Abstract
T1R taste receptors are present throughout the gastrointestinal tract. Glucose absorption comprises active absorption via SGLT1 and facilitated absorption via GLUT2 in the apical membrane. Trafficking of apical GLUT2 is rapidly up-regulated by glucose and artificial sweeteners, which act through T1R2 + T1R3/α-gustducin to activate PLC β2 and PKC βII. We therefore investigated whether non-sugar nutrients are regulated by taste receptors using perfused rat jejunum in vivo. Under different conditions, we observed a Ca2+-dependent reciprocal relationship between the H+/oligopeptide transporter PepT1 and apical GLUT2, reflecting the fact that trafficking of PepT1 and GLUT2 to the apical membrane is inhibited and activated by PKC βII, respectively. Addition of l-glutamate or sucralose to a perfusate containing low glucose (20 mm) each activated PKC βII and decreased apical PepT1 levels and absorption of the hydrolysis-resistant dipeptide l-Phe(ΨS)-l-Ala (1 mm), while increasing apical GLUT2 and glucose absorption within minutes. Switching perfusion from mannitol to glucose (75 mm) exerted similar effects. l-Glutamate induced rapid GPCR internalization of T1R1, T1R3 and transducin, whereas sucralose internalized T1R2, T1R3 and α-gustducin. We conclude that l-glutamate acts via amino acid and glucose via sweet taste receptors to coordinate regulation of PepT1 and apical GLUT2 reciprocally through a common enterocytic pool of PKC βII. These data suggest the existence of a wider Ca2+ and taste receptor-coordinated transport network incorporating other nutrients and/or other stimuli capable of activating PKC βII and additional transporters, such as the aspartate/glutamate transporter, EAAC1, whose level was doubled by l-glutamate. The network may control energy supply.
The T1R taste receptor family comprises three G-protein coupled receptors (GPCRs), which act through α-gustducin and/or transducin to activate a PLC β2-dependent pathway (McLaughlin et al. 1992; Adler et al. 2000; Montmayeur et al. 2001; Li et al. 2002; Nelson et al. 2002). T1R receptors may also activate a cAMP-dependent pathway (Margolskee, 2002). The T1R2 + T1R3 heterodimer senses sweet taste, with simple sugars (glucose, fructose and sucrose) acting at concentrations in the hundred millimolar range and artificial sweeteners (acesulfame-K, sucralose and saccharin) at a few millimolar (Li et al. 2002). The T1R1 + T1R3 heterodimer senses amino acids, notably l-aspartate and l-glutamate (umami). In 2007, four papers identified roles for T1R2 + T1R3 sweet taste receptors in the regulation of glucose absorption and enteroendocrine hormone secretion, prompting new lines of research (Jang et al. 2007; Le Gall et al. 2007; Margolskee et al. 2007b; Mace et al. 2007b).
The first paper concerned regulation of the facilitated component of glucose absorption. There are two pathways of intestinal glucose absorption. At low concentrations, the predominant pathway is classical active absorption mediated by the Na+–glucose cotransporter. However, SGLT1 becomes saturated at concentrations below those generated at the apical membrane after a meal (30–100 mm; Mace et al. 2007b). SGLT1 therefore displays an important regulatory role by providing the first essential signal to generate additional transport capacity through rapid insertion of GLUT2 into the apical membrane (Helliwell et al. 2000a,b; Kellett & Helliwell, 2000; Kellett, 2001; Kellett & Brot-Laroche, 2005; Kellett et al. 2008). SGLT1 does so by depolarizing the apical membrane to induce rapid influx of Ca2+ through the neuroendocrine L-type channel Cav1.3, so that the rate of Ca2+ absorption is increased 3-fold at 10–20 mm glucose (Morgan et al. 2003,2007). The ensuing phosphorylation of myosin II in the terminal web and the peri-junctional actomyosin ring is associated with the enterocyte cytoskeletal rearrangement necessary for apical GLUT2 insertion (Madara & Pappenheimer, 1987; Berglund et al. 2001; Mace et al. 2007a).
However, no insertion over basal levels of GLUT2 occurs until the second essential signal is provided by the activation of sweet taste receptors in the apical membrane: the activation range of T1R2 + T1R3 by glucose in a heterologous expression system coincides with that for apical GLUT2 insertion in intestine (30–100 mm glucose; Li et al. 2002; Mace et al. 2007b). At these high concentrations, apical GLUT2 then provides the major pathway of absorption. Alternatively, apical GLUT2 insertion at low glucose concentrations (20 mm) may be rapidly induced by artificial sweeteners (Mace et al. 2007b).
Simple sugars and artificial sweeteners act synergistically through a T1R2 + T1R3–α-gustducin–PLC β2 pathway to stimulate PKC βII activation, which is essential for apical GLUT2 insertion (Helliwell et al. 2000b,2003). The necessary taste reception signalling components have been detected in the enterocytes of glucose-perfused jejunum from a fed rat and in solitary chemosensory cells (SCC) labelling with α-gustducin (Gα,gust) or transducin (Gα,t1), which therefore include enteroendocrine cells, brush cells and bipolar SCCs (Sbarbati & Osculati, 2005; Bezencon et al. 2007; Mace et al. 2007b). Rapid agonist-induced receptor internalization, a characteristic of GPCRs (Tan et al. 2004), has been observed for T1R2, T1R3 and α-gustducin and occurs simultaneously with externalization of T1R1, transducin and PLC β2 (Mace et al. 2007b).
Margolskee and colleagues further reported that enteroendocrine cells in the duodenum of starved mouse secrete incretins GIP and GLP-1 from K- and L-cells, respectively, in response to sweet taste receptor stimulation by high concentrations (550 mm) of glucose (Jang et al. 2007). In addition, sucralose increased SGLT1 mRNA, SGLT1 protein and active glucose absorption of mice on a low carbohydrate diet for 4 weeks. Increased SGLT1 up-regulation and incretin secretion were both attenuated in T1R3 knockout and in α-gustducin knockout mice (Margolskee et al. 2007a).
Long-term, taste receptor-mediated up-regulation of SGLT1 was also observed in the clonal enterocytic cell line, Caco-2. Under sugar-replete conditions, Caco-2 cells contain transcripts for T1R3 and α-gustducin and also T1R2 and T1R3 protein in the plasma membrane (Le Gall et al. 2007), so that fructose-induced increases in SGLT1 mRNA and protein were blocked by the T1R3 inhibitor lactisole. GLP-2, secreted from L-cells, up-regulates apical GLUT2 (Au et al. 2002). Work from several laboratories therefore demonstrates clearly that there are both enteroendocrine and enterocyte-based mechanisms for controlling sugar absorption (for a review, see Kellett et al. 2008).
The observations in the four papers raise several interesting questions: What other nutrients, if any, do sweet taste receptors regulate? What is the function of amino acid taste receptors – do they also regulate absorption of any nutrients? If so, is there any cross-talk between sweet and amino acid taste reception pathways, that is, can nutrient absorption be coordinated by different taste receptors?
The opportunity to provide positive answers to these questions was prompted by a preliminary observation that levels of the oligopeptide transporter, PepT1, appeared to decrease under conditions that increased those of apical GLUT2. PepT1 is proton dependent (Ganapathy & Leibech, 1985; Daniel, 2004; Thwaites & Anderson, 2007). It transports di- and tri-peptides and a variety of pharmacological agents (for reviews see Meredith & Boyd, 2000; Daniel, 2004). Of interest in the present context, there is evidence that PepT1 in Caco-2 cells is inhibited by Ca2+ and PKC and that rapid regulation of PepT1 involves trafficking to the apical membrane from an intracellular pool (Brandsch et al. 1994; Thamotharan et al. 1999b; Buyse et al. 2001; D'Souza et al. 2003; Watanabe et al. 2004). Investigation of the parallels between PepT1 and apical GLUT2 regulation has now led to the first functional demonstration of amino acid taste receptors in nutrient absorption and to the discovery of a Ca2+ and taste-receptor mediated network of nutrient absorption.
Methods
Animals
Male Wistar rats (240–270 g), fed ad libitum on a standard Bantin and Kingman (Hull, UK) rat and mouse diet, had free access to water and were kept under a 12 h day–night cycle. All procedures used conformed to the UK Animals (Scientific Procedures) Act 1986 and had the approval of the Ethical Review Process Committee of the Department of Biology at the University of York. The number of animals used specifically for this paper was 36. In addition, data were obtained from 65 other animals using archived vesicle and immunocytochemical samples that were prepared for and retained after previously published work.
