Abstract
Myostatin inhibits myogenesis and there is reduced abundance of the mature protein in skeletal muscles of adult male compared with female mice. This reduction probably occurs after translation, which suggests that it is a regulated mechanism to reduce the availability of myostatin in males. Reduced myostatin may, thereby, contribute to the development of sexually dimorphic growth of skeletal muscle. Our first objective was to determine if the decrease in mature myostatin protein occurs before the linear growth phase to aid growth, or afterwards to maintain the mass of adult muscle. Mice were killed from 2 to 32 weeks and the gastrocnemius muscle was excised. Myostatin mRNA increased from 2 to 32 weeks and was higher in males than females (P < 0.001). In contrast, mature protein decreased in males after 6 weeks (P < 0.001). Our second objective was to determine if growth hormone (GH) induces the decrease in mature myostatin protein. GH increased myostatin mRNA and decreased the abundance of mature protein in hypophysectomised mice (P < 0.05). Our final objective was to determine if the decrease in mature protein occurs in skeletal muscles of male Stat5b−/− mice (Stat5b mediates the actions of GH). As expected, mature myostatin protein was not reduced in Stat5b−/− males compared with females. However, myostatin mRNA remained higher in males than females irrespective of genotype. These data suggest that: (1) the decrease in mature myostatin protein is developmentally regulated, (2) GH acting via Stat5b regulates the abundance of mature myostatin and (3) GH acts via a non-Stat5b pathway to regulate myostatin mRNA.
Myostatin is expressed in skeletal muscle where it inhibits the proliferation and differentiation of precursor muscle cells (McPherron et al. 1997; Thomas et al. 2000). Remarkable increases (2-to 3-fold) in skeletal muscle occur in myostatin null (Mstn−/−) mice and in cattle with natural mutations in the myostatin gene that render the protein inactive (Grobet et al. 1997; Kambadur et al. 1997; McPherron et al. 1997; McPherron & Lee, 1997). Therefore, the amount of myostatin expressed may have a critical role in regulating the amount of muscle mass in individuals. We and others have demonstrated that the receptor binding moiety of myostatin, known as the mature protein, is 50% lower in skeletal muscles of adult male than female mice (McMahon et al. 2003a; Reisz-Porszasz et al. 2003). Our finding suggests that myostatin is differentially regulated between the sexes and this difference in abundance of the mature protein may contribute to the development of sexually dimorphic growth of skeletal muscles.
Like other transforming growth factor-β (TGF-β) family members, myostatin is proteolytically cleaved into an N-terminal latency-associated protein (LAP) and a C-terminal mature protein, which recombine in a non-covalent manner before secretion. Despite the decrease in mature myostatin in muscle of male mice, there was an equal abundance of LAP in skeletal muscles of both sexes (McMahon et al. 2003a). Therefore, the reduced abundance of mature myostatin protein in males probably occurs after translation. At present, it is unclear how this post-translational decrease in mature myostatin protein in males is regulated. It is also unclear if the mechanism is activated before the linear growth phase to enhance development of muscle in young males or after the linear growth phase to maintain the greater musculature of adult males.
It is clear that myostatin does not solely regulate sexually dimorphic growth because size dimorphism exists in Mstn−/− mice despite the greater musculature compared with wild-type mice (McPherron et al. 1997). At present, sexually dimorphic growth is largely attributed to the actions of GH acting via the signal transducer Stat5b to regulate synthesis and secretion of IGF-1 (Udy et al. 1997; Davey et al. 2001). Sexually dimorphic growth is absent in Stat5b−/− mice and growth of males is equivalent to that of females (Udy et al. 1997). In a preliminary study, we demonstrated that mature myostatin protein was not reduced in muscles of male compared with female Stat5b−/− mice (McMahon et al. 2003b). We postulate that sexually dimorphic growth of skeletal muscle is the result of GH, acting via Stat5b, to regulate a reduction in mature myostatin protein in addition to the increased expression and action of IGF-1 in males.
The purpose of the current study was 3-fold: first, to determine when the decrease in mature myostatin occurs in male mice during postnatal development; second, to determine if GH regulates the decrease in mature myostatin protein in skeletal muscles of males; and third, to determine if the decrease in mature myostatin protein is absent in skeletal muscles of male Stat5b−/− mice.
Methods
Animals
Male and female mice of the C57 strain were killed at 2, 3, 6, 12, 20 and 32 weeks of age (n= 6 of each sex per age; experiment 1). Thirty male hypophysectomised (Hypox) and six sham-Hypox male mice of the C57 strain (12–14 weeks old) were purchased from The Jackson Laboratory (Bar Harbour, MA, USA) and allocated at random to be injected i.p. with 50 μg/100 g body mass of recombinant hGH (Serono S.A., Aubonne, Switzerland) and killed at 30, 60 and 120 min (experiment 2). This dose has previously been shown to be effective in rats (Choi & Waxman, 2000). Controls (sham-Hypox and Hypox) were injected i.p. with sterile saline (vehicle) and killed at 0 min. At death, a visual inspection was made of the sella turcica to confirm complete removal of the anterior pituitary gland. Mice with an incomplete hypophysectomy were removed from analysis to ultimately give four to six animals per group. Male and female Stat5b−/− mice acquired from AgResearch Ltd (Udy et al. 1997) were killed at 20 weeks of age (n= 6 of each sex, experiment 3).
