Abstract
Background
One strategy for improving anti-tuberculosis (TB) vaccination involves the use of recombinant Bacillus Calmette Guérin (rBCG) overexpressing protective TB antigens. rBCG30, overexpressing the Mycobacterium tuberculosis secreted antigen, Ag85b, was the first rBCG shown to induce significantly greater TB protection in animals than parental BCG.
Methods
We report the first phase I, double-blind trial of rBCG30 in 35 adults randomized to receive rBCG30 or parental Tice BCG intradermally. Clinical reactogenicity was assessed and state-of-the-art immunological assays used to study Ag85b-specific immune responses induced by both vaccines.
Results
Similar clinical reactogenicity occurred with both vaccines. rBCG30 induced significantly increased Ag85b-specific T cell lymphoproliferation, IFN-γ secretion, IFN-γ ELISPOT responses, and direct ex vivo intracellular IFN-γ responses. Additional flow cytometric studies measuring CFSE dilution and intracellular cytokine production demonstrated that rBCG30 significantly enhanced Ag85b-specific CD4+ and CD8+ T cells capable of concurrent expansion and effector function. More importantly, rBCG30 significantly increased Ag85b-specific T cells capable of inhibiting intracellular mycobacteria.
Conclusions
These results provide proof-of-principal that rBCG can safely enhance human TB immunity, and support further development of rBCG overexpressing Ag85b for TB vaccination.
Keywords: (3-10), TB Vaccination, recombinant BCG, T cell immunity
One third of the world’s population is infected with M. tuberculosis, and 2 million tuberculosis (TB) deaths occur annually (http://www.who.int/gtb/publications/globrep01/index.html). These staggering statistics persist despite the availability of a TB vaccine, M. bovis Bacille Calmette Guérin (BCG), for over 75 years. New vaccines are urgently needed to reduce this immense burden of TB disease. One potential approach for improving TB vaccination is the generation of recombinant BCG (rBCG), which may work better than standard BCG strains by overexpressing key M. tuberculosis antigens, immunoenhancers and/or proteins promoting phagosomal escape and potent CD8+ T cell stimulation [1–7]. Furthermore, rBCG are attractive options because of the extensive clinical experience, known immunogenicity protective against severe TB disease, and relative safety profile of standard BCG strains. However, rBCG TB vaccines have not been studied in humans to demonstrate safety and enhanced immunogenicity of this approach.
M. tuberculosis is an intracellular pathogen that replicates in host mononuclear phagocytes [8, 9]. Bacillary proteins secreted intracellularly are early targets of TB immunity [8–10]. Immunization of guinea pigs with purified M. tuberculosis extracellular proteins, including the 30 kDa major secretory protein (a mycolyltransferase known as α-antigen and antigen 85b [11, 12]), induces substantial protection against aerosol challenge with highly virulent M. tuberculosis [9]. Furthermore, M. tuberculosis secreted proteins [13] and DNA encoding secreted antigens [14] also induce TB immunity in mice. Although previous vaccinations in animals induced significant levels of protection, this protection was never superior and usually less than induced by BCG vaccination. Horwitz et al. generated recombinant BCG overexpressing the 30 kD Ag85b antigen of M. tuberculosis (rBCG30) in 2 distinct BCG strains. These rBCG30 vaccines were the first new TB vaccines capable of inducing protective immunity in guinea pigs significantly better than nonrecombinant BCG [1, 2].
Based upon these promising results, the Aeras Foundation initiated clinical development of rBCG30 as their first model TB vaccine candidate. We report the initial clinical and detailed immunological testing of rBCG30 in QuantiFERON PPD negative, adult volunteers. rBCG30 was as safe as nonrecombinant BCG and induced significantly increased Ag85b-specific immunity in multiple relevant subsets of mycobacteria-specific immune responses.
MATERIALS AND METHODS
BCG Vaccinations
After obtaining informed consent 35 PPD-/HIV- healthy adults were randomized in a double-blind fashion to receive ~5×105 cfu of either rBCG30 (Korean Institute of Tuberculosis, Aeras IND, Lot 200303; parental Tice BCG strain used to construct rBCG30) or parental nonrecombinant Tice BCG (Organon Teknika, Durham, NC) intradermally. Volunteers were recruited, vaccinated and followed at Saint Louis University (n=20) or Piedmont Medical Research Associates, Winston-Salem, NC (n=15). Fresh samples were studied in the Saint Louis University cohort (10/group), and frozen PBMC studied from both sites. The protocol was approved by Saint Louis University and Aeras Institutional Review Boards.
Antigen-specific lymphoproliferation
Heparinized blood was diluted 1:10 with RPMI containing optimal doses of control and mycobacterial antigens, and incubated for 7 days at 37°C with 5% CO2 prior to thymidine incorporation measurements. Tetanus toxoid control was used at 5 μg/ml (Statens Serum Institut, Copenhagen, Denmark). Recombinant Ag85b protein (prepared in Dr. Horwitz’s lab) was used at 5 μg/ml. Live Tice BCG was thawed and 100,000 cfu added/200 μl diluted blood.
Antigen-specific IFN-γ secretion
Heparinized blood diluted 10 fold with RPMI alone or with optimal doses of antigens was incubated at 37°C with 5% CO2 for 4 days. Secreted IFN-γ was measured by ELISA as described [15].