Procedures
The following procedures have been previously described (Helliwell et al. 2000b; Mace et al. 2007b): single pass perfusion of jejunum from a fed rat in vivo and in vivo/in vitro, preparation of apical membrane vesicles, and Western blotting and immunocytochemistry, including the antibodies used. We therefore emphasize just some key points here. Rats were anaesthetized by an intraperitoneal injection of a mixture of 1.0 ml Hypnorm (Janssen Animal Health, High Wycombe, UK) and 0.5 ml Hypnovel (Roche Diagnostics, Welwyn Garden City, UK) per kg body weight. For in vivo perfusions, tail pinch, foot pinch and corneal reflexes were carefully monitored throughout the duration of the perfusion. Additional anaesthetic was administered by intramuscular injection of a mixture of 0.4 ml Hypnorm and 0.2 ml Hypnovel per kg body weight when required. Rats were humanely killed by exsanguination under anaesthetic at the conclusion of the experiment. The in vivo single-pass perfusion technique uses two perfusate reservoirs to permit a paired comparison between a control (0–40 min) and experimental (40–90 min) period; for each set of conditions, data were collected from four perfusions. [3H]inulin (0.7 kBq ml−1) was added to correct for changes in water transport. When nutrient concentrations were less than 75 mm, their total concentration was made up to 75 mm by the addition of mannitol to obviate any potential osmotic effects. Each membrane vesicle preparation was made from two rats and three preparations were used for each condition. Immunocytochemistry employed spectral unmixing techniques to subtract all non-specific background contributions, including autofluorescence, reflection and non-assigned residuals. Where antigen was available for a given antibody, neutralization of antibody with excess peptide therefore resulted in a totally black image. Accordingly, we show only individual instances to avoid large areas of black print. Dual luminal and vascular perfusion (n= 4) for characterization of dipeptide transport was performed as described by Shepherd et al. (2002). Expression of PepT1 in oocytes was performed as described in Pieri et al. (2008).
Antibodies
Antibody raised in rabbit to the C-terminal region AEIEAQFDEDEKKK of PepT1 was commissioned from Invitrogen. Goat IgG to EAAC1 (EAAT3) was sc-7761 from Santa Cruz (Autogen Bioclear, UK). Other antibodies are identified in Mace et al. (2007b).
l-Phenylalanine(ΨS)-l-alanine was synthesized as described by Bailey (2005) and Bailey et al. (2006). This dipeptide is hydrolysis resistant, is transported by PepT1 and undergoes transepithelial transport at a high rate (Fig. 3). Perfusate samples were analysed for peptide by HPLC at 210 nm on a 5 μm octadecyl silane sila C18 column (Jones Chromatography, Hengoed, Glamorgan, UK). The mobile phase was 20% methanol–80% 21 mm KH2PO4 (pH 5.0).
Figure 3. Transport characteristics of the novel hydrolysis-resistant dipeptide, l-Phe(ΨS)-l-Ala, in rat jejunum.
A, rat jejunum was perfused in vivo for 90 min with KHB containing 20 mm glucose and 1 mm l-Phe(ΨS)-l-Ala. The time course depicts the rate of glucose absorption (□, left-hand axis) and rate of l-Phe(ΨS)-l-Ala transport (▪, right hand axis). Rates are measured in μmol min−1 (g dry weight)−1. B, transport characteristics of l-Phe(ΨS)-l-Ala at pH 6.8. The time course of the cumulative appearance of 1 mm l-Phe(ΨS)-l-Ala in the vascular circuit of a luminal and vascularly perfused preparation of rat jejunum in situ. The inset shows the rate of vascular appearance compared with the rat of luminal disappearance. C, specificity of l-Phe(ΨS)-l-Ala for PepT1. Competitive inhibition of the transport of 0.4 μm d-phenylalanine-l-glutamine by l-Phe(ΨS)-l-Ala, when PepT1 was expressed in oocytes.
Statistical analysis
Values are presented as means ±s.e.m. and were tested for significance using paired or unpaired Student's t test as appropriate.
Results
A reciprocal relationship between the levels of PepT1 and GLUT2 in the apical membrane
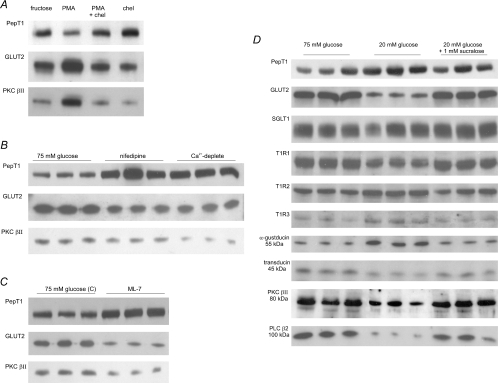
We have previously published many Western blots revealing the regulation of apical GLUT2, PKC βII and taste reception signalling components under different conditions. In Fig. 1 we now reproduce the main bands of interest for each set of conditions, in order that the reader can compare them immediately in one place with new data showing the regulation of PepT1 in each condition.
Figure 1. The levels of GLUT2 and PKC βII in the apical membrane show a reciprocal relationship with those of PepT1.
Rat jejunum was perfused in KHB for 30 min as described and apical membrane vesicles prepared. Proteins (20 μg) were then separated on 10% SDS-PAGE, transblotted onto PVDF membrane and immunoblotted for GLUT2, PKC βII and PepT1. With the exception of panel A, three separate preparations from two rats each are shown for each condition. All controls, in which antibody has been neutralized by preincubation with excess immunogenic peptide, are described in the references given. The blots for apical GLUT2 and PKC βII are reproduced with permission from the Biochemical Journal (A, Helliwell et al. 2000b), and The Journal of Physiology (B, C and D, Morgan et al. 2007; Mace et al. 2007a,b). The PepT1 blots are new; PepT1 controls were negative (data not shown). A, effect of PMA. Rat jejunum was perfused with 5 mm d-fructose in the presence or absence of 200 nm PMA and/or 2 μm chelerythrin. B, effects of nifedipine and luminal Ca2+ depletion. Jejunum was perfused with 75 mm glucose alone, 75 mm glucose and 10 μm nifedipine or 75 mm glucose in KHB from which Ca2+ was omitted (Ca2+-deplete conditions). C, effect of MLCK inhibition by ML-7. Rat jejunum was perfused with either 75 mm glucose alone or 75 mm glucose and 5 μm ML-7. D, effects glucose and artificial sweetener. Jejunum was perfused with either 75 mm glucose, 20 mm glucose or 20 mm glucose and 1 mm sucralose. Note that the first three samples of the PKC βII were inadvertently placed at the opposite end of the gel; they have been digitally transposed solely for presentational purposes; these PKC βII blots are new.
Rat jejunum was perfused in vitro with modified Krebs–Henseleit buffer (KHB) containing 5 mm fructose in the absence or presence of 200 nm PMA and/or 2 μm chelerythrin, which activate and inhibit conventional PKC isoforms, respectively. Western blots of GLUT2 and PKC βII in apical membrane vesicles prepared immediately after perfusion for 30 min are reproduced in Fig. 1A from Helliwell et al. (2000b). They show that PMA increased apical GLUT2 3.8 ± 0.6-fold compared with the control of 5 mm fructose alone. The increase was blocked by chelerythrin, which had no effect alone, and correlated with PKC βII activation, as indicated by its increased level at the apical membrane. When blots of vesicles were subsequently probed with PepT1 antibody to obtain new information, a single strong band was detected at ∼70 kDa, consistent with its reported molecular mass of 75 kDa for PepT1 (Saito et al. 1995; Ogihara et al. 1999; Hussain et al. 2002). In contrast to apical GLUT2, PMA almost halved the level of PepT1 to 55 ± 12% (n= 3, P < 0.01) of the fructose control value; the decrease was blocked with chelerythrin, which alone had no effect on PepT1 levels. PMA has no effect on PepT1 synthesis (Shiraga et al. 1999). These results demonstrate that PKC βII activation down-regulated apical PepT1 levels.
