The mean fluorescence intensity (MFI) on unactivated cells was taken as unity and compared with the MFI of cells after activation. The MFI value at the top is for unactivated T cells, the middle value (italicized) is for T cells activated with PMA + 0·1 μm I and the bottom value (bold) is for PMA + 0·5 μm I activated cells.
Increasing [Ca2+]i enhances IL-2 production and CD25 expression
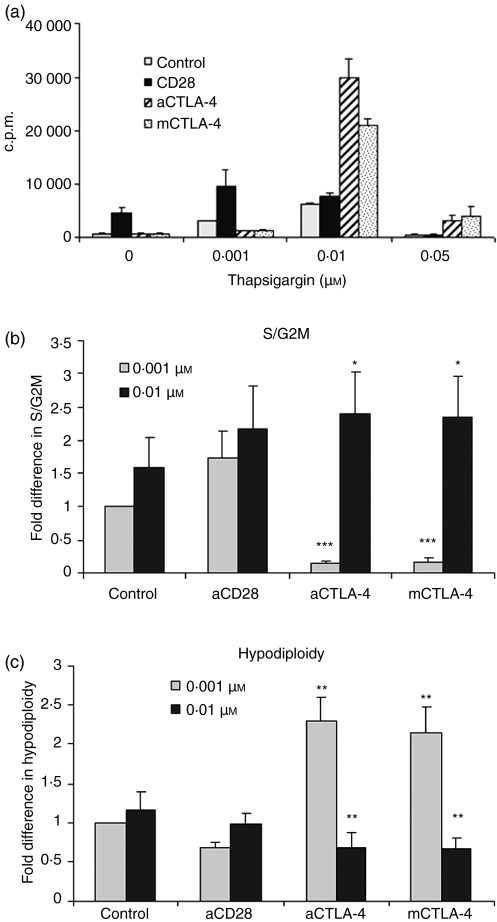
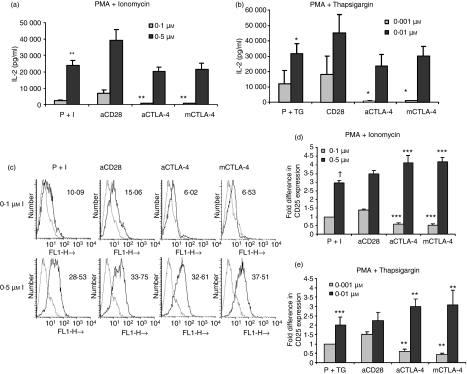
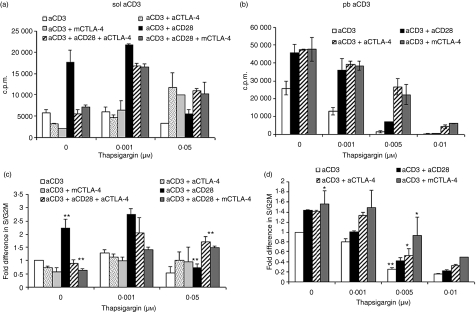
To confirm the attribution of the observed effects to an I-mediated increase in [Ca2+]i, we utilized another compound, TG, in combination with PMA (10 ng/ml). TG specifically increases [Ca2+]i by blocking Ca2+ transport to the sarcoplasmic and endoplasmic reticulum, and opens IP3 gated channels in the endoplasmic reticulum. Use of different concentrations of TG along with CTLA-4 blockade yielded similar results with respect to [3H]thymidine incorporation (Fig. 3a) and cell cycle analysis (Fig. 3b and c) to those obtained with I. As the addition of high amounts of I and TG increased cell cycling in T cells activated via blockade of CTLA-4–CD80/CD86 interactions, we investigated whether IL-2 levels were modulated. CD4+ T cells were activated with either a low dose (0·1 μm) or a high dose (0·5 μm) of I in combination with PMA (Fig. 4a). Increasing I led to a ∼10-fold increment in IL-2 in cells activated with control Ab and addition of anti-CD28 enhanced IL-2 under both conditions of activation. Blockade of CTLA-4–CD80/CD86 interactions by either anti-CTLA-4 or mCTLA-4 led to decreased amounts of IL-2 in cells activated with PMA + 0·1 μm I, as reported previously.22 However, activating T cells with increasing amounts of I (e.g. 0·5 μm) reduced the difference in IL-2 levels between the control and cells subjected to CTLA-4–CD80/CD86 blockade. Indeed, the amounts of IL-2 were comparable to those produced by cells cultured without CTLA-4 blockade (i.e. PMA + 0·5 μm I alone). Although cells cultured with a high dose of I coupled with CTLA-4 blockade proliferated to a greater extent than cells not subjected to this blockade (Fig. 1c), the amounts of IL-2 under the two culture conditions were similar. These results were consistent with respect to induction of IL-2 in T cells activated with 0·001 and 0·01 μm TG (Fig. 4b).
Figure 3.