Antigen-specific intracellular IFN-γ responses directly ex vivo
Antigen-specific, IFN-γ-producing CD4+ and CD8+ T cells were identified by whole-blood intracellular cytokine assay as described [16]. Heparinized blood was incubated with anti-CD28 and anti-CD49d alone (negative control) or with recombinant Ag85b protein (0.5 mg/ml). After 12 hours at 37°C (with brefeldin A the last 5 hours), RBC were lysed and WBC fixed with FACS Lysing Solution (BD Biosciences) before cryopreservation. For analysis cells were thawed, permeabilized, and stained with antibodies for flow cytometry (FACSCalibur and Cellquest software, BD) as described [16].
Antigen-specific IFN-γ ELISPOT responses
IFN-γ producing cells were identified by ELISPOT using ImmunoSpot plates (Cellular Technology, Ltd., Cleveland, OH) and IFN-γ-specific antibodies (BD Pharmingen, San Diego, CA). PBMC (0.2–1×105 cells/well) were stimulated with Ag85b peptide pools (1 μg/ml), recombinant Ag85b protein (5 μg/ml), live BCG (0.5 MOI) or medium alone for 24 hours at 37°C with 5% CO2. Spots were identified by CTL Analyzer and ImmunoSpot software, version 3.2 (C.T.L.).
Simultaneous detection of antigen-specific proliferation and IFN-γ production
PBMC were labeled with CFSE (Molecular Probes) and expanded with optimal doses of Ag85b peptide pools, recombinant Ag85b protein or live BCG, or rested in medium for 7 days at 37°C with 5% CO2. Then cells were incubated with 50 ng/ml PMA (Sigma), 750 ng/ml ionomycin (Sigma) and 0.7 μL/ml GolgiStop (BD) for 2 hrs before CD3, CD4 and CD8 staining, permeabilization with Cytofix/Cytoperm (BD), followed by staining for intracellular IFN-γ. Data were acquired with a FACSCalibur (BD), and analyzed using CELLQuest (BD) and FlowJo (Tree Star, Ashland, OR). Absolute numbers (AN) of effector CD4+ and CD8+ T cells (defined as both CFSElo and IFN-γ+) were calculated by multiplying total viable cells recovered times T cell subset percentages detected.
Mycobacterial growth inhibition assay
Inhibitory responses were studied as described [17, 18]. Adherent monocytes were incubated without antibiotics for 6 days to allow macrophage differentiation. Concurrently, PBMC were expanded with 5 μg/ml recombinant Ag85b protein or Tice BCG (0.5 MOI) for 7 days. One day prior to adding expanded T cells, target macrophages were infected with BCG (MOI=3). Extracellular mycobacteria were removed and effector cells added at ratios of 12.5:1 for 72 hours at 37°C. Saponin lysates were prepared and cfu of released bacilli determined. Percent inhibition was calculated as 100−[100×(experimental CFU/control CFU)]. Negative percentages were assumed to represent 0% inhibition and 4 outliers (baseline response greater than mean+SD of all baseline responses) out of the 20 volunteers available for study were excluded.
Statistics
Friedman repeated measures ANOVA were used to study overall increases post-vaccination, Wilcoxon matched pairs tests used to identify significantly increased post-vaccination compared with matched pre-vaccination responses, and Mann-Whitney U tests used to compare responses between groups. McNemar’s and Fisher’s exact tests were used to compare paired and unpaired categorical data, respectively. Analyses were completed with Statistica (Statsoft, Inc., Tulsa, OK).
RESULTS
Reactogenicity
No serious adverse event was encountered. Overall nonserious adverse events were similar between groups (Table 1). Expected local reactions at BCG intradermal vaccination sites occurred (papules followed by ulcers draining for days to weeks). Mean and median ulcer size and drainage duration were similar between rBCG30 and Tice groups. A trend for increased pain/tenderness/swelling at injection sites occurred in rBCG30 recipients, and significantly more rBCG30 volunteers experienced moderately severe erythema at vaccination sites during the first 2 weeks post-vaccination (12/18 vs 2/17; p<0.05 by 2 tailed Fisher’s exact test). However, after 2 weeks similar events were reported and all lesions healed by 3 months post-vaccination.
Table 1.
Demographics & Clinical Reactogenicity
| Parameter | Tice Group (n=17) | rBCG30 (n=18) |
|---|---|---|
| Age (Mean + SD) | 29.0 + 5.7 | 28.0 + 7.7 |
| Female Sex | 10/17 (59%) | 12/18 (67%) |
| Race (Caucasian/African-American/Asian) | 14/2/1 | 15/3/0 |
| Solicited Injection Site Pain 1st 2 weeks: | ||
| Mild | 9/17 | 11/18 |
| Moderate | 2/17 | 4/18 |
| Severe | 0/17 | 3/18 |
| Solicited Injection Site Erythema 1st 2 weeks: | ||
| Mild | 13/17 | 4/18a |
| Moderate | 2/17 | 12/18a |
| Severe | 0/17 | 2/18 |
| Solicited Injection Site Swelling 1st 2 weeks: | ||
| Mild | 14/17 | 10/18 |
| Moderate | 1/17 | 6/18 |
| Severe | 0/17 | 2/18 |
| Other AEs through Day 28 | ||
| Mild | 9/17 | 6/18 |
| Moderate | 2/17 | 4/18 |
| Severe | 1/17 | 0/18 |
| Endpoint Moderate or Severe Ulceration | 0/17 | 2/18 |
| Endpoint Moderate or Severe Scar | 1/17 | 1/18 |
| Clinical Take (development of ulcer/scar) | 17/17 | 18/18 |
| Study End PPD induration (mean +SD mm) | 3.8 + 8.0 | 4.4 + 6.5 |
| Study End PPD > 5 mm | 4/16 | 6/18 |
Note: Proportions of volunteers with adverse event (AE)/total volunteers in group are shown.