Apical GLUT2 insertion is dependent on enterocyte cytoskeletal rearrangement involving myosin contraction and on the activity of PKC βII; both these processes are Ca2+ dependent (Morgan et al. 2003; Morgan et al. 2007; Mace et al. 2007a). The blots in Fig. 1B are reproduced from Mace et al. (2007a) and show the regulation of apical GLUT2 and PKC βII by Ca2+. They reveal that when jejunum is perfused in vivo with 75 mm glucose, apical GLUT2 and PKC βII are high; however, when 10 μm nifedipine is included in the perfusate to block the glucose-dependent increase in Ca2+ absorption through Cav1.3, apical GLUT2 and PKC βII levels are halved to 44 ± 4 and 52 ± 4%, respectively, compared with control. We now find that PepT1 is doubled (2.2 ± 0.1-fold, P < 0.001). Very similar results were obtained when Ca2+ absorption was abolished by removing Ca2+ from the perfusate (Ca2+-depelete conditions, Fig. 1B). The ability of Ca2+ to induce cytoskeletal rearrangement is prevented by 20 μm ML-7, at which concentration it is a relatively selective, cell permeant inhibitor of MLCK; ML-7 therefore blocks the Ca2+-dependent increase in myosin II phosphorylation and subsequent cytoskeletal rearrangement in response to glucose. We now observe that ML-7 increases PepT1 by 1.6 ± 0.1-fold (Fig. 1C), whereas the blots for apical GLUT2 and PKC βII reproduced from Mace et al. (2007a) demonstrate that both are sharply diminished to 37 ± 4 and 37 ± 7% of the value for the glucose control, respectively.
Our early work revealed that perfusion of rat jejunum in vivo with 75–100 mm glucose doubled apical GLUT2 and increased PKC βII 1.6-fold compared with mannitol alone (Kellett & Helliwell, 2000). We have since found that PepT1 was concomitantly halved in the presence of glucose compared with mannitol; furthermore, phloridzin at high glucose concentrations strongly diminishes PepT1 (data not shown).
When we investigated the role of sweet taste receptors in regulating glucose absorption in perfused rat jejunum in vivo, we monitored changes in apical membrane levels of GLUT2, T1R1, T1R2, T1R3, α-gustducin, transducin and PLC β2 using three conditions with respect to apical GLUT2 (Mace et al. 2007b). The first was 20 mm glucose alone, at which there is a basal level of GLUT2 in the apical membrane. Since rapid glucose-induced insertion of apical GLUT2 is first detectable at 30 mm glucose, use of 20 mm glucose permitted detection of apical GLUT2 regulation by 1 mm sucralose. The results were compared with those for 75 mm glucose alone, at which there is substantial apical GLUT2 insertion above the basal level. Ca2+ absorption was maximal in each of the three conditions. We have previously presented the full Western blots for these proteins, which include bands deriving from rapid turnover products (Mace et al. 2007b). We therefore reproduce in Fig. 1D only the main bands of immediate interest, to which we now add PepT1 and PKC βII (not done previously). Apical GLUT2 is strongly enhanced at both 75 mm glucose and 20 mm glucose + 1 mm sucralose compared with 20 mm glucose alone, demonstrating a sweet taste receptor response. We now find that PKC βII is correspondingly increased to an average of 1.5 ± 0.2-fold (P < 0.05, n= 3), but, in contrast, PepT1 is decreased by an average of 48 ± 17% (P < 0.01, n= 3). There is no effect of sugar on the level of SGLT1, which appears as a broad band, but is in fact a closely spaced triplet (Balen et al. 2008). Note that 75 mm glucose or 20 mm glucose + 1 mm sucralose induce rapid internalization of T1R2, T1R3 and α-gustducin, whereas T1R1, transducin and PLC β2 are inserted into the apical membrane. For GLUT2, SGLT1, T1R2, α-gustducin, transducin, PLC β2 and PKC βII, preincubation of antibody with excess immunogenic peptide abolished all bands for that protein, confirming the specificity of detection (data not shown). For T1R1 and T1R3, no peptide was available, since the sequence was deemed ‘commercially sensitive’. However, the fact that blots and immunocytochemistry (see below) gave consistent results for the apical membrane indicates that labelling is specific.
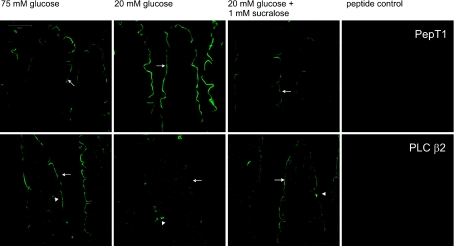
The changes revealed by Western blotting in the levels of apical GLUT2 under the three conditions have been confirmed by immunocytochemistry previously (Mace et al. 2007b). Additional immunocytochemistry in Fig. 2 now confirms the changes seen for PepT1 and PLC β2, showing how each traffics in the opposite direction, with the former being high and the latter low at 20 mm glucose compared with 75 mm glucose or 20 mm glucose + 1 mm sucralose. PepT1, an established marker for the brush-border enterocyte, is readily seen at the apical membrane over the full length of the villus, in agreement with previous reports (Ogihara et al.1996,1999; Hussain et al.2002). PLC β2 was also located over the full length of the villus at the apical membrane; however, it is essential to observe its enterocyte location under optimal conditions, when there is a strong sweet taste receptor signal (Figs 1D and 2). Note that for both proteins the peptide control for antibody labelling is completely negative. This is because all images were obtained by spectral unmixing techniques, which automatically subtract non-specific background contributions and unassigned residuals (Mace et al. 2007b); all images shown in this and the previous paper therefore display specific labelling only. Interestingly, the PLC β2 images reveal three distinct types of SCCs, which are indicated by the arrowheads in Fig. 2. The left image most likely shows an enteroendocrine cell and the middle a typical bipolar SCC (Sbarbati & Osculati, 2005); the right hand image shows a triangular cell with the apex at the basolateral membrane and very strong labelling of the base at the apical membrane. Such PLC β2-labelled triangular cells are relatively common.
Figure 2. Immunocytochemistry of PepT1 and PLC β2 regulation by glucose and artificial sweeteners in rat jejunum.
Rat jejunum was perfused with 75 mm glucose, 20 mm glucose or 20 mm glucose and 1 mm sucralose for 30 min. Sections (7 μm) were labelled with a rabbit primary antibody detecting either PepT1 or PLC β2 followed by a goat anti-rabbit secondary antibody conjugated to Alexa 488 (green). Arrows: apical membrane; arrowheads: three different types of SCC containing PLC β2. The peptide controls were obtained by neutralization of primary antibody with excess immunogenic peptide. Images were processed using spectral unmixing techniques (see Methods), which automatically subtract all background contributions to leave only specific labelling as demonstrated by the peptide controls, which are totally black.
We have used the term ‘internalization’ as shorthand to denote loss of proteins from the membrane without specifying their ultimate fate. Transporters appear to reside in intracellular compartments or vesicles from which they can recycle (Mace et al. 2007b; Tobin et al. 2008). However, signalling components such as GPCRs, G-proteins, PKC βII and PLC β2 also undergo rapid turnover and proteolytic degradation (Helliwell et al. 2003; Mace et al. 2007b).
The regulation of dipeptide transport by glucose and artificial sweetener
The viability of PepT1-mediated transport in perfused rat jejunum in vivo was assessed by determining the luminal disappearance of the novel hydrolysis-resistant dipeptide l-Phe(ΨS)-l-Ala (Bailey et al. 2005). With 1 mm dipeptide in the presence of 20 mm glucose, the high rate of dipeptide transport was maintained at a steady-state rate of 1.22 ± 0.06 μmole min−1 (g dry weight)−1 (n= 6) from 10 to 90 min, the full duration of perfusions described below (Fig. 3A); the corresponding rate of glucose absorption was 10.31 ± 0.81 μmol min−1 (g dry weight)−1, similar to that reported previously (Kellett & Helliwell, 2000; Mace et al. 2007b). Tests of the kinetic characteristics of the dipeptide transport at pH 6.8 in a luminally and vascularly perfused preparation of jejunum revealed that ∼80% of the l-Phe(ΨS)-l-Ala absorbed across the apical membrane appeared in the vascular circuit, demonstrating effective transepithelial transport (Fig. 3B). When PepT1 was expressed in oocytes, the transport of 0.4 μm d-[3H]phenylalanine-l-glutamine, a known non-hydrolysable substrate of PepT1, was competitively inhibited by l-Phe(ΨS)-l-Ala with a Ki of 0.32 ± 0.08 mm (Fig. 3C). The characteristics of l-Phe(ΨS)-l-Ala therefore make it an excellent tool with which to monitor the regulation of PepT1.