Blockade of cytotoxic T-lymphocyte antigen (CTLA)-4–CD80/CD86 interactions modulates activation of CD4+ T cells stimulated with phorbol 12-myristate 13-acetate (PMA) + Thapsigargin (TG). (a) CD4+ T cells were activated with 10 ng/ml PMA plus different concentrations of TG in the presence or absence of anti-CD28, anti-CTLA-4 or mouse CTLA-4. Cells were cultured for 36 hr and then pulsed with [3H]thymidine for an additional 12 hr. At the end of the pulse period, cells were harvested and incorporated [3H]thymidine levels were measured. (b, c) Cells activated with 10 ng/ml PMA plus 0·001 or 0·01 μm TG with or without the indicated antibodies were permeabilized for cell cycle staining after 48 hr of culture. The percentage of cells in the S/G2M (b) and hypodiploid (c) phases was determined by flow cytometry. Fold differences in percentages of S/G2M and hypodiploid cells under different activation conditions were normalized to that of cells activated with PMA + TG (0·001 μm) alone. Data shown are the mean ± standard error for three independent experiments. *P <0·1; **P< 0·05; ***P< 0·0005.c.p.m., counts per minute.
Figure 4.
Cytotoxic T-lymphocyte antigen (CTLA)-4-CD80/CD86 interactions modulate interleukin (IL)-2 production and CD25 expression. CD4+ T cells were activated with phorbol 12-myristate 13-acetate (PMA) (10 ng/ml) plus 0·1 or 0·5 μm Ionomycin (I) (a) or 0·001 or 0·01 μm Thapsigargin (TG) (b) in the presence or absence of anti-CD28, anti-CTLA-4 or mouse CTLA-4. Amounts of IL-2 were measured by enzyme-linked immunosorbent assay (ELISA) 24 hr post activation. Cell surface expression of CD25 expression was studied after 36 hr by flow cytometry (c). The numbers alongside the histograms in (c) are the mean fluorescence intensities (MFIs). The grey histograms are for controls and the black histograms show CD25 expression under the indicated activation conditions. The fold differences in MFIs under different activation conditions at 36 hr were normalized to the MFI of cells activated with PMA + 0·1 μm I (d). Similar fold differences in MFI were calculated for cells activated with PMA + 0·001 or 0·01 μm TG (e). Data shown are mean ± standard error for three independent experiments. †P < 0·0005; *P < 0·1; **P < 0·05; ***P < 0·01.
CD25 cell surface expression is routinely used as a marker for sustained T-cell activation.22 High amounts of CD25 were found after 36 hr of activation with PMA + 0·1 μm I. These amounts further increased with the addition of anti-CD28, but blockade of CTLA-4 interactions reduced expression 2-fold (Fig. 4c and d). Increasing the I concentration to 0·5 μm led to a 3-fold elevation in CD25 surface levels. The addition of anti-CD28, anti-CTLA-4 or mCTLA-4 only slightly increased CD25 expression over the control. Similar results were obtained when TG was used instead of I (Fig. 4e). These results clearly demonstrate that blockade of CTLA-4–CD80/CD86 interactions reduced T-cell activation with respect to CD25 expression and IL-2 levels during activation with low SOS. However, the increase in proliferation observed upon activation with high SOS and CTLA-4–CD80/CD86 blockade was not associated with significantly increased amounts of IL-2 or CD25 surface expression.
Role of [Ca2+]i and CTLA-4–CD80/CD86 interactions in cells activated with sol aCD3 or pb aCD3
Thus far, all experiments performed utilized the pharmacological agents PMA, I and TG, which bypass TCR–CD3 signalling when activating T cells. Therefore, we wished to confirm our observations using anti-CD3, which triggers activation via the surface TCR–CD3 complex. T cells were activated with either sol aCD3, which delivers a relatively weak signal, or pb aCD3, which delivers a relatively strong signal. Consequently, the amounts of IL-2 produced by pb aCD3-activated cells were greater than those produced by sol aCD3-activated cells.24 The addition of anti-CD28, in a dose-dependent manner, increased proliferation of T cells activated with sol anti-CD3; however, the proliferation of T cells was greater in cells activated with pb anti-CD3 and anti-CD28 did not significantly increase proliferation (Supplementary Fig. S2). Blockade of CTLA-4–CD80/CD86 interactions reduced T-cell proliferation and cell cycling in the presence of low or high amounts of anti-CD28 and sol anti-CD3. However, the reduction in proliferation and cell cycling in T cells activated with 0·1 μg/ml of anti-CD28 and blockade of CTLA-4–CD80/CD86 interactions was greater compared with activation with 0·5 μg/ml anti-CD28 (2·25- versus 1·26-fold). Therefore, suboptimal amounts of anti-CD28 (0·1 μg/ml) were used in these conditions. Blockade of CTLA-4 interactions increased proliferation of T cells activated with pb anti-CD3, and proliferation was even further enhanced upon addition of anti-CD28, probably as a result of the combination of triggering of the positive costimulatory CD28 and blockade of negative CTLA-4 interactions (Supplementary Fig. S2). First, differences in [Ca2+]i were compared between cells activated with sol and pb aCD3. Unactivated cells were used as a control and [Ca2+]i was measured at different time-points post activation (Fig. 5a). Sol aCD3-mediated activation lead to a marginal increase in [Ca2+]i compared with unactivated cells. However, [Ca2+]i was enhanced in pb aCD3-activated cells and was ∼2-fold higher at 24 hr compared with sol aCD3-activated cells. The addition of anti-CD28 only marginally increased [Ca2+]i and blockade of CTLA-4–CD80/CD86 interactions reduced [Ca2+]i in cells activated with sol aCD3, sol aCD3 + aCD28 or pb aCD3 (Figs 5b and c). The reduction upon CTLA-4 blockade was more evident in cells activated with sol aCD3 + aCD28.