At death, mice were weighed, a blood sample was obtained by cardiac puncture and the gastrocnemius muscles were excised, weighed and snap frozen in liquid nitrogen and stored at −80°C. Plasma was harvested and stored at −20°C before analysing by Western blot.
All mice were maintained under a photoperiod of 14 h light: 10 h dark and had mouse chow (Diet 86, Sharps Grain and Seed, Carterton, New Zealand) and water available ad libitum.
This study was approved by the Ruakura Animal Ethics Committee.
Protein extraction and Western blot analysis
Lysis buffer (10 mm Hepes, 10 mm KCl, 1.5 mm MgCl, pH 7.9) with 0.5% IGEPAL detergent (Sigma Chemical Co. St Louis, MO, USA) and an enzyme inhibitor (Complete, Roche Diagnostics NZ Ltd, Auckland, New Zealand) was added to muscle from each mouse (1 ml to 150 mg of muscle). Samples were homogenized (Ultra Turrax, 13 500 r.p.m.) on ice, then centrifuged at 11 000 g for 10 min. Supernatant was recovered, mixed with Laemmli loading buffer (Laemmli, 1970), boiled for 5 min, then stored at −20°C until analysis. The protein concentration of the supernatant was determined using the bicinchoninic acid assay (Sigma Chemical Co.).
Twenty micrograms of protein from each muscle sample or 2 μl of plasma (in Laemmli loading buffer) was loaded and separated in a 10% SDS–polyacrylamide gel under reducing conditions, then transferred to a nitrocellulose membrane. After transfer, membranes were stained with Ponceau S to verify transfer of protein. Membranes were blocked (0.3% bovine serum albumin, 1% polyethylene glycol and 1% polyvinylpyrrolidone) for 2 h, then incubated with rabbit anti-myostatin antibody (Sharma et al. 1999) overnight (1: 4000), washed in 0.05 m Tris-buffered saline with 0.1% Tween 20 (TBST, pH 7.6), then incubated with HRP-conjugated goat anti-rabbit antibody (DakoCytomation, Medical-Bio, Christchurch, New Zealand) at 1: 5000 for 2 h, then washed again in TBST. Bound HRP activity was detected with enhanced chemiluminescence and then blots were exposed to XOMAT AR film (Eastman Kodak Company, Rochester, NY, USA), after which the optical densities of myostatin LAP and mature bands were captured with a densitometer (GS 800, Bio-Rad Laboratories Pty, Ltd, Auckland, New Zealand) and analysed using Quantity One software (Bio-Rad Laboratories). Membranes were then stripped (0.2 m Tris, pH 7.6, 2% SDS, 0.05 mβ-mercaptoethanol, at 50°C for 30 min), and exposed to rabbit anti-actin antibody (Sigma Chemical Co.) at a dilution of 1: 10 000 and developed as above to assess uniformity of loading. The specific myostatin bands have been identified previously (Reisz-Porszasz et al. 2003; McFarlane et al. 2005).
RNA extraction and real-time PCR
Frozen samples (100 mg) of the gastrocnemius muscles were homogenized on ice in Trizol Reagent (Invitrogen NZ Ltd, Auckland, New Zealand) for 30 s at 13 500 r.p.m. using an Ultra Turrax homogenizer. Debris was removed by centrifugation for 10 min at 10 000 g and total RNA isolated using the Trizol protocol. RNA was re-suspended in diethyl pyrocarbonate-treated water and the final concentration determined by measuring absorbance at 260 nm. Total RNA (5 μg) from each sample was reverse transcribed using oligo (dt) primers and Superscript III reverse transcriptase (Invitrogen NZ Ltd) according to manufacturer's instructions. The reverse transcribed (RT) reactions were then diluted 100-fold for quantification of myostatin. Real-time PCR was carried out using a Roche Lightcycler 2.0 with 2.5 μl of the diluted RT reaction and 7.5 μl of the mastermix (2.0 μl Roche Faststart DNA master PLUS SYBR Green I mix, 4.0 μl water, 0.5 μl of 10 μm forward primer and 0.5 μl of 10 μm reverse primer). The primers used to quantify mouse myostatin were: forward 5′-ACCCATGAAAGACGGTACAAG-3′ and reverse 5′-TCATCACAGTCAAGCCCAAAG-3′. The reaction conditions were as follows: Denature for 5 min at 95°C, followed by 40 cycles of 95°C for 5 s, 60°C for 10 s and 72°C for 20 s. Results for each sample were normalized to the concentration of cDNA in the RT samples (Lundby et al. 2005).