Proportion significantly different (p<0.05) from proportion in other group by 2-tailed Fisher exact test.
Lymphoproliferative responses
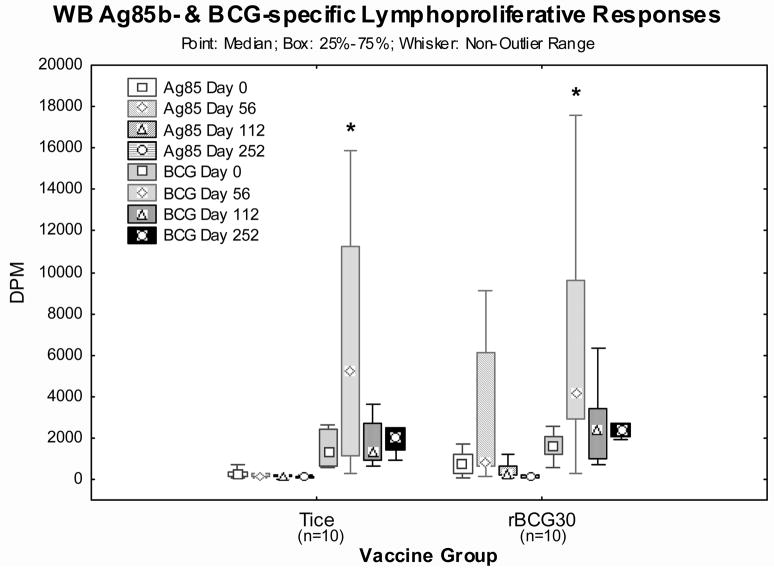
Lymphoproliferation induced by live Tice BCG and recombinant Ag85b protein were studied pre- (day 0) and post- (days 56, 112 & 252) vaccination (Figure 1). Both vaccines induced significant increases in BCG-specific lymphoproliferation detected on day 56 post-vaccination (p<0.03 by Wilcoxon matched pairs tests, n=10/group). Only rBCG30 recipients, and not Tice recipients, developed significant increases in Ag85b-specific lymphoproliferation (p<0.01 by Friedman ANOVA including day 0, 56, 112 and 252 time points, n=10/group). These results demonstrate immunogenicity of both vaccines, inducing significant increases in lymphoproliferation specific for antigens expressed by live BCG, but only rBCG30 induced significantly increased lymphoproliferation specific for the Ag85b antigen overexpressed in rBCG30.
Figure 1. Ag85b- and BCG-specific whole blood lymphoproliferative responses pre- and post-vaccination.
Heparinized whole blood harvested pre-vaccination and on days 56, 112 and 252 post-vaccination was diluted 10 fold with RPMI and stimulated for 7 days with optimal doses of Ag85b recombinant protein and live Tice strain BCG. Shown are the median values (points), mid-50% values (boxes) and non-outlier ranges (whiskers). *, p<0.03 comparing post-vaccination and pre-vaccination by Wilcoxon matched pairs test. Ag85b- and BCG-specific post-vaccination responses in the rBCG30 vaccination group were significantly increased (p<0.01 by Friedman ANOVA).
Detection of IFN-γ-producing effector T cells directly ex vivo
We measured mycobacteria-specific IFN-γ responses by intracellular cytokine staining after short-term stimulation. This assay quantitates antigen-specific effector T cells and allows for simultaneous detection of CD4+ and CD8+ T cells. Figure 2 presents CD4+ (Figure 2.a.) and CD8+ (Figure 2.b.) T cell percentages producing intracellular IFN-γ after stimulation with purified Ag85b protein. Only rBCG30 induced significantly increased Ag85b-specific CD4+ and CD8+ T cell responses. By Friedman ANOVA, overall IFN-γ-producing CD4+ T cells were significantly increased in rBCG30 recipients post-vaccination (p<0.02 analyzing days 0, 56, 112 and 252). In addition, Ag85b post-vaccination responses on day 56 in rBCG30 recipients were significantly greater than day 0 responses by Wilcoxon matched pairs testing (p<0.02). Furthermore, rBCG30-induced CD4+ T cell responses were significantly greater than Tice-induced responses on days 56 and 252 post-vaccination (p<0.05 by Mann-Whitney U tests). Ag85b-specific CD8+ T cell responses were significantly increased only in rBCG30 recipients on day 252 post-vaccination (p<0.05 by Wilcoxon matched pairs test). Both Tice and rBCG30 groups developed significant increases in total BCG-specific, CD4+ and CD8+ IFN-γ-producing T cells post-vaccination (p<0.05 by Friedman ANOVA analyses including days 0, 56, 112 and 252). By Wilcoxon matched pairs testing, BCG-specific CD4+ IFN-γ-producing T cells were significantly increased in both Tice and rBCG30 groups on days 56 and 112 post-vaccination (p<0.04), and BCG-specific CD8+ IFN-γ-producing T cells were significantly increased in both Tice and rBCG30 groups on day 112 post-vaccination (p<0.03). Overall, these results demonstrate that both vaccines induced BCG-specific responses while only rBCG30 vaccination induced significant Ag85b-specific responses.
Figure 2. Flow cytometric detection of Ag85b-specific IFN-γ-producing T cells directly ex vivo.
