Abstract
Neuropathologic and genetics studies as well as transgenic animal models have provided strong evidence linking misfolding and aggregation of α-synuclein to the progression of Parkinson disease (PD) and other related disorders. A growing body of evidence implicates various oligomeric forms of α-synuclein as the toxic species responsible for neurodegeneration and neuronal cell death. Although numerous different oligomeric forms of α-synuclein have been identified in vitro, it is not known which forms are involved in PD or how, when, and where different forms contribute to the progression of PD. Reagents that can interact with specific aggregate forms of α-synuclein would be very useful not only as tools to study how different aggregate forms affect cell function, but also as potential diagnostic and therapeutic agents for PD. Here we show that a single chain antibody fragment (syn-10H scFv) isolated from a phage display antibody library binds to a larger, later stage oligomeric form of α-synuclein than a previously reported oligomeric specific scFv isolated in our laboratory. The scFv described here inhibits aggregation of α-synuclein in vitro, blocks extracellular α-synuclein-induced toxicity in both undifferentiated and differentiated human neuroblastoma cell lines (SH-SY5Y), and specifically recognizes naturally occurring aggregates in PD but not in healthy human brain tissue.
Parkinson disease (PD)2 is the second most common neurodegenerative disorder of the elderly, affecting more than 500,000 people in the United States (1), with 50,000 new cases reported each year at an annual cost estimated at 10 billion dollars per year. Pathologically, PD is characterized by the progressive loss of dopaminergic neurons in the substantia nigra and formation of fibrillar cytoplasmic inclusions known as Lewy bodies and Lewy neurites (2, 3). The protein α-synuclein has been strongly linked to PD (4, 5) and other related neurodegenerative disorders (6, 7) by several lines of evidence. 1) It is the major component of the hallmark Lewy body aggregates associated with PD. 2) Mutations (A53T, A30P, and E46K, where A30P is human A30P α-synuclein; A53T is human A53T α-synuclein; E46K is human E46K α-synuclein) or multiplication in the α-synuclein gene have been linked to familial PD (8–10). 3) Overexpression of α-synuclein in transgenic mice and Drosophila has been shown to induce the formation of PD-like pathological phenotypes and behavior, although the animal models do not in general replicate neuronal loss patterns (11, 12).
α-Synuclein is a small protein (14 kDa) expressed mainly in brain tissues and is primarily localized at the presynaptic terminals of neurons (13). The primary structure of α-synuclein consists of three distinct regions. The N-terminal region of α-synuclein includes the mutation sites associated with familial PD (A53T, A30P, and E46K) and contains six imperfectly conserved repeats (KTKEGV) that may facilitate protein-protein binding. This repeat section is predicted to form amphipathic α-helices, typical of the lipid-binding domain of apolipoproteins (14). The central region, non-amyloid component, is extremely hydrophobic and includes a 12-residue stretch (VTGVTAVAQKTV) that is essential for aggregation (15). The C-terminal region is enriched with acidic glutamate and aspartate residues and is responsible for the chaperone function of α-synuclein (16).
α-Synuclein normally exists as an unfolded protein, but it can adopt several different folded conformations depending on the environment, including small aggregates or oligomers, spherical and linear protofibrils, as well as the fibrillar structure found in Lewy bodies (14, 15). A growing body of evidence implicates the oligomeric forms of α-synuclein as the toxic species responsible for neurodegeneration and neuronal cell death (16–18). Several different oligomeric forms of α-synuclein including spherical, annular (19), pore-like (20), and dopamine-stabilized structures have been identified in vitro (21).
α-Synuclein is considered a cytosolic protein, and consequently its pathogenic effect was assumed to be limited to the cytoplasm of single cells. However, recent studies have suggested that α-synuclein also has extracellular pathogenic effects (22–25). α-Synuclein was detected in blood plasma and cerebrospinal fluid in both monomeric and oligomeric forms (22–25), and the presence of significantly elevated levels of oligomeric species of α-synuclein has been reported extracellularly in plasma and cerebrospinal fluid samples from patients with PD (23). Furthermore, various studies have shown that aggregated α-synuclein added extracellularly to the culture medium is cytotoxic (26–32).
Despite all these studies, it is still not clear how the various aggregate forms of α-synuclein are involved in the progression of PD. Therefore, reagents that can interact with specific aggregate forms of α-synuclein would be very useful not only for fundamental studies of how α-synuclein aggregates affect cell function but also as potential diagnostic and therapeutic agents for PD.