Statistical analysis
Data in experiment 1 were analysed by two-way ANOVA with sex, age and their interaction as the treatment effects. Data in experiment 2 were analysed by ANOVA using a randomised design with Hypox, sham-Hypox and time after GH treatment as the treatment effect. Data in experiment 3 were analysed by two-way ANOVA with sex, genotype and their interaction as treatment effects. Post hoc analysis among groups was done using the method of Tukey (Sokal & Rohlf, 1995). Data are presented as means ± standard error of the mean (s.e.m.).
Results
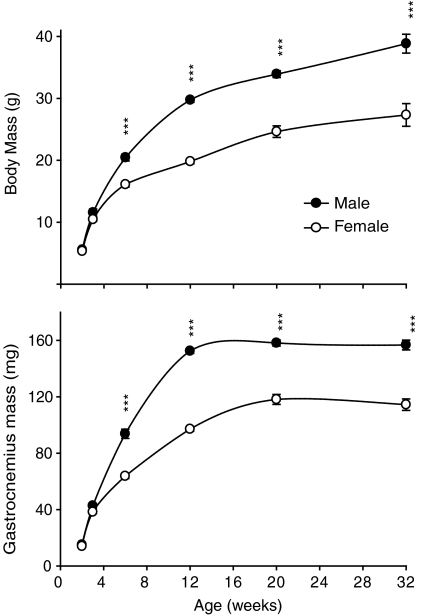
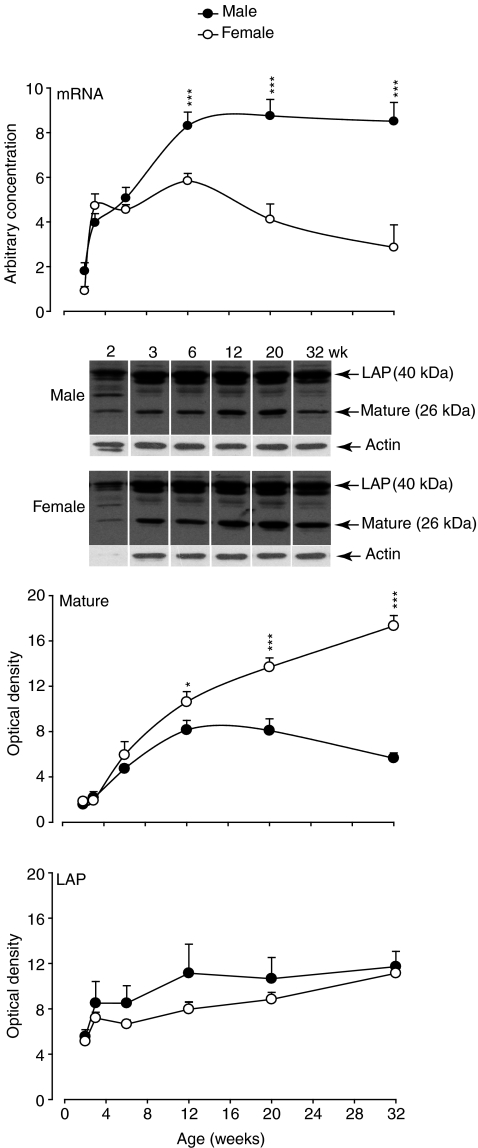
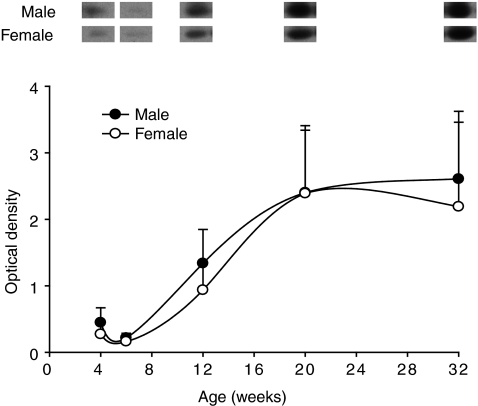
Sexual dimorphism of body mass and of gastrocnemius muscles was apparent from 6 weeks of age in mice (Fig. 1). Myostatin mRNA increased progressively from 2 weeks to maturity in both sexes and remained significantly higher in males than in females from 12 weeks of age (Fig. 2). The opposite pattern was evident for mature myostatin protein, which increased in female mice from 2 to 32 weeks, while it decreased and remained lower in male than in female muscles from 12 weeks (Fig. 2). In contrast, the abundance of LAP protein progressively increased in both male and female mice from 2 to 32 weeks, with no difference between the sexes (Fig. 2). The abundance of mature myostatin protein in blood increased from 6 to 32 weeks and was not different between male and female mice (Fig. 3).
Figure 1. Body mass and gastrocnemius mass in male and female mice of the C57 strain from 2 to 32 weeks of age.
Values are mean (±s.e.m.) (n= 6 per group). Asterisks indicate significance between sexes at each time point (***P < 0.001).
Figure 2. Myostatin mRNA, LAP and mature protein in gastrocnemius muscles of male and female C57 mice from 2 to 32 weeks of age.