In order to investigate the effect of glucose on dipeptide transport, so-called ‘paired’ perfusions were performed in which the sugar conditions were changed half way through to provide a direct comparison between two sets of conditions within a single perfusion; this approach minimizes interanimal variations in data. Thus jejunum was perfused initially in vivo with 1 mm l-Phe(ΨS)-l-Ala and 75 mm mannitol for 40 min (arrow, Fig. 4A). A steady-state rate of peptide transport of 1.56 ± 0.12 μmole min−1 (g dry weight)−1 was achieved after a marked up-regulation over the first 15–20 min. After 40 min, the perfusate was switched to an identical one containing 75 mm glucose instead of mannitol, when the rate of peptide transport decreased within 20 min to 0.43 ± 0.06 μmole min−1 (g dry weight)−1 (n= 4; P < 0.001). The 72 ± 4% decrease in peptide transport compares with a decrease in PepT1 levels of 57 ± 16% determined by Western blotting of apical membrane vesicles (data not shown).
Figure 4. Regulation of glucose and peptide absorption by intestinal taste receptors.
A, regulation by glucose. Jejunum was perfused in vivo with 75 mm mannitol (0 mm glucose) and 1 mm l-Phe(ΨS)-l-Ala for 40 min, when the perfusate was switched (arrow) to an identical one containing 75 mm glucose (0 mm mannitol). The time course depicts the rate of glucose absorption (□, left-hand axis) and the rate of l-Phe(ΨS)-l-Ala transport (▪, right-hand axis). B, regulation by sucralose. Jejunum was perfused in vivo with 20 mm glucose, 1 mm d-Phe(ΨS)-l-Ala and 1 mm sucralose for 40 min, when the perfusate was switched to an otherwise identical perfusate without sucralose. C, regulation by l-glutamate. Jejunum was perfused in vivo with 20 mm glucose, 1 mm l-Phe(ΨS)-l-Ala and 25 mm l-glutamate for 40 min, when the perfusate was changed to an otherwise identical perfusate in which l-glutamate was replaced by d-glutamate.
When 20 mm glucose was perfused in vivo with 1 mm sucralose and 1 mm l-Phe(ΨS)-l-Ala (Fig. 4B), there was a large increase in the rate of glucose absorption over the first 15 min to give a steady-state rate of glucose absorption of 23.95 ± 1.94 μmol min−1 (g dry wt)−1, similar to that reported by Mace et al. (2007b); the corresponding rate of dipeptide absorption was 0.73 ± 0.15 μmol min−1 (g dry wt)−1. When sucralose was omitted from the perfusate at 40 min, the rate of glucose absorption rapidly fell to 9.08 ± 0.67 μmol min−1 g dry wt−1 (n= 4; P < 0.001), caused entirely by trafficking of GLUT2 away from the apical membrane (Mace et al. 2007b): in the presence of sucralose, apical GLUT2 is some 3-fold greater than in its absence (Fig. 1D). In contrast, omission of sucralose resulted in a concomitant 1.8 ± 0.2-fold increase in peptide transport to 1.33 ± 0.16 μmol min−1 (g dry wt)−1 (n= 4; P < 0.001), compared with the 1.9 ± 0.2-fold increase in PepT1 level (n= 3, P < 0.001; Fig. 1D). The rates of glucose absorption in the presence and absence of sucralose in the present experiments were not significantly different from those reported previously in experiments without dipeptide, showing that 1 mm l-Phe(ΨS)-l-Ala had no effect on glucose absorption.
The regulation of dipeptide and glucose absorption by l-glutamate
The experiments above show that glucose and artificial sweetener inhibit PepT1-mediated transport by activation of PKC βII through a sweet taste receptor- and PLC β2-dependent pathway. Moreover, enterocytes contain both T1R1 and T1R3 (Mace et al. 2007b) and the T1R1 + T1R3 amino acid receptor has the potential to regulate PKC βII. We therefore investigated whether l-glutamate, one of the two cognate amino acids for the rat T1R1 + T1R3 amino acid taste receptor (Li et al. 2002), might also regulate PepT1 and apical GLUT2.
A concentration of 25 mm was chosen, because it produces a near-maximal increase in intracellular Ca2+ when rat T1R1 and T1R3 are coexpressed in a heterologous system (Li et al. 2002). Rat jejunum was perfused in vivo with KHB containing 25 mm l-glutamate, 1 mm d-Phe(ΨS)-l-Ala and 20 mm glucose. At 40 min, the perfusate was changed for an otherwise identical one in which l-glutamate was replaced by its inactive analogue, d-glutamate (arrow, Fig. 4C). The rate of l-Phe(ΨS)-l-Ala transport in the presence of l-glutamate reached a steady-state rate of 0.69 ± 0.011 μmol min−1 (g dry weight)−1 (n= 4), which was increased by 1.9-fold to 1.28 ± 0.16 μmol min−1 (g dry weight)−1 (n= 4; P < 0.01; Fig. 4C) in the absence of l-glutamate. This was not significantly different from the rate of l-Phe(ΨS)-l-Ala transport in the presence of 20 mm glucose only (P= 0.13; Fig. 4Bvs.4C). The rate of glucose absorption in the presence of l-glutamate reached a steady-state rate of 16.43 ± 0.59 μmol min−1 (g dry weight)−1 (n= 4; Fig. 4C), which rapidly diminished some 42% to 9.47 ± 0.43 μmol min−1 (g dry weight)−1 in its absence (n= 4; P < 0.01 compared to l-glutamate).
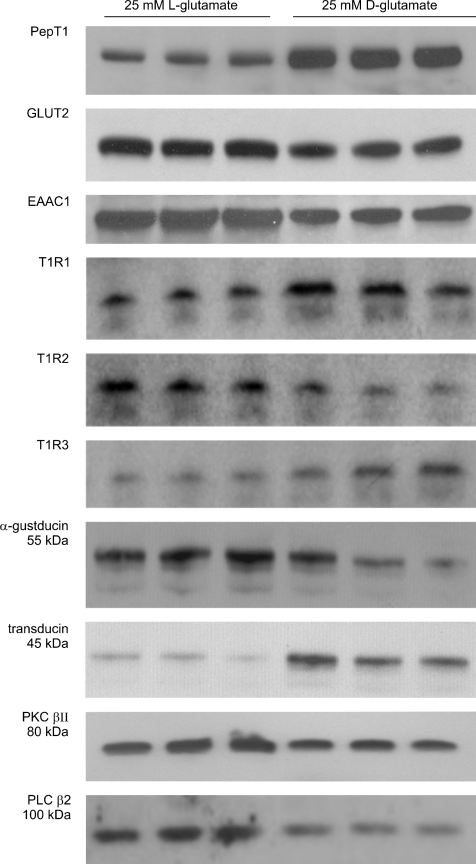
In order to correlate these changes in absorption rates with those of transporter levels, rat jejunum was perfused in vivo with either 25 mm l-glutamate or d-glutamate in the presence of 20 mm glucose for 30 min, when apical membrane vesicles were prepared for Western blotting. Figure 5 shows that d-glutamate increased the level of PepT1 by 1.8 ± 0.2-fold compared with l-glutamate (n= 3; P < 0.001) to correlate with the change in peptide transport. In contrast, l-glutamate increased PKC βII 1.6 ± 0.1-fold (n= 3; P < 0.01) and apical GLUT2 2.0 ± 0.1 -fold compared with d-glutamate (n= 3; P < 0.001). Given these marked effects of l-glutamate, we also probed the Western blot for EAAC1, which has been identified as the l-glutamate/aspartate transporter in intestine (Iwanaga et al. 2005). When viewed on a light box, EAAC1 appeared as a tight doublet at ∼70 kDa; there were no other bands. However, as shown in Fig. 5, the resolution of the scanner used to scan the image for publication was not sufficient to resolve the two bands, which were merged to appear as a single band. For l-glutamate, the apical levels of EAAC1 were increased to 1.7 ± 0.1-fold (n= 3; P < 0.01) compared with those in its absence.
