Abstract
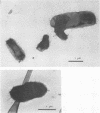
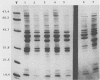
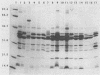
Over a period of 6 years, 114 strains of Haemophilus influenzae and Haemophilus parainfluenzae were isolated from genital, mother-infant, or neonatal infections. Their serotypes, biotypes, antibiotic resistance phenotypes, and outer membrane protein (OMP) electrophoretic patterns were characterized and correlated with the various clinical outcomes. Genital H. influenzae and H. parainfluenzae appeared to behave mostly as opportunistic pathogens; for instance, 62% of the cases of endometritis or pelvic inflammatory disease were related to the presence of an intrauterine device. However, as seen clearly in one case, the strains may be sexually transmitted. The analysis of OMP patterns proved to be a very convenient method to seek evidence for the sexual origin of the infection. H. influenzae was more often involved in complicated genital infections than was H. parainfluenzae. Nontypeable and biotype II H. influenzae strains were the more frequent isolates, except in pelvic inflammatory diseases, in which biotype I prevailed, and in mother-infant infections, in which one-fourth of the cases were due to biotype IV. Characterization of H. influenzae isolates did not support a general concept of specific genital strains. However, strains of biotype IV clearly stood out with two characteristics: (i) a peritrichous fimbriation and (ii) a very peculiar homogeneous OMP pattern comprising an OMP of molecular weight approximately 18,000 unique to this biotype. These characteristics were also found in H. influenzae biotype IV strains isolated from genital infections in the United States and used as controls. H. influenzae biotype IV strains may thus correspond to a group somewhat adapted to the genital tract.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L., Brunton J. L., Meier M., Bowman M. N., Slaney L. A. Haemophilus influenzae: comparison of respiratory tract isolates with genitourinary tract isolates. J Clin Microbiol. 1982 Nov;16(5):826–831. doi: 10.1128/jcm.16.5.826-831.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton W. L., Hammond G. W., Ronald A. R. Bacteremic Haemophilus influenzae genitourinary tract infections in adults. Arch Intern Med. 1978 Dec;138(12):1819–1821. [PubMed] [Google Scholar]
- Antiphon P. Résultats cliniques de l'enquête multicentrique sur l'infection a Haemophilus. Pathol Biol (Paris) 1983 Feb;31(2):81–85. [PubMed] [Google Scholar]
- Apicella M. A., Shero M., Dudas K. C., Stack R. R., Klohs W., LaScolea L. J., Murphy T. F., Mylotte J. M. Fimbriation of Haemophilus species isolated from the respiratory tract of adults. J Infect Dis. 1984 Jul;150(1):40–43. doi: 10.1093/infdis/150.1.40. [DOI] [PubMed] [Google Scholar]
- Aujard Y., Lambert-Zechovsky N., Proux M. C., Bingen E., Helias J. P., Mathieu H. Infection à Haemophilus para influenzae puis infection à Enterobacter cloacae chez un nouveau-né. Arch Fr Pediatr. 1982 May;39(5):315–316. [PubMed] [Google Scholar]
- Barenkamp S. J., Granoff D. M., Munson R. S., Jr Outer-membrane protein subtypes of Haemophilus influenzae type b and spread of disease in day-care centers. J Infect Dis. 1981 Sep;144(3):210–217. doi: 10.1093/infdis/144.3.210. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Outer membrane protein and biotype analysis of pathogenic nontypable Haemophilus influenzae. Infect Immun. 1982 May;36(2):535–540. doi: 10.1128/iai.36.2.535-540.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Berczy J., Fernlund K., Kamme C. Haemophilus influenzae in septic abortion. Lancet. 1973 May 26;1(7813):1197–1197. doi: 10.1016/s0140-6736(73)91212-9. [DOI] [PubMed] [Google Scholar]