Values are mean (±s.e.m.) (n= 6 per group). Asterisks indicate significance at each time point (*P < 0.05, ***P < 0.001). A representative Western blot is shown for myostatin LAP and mature protein for male and female mice at each age. The abundance of actin is shown to demonstrate the similarity in loading of protein per lane. Note, there was low protein in the samples collected at 2 weeks and actin was not detected in the representative female sample at this age.
Figure 3. Abundance of mature myostatin protein in plasma of male and female C57 mice from 4 to 32 weeks of age as measured by Western blotting.
Values are mean (±s.e.m.) (n= 6 per group).
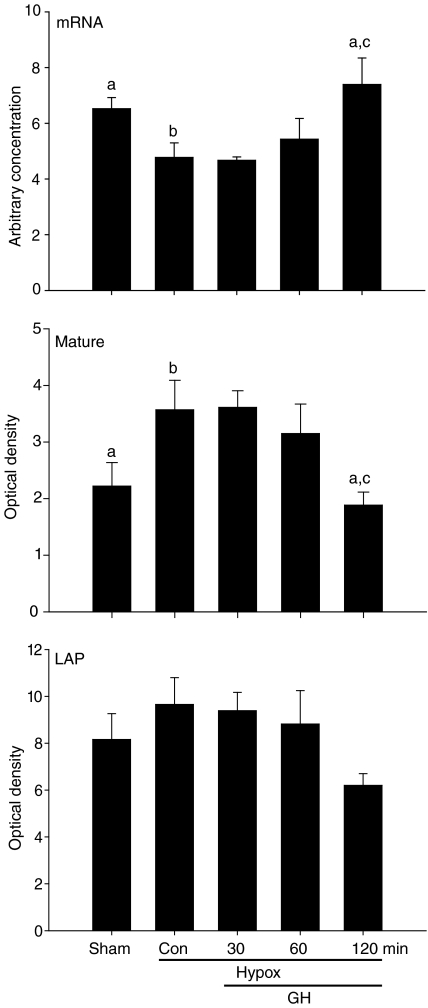
Myostatin mRNA was reduced (P < 0.05) in gastrocnemius muscles of control Hypox mice and was restored to that of sham-Hypox controls 120 min after injection of GH (Fig. 4). In contrast, abundance of mature myostatin protein was higher in muscles of control Hypox mice and was reduced to that of sham-Hypox controls 120 min after injection of GH (Fig. 4). The abundance of LAP had a similar pattern to that of mature myostatin, but differences were not significant (Fig. 4).
Figure 4. Myostatin mRNA, LAP and mature protein in gastrocnemius muscles of sham-Hypox or Hypox male C57 mice at 12 weeks of age.
Values are mean (±s.e.m.) (n= 4–6 per group). Mice were injected i.p. with vehicle (sham-Hypox and Hypox-controls), or with GH (50 μg/100 g body mass) and then killed at 30, 60 or 120 min. Unlike letters indicate significance among groups (a,bP < 0.05, b,cP < 0.01).
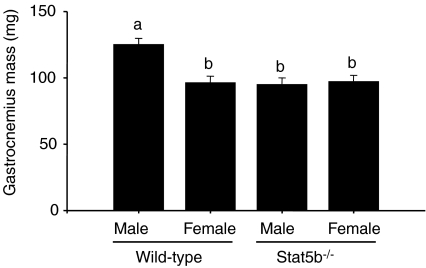
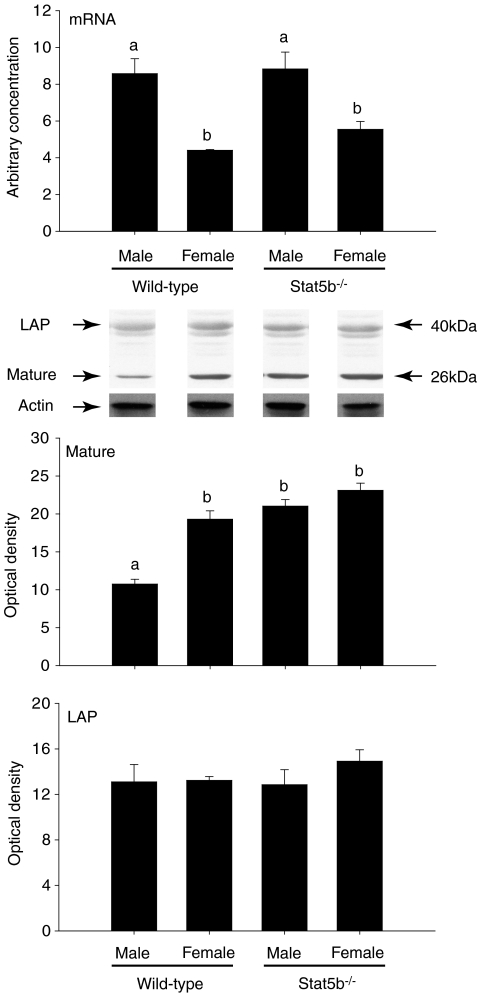
Sexually dimorphic growth of the gastrocnemius muscle did not occur in the absence of Stat5b (Fig. 5). However, myostatin mRNA was higher (P < 0.001) in male wild-type and Stat5b−/− mice, but only wild-type males had a lower abundance of mature myostatin protein than females (P < 0.001) (Fig. 6).