Figure 5. Regulation of transporters and taste reception signalling components by l-glutamate.
Rat jejunum was perfused for 30 min with 20 mm glucose and either 25 mm l-glutamate or 25 mm d-glutamate. Apical membrane vesicles (20 μg) were immunoblotted for the proteins shown.
Western blotting also revealed that l-glutamate induced profound changes in the pattern of rapid trafficking of taste receptor and signalling components compared with d-glutamate. All taste receptor components (T1R1, T1R2 and T1R3) show some evidence of proteolysis in Western blots; moreover, the signalling components, α-gustducin, transducin, PLC β2 and PKC βII, undergo some combination of cleavage, turnover and ubquitylation to produce multiple species on activation. We have described the changes induced by sugars in some detail (Helliwell et al. 2003; Mace et al. 2007b) and find now that l-glutamate induces similar changes. It is apparent that, compared with d-glutamate, l-glutamate causes the rapid internalization of T1R1, T1R3 and transducin, whereas it causes apical membrane insertion of T1R2, α-gustducin, PLC β2 and PKC βII.
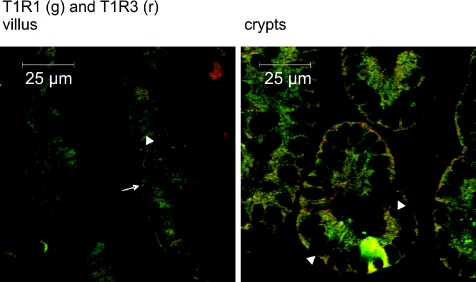
In the jejunum of a fed rat, T1R1 and T1R3 are localized in enterocytes, SCCs and in Paneth cells (Mace et al. 2007b). Figure 6 shows T1R1 and T1R3 colocalized in the apical membrane (arrow) over large parts of the villus and in the enterocyte cytoplasm. Note particularly the absence of T1R1 and T1R3 in the basolateral membrane in the villus as indicated by the membrane's appearance as a dark line between neighbouring enterocytes (arrowhead). In the crypts, T1R1 and T1R3 are again to be found in the Paneth cells and cytosolic vesicles. However, in contrast to the villus, the basolateral boundary of the cell is clearly seen (arrowheads). Supplementary Figs 1A and B confirm T1R1, T1R3 and transducin expression in Paneth cell granules by colocalization of each with lysozyme, an established Paneth cell marker. Supplementary Fig. 1C shows that T1R3 appears to be secreted from Paneth cell granules in fed and glucose-perfused conditions.
Figure 6. Colocalization of T1R1 and T1R3 in villus and crypts.
Sections (7 μm) were dual-labelled with primary antibodies detecting T1R1 (green) and T1R3 (red) using Alexa 488- and 568-conjugated secondary antibodies. In the merged images for villus, T1R1 and T1R3 are colocalized in the apical membrane (arrow), but not in the basolateral membranes, which appear as dark lines (no labelling, arrowhead) where the membrane separates enterocytes. Note also the contrast with crypts, in which T1R1 and T1R3 are colocalized in the clearly visible basolateral membrane (arrowheads).
Discussion
A general model for the coordination of nutrient absorption by Ca2+ and taste receptors
In a remarkable display of coordinated regulation, l-glutamate, d-glucose, sucralose and Ca2+ regulate peptide, l-glutamate and glucose absorption. The fundamental concept underlying the working model for regulation in Fig. 7 is that sweet and amino acid taste receptors located in enterocytes target a common pool of PKC βII in an SGLT1- and Ca2+-dependent manner (left hand enterocyte); PKC βII in turn coordinates transporter trafficking (right hand enterocyte).
Figure 7. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine.
The left hand enterocyte depicts the signalling mechanisms for the activation of a common PKC βII pool by Ca2+ absorption and by sweet and amino acid taste receptors; the right hand enterocyte depicts the regulation of transporters by PKC βII.
The first signal for PKC βII activation arises from glucose absorption through SGLT1, which depolarizes the apical membrane to stimulate Cav1.3 and increase Ca2+ absorption 3-fold (Morgan et al. 2007; Mace et al. 2007a). The resulting increase in intracellular Ca2+ induces terminal web and cytoskeletal rearrangement, which changes the pattern of protein trafficking to the apical membrane (see ML-7 data, Fig. 1C). Increased Ca2+ further promotes the translocation of inactive, but competent (phosphorylated) PKC βII from the cytosol to the apical membrane by increasing its affinity for phosphatidylserine, but PKC βII is not yet fully activated (Newton, 1997). For the latter, a taste reception signal is now required. While Ca2+ absorption is maximal at 20 mm glucose, the sweet taste receptor signal is provided by T1R2 + T1R3, which operates over the range 30–100 mm glucose, identical to that for apical GLUT2 trafficking (Kellett & Helliwell, 2000; Li et al. 2002). The sweet taste receptor is also maximally stimulated by 1 mm sucralose to double glucose absorption at 20 mm glucose by a 3-fold increase in apical GLUT2 (Mace et al. 2007b). Stimulation of T1R2 + T1R3 activates a heterotrimeric G-protein containing α-gustducin to which it is preferentially coupled (see below), resulting in dissociation of the Gα,gust subunit from the membrane into the cytosol. The βγ subunits remain anchored in the membrane, where they bind and activate PLC β2, thereby promoting the translocation of inactive PLC β2 from the cytosol to the membrane (Rhee, 2001). PLC β2 now generates diacylglycerol (DAG), which completes the activation of PKC βII by promoting removal of the N-terminal pseudosubstrate region from the active site (Newton, 1997). Additional translocation of PKC βII may occur as the affinity of PKC βII for membrane phospholipid increases further on full activation; see the effect of sucralose in Fig. 1D.
The right hand enterocyte of Fig. 7 now shows that active PKC βII inhibits trafficking of PepT1 to the apical membrane and promotes that of apical GLUT2, accounting for their reciprocal relationship in all blots presented above. Dipeptide undergoes hydrolysis within the enterocyte or exits across the as yet unidentified basolateral peptide transporter (BLPT) (Saito & Inui, 1993; Thwaites et al. 1993; Shepherd et al. 2002). The left hand enterocyte shows that the amino acid taste receptor, T1R1 + T1R3, is also coupled through a G-protein, in this case transducin (Gα,t1), to activate PLC β2 and hence PKC βII, thereby inducing changes in transporter regulation similar to those of T1R2 + T1R3.
The mechanism in Fig. 7 illustrates how the separate components can be distinguished experimentally by the use of five specific conditions in rat jejunum perfused in vivo: (i) 20 mm glucose alone, where sweet taste receptors are not effectively stimulated, so that PepT1 and apical GLUT2 are at maximal and basal level levels, respectively; (ii) 20 mm glucose plus 1 mm sucralose, where sweet taste receptors are maximally stimulated by sucralose, which is neither absorbed nor metabolized; (iii) 75 mm glucose alone, so that sweet taste receptors are approaching maximal stimulation by glucose; 20 mm glucose plus either (iv) 25 mm l-glutamate or (v) 25 mm d-glutamate, so that amino acid taste receptors are maximally or not effectively stimulated, respectively. In addition, the Ca2+ and taste receptor components of the mechanism in Fig. 7 can be bypassed completely by perfusion with PMA to activate PKC βII directly.
The inclusion of glucose at a concentration of 20 mm glucose or more in each of the conditions ensures that Ca2+ absorption through Cav1.3 is always maximal (Morgan et al. 2003,2007). The upstream signal from SGLT1/Ca2+ can be blocked by phloridzin, nifedipine or use of Ca2+-deplete perfusate. In each case, the ensuing downstream taste reception signals are prevented, so that at 75 mm glucose, for example, both apical GLUT2 and PKC βII are diminished as PepT1 is simultaneously increased (Fig. 1B). Such reciprocal control of intracellular free Ca2+ and PepT1 has been reported by Daniel and colleagues in Caco-2 cells (Wenzel et al. 2002); in particular, their use of nifedipine and other antagonists is in agreement with our finding that L-type channels play an important role in epithelial Ca2+ entry in rat jejunum (Morgan et al. 2003,2007; Mace et al. 2007a).