Figure 5.
Cytotoxic T-lymphocyte antigen (CTLA)-4–CD80/CD86 interactions modulate intracellular Ca2+ concentration ([Ca2+]i) in CD4+ T cells activated with antiCD3. [Ca2+]i was measured after different times of activation with soluble anti-CD3 (sol aCD3) or plate-bound anti-CD3 (pb aCD3). Mean fluorescence intensities (MFIs) normalized to the baseline level in unactivated cells, which was taken as unity, are shown in (a). [Ca2+]i was measured at 12 and 24 hr in the presence or absence of CTLA-4–CD80/CD86 blockade. The MFIs were normalized to that of cells activated with anti-CD3, which was taken as unity (b and c). Data shown are mean ± standard error for three independent experiments. **P < 0·05.
Next, the functional effect of [Ca2+]i was studied in cells activated with sol aCD3 or sol aCD3+ aCD28, in the presence or absence of blockade. [Ca2+]i was increased under these culture conditions by addition of TG (Fig. 6a) and addition of anti-CD28 greatly enhanced proliferation (Figs 6a and c). In cells cultured without TG, CTLA-4 blockade reduced [3H]thymidine incorporation and also the percentage of S/G2M cells, especially in cells activated with sol aCD3+ aCD28 (Figs 6a and c), which was reversed by the addition of TG. At the highest concentration of TG, proliferation of cells activated with sol aCD3 or sol aCD3+ aCD28 was substantially compromised. However, cells cultured under the same conditions with CTLA-4–CD80/CD86 blockade demonstrated greater proliferation. Similar experiments were performed using pb aCD3 for activation (Fig. 6b and d). Cells cultured with CTLA-4 blockade proliferated to a greater extent than cells cultured with pb aCD3 and control mAb, as reported previously.24 Addition of TG greatly reduced proliferation in pb aCD3, in the presence of control mAb or anti-CD28 (Fig. 6b and d). Our observations are similar to previous reports where the addition of TG triggered apoptosis.33 Cells activated with pb aCD3 had high [Ca2+]i and a further increase resulted in growth suppression. However, cells cultured with blockade were better able to resist this inhibition of proliferation caused by addition of TG (Fig. 6d).
Figure 6.
Increasing intracellular Ca2+ concentration ([Ca2+]i) changes the strength of signal (SOS) and the profile of cytotoxic T-lymphocyte antigen (CTLA)-4 interactions. Increasing concentrations of Thapsigargin (TG) were added to soluble anti-CD3 (sol aCD3) or plate-bound anti-CD3 (pb aCD3) in the absence and presence of anti-CD28, anti-CTLA-4 or mouse CTLA-4. Proliferation was measured by [3H]thymidine incorporation (a) to cells activated with sol aCD3 and (b) to cells activated with pb aCD3. Also, at the end of 48 hr, cells were stained with propidium iodide and the percentage of cells in the S/G2M phase was determined by flow cytometry. The percentages were normalized to that of cells activated with just sol aCD3 or pb aCD3 and fold differences are shown in (c) and (d). The left-hand panel shows data for sol aCD3 and the right-hand panel data for pb aCD3 activation. *P < 0·1; ***P < 0·05. c.p.m., counts per minute.
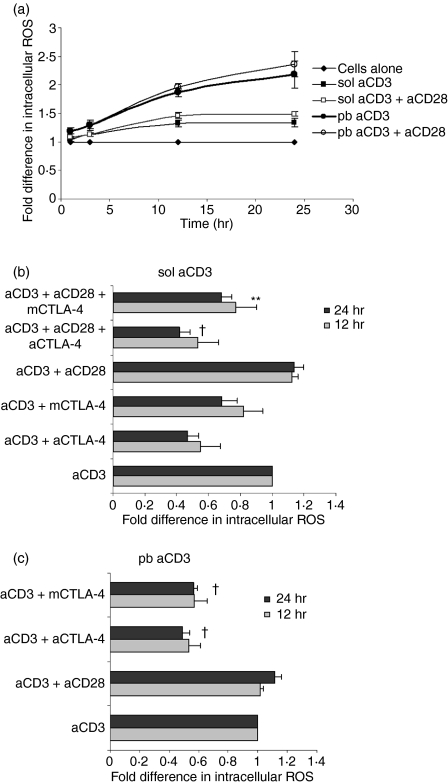
CTLA-4–CD80/CD86 interactions modulate intracellular amounts of ROS
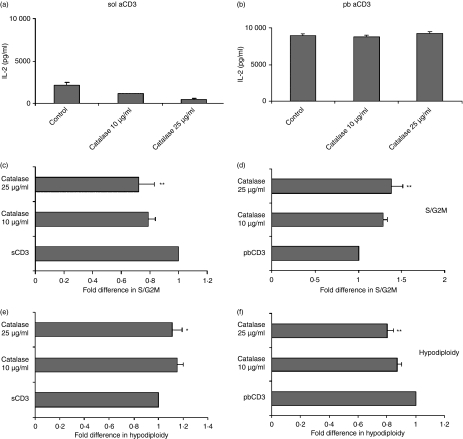
Our earlier study using concanavalin A (ConA) showed that CTLA-4–CD80/CD86 interactions modulated intracellular ROS levels, which directly affected cell cycling.23 In the next set of experiments, we attempted to investigate whether amounts of ROS were modulated by CTLA-4 blockade in cells activated with anti-CD3. ROS-dependent conversion of DCFDA was higher in cells activated with pb aCD3 compared with those activated with sol aCD3 after different times of activation (Fig. 7a). The maximum difference in DCFDA fluorescence between sol aCD3- and pb aCD3-activated cells was observed at 24 hr post activation. The addition of anti-CD28 did not have any significant effect but blockade of CTLA-4 interactions reduced ROS in cells activated with sol aCD3 or pb aCD3 (Fig. 7b and c). To elucidate the functional role of ROS in this system, IL-2 levels (Figs 8a and b) and the percentage of S/G2M and hypodiploid cells were quantified (Fig. 8c–f) in cells activated with sol or pb aCD3 cultured in the presence and absence of the H2O2 scavenger catalase. Scavenging of H2O2 in cells activated with sol aCD3 reduced IL-2 and cell cycling and marginally increased hypodiploidy, whereas similar culture conditions with pb aCD3 had no effect on IL-2 but increased cell cycling and decreased hypodiploidy. These data suggested that the functional roles of ROS produced during CD4+ T-cell activation were distinct for low and high signal strengths of activation.