Figure 5. Mass of the gastrocnemius muscle in wild-type (C57) and Stat5b−/− mice.
Values are mean (±s.e.m.). Unlike letters indicate a significant difference among groups (a,bP < 0.001, n= 6 per sex and genotype).
Figure 6. Myostatin mRNA, LAP and mature protein in gastrocnemius muscles of male and female wild-type (C57) and Stat5b−/− mice at 20 weeks of age.
Values are mean (±s.e.m.) (n= 6 per group). Unlike letters indicate a significant difference among groups (a,bP < 0.001). A representative Western blot is shown for myostatin LAP and mature protein for each sex and genotype. The abundance of actin was assessed to demonstrate the similarity in loading of protein per lane.
Discussion
We have demonstrated here that the reduction in mature myostatin protein in skeletal muscles of male mice is initiated between 6 and 12 weeks of age. From 6 weeks, the divergence in growth and the abundance of mature myostatin protein became more apparent between the sexes, reaching the greatest difference when mice were 32 weeks of age. These data confirm and extend the observations in previous reports and support the postulate that a reduction in mature myostatin protein is initiated during the linear growth phase (McMahon et al. 2003a; Reisz-Porszasz et al. 2003). Therefore, this mechanism may contribute to the genesis of sexually dimorphic growth of skeletal muscle and maintenance of the greater mass of skeletal muscle in adult males. We speculate that the decrease occurs locally to regulate development of skeletal muscle in a paracrine or autocrine manner because the lower abundance of mature myostatin is observed in skeletal muscle, but not in blood. We speculate that the higher abundance of mature myostatin in females may be degraded before it reaches circulation.
We speculate that the decrease in mature myostatin protein occurs after translation because there is less in male gastrocnemius, but an equal abundance of LAP in males and females. However, we do not discount the possibility that a decrease in translation is also occurring. Indeed, while there is increased myostatin mRNA in male gastrocnemius, there is an equal amount of LAP in males and females, which could be explained by a decrease in translation in males. To address the potential mechanism, we provide evidence that GH induces the decrease in mature myostatin protein. It is interesting to note that the abundance of LAP tended to have a similar pattern to that of mature myostatin after injection of GH in hypophysectomised mice, which suggests that GH may mediate a similar reduction in the abundance of LAP, albeit to a lesser extent than the mature protein. The similar abundance of LAP between the sexes suggests that LAP is maintained at a constant amount in an age-dependent manner. The reason for this is unclear, but may relate to the fact that LAP also promotes the development of skeletal muscle in male and female mice (Yang et al. 2001). Therefore, a decrease in LAP would be detrimental to development of skeletal muscle and sexually dimorphic growth.
It is unclear why myostatin mRNA is higher in adult male compared with adult female mice. Given that the abundance of mature myostatin protein is much lower in males than in females, the higher mRNA in males may reflect a failure of myostatin to induce negative feedback to limit its own expression via increased expression of Smad7 (Zhu et al. 2004; Forbes et al. 2006). However, we show that in skeletal muscles of Stat5b−/− males, mature myostatin protein is not reduced to the level of wild-type males, yet myostatin mRNA is equally high in males of both genotypes compared with females. Therefore, these data suggest that the higher myostatin mRNA in adult C57 mice is not due to reduced negative feedback resulting from low concentrations of mature myostatin protein. The consequence of this is not clear at present.
The development of the sexually dimorphic pattern of secretion of GH occurs between 4 and 6 weeks of age in rats, and presumably also in mice (Eden, 1979; Gabriel et al. 1992). Before 4 weeks of age, circulating concentrations of GH are low. After 6 weeks of age, GH is secreted in episodic bursts in males, while females have higher basal concentrations with fewer and lower amplitude secretory episodes. The interpulse interval may be more critical than the amplitude because adult mice have similar peak amplitude pulses, but the interval between them is twice as long in males (MacLeod et al. 1991). Binding of GH to its receptors recruits the tyrosine kinase JAK2, which, in turn, phosphorylates Stat5b (Xu et al. 1996; Davey et al. 1999). In rats, transcription of Stat5b is maintained in males by episodic secretion of GH and is repressed in females by the more continuous secretion of GH with higher basal concentrations (Choi & Waxman, 1999; Gebert et al. 1999; Choi & Waxman, 2000). In fact, the binding activity of Stat5b is increased in male rats in conjunction with increased secretion of GH and decreased as concentrations of GH decline after a pulse (Tannenbaum et al. 2001). The continuous secretion of GH in female rats leads to a reduced abundance of Stat5b and renders them partially refractory to GH (Choi & Waxman, 1999,2000). The divergence of myostatin mRNA and mature protein occurred around 6 weeks of age, which is also consistent with the genesis of pulsatile secretion of GH. This observation prompted us to treat hypophysectomised mice (to remove endogenous GH) with GH to determine if GH reduces the abundance of mature myostatin. As expected, GH reduced the abundance of mature myostatin protein, but unexpectedly increased myostatin mRNA in skeletal muscle. This observation is consistent with the increased mRNA and decreased mature myostatin protein measured in male mouse gastrocnemius muscle in vivo. Given that myostatin mRNA is higher in gastrocnemius muscles of male Stat5b−/− mice than in females, these data suggest that GH induces transcription of myostatin mRNA via a Stat5b-independent pathway and induces the decrease in mature myostatin protein (and partially of LAP) via a Stat5b-mediated pathway.