Inhibition of PepT1 by Ca2+ in Caco-2 cells or the mutual inhibition of amino acid and active sugar transport is membrane potential dependent (Hindmarsh et al. 1966; Murer et al. 1975). Several lines of evidence, however, indicate that the changes in dipeptide and sugar absorption described above are caused by PepT1 and apical GLUT2 trafficking. Thus changes in dipeptide absorption correlate with changes in PepT1 levels and changes in glucose absorption are caused solely by change in apical GLUT2 without change in active transport (Mace et al. 2007b). All solutions contain 20 mm glucose at which concentration Ca2+ absorption through Cav1.3 is maximal; nevertheless, sucralose and l-glutamate independently and rapidly induce changes in PepT1, apical GLUT2 and EAAC1 levels at 20 mm glucose (Figs 1D and 5). Related changes induced by ML-7 (Fig. 1C) have no effect on the upstream signal of Ca2+ absorption (Mace et al. 2007a). PMA promotes PepT1 trafficking independently of membrane potential in Caco-2 cells (Brandsch et al. 1994). PepT1 contains either two (human) or one (rat) consensus sites for PKC (Liang et al. 1995; Saito et al. 1995); phosphorylation of the latter site might provide a basis for direct effects on PepT1 trafficking.
Taste receptor regulation of PepT1, apical GLUT2 and EAAC-1
At 20 mm glucose, apical GLUT2 is at basal and PepT1 at maximal levels. Increasing the glucose concentration to 75 mm or the addition of 1 mm sucralose each provides a potent sweet taste receptor signal, which strongly increases apical GLUT2 through T1R2 + T1R3 (Fig. 1D). We have now shown that both signals activate PKC βII, while at the same time halving PepT1. Of note, new steady-state rates of dipeptide transport are attained within 15 min and are reciprocal to changes in the rates of glucose absorption mediated by apical GLUT2. As argued in detail elsewhere, sucralose and other artificial sweeteners of different potencies act through sweet taste receptors (Mace et al. 2007b).
We had previously established that the amino acid taste receptor, T1R1 + T1R3, is present in the apical membrane. Since T1R1 + T1R3 also targets PLC β2, we investigated the possibility that T1R1 + T1R3 might also regulate peptide and glucose absorption. In the presence of 20 mm glucose, 25 mm l-glutamate rapidly up-regulated glucose absorption while dipeptide absorption remained low, but the situation was reversed within 15 min of switching to 25 mm d-glutamate (Fig. 4C).
On this basis, we predicted all the changes in transporters and signalling proteins induced by l-glutamate (Fig. 5). The changes in steady state rates of glucose and dipeptide absorption were again reflected in changes in apical GLUT2 and PepT1 levels. Confirmation that l-glutamate acts through the amino acid taste receptor is provided by its effect on receptor trafficking. A recognized characteristic of GPCRs is that activation by agonist results in rapid internalization from the target membrane to cytosolic vesicles (Tan et al. 2004; Scherrer et al. 2006). l-Glutamate simultaneously internalizes T1R1, T1R3 and transducin and induces trafficking of T1R2 and α-gustducin to the apical membrane, as well as activating and externalizing the downstream signalling components of amino acid taste reception, namely PLC β2 and PKC βII (Fig. 5). These changes contrast with those induced by high glucose or low glucose plus sucralose, which internalize T1R2, T1R3 and α-gustducin while externalizing T1R1 and transducin together with PLC β2 and PKC βII. The two sets of results are consistent with the view that T1R3 is a required partner for both T1R1 and T1R2 to be functional as amino acid and sweet taste receptors, respectively. The concerted trafficking of PLC β2 and PKC βII to the apical membrane is consistent with the regulation of the latter by the former. The trafficking patterns indicate that transducin is the preferred partner for functional T1R1 + T1R3 and α-gustducin that for T1R2 + T1R3. This pattern is repeated in the secretory granules of the crypts, where T1R1 and T1R3 are routinely colocalized with transducin (Fig. 6, Supplementary Fig. 1A and B).
Apical GLUT2, PepT1 and PLC β2 are distributed throughout the full length of the jejunal villus; in addition T1R1 and T1R3, which target PLC β2, were detected over the whole of the villus, as were T1R2, α-gustducin, transducin and Cav1.3 (Fig. 6; see also Mace et al. 2007b). The changes in transporter levels and in glucose and dipeptide absorption in the l-glutamate perfusions correlate with those observed in the glucose and sucralose perfusions. We therefore conclude that l-glutamate can act via amino acid taste receptors and sugars via sweet taste receptors to coordinate the regulation PepT1 and apical GLUT2 reciprocally and to achieve a similar distribution of transporters through a common enterocytic pool of PKC βII, which is activated by receptor-mediated activation of PLC β2.
In principle, any transporter with access to PKC βII in enterocytes could be part of a coordinated network with PepT1 and apical GLUT2. Thus the network might be larger than the core revealed so far. In order to test this possibility, we looked to l-glutamate, which is transported by EAAC1 (system XAG− or EAAT3; Kanai & Hediger, 1992). Interestingly, EAAC1 specificity for aspartate and glutamate appears to be the same as that for T1R1 + T1R3 (Li et al. 2002). Moreover, PKC-dependent EAAC1 trafficking results in a doubling of l-glutamate transport in C6 glioma cells (Fournier et al. 2004). Figure 5 reveals that the level of EAAC-1 in the intestinal apical membrane was also doubled by l-glutamate compared with d-glutamate. Trafficking correlated with increased PKC βII and increased apical GLUT2 and glucose absorption, whereas PepT1 and dipeptide absorption were decreased. Although EAAC-1 is present in the villus, it is predominant in lower villus and crypts and in ileum compared with jejunum, in contrast to apical GLUT2 and PepT1 (Fan et al. 2004; Iwanaga et al. 2005). The balance of signalling pathways for different transporters within the network will therefore depend on regional differences.
A network of nutrient absorption for the control of energy supply
What is the physiological significance of the coordinated regulation of PepT1, EAAC-1 and apical GLUT2? Part of the answer, at least, seems to be control of energy supply, for the substrates of apical GLUT2 (glucose) and EEAC-1 (the non-essential amino acids l-glutamate and l-aspartate) are important energy sources for enterocytes, while PepT1 provides an alternative to amino acid transport. Aspartate and glutamate are also readily formed within the cell from their immediate precursors, glutamine and asparagine. Glutamine is taken up by intestine so efficiently as an energy source that it is extracted from the circulation in starvation and converted to glutamate.
Intestinal mucosa is a rapidly dividing tissue requiring amino acids and energy to sustain a high rate of amino acid synthesis. At the same time, an important function of mucosal metabolism is to deliver the carbon skeleton of glucose across the intestine in the form of alanine and lactate (the phenomenon of aerobic lactate production). This is achieved by transamination of pyruvate by glutamate to alanine and 2-oxoglutarate, a key TCA cycle intermediate. 2-Oxoglutarate is converted ultimately to oxaloacetate, which is derived also from transamination of 2-oxoglutarate by aspartate. Replenishment of TCA cycle intermediates from aspartate and glutamate not only generates ATP by oxidative phosphorylation, but also diverts pyruvate away from oxaloacetate to lactate or alanine, which enter the circulation. Glucose and glutamate/aspartate absorption therefore go hand in hand, assisted by complementary signals for mutual up-regulation by PKC βII activation through sweet and amino acid taste receptors. Energy, of course, is also required to power Na+- and H+-dependent nutrient absorption. Up-regulation of apical GLUT2 rather than SGLT1 has the advantage that absorption by apical GLUT2 is facilitated and therefore energetically cost-free, which is desirable when energy demand is high during postprandial absorption (Walker et al. 2005).