Heparinized whole blood harvested pre-vaccination and on days 56, 112 and 252 post-vaccination was stimulated for 12 hours with Ag85b recombinant protein (Brefeldin A added for the last 5 hours of incubation) before freezing in liquid nitrogen. Matching sets of pre- and post-vaccination samples from individual volunteers were thawed, processed for staining of T cell surface markers and intracellular IFN-γ and then analyzed by flow cytometry. The percentages of CD4+ (a.) and CD8+ (b.) T cells positive for intracellular IFN-γ staining are presented. Shown are the median values (points), mid-50% values (boxes) and non-outlier ranges (whiskers). *, p<0.05 comparing pre- and post-vaccination responses by Wilcoxon matched pairs test. **, p<0.05 comparing rBCG30 and Tice vaccination groups by Mann-Whitney U test. In addition, post-vaccination CD4+IFN-γ+ T cell responses in the rBCG30 group were significantly increased (p<0.02) by Friedman ANOVA.
Partial epitope mapping of major Ag85b-specific T cell epitopes
We generated 55 individual 15mer peptides overlapping by 10aa and spanning the entire Ag85b sequence (M. tuberculosis gene Rv1886c). Epitope mapping studies of immunodominant Ag85b-specific T cell epitopes were initiated using 3 separate pools of these peptides to stimulate IFN-γ ELISPOT responses in thawed PBMC. Each Ag85b peptide pool contained 19 peptides: peptide pool 1 (PP1) covered the amino-terminal 1–105aa, PP2 covered the central 91–195aa and PP3 covered the carboxy-terminal 181–285aa. Figure 3 demonstrates that only rBCG30 recipients developed significant increases in Ag85b-specific responses post-vaccination (days 7 and 56). rBCG30-induced increases in Ag85b-specific IFN-γ responses were stimulated with PP1 (p<0.01 by Friedman ANOVA including results from days 0, 7 and 56). In addition, rBCG30-induced PP1-specific responses were significantly greater than day 0 responses on both days 7 and 56 post-vaccination (p<0.01, by Wilcoxon matched pairs tests). Furthermore, rBCG30 day 7 PP1 responses were significantly greater than Tice day 7 PP1 responses (p<0.05, by Mann-Whitney U test). Only PP1 stimulations detected vaccine-induced responses in rBCG30 recipients. This suggests that the amino-terminal Ag85b sequence contains immunodominant epitopes. Figure 3.b. presents proportions of positive IFN-γ ELISPOT responses (greater than the mean+SD of matching baseline responses). Only rBCG30 recipients developed significantly increased positive responses post-vaccination, and only after stimulation with PP1. On day 7 post-vaccination, 10 of 16 rBCG30 recipients had PP1 positive responses compared with only 1 of 16 on day 0 (p<0.01 by McNemar’s test), and only 3 of 15 Tice recipients positive on day 7 (p<0.03 by Fisher’s exact test). On day 56 post-vaccination, 8 of 17 rBCG30 recipients had PP1 positive responses compared with only 1 of 16 on day 0 (p<0.05 by McNemar’s test).
Figure 3. Peptide epitope mapping of Ag85b-specific IFN-γ-producing T cell responses.
Frozen PBMC harvested pre-vaccination and on days 7 and 56 post-vaccination were stimulated with pools of peptides representing the entire Ag85b protein sequence (15mer peptides overlapping by 10 aa) in IFN-γ ELISPOT assays. Peptide pools 1, 2 and 3 correspond to the initial amino-terminal third, middle third and carboxy-terminal third of the Ag85b molecule, respectively. Shown in a. are the median values of IFN-γ spot forming cells (points), mid-50% values (boxes) and non-outlier ranges (whiskers). *, p<0.01 comparing pre- and post-vaccination responses by Wilcoxon matched pairs test. **, p<0.05 comparing rBCG30 and Tice vaccination groups by Mann-Whitney U test. In addition, post-vaccination peptide pool 1-specific IFN-γ-producing T cell responses in the rBCG30 group were significantly increased (p<0.01) by Friedman ANOVA. Shown in b. are the proportions of positive IFN-γ responses (with a positive response defined as a response ≥ the mean plus SD of matching baseline responses). *p<0.01 and p<0.03 by McNemar’s and Fisher’s exact tests compared with Day 0 rBCG30 responses and Day 7 Tice responses, respectively. **p<0.05 by McNemar’s test comparing Day 56 and Day 0 responses within the rBCG30 group.