We speculate that the pulsatile pattern of secreted GH is critical to regulate transcription, translation and post-translational processing of myostatin. This close relationship with the pattern of secreted GH may explain why others did not see an effect of administered GH on the abundance of myostatin mRNA or protein in GH-deficient rats (Kirk et al. 2000), pigs (Ji et al. 1998) and hypogonadal young men (Hayes et al. 2001) or elderly men (Brill et al. 2002). In those studies, GH was administered as a single daily injection, with tissues or biopsy samples taken long after injection, e.g. approximately 12 h later (Brill et al. 2002). In the current study, we investigated the effect of GH on myostatin in a time-dependent manner acutely after administration. In addition, with the exception of GH-deficient rats (Kirk et al. 2000), a further complication in those studies was the administration of GH to pituitary-intact males, in which the pulsatile secretion of GH was probably already influencing phosphorylation of Stat5b. Additional GH could down-regulate binding activity of Stat5b (as noted above) and we suggest that it would be unlikely for additional GH to elicit a further effect on the abundance of GH mRNA or protein, particularly in the study of Ji et al. (1998), where young, pituitary-intact pigs were used. Alternatively, the phenomenon of reduced myostatin protein in skeletal muscles of males may be restricted to rodents. We have observed that mature myostatin protein is also lower in skeletal muscles of adult male compared with female rats, but found no differences between skeletal muscles of adult male and female sheep or biopsy samples collected from skeletal muscles of men and women (range 18–30 year) (D. F. Gerrard, J. M. Oldham & C. D. McMahon, unpublished observations). The reason for this may be related to the reduced difference in secretion of GH between sexes in humans (Pincus et al. 1996; Veldhuis & Bowers, 2003), sheep (Gatford et al. 1997) and pigs (Dubreuil et al. 1987) compared with the more marked differences in rodents (Eden, 1979; MacLeod et al. 1991). To avoid these problems, we took care in the current study to remove the anterior pituitary gland, which provides the endogenous source of GH, and used mice over 10 weeks of age when the pattern of sexually dimorphism in secretion of GH had become established. Our rationale was that although exogenous GH can activate Stat5b in young rat pups (2–3 weeks), some downstream target genes are not responsive to Stat5b binding until after puberty (Choi & Waxman, 2000). This may explain why the abundance of myostatin mRNA was unchanged in the plantaris and masseter muscles after hypophysectomy of young (5 week old) male rats (Yamaguchi et al. 2006). Interestingly, hypophysectomy did lead to increased myostatin mRNA in the soleus muscle in that study, which contrasts with our data and, perhaps, highlights potential differences between slow-and fast-twitch muscles.
While the current data support a role for myostatin in regulating postnatal growth, the GH–IGF-1 axis is widely recognized as the major regulator of postnatal growth and is required for the development of sexual dimorphism (Le Roith et al. 2001). Global deletion of IGF-1 results in mice that are 70% smaller than wild-type controls as adults with no difference in size between males and females (Liu & LeRoith, 1999; Lupu et al. 2001). Local expression of IGF-1 is now thought to be the major contributor to growth since targeted deletion of liver-specific IGF-1 had no effect on postnatal growth of mice, despite reducing circulating concentrations of IGF-1 by 75% (Sjogren et al. 1999; Yakar et al. 1999). In support, muscle-specific deletion of Stat5a and 5b, which normally signal the actions of GH to induce transcription of IGF-1, resulted in reduced body and muscle mass in mice and is consistent with local regulation of muscle growth by autocrine/paracrine-derived IGF-1 (Klover & Hennighausen, 2007). An important observation in this study is that GH acts via Stat5b to regulate the reduction in mature myostatin protein. Therefore, it remains unclear what contribution IGF-1 and mature myostatin protein each make in regulating postnatal growth because elimination of Stat5b alone or Stat5a and 5b together will affect expression of both IGF-1 and myostatin. Potentially, the actions of GH are mediated via Stat5a and 5b to regulate the local availability of both IGF-1 and mature myostatin protein for co-ordination of postnatal growth in specific tissues.