Significant absorption of amino acids occurs in the form of dipeptides; once in the enterocyte, they are rapidly cleaved by cytosolic peptidases, so that dipeptide absorption occurs down the concentration gradient and avoids amino acid absorption against the concentration gradient. However, PepT1 transports primarily dipeptides and, to a lesser extent, tripeptides; longer peptides are not effectively transported. In the fed state therefore the presence of elevated membrane peptidases at the luminal surface ensures significant free amino acid production, especially when there is a large dietary load during a meal and as longer peptides are slowly cleaved on their way to the ileum. The generation of glutamate in the lumen can again provide a signal to both up-regulate its own absorption and stimulate glucose uptake to assist in transamination. As the proportion of amino acids and therefore glutamate absorbed by Na+-dependent transporters increases, PKC βII activation signals diminished requirement for peptide absorption and therefore PepT1. When sodium-dependent transporters are working near their maximum capacity, the AMP/ATP ratio is high, which may activate AMPK to result in stimulation of apical GLUT2 (Walker et al. 2005) and inhibition PepT1 (E. L. Morgan and M. Pieri, unpublished observations). A relation between AMPK and PKC βII remains to be established.
In overnight starvation, apical GLUT2 is decreased (P. A. Helliwell, unpublished observation), as absorption switches to the scavenging role of SGLT1 at luminal glucose concentrations less than those of plasma. Figures 2 and 5 show that PepT1 and apical GLUT2 levels in the apical membrane of jejunum from fed rats increase and decrease, respectively, within minutes as the concentrations of glucose or l-glutamate in the lumen decrease. These changes appear to prepare the intestine for a 3-fold increase in apical membrane PepT1 following a one-day fast (Thamotharan et al. 1999a), which is attenuated somewhat by longer term starvation for 4 days (Ogihara et al. 1999; Ihara et al. 2000). Indeed, in phase 3 starvation (4 days, protein catabolism), where there is marked villus atrophy, apical GLUT2 is abolished and SGLT1 tripled (Habold et al. 2005). However, on refeeding, large amounts of apical GLUT2 appear almost exclusively at the apical membrane within just 2 h and SGLT1 is normalized. In starvation, recycling of peptides from villus desquamation is favoured by decreased membrane peptidase activity and increased uptake by PepT1, which is inherently more energy efficient than Na+-dependent amino acid uptake. As in starvation, the reciprocal relationship of apical GLUT2 and PepT1 is preserved in streptozotocin-diabetes, which results in strong activation of PKC βII, persistently high GLUT2 and diminished PepT1 in the apical membrane (Corpe et al. 1996; Bikhazi et al. 2004).
Consistent with these findings, insulin rapidly doubles dipeptide transport and PepT1 trafficking to the apical membrane of Caco-2 cells (Thamotharan et al. 1999b; Watanabe et al. 2004), while in mice, circulating insulin promotes rapid trafficking of both basolateral and apical GLUT2 to an intracellular pool (Tobin et al. 2008). Since insulin secretion is augmented by incretins, these findings implicate taste receptor-dependent GLP-1 and GIP secretion from L- and K-cells, respectively, in attenuation of glucose absorption by insulin to limit postprandial glucose excursions (Jang et al. 2007; Kellett et al. 2008).
Interestingly, vesicular l-glutamate is colocalized with GLP-1 in mouse L-cells and the GLUTag cell line, from which release of both is induced by glucose (Uehara et al. 2006); GLP-2 from L-cells also promotes apical GLUT2 (Au et al. 2002). l-Glutamate from L-cells might therefore play a role in regulating PepT1, apical GLUT2 and EAAC1, through T1R1 + T1R3 in the basolateral membrane of crypts (Fig. 6), especially in starvation when villus atrophy occurs. It is further conceivable that T1R1, T1R3 and transducin are secreted into the crypt lumen for insertion into the apical membrane during the starved to fed transition (Supplementary Fig. 1C).
Conclusion
Studies in intestinal absorption have in the past been largely advanced separately by specialists in the fields of individual nutrients. Our data reveal, however, that absorption of major energy nutrients is in fact mediated by an integrated network – systems biology. The absorption of sugars, peptides and amino acids is coordinated by newly discovered pathways of Ca2+ absorption and the signalling of intestinal sweet and amino acid taste receptors to target a common enterocytic pool of PKC βII; enteroendocrine hormones and metabolites also have important roles in some conditions. Any transporter that can be regulated by PKC βII or, indeed, any other nutrient that can regulate PKC βII, has the potential to be part of this network.
Acknowledgments
This work was supported by the Wellcome Trust.
Supplemental material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.159616/DC1
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Au A, Gupta A, Schembri P, Cheeseman CI. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J. 2002;367:247–254. doi: 10.1042/BJ20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P. Drug delivery system. 2005. Appl. No. PCT/Gb2005/0001. Pub. No. WO2005/0067978.
- Bailey PD, Boyd CA, Collier ID, George JP, Kellett GL, Meredith D, Morgan KM, Pettecrew R, Price RA. Affinity prediction for substrates of the peptide transporter PepT1. Chem Commun (Camb) 2006:323–325. doi: 10.1039/b511996k. [DOI] [PubMed] [Google Scholar]
- Bailey PD, Boyd CA, Collier ID, Kellett GL, Meredith D, Morgan KM, Pettecrew R, Price RA. Probing dipeptide trans/cis stereochemistry using pH control of thiopeptide analogues, and application to the PepT1 transporter. Org Biomol Chem. 2005;3:4038–4039. doi: 10.1039/b513274f. [DOI] [PubMed] [Google Scholar]
- Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I. Revised immunolocalization of the Na+-D-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol. 2008;295:C475–C489. doi: 10.1152/ajpcell.00180.2008. [DOI] [PubMed] [Google Scholar]
- Berglund JJ, Riegler M, Zolotarevsky Y, Wenzl E, Turner JR. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na+-glucose cotransport. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1487–G1493. doi: 10.1152/ajpgi.2001.281.6.G1487. [DOI] [PubMed] [Google Scholar]
- Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- Bikhazi AB, Skoury MM, Zwainy DS, Jurjus AR, Kreydiyyeh SI, Smith DE, Audette K, Jacques D. Effect of diabetes mellitus and insulin on the regulation of the PepT 1 symporter in rat jejunum. Mol Pharm. 2004;1:300–308. doi: 10.1021/mp049972u. [DOI] [PubMed] [Google Scholar]
- Brandsch M, Miyamoto Y, Ganapathy V, Leibach FH. Expression and protein kinase C-dependent regulation of peptide/H+ co-transport system in the Caco-2 human colon carcinoma cell line. Biochem J. 1994;299:253–260. doi: 10.1042/bj2990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, Peranzi G, Merlin D, Laburthe M, Lewin MJ, Roze C, Bado A. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest. 2001;108:1483–1494. doi: 10.1172/JCI13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpe CP, Basaleh MM, Affleck J, Gould G, Jess TJ, Kellett GL. The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflugers Arch. 1996;432:192–201. doi: 10.1007/s004240050124. [DOI] [PubMed] [Google Scholar]
- D'Souza VM, Buckley DJ, Buckley AR, Pauletti GM. Extracellular glucose concentration alters functional activity of the intestinal oligopeptide transporter (PepT-1) in Caco-2 cells. J Pharm Sci. 2003;92:594–603. doi: 10.1002/jps.10325. [DOI] [PubMed] [Google Scholar]
- Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- Fan MZ, Matthews JC, Etienne NM, Stoll B, Lackeyram D, Burrin DG. Expression of apical membrane L-glutamate transporters in neonatal porcine epithelial cells along the small intestinal crypt-villus axis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G385–G398. doi: 10.1152/ajpgi.00232.2003. [DOI] [PubMed] [Google Scholar]
- Fournier KM, Gonzalez MI, Robinson MB. Rapid trafficking of the neuronal glutamate transporter, EAAC1: evidence for distinct trafficking pathways differentially regulated by protein kinase C and platelet-derived growth factor. J Biol Chem. 2004;279:34505–34513. doi: 10.1074/jbc.M404032200. [DOI] [PubMed] [Google Scholar]
- Ganapathy, Leibach FH. Is intestinal peptide transport energized by a proton gradient? Am J Physiol Gastrointest Liver Physiol. 1985;249:G153–G160. doi: 10.1152/ajpgi.1985.249.2.G153. [DOI] [PubMed] [Google Scholar]