- Blum F., Tessier F., Pathier D., Treisser A., Faguer C., Barrat J. Infections génitales hautes aiguës. II. Etude bactériologique et conséquences thérapeutiques. J Gynecol Obstet Biol Reprod (Paris) 1980;9(2):229–242. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burns T. R., Hinds D. B., Hawkins E. Haemophilus organisms: urinary tract pathogens in children? Diagn Microbiol Infect Dis. 1984 Jun;2(3):251–253. doi: 10.1016/0732-8893(84)90038-5. [DOI] [PubMed] [Google Scholar]
- Campognone P., Singer D. B. Neonatal sepsis due to nontypable Haemophilus influenzae. Am J Dis Child. 1986 Feb;140(2):117–121. doi: 10.1001/archpedi.1986.02140160035025. [DOI] [PubMed] [Google Scholar]
- Chen W. N., Richards R., Carpenter R., Ramachander N. Haemophilus influenzae as an agent of urinary tract infectio.?W0C. West Indian Med J. 1976 Sep;25(3):158–161. [PubMed] [Google Scholar]
- Chesney P. J., Saari T. N., Mueller G. Acute epididymo-orchitis due to Hemophilus influenzae type b. J Pediatr. 1977 Oct;91(4):685–685. doi: 10.1016/s0022-3476(77)80540-4. [DOI] [PubMed] [Google Scholar]
- Controni G., Chang M. J., Gold B. G., Rodriguez W. J. Haemophilus influenzae biotype III infections in children and report of three unusual cases. Am J Clin Pathol. 1981 Nov;76(5):718–720. doi: 10.1093/ajcp/76.5.718. [DOI] [PubMed] [Google Scholar]
- Courtney S. E., Hall R. T. Haemophilus influenzae sepsis in the premature infant. Am J Dis Child. 1978 Oct;132(10):1039–1040. doi: 10.1001/archpedi.1978.02120350107022. [DOI] [PubMed] [Google Scholar]
- Dabernat H., Delmas C., Lareng M. B. Prévalence de la résistance aux antibiotiques des Haemophilus influenzae isolés en France: un an d'activité du réseau de surveillance des infections à H. influenzae. Pathol Biol (Paris) 1986 May;34(5):372–378. [PubMed] [Google Scholar]
- DePass E. E., Fardy P. W., Boulos J. B., Abear E. M. Haemophilus influenzae pyosalpingitis. Can Med Assoc J. 1982 Jun 15;126(12):1417–1418. [PMC free article] [PubMed] [Google Scholar]
- Eschenbach D. A., Buchanan T. M., Pollock H. M., Forsyth P. S., Alexander E. R., Lin J. S., Wang S. P., Wentworth B. B., MacCormack W. M., Holmes K. K. Polymicrobial etiology of acute pelvic inflammatory disease. N Engl J Med. 1975 Jul 24;293(4):166–171. doi: 10.1056/NEJM197507242930403. [DOI] [PubMed] [Google Scholar]
- Farrand R. J. Haemophilus influenzae infections of the genital tract. J Med Microbiol. 1971 Aug;4(3):357–358. doi: 10.1099/00222615-4-3-357. [DOI] [PubMed] [Google Scholar]
- Goplerud C. P., Ohm M. J., Galask R. P. Aerobic and anaerobic flora of the cervix during pregnancy and the puerperium. Am J Obstet Gynecol. 1976 Dec 1;126(7):858–868. doi: 10.1016/0002-9378(76)90674-8. [DOI] [PubMed] [Google Scholar]
- Granato P. A., Jurek E. A., Weiner L. B. Biotypes of Haemophilus influenzae: relationship to clinical source of isolation, serotype, and antibiotic susceptibility. Am J Clin Pathol. 1983 Jan;79(1):73–77. doi: 10.1093/ajcp/79.1.73. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Roskes S. Urinary tract infection due to Hemophilus influenzae, type b. J Pediatr. 1974 Mar;84(3):414–416. doi: 10.1016/s0022-3476(74)80729-8. [DOI] [PubMed] [Google Scholar]