Recently, we reported inhibition of both aggregation and extracellular toxicity of α-synuclein in vitro by a single chain variable domain antibody fragment (scFv) that specifically recognized an oligomeric form of α-synuclein (32). In this study, we describe a second scFv (syn-10H) that binds a larger later stage oligomeric form of α-synuclein than the previously reported scFv. The syn-10H scFv neutralizes α-synuclein-induced toxicity in both undifferentiated and differentiated SH-SY5Y human neuroblastoma cell line and inhibits α-synuclein aggregation in vitro. The syn-10H scFv reacts specifically with homogenized PD brain tissue but does not cross-react with similarly treated samples taken from Alzheimer disease (AD) or healthy brain samples. Such scFvs therefore have potential value as diagnostic reagents to identify the presence of specific oligomeric species in PD tissue and fluid samples. The scFvs also have value as therapeutic agents as they can be used either extracellularly or expressed intracellularly (intrabodies) to prevent formation of toxic aggregates in vivo whether inside or outside of cells. Intrabodies have been used efficiently to neutralize toxic effects of different pathogenic agents, including α-synuclein (33–36). Moreover, immunization studies in mouse models of PD have shown that extracellular antibodies can reduce accumulation of intracellular aggregates of α-synuclein (37), thereby providing precedent for the use of scFvs in potential passive vaccination strategies for treating PD.
EXPERIMENTAL PROCEDURES
Materials
The Tomlinson I and J phage libraries, helper phage KM13, Escherichia coli TG1, and HB2151 were obtained from Medical Research Council (Cambridge, UK).
Phage Display Antibody Library
Each phage library has a diversity of greater than 108 and are comprised of a single polypeptide with the VH and VL domains connected by a flexible (Gly4Ser)3 linker. The phage library was propagated essentially as described (32, 38). Libraries were grown separately and mixed in equal titers for the panning experiments.
Production and Purification of α-Synuclein
α-Synuclein plasmid provided by Dr. Michael J. Volles (Brigham and Women's Hospital, Harvard Medical School) was transformed into BL-21 competent cells, plated onto LB-agar plates (supplemented with 100 μg/ml ampicillin), and grown overnight at 37 °C. Single colonies of BL21 (DE3) were grown and purified essentially as described (39). α-Synuclein was lyophilized and stored at –80 °C until further use.
Production of Oligomeric and Fibrillar α-Synuclein
The lyophilized α-synuclein stock was dissolved in Buffer A (25 mm Tris-HCl and 150 mm NaCl, pH 7.4) to a concentration of 70 μm. Oligomeric aggregates of α-synuclein were obtained by incubating at 37 °C for 7–10 days without shaking. Fibrils were obtained upon longer incubation up to 35 days.
Production of Aβ Oligomers
Aβ40 oligomers (BIOSOURCE) were dissolved in 100% 1,1,1,3,3,3-hexafluoro-2-propanol at a concentration of 1 mg/ml in 250-μl aliquots, air-dried, and stored at –20 °C until further use. Aliquots were resuspended in 100% dimethyl sulfoxide (DMSO) and further diluted in Buffer A to a 20 μm concentration and incubated at 37 °C without shaking for 5 days. The oligomeric morphologies of Aβ40 were verified by atomic force microscopy (AFM) prior to study (40).
Bio-panning against α-Synuclein
A diluted sample of α-synuclein (20 μm) containing predominantly larger aggregate forms was used for bio-panning studies. Three rounds of bio-panning against the α-synuclein mixture were performed essentially as described (41). Briefly, immunotubes (Maxisorb, Nunc) were coated overnight at 4 °C with 20 μm sample of α-synuclein mixture in 50 mm carbonate/bicarbonate, pH 9.6. The tube was then blocked with 3% BSA in phosphate-buffered saline (PBS: 10 mm phosphate and 150 mm NaCl, pH 7.4) for 2 h at 37 °C. An aliquot of phage library (1012 plaque-forming unit/ml) in 4 ml of 1% BSA/PBS was added to the immunotube and incubated for 30 min with continuous rocking, followed by 90 min without rocking at 22 °C. The sample was subsequently washed 10 times with 1 ml of PBS/Tween 0.1% and 15 times with PBS to remove the nonspecifically bound phage. The bound phage were eluted from the immunotube by incubation with triethylamine (100 mm, pH 11) for 10 min, followed by neutralization with Tris-HCl (1 m, pH 7.4) and hydrolysis with trypsin (1 mg/ml) and calcium (1 mm) for 30 min with continuous rocking at room temperature. A 1-ml aliquot of eluted phage was added to 10 ml of E. coli TG1 (A600 of 0.4) and incubated for 30 min at 37 °C. Eluted phage were amplified by infecting fresh TG1 cells in the presence of helper phage KM13 (5 × 1010) and purified according to standard protocols (Cambridge, UK). Phage titers were determined by serial dilutions on agar plates containing ampicillin (100 μg/ml).