Our findings are consistent with those of others who show that GH induces a decrease in myostatin protein in C2C12 myotubes (Liu et al. 2003). However, GH also reduced myostatin mRNA in biopsy samples collected from V. lateralis muscles of adult hypopituitary patients treated with GH and in differentiating C2C12 myoblasts in that study, which departs from our observations in vivo. An explanation for these differences between in vitro and in vivo studies is unclear. It should be noted that in the present study, we show that myostatin mRNA is consistently greater in males compared with females of two genotypes of mice (C57 and Stat5b−/−) and that this difference is developmentally regulated. Therefore, we suggest that different autocrine and endocrine factors might be acting in vivo that may not be present in vitro, at different stages of development or in different pathological states.
In conclusion, these data suggest that: (1) the decrease in mature myostatin protein is developmentally regulated, (2) GH acting via Stat5b regulates the decrease in mature myostatin protein and (3) GH acts via a non-Stat5b pathway to regulate myostatin mRNA.
Acknowledgments
We are indebted to the late Helen Davey for Stat5b−/− mice. We thank Ric Broadhurst, Bobby Smith and Glenda Smith for care and maintenance of mice during this study, Dr Neil Cox for statistical advice and Drs Ravi Kambadur and Mridula Sharma for myostatin antibody.
References
- Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, Hanks JB, Urban RJ, Veldhuis JD. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab. 2002;87:5649–5657. doi: 10.1210/jc.2002-020098. [DOI] [PubMed] [Google Scholar]
- Choi HK, Waxman DJ. Growth hormone, but not prolactin, maintains, low-level activation of STAT5a and STAT5b in female rat liver. Endocrinology. 1999;140:5126–5135. doi: 10.1210/endo.140.11.7106. [DOI] [PubMed] [Google Scholar]
- Choi HK, Waxman DJ. Plasma growth hormone pulse activation of hepatic JAK-STAT5 signaling: developmental regulation and role in male-specific liver gene expression. Endocrinology. 2000;141:3245–3255. doi: 10.1210/endo.141.9.7638. [DOI] [PubMed] [Google Scholar]
- Davey HW, Wilkins RJ, Waxman DJ. STAT5 signaling in sexually dimorphic gene expression and growth patterns. Am J Hum Genet. 1999;65:959–965. doi: 10.1086/302599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology. 2001;142:3836–3841. doi: 10.1210/endo.142.9.8400. [DOI] [PubMed] [Google Scholar]
- Dubreuil P, Pelletier G, Petitclerc D, Lapierre H, Couture Y, Brazeau P, Gaudreau P, Morisset J. Influence of age and sex on basal secretion of growth hormone (GH) and on GH-induced release by porcine GH-releasing factor pGRF (1-29NH2) in growing pigs. Domest Anim Endocrinol. 1987;4:299–307. doi: 10.1016/0739-7240(87)90026-9. [DOI] [PubMed] [Google Scholar]
- Eden S. Age-and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology. 1979;105:555–560. doi: 10.1210/endo-105-2-555. [DOI] [PubMed] [Google Scholar]
- Forbes D, Jackman M, Bishop A, Thomas M, Kambadur R, Sharma M. Myostatin auto-regulates its expression by feedback loop through Smad7 dependent mechanism. J Cell Physiol. 2006;206:264–272. doi: 10.1002/jcp.20477. [DOI] [PubMed] [Google Scholar]
- Gabriel SM, Roncancio JR, Ruiz NS. Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology. 1992;56:619–625. doi: 10.1159/000126284. [DOI] [PubMed] [Google Scholar]
- Gatford KL, Fletcher TP, Rao A, Egan AR, Hosking BJ, Clarke IJ. GH, GH-releasing factor and somatostatin in the growing lamb: sex differences and mechanisms for sex differences. J Endocrinol. 1997;152:19–27. doi: 10.1677/joe.0.1520019. [DOI] [PubMed] [Google Scholar]
- Gebert CA, Park SH, Waxman DJ. Down-regulation of liver JAK2-STAT5b signaling by the female plasma pattern of continuous growth hormone stimulation. Mol Endocrinol. 1999;13:213–227. doi: 10.1210/mend.13.2.0238. [DOI] [PubMed] [Google Scholar]
- Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset R, Georges M. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17:71–74. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- Hayes VY, Urban RJ, Jiang J, Marcell TJ, Helgeson K, Mauras N. Recombinant human growth hormone and recombinant human insulin-like growth factor I diminish the catabolic effects of hypogonadism in man: metabolic and molecular effects. J Clin Endocrinol Metab. 2001;86:2211–2219. doi: 10.1210/jcem.86.5.7517. [DOI] [PubMed] [Google Scholar]
- Ji S, Losinski RL, Cornelius SG, Frank GR, Willis GM, Gerrard DE, Depreux FF, Spurlock ME. Myostatin expression in porcine tissues: tissue specificity and developmental and postnatal regulation. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1265–R1273. doi: 10.1152/ajpregu.1998.275.4.R1265. [DOI] [PubMed] [Google Scholar]