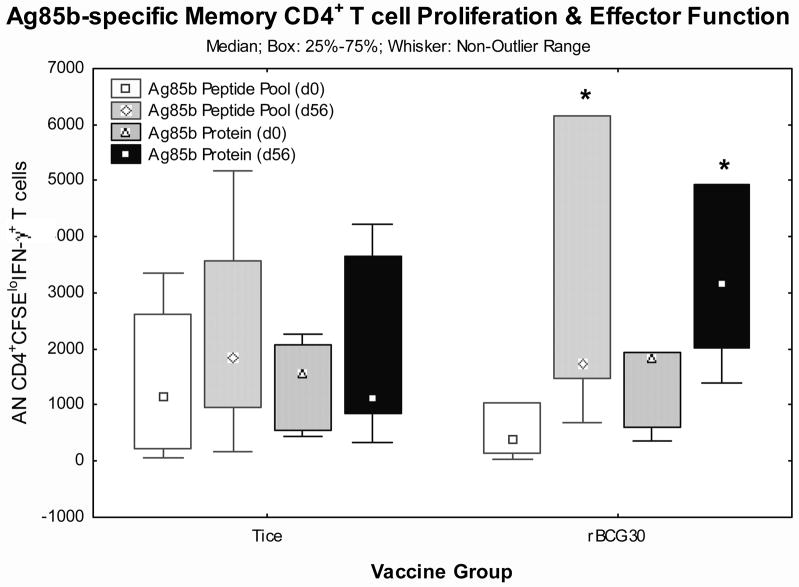
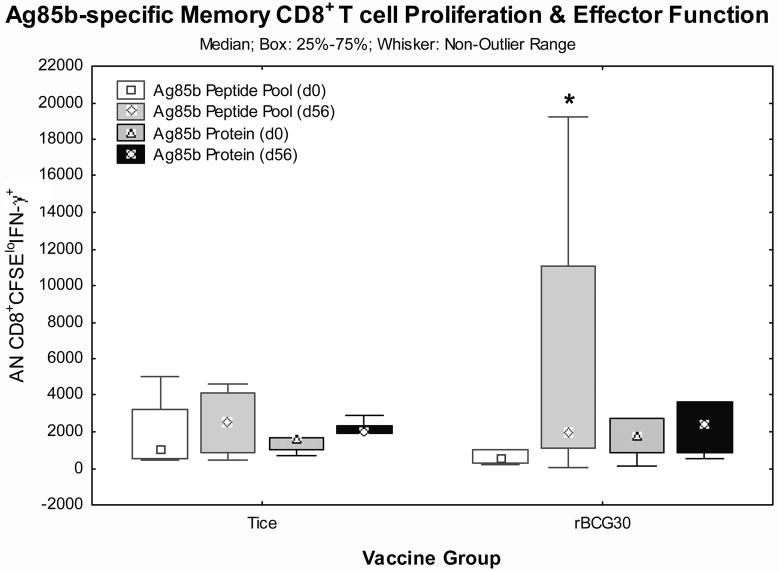
Detection of IFN-γ responses in vaccine-induced memory T cells capable of antigen-specific expansion
Long term protection requires memory T cells that can both expand and produce effector functions in response to later infectious challenge [19–21]. To determine whether Tice and/or rBCG30 vaccinations induced memory T cells capable of both expansion and IFN-γ effector responses, we developed a flow-based assay that measures CFSE dilution to track lymphoproliferation coupled with intracellular staining to detect IFN-γ effector responses. With this assay we studied frozen PBMC harvested from days 0 and 56 post-vaccination in 12 volunteers (5 Tice and 7 rBCG30 recipients). Figures 4.a. and 4.b. present CD4+ and CD8+ T cell responses, respectively. Both Ag85b PP1 and whole recombinant protein induced significantly increased numbers of CD4+ T cells to proliferate (become CFSE low) and produce IFN-γ in PBMC from rBCG30 recipients on day 56 post-vaccination (p<0.05 by Wilcoxon matched pairs test). In addition, Ag85b PP1-induced CD8+ T cell responses were significantly increased in rBCG30 recipients post-vaccination (p<0.05 by Wilcoxon matched pairs test). Ag85b-specific responses were not increased post-vaccination in Tice recipients. These results indicate that rBCG30 uniquely induced increases in Ag85b-specific memory CD4+ and CD8+ T cells capable of both antigen-specific expansion and IFN-γ effector function.
Figure 4. Detection of Ag85b-specific memory T cells capable of both expansion and IFN-γ effector function.
Matching pre- and post-vaccination frozen PBMC from individual volunteers were thawed, labeled with CFSE, stimulated with Ag85b peptide pool 1 or recombinant protein for 7 days, and then stimulated with PMA/ionomycin for 2 hours in the presence of Golgistop to maximize detection of intracellular IFN-γ before surface and intracellular staining for flow cytometry. Presented are the absolute numbers of CD4+ (a.) and CD8+ (b.) T cells that both proliferated (became CFSElo) and produced detectable intracellular IFN-γ. Shown are the median values (points), mid-50% values (boxes) and non-outlier ranges (whiskers). *, p<0.05 comparing pre- and post-vaccination responses by Wilcoxon matched pairs test.
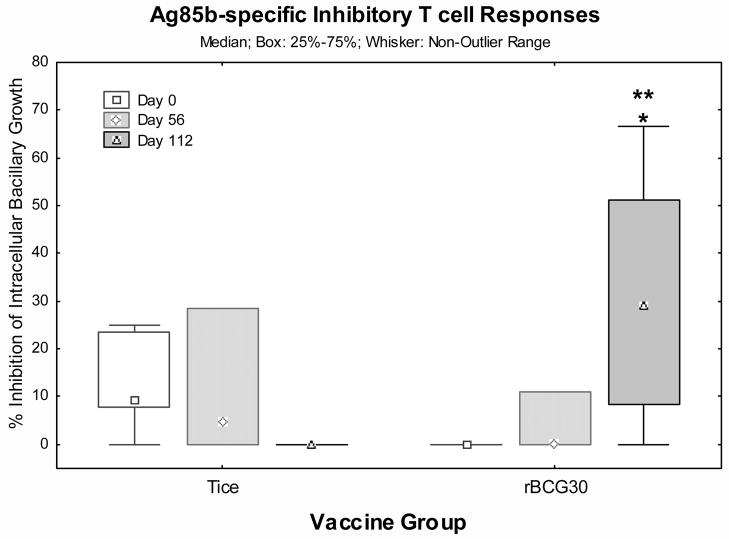
Detection of vaccine-induced T cell responses capable of inhibiting intracellular mycobacteria
Mycobacteria predominantly replicate within macrophages in vivo and T cells capable of inhibiting intracellular replication are critical for protective TB immunity. We have developed an assay that measures T cell-mediated inhibition of intracellular mycobacteria [17, 18]. Figure 5 presents T cell-mediated inhibitory responses by Ag85b protein-expanded PBMC (rBCG30 n=9, Tice n=7). rBCG30 recipients demonstrated progressive increases in Ag85b-specific inhibitory responses which were significantly increased on day 112 post-vaccination (p<0.05 by Wilcoxon matched pairs test), and significantly greater than Tice day 112 responses (p<0.05 by Mann-Whitney U test). Similarly increased inhibitory effects were mediated by T cells expanded with live BCG in both groups post-vaccination (not shown).