Figure 7.
Intracellular reactive oxygen species (ROS) increases with signal strength and cytotoxic T-lymphocyte antigen (CTLA)-4–CD80/CD86 interactions. Amounts of ROS were measured by 2′, 7′-dichlorofluorescein diacetate (DCFDA) fluorescence conversion at different times in cells activated with soluble anti-CD3 (sol aCD3) and plate-bound anti-CD3 (pb aCD3) ± anti-CD28. Mean fluorescence intensities (MFIs) under different conditions were normalized to the MFI of unactivated cells, which was taken as unity (a). A similar experiment was performed with blockade of CTLA-4–CD80/CD86 interactions and ROS was measured after 12 and 24 hr of activation. MFIs were normalized to that of cells activated with anti-CD3, which was taken as unity (b and c). Data shown are mean ± standard error for three independent experiments. †P< 0·0005; **P< 0·05.
Figure 8.
Opposing roles of reactive oxygen species (ROS) in cells activated with soluble anti-CD3 (sol aCD3) and those activated with plate-bound anti-CD3 (pb aCD3). Sol and pb aCD3-activated cells were cultured with the indicated concentrations of catalase. Interleukin (IL)-2 was measured after 36 hr (a and b). At the end of 48 hr, cells were stained with propidium iodide and cell cycle analysis was performed. The fold differences in the percentages of cells in the S/G2M (c and d) and hypodiploid (e and f) phases under different activation conditions were calculated with respect to that of cells activated with sol aCD3 or pb aCD3, which was taken as unity. The left-hand panel shows data for sol aCD3 and the right-hand data for pb aCD3. Data shown are the mean ± standard error for three independent experiments. *P< 0·1; **P < 0·05.
Discussion
Multiple pathways have been proposed to explain the mechanisms by which CTLA-4 inhibits T-cell activation: for example, competition with CD28 to bind to CD80/CD86; interference with lipid raft formation; involvement of the phosphatases, protein phosphatase 2A (PP2A) and src homology protein 2 (SHP-2) (intrinsic factors); and CTLA-4 ligation leading to enhanced production of transforming growth factor (TGF)-β and indoleamine dioxygenase (extrinsic factors).5–7 In addition, several models have been proposed to explain CTLA-4 function. According to one model, CTLA-4 raises the threshold for T-cell activation. Therefore, in the absence of CTLA-4, the threshold for T-cell activation is lowered, resulting in uncontrolled proliferation of CD4+ T cells in response to self antigens. The attenuator model predicts that CTLA-4 functions as a brake to a strong primary activating signal; therefore, in the absence of CTLA-4, cells divide more frequently.5 Recently, the reverse stop-signal model has proposed that surface CTLA-4 reduces the long contact time between T cells and APCs which is required for optimal T-cell activation.7 Although it is likely that multiple pathways are involved, there is no consensus on the contributions of the different pathways by which CTLA-4 functions in regulating T-cell function. In this study, the consequences of blockade of CTLA-4–CD80/CD86 interactions on purified CD4+ T cells were studied in the context of varying SOS, using different amounts of I or TG. These effects were observed with the use of two independent reagents: anti-CTLA-4, which, under soluble conditions, blocks binding of CTLA-4 to CD80/CD86,25,34 and mCTLA-4hIgG1, the non-crosslinkable single chain fusion protein, which binds to CD80/CD86 and blocks their interactions with both CD28 and CTLA-4.35 Previous studies have demonstrated the role of SOS and CTLA-4–CD80/CD86 interactions in modulating T-cell activation.16,17,23,24 Here, we show that CTLA-4–CD80/CD86 interactions increased the amounts of two key intracellular mediators of T-cell activation, [Ca2+]i and ROS. Importantly, the consequences of CTLA-4–CD80/CD86-induced increases in [Ca2+]i and ROS were consistent for low and high signal strengths of activation via TCR–CD3 signalling (Fig. 9).