- Kambadur R, Sharma M, Smith TPL, Bass JJ. Mutations in myostatin (GDF8) in double-muscle Belgian blue and Piedmontese cattle. Genome Res. 1997;7:910–915. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- Kirk S, Oldham J, Kambadur R, Sharma M, Dobbie P, Bass J. Myostatin regulation during skeletal muscle regeneration. J Cellular Physiol. 2000;184:356–363. doi: 10.1002/1097-4652(200009)184:3<356::AID-JCP10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Klover P, Hennighausen L. Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology. 2007;148:1489–1497. doi: 10.1210/en.2006-1431. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu J-L, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- Liu J-L, LeRoith D. Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology. 1999;140:5178–5184. doi: 10.1210/endo.140.11.7151. [DOI] [PubMed] [Google Scholar]
- Liu W, Thomas SG, Asa SL, Gonzalez-Cadavid N, Bhasin S, Ezzat S. Myostatin is a skeletal muscle target of growth hormone anabolic action. J Clin Endocrinol Metab. 2003;88:5490–5496. doi: 10.1210/jc.2003-030497. [DOI] [PubMed] [Google Scholar]
- Lundby C, Nordsborg N, Kusuhara K, Kristensen KM, Neufer PD, Pilegaard H. Gene expression in human skeletal muscle: alternative normalization method and effect of repeated biopsies. Eur J Appl Physiol. 2005;95:351–360. doi: 10.1007/s00421-005-0022-7. [DOI] [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- McFarlane C, Langley B, Thomas M, Hennebry A, Plummer E, Nicholas G, McMahon C, Sharma M, Kambadur R. Proteolytic processing of myostatin is auto-regulated during myogenesis. Dev Biol. 2005;283:58–69. doi: 10.1016/j.ydbio.2005.03.039. [DOI] [PubMed] [Google Scholar]
- MacLeod JN, Pampori NA, Shapiro BH. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol. 1991;131:395–399. doi: 10.1677/joe.0.1310395. [DOI] [PubMed] [Google Scholar]
- McMahon CD, Popovic L, Jeanplong F, Oldham JM, Kirk SP, Osepchook CC, Wong KW, Sharma M, Kambadur R, Bass JJ. Sexual dimorphism is associated with decreased expression of processed myostatin in males. Am J Physiol Endocrinol Metab. 2003a;284:E377–E381. doi: 10.1152/ajpendo.00282.2002. [DOI] [PubMed] [Google Scholar]
- McMahon CD, Wilkins RJ, Davey HW, Xie T, Jeanplong F, Oldham JM, Kirk SP, Osepchook CC, Sharma M, Kambadur R, Bass JJ. Stat5b may mediate the post-translational decrease of mature myostatin peptide in male mice (Abstract). The Endocrine Society, 85th Annual Meeting; Philadelphia. 2003b. [Google Scholar]
- McPherron AC, Lawler AM, Lee S-J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM, Gevers EF, Robinson IC, van den Berg G, Roelfsema F, Hartman ML, Veldhuis JD. Females secrete growth hormone with more process irregularity than males in both humans and rats. Am J Physiol Endocrinol Metab. 1996;270:E107–E115. doi: 10.1152/ajpendo.1996.270.1.E107. [DOI] [PubMed] [Google Scholar]
- Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003;285:E876–E888. doi: 10.1152/ajpendo.00107.2003. [DOI] [PubMed] [Google Scholar]
- Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ. Myostatin, a transforming growth factor-β superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999;180:1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO, Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. 3rd edn. New York: W.H. Freeman; 1995. pp. 1–887. [Google Scholar]
- Tannenbaum GS, Choi HK, Gurd W, Waxman DJ. Temporal relationship between the sexually dimorphic spontaneous GH secretory profiles and hepatic STAT5 activity. Endocrinology. 2001;142:4599–4606. doi: 10.1210/endo.142.11.8480. [DOI] [PubMed] [Google Scholar]
- Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Bowers CY. Human GH pulsatility: an ensemble property regulated by age and gender. J Endocrinol Invest. 2003;26:799–813. doi: 10.1007/BF03345229. [DOI] [PubMed] [Google Scholar]
- Xu BC, Wang X, Darus CJ, Kopchick JJ. Growth hormone promotes the association of transcription factor STAT5 with the growth hormone receptor. J Biol Chem. 1996;271:19768–19773. [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Fujikawa T, Tateoka M, Soya H, Sakuma K, Sugiura T, Morita I, Ikeda Y, Hirai T. The expression of IGF-I and myostatin mRNAs in skeletal muscle of hypophysectomized and underfed rats during postnatal growth. Acta Physiol (Oxf) 2006;186:291–300. doi: 10.1111/j.1748-1716.2006.01569.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Ratovitski T, Brady JP, Solomon MB, Wells KD, Wall RJ. Expression of myostatin pro domain results in muscular transgenic mice. Mol Reprod Dev. 2001;60:351–361. doi: 10.1002/mrd.1097. [DOI] [PubMed] [Google Scholar]
- Zhu X, Topouzis S, Liang LF, Stotish RL. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine. 2004;26:262–272. doi: 10.1016/j.cyto.2004.03.007. [DOI] [PubMed] [Google Scholar]