Figure 5. Ag85b-specific inhibitory T cells responses.
PBMC harvested pre-vaccination and on days 56 and 112 post-vaccination were stimulated with recombinant Ag85b protein for 7 days and then Ag85b-specific expanded T cells were co-cultured with BCG-infected autologous macrophages for 3 days. After co-culture, mammalian membranes were disrupted by saponin lysis and released viable CFU of BCG enumerated on Middlebrook agar plates. The percent inhibition mediated by Ag85b-specific T cells compared with medium rested T cells is graphically presented. Shown are the median values (points), mid-50% values (boxes) and non-outlier ranges (whiskers). *, p<0.05 comparing pre- and post-vaccination responses by Wilcoxon matched pairs test. **, p<0.05 comparing rBCG30 and Tice vaccination groups by Mann-Whitney U test.
DISCUSSION
Our results provide proof-of-principle that rBCG can safely induce enhanced immunity in humans. rBCG30 was the first rBCG shown to induce significantly better protection against aerosolized TB challenge in the stringent guinea pig model of pulmonary TB [1, 2]. The current results extend these observations providing the first demonstration in humans that rBCG30 vaccination is not only well-tolerated but also capable of inducing significantly enhanced Ag85b-specific immunity compared with the parental Tice BCG. Our results should encourage continued work on rBCG vaccines currently in differing stages of clinical development.
We carefully evaluated local and systemic reactions after Tice and rBCG30 intradermal vaccination and found both had similar clinical effects (Table 1). Except for increased injection site erythema early after rBCG30 vaccination, local responses to Tice and rBCG30 were very similar. rBCG30 recipients tended to report more injection site pain and swelling early post-vaccination, but these differences were not statistically significant. Local effects after 2 weeks and systemic adverse events post-vaccination were not different (data not shown). These results demonstrate that intradermal rBCG30 vaccination was as well-tolerated as nonrecombinant BCG.
Human responses to BCG vaccination are complex involving numerous components of immunity. We therefore compared a number of immune functions induced by rBCG30 and wild type BCG including antigen-specific T cell expansion capacity, IFN-γ secretion, phenotypes of CD4+ and CD8+ T cells consistent with effector and memory T cell populations, and direct inhibitory effects of vaccine-induced T cells on intracellular mycobacteria. We demonstrate that rBCG30 significantly induced all of these responses predicted to be critical for protective TB immunity. Our finding that humans develop enhanced Ag85b-specific responses after immunization with rBCG30 but not parental wild type BCG mirrors previous guinea pig results, where enhanced cutaneous delayed-type hypersensitivity and antibody responses developed after rBCG30 but not BCG vaccination [1, 2, 22]. For both hosts, these differences in immune responses likely reflect the low levels of Ag85b produced by standard BCG strains compared with rBCG30 [1, 2].
CD4+ Th1 cells produce IFN-γ, IL-2 and TNF-α, cytokines involved in the activation of intracellular microbicidal activities, as well as initiation and maintenance of protective granulomatous inflammation [23, 24]. CD4+ Th1 cells are of predominant importance for protection in animal models of TB [25]. In humans, natural genetic polymorphisms resulting in deficient IFN-γ signaling result in extreme susceptibility to overwhelming disseminated mycobacterial infections [26]. We demonstrate that rBCG30 induced significantly increased Ag85b-specific CD4+ Th1 cell responses in humans (Figures 1. and 2.a.) predicted to be important for protective TB immunity.
CD8+ T cells can recognize and destroy target cells infected with intracellular pathogens including M. tuberculosis. Peptides are presented to CD8+ T cells by MHC class I molecules which generally bind peptide fragments synthesized in the cytoplasm of the cell. Therefore, intracellular pathogens that escape phagosomal compartments and replicate freely within cytoplasm are potent stimulators of CD8+ T cell responses and protective immunity against these pathogens generally depends more on these responses. However, M. tuberculosis prevents phagosome-lysome fusion, preferring to replicate within the original phagosomal/endosomal compartments it infects. These intracellular compartments favor presentation of MHC class II-restricted peptide antigens to CD4+ T cells which have classically been considered the more important T cell subset for protective TB immunity. Despite this dogma, it has recently been shown that CD8+ T cells do contribute to protective TB immunity, and may be particularly important for prevention of TB reactivation [27–32]. Peptide antigens not synthesized in cytoplasmic locations can be presented by activated dendritic cells to CD8+ T cells via a mechanism known as cross-presentation [33–35]. In addition, M. tuberculosis-specific CD8+ cytolytic T cells can recognize mycobacterial lipid antigens presented by nonclassical MHC Ib restriction elements [36–38]. Furthermore, human CD8+ T cells produce granulysin, a component of their cytolytic granules, which has been shown to have direct microbicidal effects against both extracellular and intracellular mycobacteria [39]. In this report, we demonstrate that rBCG30 significantly enhanced Ag85b-specific CD8+ T cell responses in humans (Figures 2.b. ) predicted to contribute to protective TB immunity.