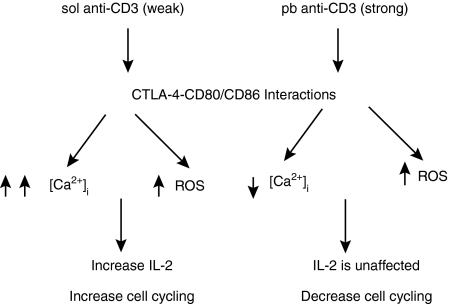
Figure 9.
Model depicting the distinct effects of cytotoxic T-lymphocyte antigen (CTLA)-4–CD80/CD86 interactions under low and high signal strengths of T-cell activation. Activation of T cells with plate-bound anti-CD3 (pb aCD3), compared with soluble anti-CD3 (sol aCD3), demonstrates higher intracellular Ca2+ concentration ([Ca2+]i), reactive oxygen species (ROS) level and interleukin (IL)-2 production. The reduction in [Ca2+]i was greater in T cells activated with sol aCD3, compared with pb aCD3, and blockade of CTLA-4–CD80/CD86 interactions. Under activation with both pb and sol aCD3 and CTLA-4 blockade, the reductions in ROS amounts were similar. Despite the role of CTLA-4–CD80/CD86 interactions in enhancing [Ca2+]i and ROS levels, the functional outcomes with respect to proliferation are distinct.
The SOS regulates recruitment of CTLA-4 into the immunological synapse.15 Previous studies with T cells activated with different doses of ConA23 or different modes of anti-CD324 have shown that the surface expression of CD28, CTLA-4, CD80 and CD86 is increased upon activation. Furthermore, the cell surface expression of CTLA-4 is higher in cells activated with pb aCD3 compared with cells activated with sol aCD3.24 Similarly, CTLA-4 and CD28 surface expression was increased post activation with increased amounts of I (Fig. 2). Also, the fold increase in the cell surface expression of CTLA-4 was higher compared with CD28. CD80 also increased with I concentration after 12 hr of activation but the fold induction of CD86 was lower compared with CD80 (Fig. 2). This observation is relevant in the light of the fact that CD80 selectively recruits CTLA-4 to the immunological synapse.36 Although peak surface expression of CTLA-4 was observed 36 hr after activation, the functional effects of blocking CTLA-4–CD80/CD86 interactions in modulating [Ca2+]i and ROS were evident at 12 and 24 hr. Also, effects of CTLA-4 blockade on proliferation were observed upon adding the blocking reagents within 6 hr of activation (data not shown). This suggests that low but functional amounts of CTLA-4, not detectable by flow cytometry, were expressed early during activation.
[Ca2+]i plays important roles in T-cell biology and a sustained Ca2+ signal is necessary to activate the nuclear factor of activated T cells, a critical transcription factor that regulates the expression of several important genes, including IL-2.37,38 The effects of [Ca2+]i on T-cell function have been clearly demonstrated in patients with mutations in genes encoding Ca2+ release-activated Ca2+ channels, who show severe combined immunodeficiency with severe impairment of nuclear factor of activated T cells (NFAT)-dependent gene activation.39,40 Also, high [Ca2+]i triggers several pathways, resulting in cell death by apoptosis and necrosis.32,33 In addition, the relationship between TCR signal strength and [Ca2+]i is well established. A weak TCR signal induces a Ca2+ signal that induces IL-4 production while a strong TCR signal leads to interferon-γ (IFN-γ) production.41 Also, strong TCR activation during thymic education leads to elevated [Ca2+]i, which leads to apoptosis, while weak TCR activation leads to [Ca2+]i oscillations that do not lead to apoptosis.42 In human T lymphocytes, increased [Ca2+]i and the calcineurin signalling pathway are implicated in the regulation of CTLA-4 gene expression.43 A recent study has demonstrated that high expression of CTLA-4 in a T-cell hybridoma reduces the rapid increase in [Ca2+]i in the presence of APC and cognate antigen.44 In our system of T cell–T cell interactions, [Ca2+]i increased with the SOS, and this increased [Ca2+]i was sustained for 24 hr (Fig. 5a). Most studies on Ca2+ influx in T cells concentrate only on the initial few minutes to 1 hr of activation, which provides information about the initial spike in [Ca2+]i. However, we were interested in studying Ca2+ levels at later time-points, as CTLA-4 blockade did not modulate Ca2+ levels earlier than 3 hr, probably as a result of the inducible expression of CTLA-4. Initial experiments demonstrated that [Ca2+]i was greatly reduced in CD4+ T cells activated with PMA + I (0·1 μm) together with blockade of CTLA-4–CD80/CD86 interactions (Fig. 1), which led to the investigation of the role of [Ca2+]i in the PMA +I/TG and anti-CD3 systems of activation.
CTLA-4–CD80/CD86 interactions enhanced [Ca2+]i under activation with low or high SOS. However, the functional effects were more apparent upon activation of T cells with a low SOS. In fact, blockade of CTLA-4–CD80/CD86 interactions and activation with PMA + I (0·1 μm) decreased the amounts of IL-2 (Fig. 4a). Also, the decrease in CD25 expression, a marker for T-cell activation, upon blockade was enhanced compared with cells activated with high amounts of I or TG (Fig. 4d and e). Increased T-cell activation also enhanced cell cycling with increased amounts of I or TG and blockade of CTLA-4–CD80/CD86 interactions (Figs 1c, 3a and 6a). However, in T cells activated with a strong signal it is unlikely that T-cell activation was affected, as CD25 induction (Fig. 4c–e) and IL-2 production (Fig. 4a and b) were not significantly modulated. T-cell activation with a strong signal led to accumulation of high [Ca2+]i, which may inhibit cell proliferation (Fig. 6b and d), whereas the opposite effect was observed in T cells activated with a comparatively weak signal (Figs 6a and c). The growth suppression observed in T cells activated with a strong signal was probably a result of the production of excess ROS.