- Habold C, Foltzer-Jourdainne C, Le Maho Y, Lignot JH, Oudart H. Intestinal gluconeogenesis and glucose transport according to body fuel availability in rats. J Physiol. 2005;566:575–586. doi: 10.1113/jphysiol.2005.085217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck J, Kellett GL. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J. 2000a;350:163–169. [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck J, Kellett GL. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000b;350:149–154. [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Rumsby MG, Kellett GL. Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C βII mediated by phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent pathways. J Biol Chem. 2003;278:28644–28650. doi: 10.1074/jbc.M301479200. [DOI] [PubMed] [Google Scholar]
- Hindmarsh JT, Kilby D, Wiseman G. Effect of amino acids on sugar absorption. J Physiol. 1966;186:166–174. doi: 10.1113/jphysiol.1966.sp008026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I, Kellett L, Affleck J, Shepherd J, Boyd R. Expression and cellular distribution during development of the peptide transporter (PepT1) in the small intestinal epithelium of the rat. Cell Tissue Res. 2002;307:139–142. doi: 10.1007/s00441-001-0473-z. [DOI] [PubMed] [Google Scholar]
- Ihara T, Tsujikawa T, Fujiyama Y, Bamba T. Regulation of PepT1 peptide transporter expression in the rat small intestine under malnourished conditions. Digestion. 2000;61:59–67. doi: 10.1159/000007736. [DOI] [PubMed] [Google Scholar]
- Iwanaga T, Goto M, Watanabe M. Cellular distribution of glutamate transporters in the gastrointestinal tract of mice: an immunohistochemical and in situ hybridization approach. Biomed Res. 2005;26:271–278. doi: 10.2220/biomedres.26.271. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gutexpressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:10569–10574. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett GL, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54:3056–3062. doi: 10.2337/diabetes.54.10.3056. [DOI] [PubMed] [Google Scholar]
- Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutrition. 2008;28:35–54. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350:155–162. [PMC free article] [PubMed] [Google Scholar]
- Le Gall M, Tobin V, Stolarczyk E, Dalet V, Leturque A, Brot-Laroche E. Sugar sensing by enterocytes combines polarity, membrane bound detectors and sugar metabolism. J Cell Physiol. 2007;213:834–843. doi: 10.1002/jcp.21245. [DOI] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Fei YJ, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem. 1995;270:6456–6463. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007b;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace OJ, Morgan EL, Affleck JA, Lister N, Kellett GL. Calcium absorption by Cav1.3 induces terminal web myosin II phosphorylation and apical GLUT2 insertion in rat intestine. J Physiol. 2007a;580:605–616. doi: 10.1113/jphysiol.2006.124784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- Meredith D, Boyd CA. Structure and function of eukaryotic peptide transporters. Cell Mol Life Sci. 2000;57:754–778. doi: 10.1007/s000180050040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- Morgan EL, Mace OJ, Affleck J, Kellett GL. Apical GLUT2 and Cav1.3: regulation of rat intestinal glucose and calcium absorption. J Physiol. 2007;580:593–604. doi: 10.1113/jphysiol.2006.124768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EL, Mace OJ, Helliwell PA, Affleck J, Kellett GL. A role for Cav1.3 in rat intestinal calcium absorption. Biochem Biophys Res Commun. 2003;312:487–493. doi: 10.1016/j.bbrc.2003.10.138. [DOI] [PubMed] [Google Scholar]
- Murer H, Sigrist-Nelson K, Hopfer U. On the mechanism of sugar and amino acid interaction in intestinal transport. J Biol Chem. 1975;250:7392–7396. [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- Ogihara H, Saito H, Shin BC, Terado T, Takenoshita S, Nagamachi Y, Inui K, Takata K. Immunolocalization of H+/peptide cotransporter in rat digestive tract. Biochem Biophys Res Commun. 1996;220:848–852. doi: 10.1006/bbrc.1996.0493. [DOI] [PubMed] [Google Scholar]
- Ogihara H, Suzuki T, Nagamachi Y, Inui K, Takata K. Peptide transporter in the rat small intestine: ultrastructural localization and the effect of starvation and administration of amino acids. Histochem J. 1999;31:169–174. doi: 10.1023/a:1003515413550. [DOI] [PubMed] [Google Scholar]
- Pieri M, Hall D, Price R, Bailey P, Meredith D. Site-directed mutagenesis of Arginine282 suggests how protons and peptides are co-transported by rabbit PepT1. Int J Biochem Cell Biol. 2008;40:721–730. doi: 10.1016/j.biocel.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Inui K. Dipeptide transporters in apical and basolateral membranes of the human intestinal cell line Caco-2. Am J Physiol Gastrointest Liver Physiol. 1993;265:G289–G294. doi: 10.1152/ajpgi.1993.265.2.G289. [DOI] [PubMed] [Google Scholar]
- Saito H, Okuda M, Terada T, Sasaki S, Inui K. Cloning and characterization of a rat H+/peptide cotransporter mediating absorption of beta-lactam antibiotics in the intestine and kidney. J Pharmacol Exp Ther. 1995;275:1631–1637. [PubMed] [Google Scholar]
- Sbarbati A, Osculati F. The taste cell-related diffuse chemosensory system. Prog Neurobiol. 2005;75:295–307. doi: 10.1016/j.pneurobio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd EJ, Lister N, Affleck JA, Bronk JR, Kellett GL, Collier ID, Bailey PD, Boyd CA. Identification of a candidate membrane protein for the basolateral peptide transporter of rat small intestine. Biochem Biophys Res Commun. 2002;296:918–922. doi: 10.1016/s0006-291x(02)02021-1. [DOI] [PubMed] [Google Scholar]
- Shiraga T, Miyamoto K, Tanaka H, Yamamoto H, Taketani Y, Morita K, Tamai I, Tsuji A, Takeda E. Cellular and molecular mechanisms of dietary regulation on rat intestinal H+/peptide transporter PepT1. Gastroenterology. 1999;116:354–362. doi: 10.1016/s0016-5085(99)70132-0. [DOI] [PubMed] [Google Scholar]
- Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2004;44:559–609. doi: 10.1146/annurev.pharmtox.44.101802.121558. [DOI] [PubMed] [Google Scholar]
- Thamotharan M, Bawani SZ, Zhou X, Adibi SA. Functional and molecular expression of intestinal oligopeptide transporter (Pept-1) after a brief fast. Metabolism. 1999a;48:681–684. doi: 10.1016/s0026-0495(99)90164-6. [DOI] [PubMed] [Google Scholar]
- Thamotharan M, Bawani SZ, Zhou X, Adibi SA. Hormonal regulation of oligopeptide transporter Pept-1 in a human intestinal cell line. Am J Physiol Cell Physiol. 1999b;276:C821–C826. doi: 10.1152/ajpcell.1999.276.4.C821. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Anderson CM. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol. 2007;92:603–619. doi: 10.1113/expphysiol.2005.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, Brown CD, Hirst BH, Simmons NL. H+-coupled dipeptide (glycylsarcosine) transport across apical and basal borders of human intestinal Caco-2 cell monolayers display distinctive characteristics. Biochim Biophys Acta. 1993;1151:237–245. doi: 10.1016/0005-2736(93)90108-c. [DOI] [PubMed] [Google Scholar]
- Tobin V, Le Gall M, Fioramonti X, Stolarczyk E, Blazquez AG, Klein C, Prigent M, Serradas P, Cuif MH, Magnan C, Leturque A, Brot-Laroche E. Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice. Diabetes. 2008;57:555–562. doi: 10.2337/db07-0928. [DOI] [PubMed] [Google Scholar]
- Uehara S, Jung SK, Morimoto R, Arioka S, Miyaji T, Juge N, Hiasa M, Shimizu K, Ishimura A, Otsuka M, Yamamoto A, Maechler P, Moriyama Y. Vesicular storage and secretion of L-glutamate from glucagon-like peptide 1-secreting clonal intestinal L cells. J Neurochem. 2006;96:550–560. doi: 10.1111/j.1471-4159.2005.03575.x. [DOI] [PubMed] [Google Scholar]
- Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL. 5-Aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J. 2005;385:485–491. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Terada K, Jinriki T, Sato J. Effect of insulin on cephalexin uptake and transepithelial transport in the human intestinal cell line Caco-2. Eur J Pharm Sci. 2004;21:87–95. doi: 10.1016/j.ejps.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Wenzel U, Kuntz S, Diestel S, Daniel H. PEPT1-mediated cefixime uptake into human intestinal epithelial cells is increased by Ca2+ channel blockers. Antimicrob Agents Chemother. 2002;46:1375–1380. doi: 10.1128/AAC.46.5.1375-1380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.