Selection by Phage ELISA
Polyclonal ELISA—The polyclonal phage ELISA was performed as described previously (38). High binding polystyrene microtiter plates were coated with 5 μm of the α-synuclein mixture in carbonate/bicarbonate, pH 9.6, and 1010 plaque-forming unit aliquots of PEG-precipitated phage was added. Bound phage was detected after 1 h of incubation with a 1:5000 dilution of anti-M13 antibody horseradish peroxidase (HRP) conjugate and detected with HRP substrate 3,3′,5,5′-tetramethyl-benzidine (Sigma) after 20 min. The activity was determined by subtracting A650 from A450 using a Wallac 1420 plate reader (PerkinElmer Life Sciences).
Monoclonal ELISA—Individual clones obtained from panning against α-synuclein mixture were grown as described (32). The high binding microtiter plates (Corning Glass) were coated with 5 μm α-synuclein mixture and blocked as described above. Bacterial supernatant containing antibody fragments was added to each well (100 μl/well). Bound phage were detected as described above.
Soluble scFv ELISA
Soluble scFv was produced by expressing recovered phagemid samples in the nonsuppressor E. coli strain HB2151 (42). Individually selected clones were grown as described previously (32), and scFv production was induced by the addition of 1 mm isopropyl β-d-thiogalactopyranoside. Supernatant and periplasmic fractions were assayed for antigen binding by ELISA as described (32). High affinity microtiter plates were coated with 5 μm α-synuclein mixture. After blocking and washing, an aliquot (100 μl) of supernatant and periplasmic fractions containing antibody fragments was added to each well, and the plate was incubated for 2 h at room temperature. Bound antibodies were detected after a 1-h incubation using 1:500 dilution of anti-c-Myc tag (9E10) HRP conjugate (Santa Cruz Biotechnology).
Plasmid Preparation and PCR Amplification
Plasmid was isolated from E. coli TG1 using a plasmid mini-prep kit (Qiagen, Valencia, CA) according to the manufacturer's protocols. The presence of a full-length 935-bp scFv insert was confirmed by PCR as described (32, 43).
Site-directed Mutagenesis
The false stop codon (TGA) in the constant region of scFv was corrected and replaced with a tryptophan (TGG) using site-directed mutagenesis as described (44).
Purification of scFv
Selected individual clones were purified using a protein A-Sepharose column (GE Healthcare) as described previously (32).
Dot Blot Assay
Lyophilized α-synuclein was dissolved to a final concentration of 70 μm in Buffer A, filtered through a 0.2-μm filter, and incubated at 37 °C for 35 days. Aliquots (3 μl) of incubated solution taken at different time points were applied to nitrocellulose membranes (Bio-Rad) and air-dried. The membrane was blocked with phosphate-buffered saline containing 2% milk (PMSM) and probed with 0.3 mg/ml syn-10H for 2 h followed by overnight incubation with 1:500 dilution of 9E10. The immunoreactivity was detected after a 1-h incubation using a 1:1000 dilution of anti-mouse HRP.
Tris/Tricine SDS-PAGE and Western Blot
Lyophilized α-synuclein was dissolved to a final concentration of 100 μm in Buffer A and incubated at 37 °C for 7 days without shaking. An aliquot (20 μl) of incubated solution was analyzed by SDS-PAGE using a Tris/Tricine buffer system (45) and developed using silver staining according to the manufacturer's protocols. The sample was then transferred to a nitrocellulose membrane as described (32) and probed with 0.3 mg/ml syn-10H as described above.
Thioflavin T Aggregation Assay
α-Synuclein was dissolved to a final concentration of 70 μm in Buffer A, filtered as described above, and incubated at 37 °C either with or without the addition of 15 μm scFv. Aggregation of α-synuclein was measured in triplicate at various time points as described previously (32, 46).