T-cell activation results in increased ROS production, which plays important roles in cellular functions. Low doses of ROS, especially H2O2, are mitogenic: it acts as a second messenger mediating its effects by inactivating phosphatases and activating signalling pathways leading to the upregulation of different transcription factors.45–46 However, excess ROS leads to oxidative stress, resulting in reduced TCR-induced extracellular related kinase phosphorylation, increased production of inflammatory products and T-cell hyporesponsiveness.47–48 The amounts of ROS increase upon activation of T cells with suppressive doses of ConA. In these conditions, free radicals play functional roles and CTLA-4–CD80/CD86 interactions reduce T-cell cycle progression and survival.23 In this study, increased amounts of ROS were observed after activation with pb aCD3 compared with sol aCD3 (Fig. 7a). Also, blockade of CTLA-4–CD80/CD86 interactions decreased ROS, although with differing consequences in cells activated with sol aCD3 and pb aCD3 (Figs 7, 8 and 9). Reducing the amounts of H2O2 post activation with sol aCD3 reduced IL-2 production and cell proliferation and increased hypodiploidy (Fig. 8a, c and e). These effects were probably attributable to reduction in amounts of ROS that are mitogenic.45–46 However, scavenging of excess ROS produced after activation with a strong primary signal (e.g. pb aCD3) increased proliferation and reduced hypodiploidy (Fig. 8d and f). In one of the first reports on anti-CTLA-4, blockade of CTLA-4 interactions enhanced in vitro T-cell proliferation upon activation with pb aCD325,34 However, the reasons for this were not known. We show here that IL-2 production was not significantly affected but a functional role for ROS was revealed. In fact, a reduction in the amounts of ROS did not affect IL-2 levels but increased T-cell proliferation (Fig. 8b and d).
These results demonstrating the roles of SOS and CTLA-4–CD80/CD86 interactions in modulating T-cell activation and cell cycle progression may be of immunological importance. The frequency of some TCRs was reduced whereas that of others was increased in a patient with multiple sclerosis upon activation with MBP and CTLA-4 blockade. These results clearly demonstrate the differential effects of CTLA-4 on distinct MBP reactive clones.16 In a mouse model of experimental autoimmune encephalomyelitis, priming with a disease antagonist peptide and CTLA-4 blockade reduce the frequency of clones reactive to the disease agonist peptide. In other words, CTLA-4–CD80/CD86 interactions enhance the generation of cross-reactive clones in this model.17 It has been proposed that, by inhibiting T-cell clones possessing a high-affinity TCR, CTLA-4 may inhibit the generation of T cells containing exclusively high-affinity TCRs which may dominate the immune response. Alternatively, CTLA-4 may broaden the T-cell response to cross-reactive antigens by encouraging the generation of T cells with low-affinity TCRs. This may be important in tackling pathogens, which mutate to overcome the host immune response.5,17 Our results are consistent with roles of SOS and CTLA-4 in regulating the primary CD4+ T-cell response.15–17,23,24 Importantly, this study identifies and demonstrates functional roles of [Ca2+]i and ROS generated by SOS and CTLA-4–CD80/CD86 interactions in modulating T-cell activation and cell cycle progression.
Acknowledgments
We thank Dr Kanury Rao for his talk on the role of Ca2+ capacitance during B-cell activation which inspired this study. In addition, we are grateful to Professor T. Ramasarma, Professor S. Sikdar and Dr. A. Sarin for extensive discussions and support. We appreciate the generosity of Dr V. Bal and Dr S. Rath for access to the lpr strain of mice. Dr S. Rath is also thanked for his patient reading of and comments on the manuscript. The expertise of Dr O. Joy, H. Krishnan and M. Vamsi at the IISc FACS facility is greatly appreciated. SM was awarded a research fellowship from CSIR. This study was supported by a grant from the Department of Biotechnology, Government of India.