- Guinet R., Boude M., Guillermet F. N., Palayer C., Lanazou-Betbeder J. Pyosalpingite aiguë à Haemophilus influenzae type b biotype I. Nouv Presse Med. 1979 Sep 24;8(36):2904–2904. [PubMed] [Google Scholar]
- Hall G. D., Washington J. A., 2nd Haemophilus influenzae in genitourinary tract infections. Diagn Microbiol Infect Dis. 1983 Mar;1(1):65–70. doi: 10.1016/0732-8893(83)90034-2. [DOI] [PubMed] [Google Scholar]
- Herva E., Pokela R., Ylikorkala O. Haemophilus influenzae as a cause of salpingitis. Ann Chir Gynaecol Fenn. 1975;64(5):317–319. [PubMed] [Google Scholar]
- Holmes R. L., DeFranco L. M., Otto M. Novel method of biotyping Haemophilus influenzae that uses API 20e. J Clin Microbiol. 1982 Jun;15(6):1150–1152. doi: 10.1128/jcm.15.6.1150-1152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne R. W., Ronchetti I. P. A negative staining-carbon film technique for studying viruses in the electron microscope. I. Preparative procedures for examining icosahedral and filamentous viruses. J Ultrastruct Res. 1974 Jun;47(3):361–383. doi: 10.1016/s0022-5320(74)90015-x. [DOI] [PubMed] [Google Scholar]
- Hurley R. Haemophilus endometritis in woman fitted with Lippes loop. Br Med J. 1970 Feb 28;1(5695):566–566. doi: 10.1136/bmj.1.5695.566-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuri-Bulos N., McIntosh K. Neonatal Haemophilus influenzae infection. Report of eight cases and review of the literature. Am J Dis Child. 1975 Jan;129(1):57–62. doi: 10.1001/archpedi.1975.02120380037009. [DOI] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Kilian M., Sørensen I., Frederiksen W. Biochemical characteristics of 130 recent isolates from Haemophilus influenzae meningitis. J Clin Microbiol. 1979 Mar;9(3):409–412. doi: 10.1128/jcm.9.3.409-412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman M. B., Reynolds J. K., Schreiner R. L., Smith J. W. Failure to demonstrate special virulence of nontypable Haemophilus influenzae biotype 4 in neonatal sepsis. J Infect Dis. 1983 Sep;148(3):615–615. doi: 10.1093/infdis/148.3.615. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Marston G., Wald E. R. Hemophilus influenzae type b sepsis in infant and mother. Pediatrics. 1976 Dec;58(6):863–864. [PubMed] [Google Scholar]
- Murphy T. F., Apicella M. A. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis. 1987 Jan-Feb;9(1):1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Dudas K. C., Mylotte J. M., Apicella M. A. A subtyping system for nontypable Haemophilus influenzae based on outer-membrane proteins. J Infect Dis. 1983 May;147(5):838–846. doi: 10.1093/infdis/147.5.838. [DOI] [PubMed] [Google Scholar]
- Musher D. M. Haemophilus influenzae infections. Hosp Pract (Off Ed) 1983 Aug;18(8):158-61, 166, 169-70. doi: 10.1080/21548331.1983.11702617. [DOI] [PubMed] [Google Scholar]
- Mårdh P. A., Weström L. Tubal and cervical cultures in acute salpingitis with special reference to Mycoplasma hominis and T-strain mycoplasmas. Br J Vener Dis. 1970 Jun;46(3):179–186. [PMC free article] [PubMed] [Google Scholar]
- Oberhofer T. R., Back A. E. Biotypes of Haemophilus encountered in clinical laboratories. J Clin Microbiol. 1979 Aug;10(2):168–174. doi: 10.1128/jcm.10.2.168-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden E., Amstey M. S. Hemophilus influenza septicemia and midtrimester abortion. J Reprod Med. 1979 Feb;22(2):106–108. [PubMed] [Google Scholar]