Two subpopulations of memory T cells have been identified, described as central and effector memory T cells [19, 40, 41]. Central memory T cells reside within lymph nodes and spleen due at least in part to expression of the lymph node homing molecule CCR7, have capacity for long-term homeostatic proliferation in the absence of residual antigen, expand robustly in response to new antigenic challenge and then differentiate into effector T cells with protective functions. On the other hand, effector memory T cells do not express CCR7 and reside predominantly within peripheral tissues. Effector memory T cells rapidly provide protective cytokine and cytolytic responses, but have reduced lymphoproliferative capacity and may depend on persistence of at least low levels of antigen. Induction of both memory T cell populations may be necessaryfor optimal long-term, vaccine-induced protection. IFN-γ production stimulated by mycobacterial antigens directly ex vivo can be used to assess the relative functional levels of effector memory T cell responses. Identification of cells with capacity for both expansion and effector function can assess the relative functional levels of central memory T cell responses. Our combined results (Figures 2–4) suggest that rBCG30 induced significant increases in both effector and central memory Ag85b-specific T cells predicted to be important for protective TB immunity. Future studies will need to investigate whether these putative central and peripheral effector memory populations can be distinguished in human blood based on the presence or absence of CCR7 expression, respectively.
Mycobacteria replicate within macrophages, and defects in cell mediated immunity result in increased susceptiblity to these intracellular pathogens. Therefore, we predict that T cells capable of inhibiting intracellular mycobacterial growth are important for in vivo protection against TB, and immune-mediated inhibition of mycobacterial growth would more directly correlate with protective TB immunity than other immunological responses. For these reasons, we have developed an in vitro assay capable of measuring T cell mediated inhibition of intracellular mycobacterial growth [17, 18]. In this study, we found that rBCG30 but not Tice BCG induced significant increases in Ag85b-specific inhibitory T cell responses (Figure 5). These results further indicate that rBCG vaccine can enhance responses relevant for protective immunity. However, only future phase III efficacy trials can prove whether or not rBCG30 or other rBCG vaccines can enhance protection against TB infection and disease.
rBCG30 is the first TB vaccine intended as a replacement for BCG, the current vaccine used worldwide. Other vaccines currently in clinical development are intended primarily as booster vaccines for BCG-immunized people. Only one such vaccine, MVA85A, has been tested in humans and the results reported. MVA85A is a modified vaccinia virus expressing Ag85a, a close relative of Ag85b, the antigen overexpressed in rBCG30. In humans, MVA85A induced significantly enhanced Ag85a-specific IFN-γ secreting T cells [42]. Although such a response has generally been thought to indicate important immune induction, in a recent study increased Ag85a-specific IFN-γ responses produced by CD4+ T cells alone did not result in enhanced protection [43]. The capacity of T cells to both proliferate and mediate effector functions, or mediate inhibitory effects against intracellular mycobacteria, have not been reported after MVA85A vaccination. It is of interest to compare preclinical studies completed with Ag85a and Ag85b vaccines. Boosting BCG with MVA85A was not more efficacious than administering BCG alone in the guinea pig model of pulmonary TB [44]. In contrast, boosting BCG with purified Ag85b in adjuvant significantly enhanced protective immunity in guinea pigs compared with BCG alone [22], and the use of rBCG30 overexpressing Ag85b also significantly enhanced protection in guinea pigs compared with wild type BCG [1, 2]. It will be important to make direct comparisons between the levels of memory T cells capable of proliferation, IFN-γ production and inhibition of intracellular mycobacteria induced by these different experimental TB vaccines in future trials.
In summary, we have shown that rBCG30 is safe and induces significantly increased CD4+ and CD8+ Type 1 immunity, central and effector memory T cell subsets, and inhibitory T cells specific for the overexpressed Ag85b protective antigen. rBCG30 is thus the first TB vaccine to achieve three key developmental milestones. First, it demonstrated superior efficacy in the guinea pig TB model. Second, it is safe and well-tolerated in humans. Third, it has the capacity to induce relevant TB immunity in humans by several criteria. This provides optimism that rBCG30 and/or similar vaccines modeled after it will be safe and provide superior protection against TB in humans.
Acknowledgments
We thank the volunteers who participated in this trial for their magnanimity and the nurses from the Saint Louis University Vaccine Center for their tireless devotion to their work. We thank Barbara Jane Dillon for technical assistance with protein purification. Funding for this trial was provided by the Aeras Global TB Vaccine Foundation, the Saint Louis University Vaccine and Treatment Evaluation Unit (NIH NO1-AI-25464), NIH RO1-AI-48391 (D.F.H.) and NIH RO1-AI-31338 (M.A.H.).
Financial support: This work was funded by the Aeras Global TB Vaccine Foundation.
Footnotes
Potential conflicts of interest: none to report.
Not previously presented.