Glossary
Abbreviations
- [Ca2+]i
intracellular concentration of Ca2+
- APC
antigen-presenting cell
- ConA
concanavalin A
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- DCFDA
2′, 7′-dichlorofluorescein diacetate
- H2O2
hydrogen peroxide
- I
Ionomycin
- IL-2
interleukin-2
- mCTLA-4-hIgG/mCTLA-4
mouse CTLA-4 human immunoglobulin G
- MBP
myelin basic protein
- MFI
mean fluorescence intensity
- pb aCD3
plate-bound anti-CD3
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
- sol aCD3
soluble anti-CD3
- SOS
strength of signal
- TCR
T-cell receptor
- TG
Thapsigargin
Supplementary material
The following supplementary material is available for this article:
Figure S1. Inhibition of proliferation as a result of cytotoxic T-lymphocyte antigen (CTLA)-4 blockade is independent of caspase activation and Fas—FasL interactions. CD4+ T cells were activated with 10 ng/ml phorbol 12-myristate 13-acetate (PMA) + 0·1 μM Ionomycin (I) in the presence or absence of anti-CD28, anti-CTLA-4 or mouse CTLA-4. The pan caspase inhibitor Boc-d-fluoromethyl ketone (BDfmk) and antibodies against Fas were added to the cultures at the indicated concentrations. N-benzyloxycarbonyl phe-ala-fluoromethane (ZFAfmk) was used as a control. Proliferation was measured by [3H]thymidine incorporation (a). Also, proliferation in the presence of anti-CD28, anti-CTLA-4 and mCTLA-4 was measured in cells isolated from wild type as well as lpr mice (CD95-deficient) (b). As a control, CD4+ T cells were activated with PMA + I for 36 hr, washed and rested for 36 hr. Activation of these cells with plate-bound anti-CD3 (pb aCD3) resulted in 41% of hypodiploid cells in the wild type and death was reduced to 23% in lpr CD4+T cells, clearly demonstrating the requirement for Fas during activation-induced death (data not shown). Similarly, experiments were performed with the irreversible pan-caspase inhibitor BDfmk. In the same model of activationinduced cell death, BDfmk reduced hypodiploid from 62% in the wild type to 37%, demonstrating the requirements for caspases (data not shown).
Figure S2. Effect of increasing amounts of anti-CD28 on T-cell proliferation and cell cycling after activation with soluble (sol) or plate-bound (pb) anti-CD3. Increasing concentrations of anti-CD28 were added to CD4+ T cells activated with either sol or pb anti-CD3 (0·1 lg/ml) and cells were cultured with or without cytotoxic T-lymphocyte antigen (CTLA)-4—CD80/CD86 blockade. Proliferation was measured by [3H]thymidine incorporation (a and b). Also, after 48 hr of culture, cell cycle analysis was performed and the percentage of cells in the S/G2M phase was determined. The percentages were normalized to that for cells activated with anti-CD3 alone, which was taken as unity (c and d). Data shown are representative of two independent experiments.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/full/ 10.1111/ j.1365-2567.2008.02902.x.
Please note: Blackwell publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than about missing material) should be directed to the corresponding author for the article.
References
- 1.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci USA. 1999;96:185–90. doi: 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 3.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–9. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 4.Lucas PJ, Negishi I, Nakayama K, Fields LE, Loh DY. Naive CD28-deficient T cells can initiate but not sustain an in vitro antigen-specific immune response. J Immunol. 1995;15:5757–68. [PubMed] [Google Scholar]
- 5.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–8. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 6.Teft WA, Kirchof MG, Madrenas J. A molecular perspective of CTLA4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 7.Rudd CE. The reverse stop-signal model for CTLA4 function. Nat Rev Immunol. 2008;8:153–60. doi: 10.1038/nri2253. [DOI] [PubMed] [Google Scholar]
- 8.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 9.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Mathis D, Benoist C. Modeling CTLA4 linked autoimmunity with RNA interference in mice. Proc Natl Acad Sci USA. 2006;103:16400–5. doi: 10.1073/pnas.0607854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda H, Howson JMM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–55. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 13.Walker LSK, Ausubel LJ, Chodos A, Bekarian N, Abbas AK. CTLA4 differentially regulates T cell responses to endogenous tissue protein versus exogenous immunogen. J Immunol. 2002;169:6202–9. doi: 10.4049/jimmunol.169.11.6202. [DOI] [PubMed] [Google Scholar]
- 14.Chambers CA, Kuhns MS, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4+ T cell responses. Proc Natl Acad Sci USA. 1999;96:8603–8. doi: 10.1073/pnas.96.15.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egen JG, Allison JP. CTLA4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 16.Anderson DE, Bieganowska KD, Bar-Or A, Oliveira EML, Carreno B, Collins M, Hafler DA. Paradoxical inhibition of T-cell function in response to CTLA-4 blockade; heterogeneity within the human T-cell population. Nat Med. 2000;6:211–4. doi: 10.1038/72323. [DOI] [PubMed] [Google Scholar]
- 17.Kuhns MS, Epshteyn V, Sobel RA, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates the size, reactivity, and function of a primed pool of CD4+ T cells. Proc Natl Acad Sci USA. 2000;97:12711–6. doi: 10.1073/pnas.220423597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon H, Jun HS, Khil LY, Yoon JW. Role of CTLA4 in the activation of single and double positive thymocytes. J Immunol. 2004;173:6645–53. doi: 10.4049/jimmunol.173.11.6645. [DOI] [PubMed] [Google Scholar]