- Ohm M. J., Galask R. P. Bacterial flora of the cervix from 100 prehysterectomy patients. Am J Obstet Gynecol. 1975 Jul 15;122(6):683–687. doi: 10.1016/0002-9378(75)90571-2. [DOI] [PubMed] [Google Scholar]
- Paavonen J., Lehtinen M., Teisala K., Heinonen P. K., Punnonen R., Aine R., Miettinen A., Grönroos P. Haemophilus influenzae causes purulent salpingitis. Am J Obstet Gynecol. 1985 Feb 1;151(3):338–339. doi: 10.1016/0002-9378(85)90299-6. [DOI] [PubMed] [Google Scholar]
- Palmer G. G. Haemophili in faeces. J Med Microbiol. 1981 Feb;14(1):147–150. doi: 10.1099/00222615-14-1-147. [DOI] [PubMed] [Google Scholar]
- Pinon G., Quentin R., Lebret P., Zephyr D. Orchite de l'adulte à Haemophilus influenzae. Ann Urol (Paris) 1984 Feb;18(1):40–41. [PubMed] [Google Scholar]
- Quentin R., Goudeau A., Burfin E., Pinon G., Berger C., Laugier J., Soutoul J. H. Infections materno-foetales à Haemophilus influenzae. Presse Med. 1987 Jun 20;16(24):1181–1184. [PubMed] [Google Scholar]
- Sackel S. G., Alpert S., Rosner B., McCormack W. M., Finland M. In vitro activity of p-hydroxybenzyl penicillin (penicillin X) and five other penicillins against Neisseria gonorrhoeae: comparisons of strains from patients with uncomplicated infections and from women with pelvic inflammatory disease. Antimicrob Agents Chemother. 1977 Jul;12(1):31–36. doi: 10.1128/aac.12.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit K. E. Isolation of Haemophilus in urine cultures from children. J Pediatr. 1979 Oct;95(4):565–566. doi: 10.1016/s0022-3476(79)80769-6. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Southwick F. S., Moellering R. C., Jr, Sherman E. Hemophilus influenzae in hospitalized adults: current perspectives. Am J Med. 1980 Aug;69(2):219–226. doi: 10.1016/0002-9343(80)90381-2. [DOI] [PubMed] [Google Scholar]
- Sweet R. L., Mills J., Hadley K. W., Blumenstock E., Schachter J., Robbie M. O., Draper D. L. Use of laparoscopy to determine the microbiologic etiology of acute salpingitis. Am J Obstet Gynecol. 1979 May 1;134(1):68–74. doi: 10.1016/0002-9378(79)90798-1. [DOI] [PubMed] [Google Scholar]
- Trollfors B. Two cases of septicemia caused by noncapsulated strains of Haemophilus influenzae. Scand J Infect Dis. 1978;10(3):247–248. doi: 10.3109/inf.1978.10.issue-3.16. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Waldman L. S., Kosloske A. M., Parsons D. W. Acute epididymoorchitis as the presenting manifestation of Hemophilus influenzae septicemia. J Pediatr. 1977 Jan;90(1):87–89. doi: 10.1016/s0022-3476(77)80773-7. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Baker C. J., Quinones F. J., Hollis D. G., Weaver R. E., Wiss K. Nontypable Haemophilus influenzae (biotype 4) as a neonatal, maternal, and genital pathogen. Rev Infect Dis. 1983 Jan-Feb;5(1):123–136. doi: 10.1093/clinids/5.1.123. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musher D. M., Septimus E. J., McGowan J. E., Jr, Quinones F. J., Wiss K., Vance P. H., Trier P. A. Haemophilus influenzae infections in adults: characterization of strains by serotypes, biotypes, and beta-lactamase production. J Infect Dis. 1981 Aug;144(2):101–106. doi: 10.1093/infdis/144.2.101. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Hopman C., Zanen H. C. Homogeneity of cell envelope protein subtypes, lipopolysaccharide serotypes, and biotypes among Haemophilus influenzae type b from patients with meningitis in The Netherlands. J Infect Dis. 1983 Jul;148(1):75–81. doi: 10.1093/infdis/148.1.75. [DOI] [PubMed] [Google Scholar]