References
- 1.Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic’ S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A. 2000;97:13853–8. doi: 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun. 2003;71:1672–9. doi: 10.1128/IAI.71.4.1672-1679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Donnell MA, Aldovini A, Duda RB, et al. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. 1994;62:2508–14. doi: 10.1128/iai.62.6.2508-2514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray PJ, Aldovini A, Young RA. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guerin strains that secrete cytokines. Proc Natl Acad Sci U S A. 1996;93:934–9. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young SL, O’Donnell MA, Buchan GS. IL-2-secreting recombinant bacillus Calmette Guerin can overcome a Type 2 immune response and corticosteroid-induced immunosuppression to elicit a Type 1 immune response. Int Immunol. 2002;14:793–800. doi: 10.1093/intimm/dxf050. [DOI] [PubMed] [Google Scholar]
- 6.Conradt P, Hess J, Kaufmann SH. Cytolytic T-cell responses to human dendritic cells and macrophages infected with Mycobacterium bovis BCG and recombinant BCG secreting listeriolysin. Microbes Infect. 1999;1:753–64. doi: 10.1016/s1286-4579(99)80077-x. [DOI] [PubMed] [Google Scholar]
- 7.Grode L, Seiler P, Baumann S, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005;115:2472–9. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pal PG, Horwitz MA. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–92. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz MA, Lee BE, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–4. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blander SJ, Horwitz MA. Vaccination with the major secretory protein of Legionella pneumophila induces cell-mediated and protective immunity in a guinea pig model of Legionnaires’ disease. J Exp Med. 1989;169:691–705. doi: 10.1084/jem.169.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–61. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belisle JT, Varalakshmi DV, Sievert T, Takayama K, Brennan PJ, Besra GS. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–2. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 13.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–44. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huygen K, Content J, Denis O, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–8. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 15.Hoft DF, Brown R, Roodman S. Bacille Calmette-Guerin vaccination enhances human γδT cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–54. [PubMed] [Google Scholar]
- 16.Hanekom WA, Hughes J, Mavinkurve M, et al. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods. 2004;291:185–95. doi: 10.1016/j.jim.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Hoft DF, Worku S, Kampmann B, et al. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J Infect Dis. 2002;186:1448–57. doi: 10.1086/344359. [DOI] [PubMed] [Google Scholar]
- 18.Worku S, Hoft DF. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect Immun. 2003;71:1763–73. doi: 10.1128/IAI.71.4.1763-1773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Lauvau G, Vijh S, Kong P, et al. Priming of memory but not effector CD8+ T cells by a killed bacterial vaccine. Science. 2001;294:1735–9. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- 21.Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–8. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Enhancing the protective efficacy of Mycobacterium bovis BCG vaccination against tuberculosis by boosting with the Mycobacterium tuberculosis major secretory protein. Infect Immun. 2005;73:4676–83. doi: 10.1128/IAI.73.8.4676-4683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone: I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 24.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 25.Wangoo A, Sparer T, Brown IN, et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous lung disease. J Immunol. 2001;166:3432–9. doi: 10.4049/jimmunol.166.5.3432. [DOI] [PubMed] [Google Scholar]
- 26.Newport MJ, Huxley CM, Huston S, et al. A mutation in the IFN-gamma receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–9. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 27.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–7. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloom BR, Flynn J, McDonough K, Kress Y, Chan J. Experimental approaches to mechanisms of protection and pathogenesis in M. tuberculosis infection. Immunobiology. 1994;191:526–36. doi: 10.1016/S0171-2985(11)80459-6. [DOI] [PubMed] [Google Scholar]
- 29.Ladel CH, Daugelat S, Kaufmann SH. Immune response to Mycobacterium bovis bacille Calmette Guerin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–84. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 30.Feng CG, Britton WJ. CD4+ and CD8+ T cells mediate adoptive immunity to aerosol infection of Mycobacterium bovis bacillus Calmette-Guerin. J Infect Dis. 2000;181:1846–9. doi: 10.1086/315466. [DOI] [PubMed] [Google Scholar]
- 31.Cho S, Mehra V, Thoma-Uszynski S, et al. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc Natl Acad Sci U S A. 2000;97:12210–5. doi: 10.1073/pnas.210391497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8+ T cells. Eur J Immunol. 2000;30:3689–98. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–57. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 34.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–83. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 35.Bevan MJ. Cross-priming. Nat Immunol. 2006;7:363–5. doi: 10.1038/ni0406-363. [DOI] [PubMed] [Google Scholar]
- 36.Lewinsohn DM, Alderson MR, Briden AL, Riddell SR, Reed SG, Grabstein KH. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J Exp Med. 1998;187:1633–40. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalvani A, Brooks R, Wilkinson R, et al. Human cytolytic and IFN-γsecreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–5. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewinsohn DM, Briden AL, Reed SG, Grabstein KH, Alderson MR. Mycobacterium tuberculosis-reative CD8+ T lynphocytes: the realtive contribution of classical versus nonclassical HLA restriction. J Immunol. 2000;165:925–30. doi: 10.4049/jimmunol.165.2.925. [DOI] [PubMed] [Google Scholar]
- 39.Stenger S, Hanson DA, Teitlbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–5. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 40.Campbell JJ, Murphy KE, Kunkel EJ, et al. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001;166:877–84. doi: 10.4049/jimmunol.166.2.877. [DOI] [PubMed] [Google Scholar]
- 41.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–9. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–4. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 43.Majlessi L, Simsova M, Jarvis Z, et al. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect Immun. 2006;74:2128–37. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams A, Hatch GJ, Clark SO, et al. Evaluation of vaccines in the EU TB Vaccine Cluster using a guinea pig aerosol infection model of tuberculosis. Tuberculosis (Edinb ) 2005;85:29–38. doi: 10.1016/j.tube.2004.09.009. [DOI] [PubMed] [Google Scholar]