- 19.Madrenas J, Chau LA, Teft WA, Wu PW, Jussif J, Kasaian M, Carreno BM, Ling V. Conversion of CTLA4 from inhibitor to activator of T cells with a bispecific tandem single chain Fv ligand. J Immunol. 2004;172:5948–56. doi: 10.4049/jimmunol.172.10.5948. [DOI] [PubMed] [Google Scholar]
- 20.Yin L, Schneider H, Rudd CE. Short cytoplasmic SDYMNM segment of CD28 is sufficient to convert CTLA4 to a positive signaling receptor. J Leukoc Biol. 2003;73:178–82. doi: 10.1189/jlb.0702365. [DOI] [PubMed] [Google Scholar]
- 21.Hueber AJ, Matzkies FG, Rahmeh M, Manger B, Kalden JR, Nagel T. CTLA4 lacking the cytoplasmic domain costimulates IL-2 production in T cell hybridomas. Immunol Cell Biol. 2006;84:51–8. doi: 10.1111/j.1440-1711.2005.01402.x. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee S, Maiti PK, Nandi D. Role of CD80, CD86 and CTLA4 on mouse CD4+ T lymphocytes in enhancing cell cycle progression and survival after activation with PMA and Ionomycin. J Leukoc Biol. 2002;72:921–31. [PubMed] [Google Scholar]
- 23.Mukherjee S, Ahmed A, Nandi D. CTLA4-CD80/CD86 interactions on primary mouse CD4+ T cells integrate signal-strength information to modulate activation with Concanavalin A. J Leukoc Biol. 2005;78:144–57. doi: 10.1189/jlb.1104644. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee S, Ahmed A, Malu S, Nandi D. Modulation of cell cycle progression by CTLA4-CD80/CD86 interactions on CD4+ T cells depends on strength of the CD3 signal: critical role for IL-2. J Leukoc Biol. 2006;80:66–74. doi: 10.1189/jlb.0505260. [DOI] [PubMed] [Google Scholar]
- 25.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Höllsberg P, Scholz C, Anderson DE, Greenfield EA, Kuchroo VK, Freeman GJ, Hafler DA. Expression of a hypoglycosylated form of CD86 (B7.2) on human T cells with altered binding properties to CD28 and CTLA4. J Immunol. 1997;159:4799–805. [PubMed] [Google Scholar]
- 27.van Parijs L, Sethna MP, Schweitzer N, Borriello F, Sharpe AH, Abbas AK. Functional consequences of dysregulated B7.1 (CD80) and B7.2 (CD86) expression in B or T lymphocytes of transgenic mice. J Immunol. 1997;159:5336–44. [PubMed] [Google Scholar]
- 28.Schweitzer AN, Sharpe AH. Mutual regulation between B7-1 (CD80) expressed on T cells and IL-4. J Immunol. 1999;163:4819–25. [PubMed] [Google Scholar]
- 29.Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells downregulates immune responses through CTLA4 ligation via T–T interactions. J Immunol. 2004;172:34–9. doi: 10.4049/jimmunol.172.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 31.Jäättelä M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2003;4:416–23. doi: 10.1038/ni0503-416. [DOI] [PubMed] [Google Scholar]
- 32.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Youn H, Liu JO. Thapsigargin-induced apoptosis involves Cabin 1 – MEF2-mediated induction of Nur77. Eur J Immunol. 2001;31:1757–64. doi: 10.1002/1521-4141(200106)31:6<1757::aid-immu1757>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 34.Walunas T, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 35.Lane P, Gerhard W, Hueble S, Lanzavecchia A, McConnell F. Expression and functional properties of mouse B7/BB1 using a fusion protein between mouse CTLA4 and human γ1. Immunology. 1993;80:56–61. [PMC free article] [PubMed] [Google Scholar]
- 36.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;2:401–13. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–40. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 38.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin and NFAT. Genes Dev. 2003;17:2205–32. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 39.Fanger CM, Hoth M, Crabtree GR, Lewis RS. Characterization of T cell mutants with defects in capacitative calcium entry: genetic evidence for the physiological roles of CRAC channels. J Cell Biol. 1995;131:655–67. doi: 10.1083/jcb.131.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feske S, Gwack Y, Prakriya M, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 41.Badou A, Savignac M, Moreau M, et al. Weak TCR stimulation induces a calcium signal that triggers IL-4 synthesis, stronger TCR stimulation induces MAP kinases that control IFN-gamma production. Eur J Immunol. 2001;31:2487–96. doi: 10.1002/1521-4141(200108)31:8<2487::aid-immu2487>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 42.Davis MC, Distelhorst CW. Live free or die: an immature T cell decision encoded in distinct Bcl-2 sensitive and insensitive Ca2+ signals. Cell Cycle. 2006;5:1171–4. doi: 10.4161/cc.5.11.2778. [DOI] [PubMed] [Google Scholar]
- 43.Vendetti S, Riccomi A, Sacchi A, Gatta L, Pioli C, de Magistris MT. Cyclic adenosine 5′-monophosphate and calcium induce CD152 (CTLA4) upregulation in resting CD4+ T lymphocytes. J Immunol. 2002;169:6231–5. doi: 10.4049/jimmunol.169.11.6231. [DOI] [PubMed] [Google Scholar]
- 44.Schneider H, Smith X, Liu H, Bismuth G, Rudd CE. CTLA-4 disrupts ZAP70 microcluster formation with reduced T cell/APC dwell times and calcium mobilization. Eur J Immunol. 2008;38:40–7. doi: 10.1002/eji.200737423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species and cell signaling. Free Radic Biol Med. 2004;37:1144–51. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–34. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 47.Cayota A, Vuillier F, Gonzalez G, Dighiero G. In vitro antioxidant treatment recovers proliferative responses of anergic CD4+ lymphocytes from human immunodeficiency virus-infected individuals. Blood. 1996;87:4746–53. [PubMed] [Google Scholar]
- 48.Remans PHJ, Gringhuis SI, van Laar JM, et al. Rap1 signaling is required for suppression of Ras-generated reactive oxygen species and protection against oxidative stress in T lymphocytes. J Immunol. 2004;173:920–31. doi: 10.4049/jimmunol.173.2.920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.