Cell Culture and Viability Assay
Human neuroblastoma cells (SH-SY5Y) were maintained in culture flasks in medium containing 50% (v/v) minimal essential medium, 50% (v/v) Ham's modification of F-12, 10% (v/v) fetal bovine serum, 1% (w/v) l-glutamine (3.6 mm), and 1% penicillin/streptomycin antibiotic and grown in a 5% CO2 atmosphere at 37 °C.
Differentiated SH-SY5Y Cell
Cells were harvested from flasks and plated in 48-well polystyrene plates (Corning Glass) with 3 × 105 cells per 300 μl of medium per well. The cells were differentiated by adding 10 μm retinoic acid to each well and incubating at 37 °C for 4 days. As reported previously, SH-SY5Y cells treated with such a protocol exhibit several characteristics of cholinergic neurons, including expression of ChAT and VMAT (47, 48). After 4 days, the medium was exchanged with 300 μl of serum-free media, followed by the addition of the preincubated mixtures of α-synuclein with or without scFv to individual wells. The same volume of medium was added to the control cultures. Plates were then incubated at 37 °C for 48 h.
Cell Viability
Cell viability was measured by lactic acid dehydrogenase (LDH) assay. Briefly, cells were centrifuged, and aliquots (50 μl) of the media from each well were transferred to a 96-well plate. The supplied buffer and substrate were then added to the supernatant as described by the manufacturer. Absorbance was measured by subtracting A650 from A480 using a Wallac 1420 plate reader. LDH release was determined by dividing the absorbance of treated wells by the absorbance of untreated wells. The data are reported as percentage of control value obtained from three independent experiments. The cell viability was also measured using trypan blue assay as described previously (32).
Detection of Oligomeric α-synuclein on the Surface of SH-SY5Y Cell by Flow Cytometry
SH-SY5Y cells were detached from flasks using trypsin, washed with PBS, and plated in a 96-well plate with the density of 2 × 104 cells per well per 100 μl of PBS. The cell was centrifuged, and the pellet was washed twice with buffer containing PBS and BSA (0.5%). The cells were then labeled with or without syn-10H scFv (0.3 mg/ml) for 1 h at 4 °C. After washing, the pellet was incubated with 9E10 antibody (Roche Applied Science) for 1 h at 4 °C followed by the addition of fluorescein conjugated goat anti-mouse antibody (Invitrogen). The percentage and mean fluorescence intensity (MFI) of positive cells were analyzed with a flow cytometer (FACSCalibur system). Control samples stained with secondary antibody alone were included in each experiment.
Atomic Force Microscopy
Topographic AFM images were obtained in air at room temperature using a Tapping Mode AFM with a Nanoscope IIIa controller (Veeco, Santa Barbara, CA). Images were acquired using oxide sharpened Si3N4 AFM tips (k = 40 n/m, ∼300-kHz) (model OTESPA, Veeco, Santa Barbara, CA) at scan rates of 2–3 Hz and at scan resolution of 512 samples per line. AFM images were analyzed with the scanning probe imaging processor software (Image Metrology) to generate height distribution histograms for each sample.
Size Exclusion Chromatography
A 600 × 7.8-mm BioSep-SEC S-2000 column (Phenomenex) on a System Gold high pressure liquid chromatograph (Beckman Coulter) was washed and equilibrated with PBS. An aliquot of purified α-synuclein (100 μm) was incubated in Buffer A at 37 °C for 7 days and was run on the size exclusion column at a 0.8 ml/min flow rate. After washing the column, 0.5-ml fractions were collected and analyzed by electrophoresis on a 10% Tris/Tricine SDS-PAGE (45).
Human Brain Tissue Processing and Analysis
Human brain sections were a generous donation from Dr. Thomas Beach (Sun Health Research Institute, Sun City, AZ). The human brain tissue samples were homogenized in a buffer containing 50 mm Tris-HCl, pH 7.5, 5 mm EDTA, and 1% SDS supplemented with protease inhibitor mixture (Sigma). The samples were sonicated on ice using 10-s bursts followed by 15-s pauses for a total of 5 min using a Fisher sonic dismembrator. Homogenates were centrifuged for 10 min at 13,000 rpm at 4 °C. Supernatants of brain homogenates were aliquoted and stored at –80 °C until further use.
Dot Blot Analysis of Brain Samples with syn-10H scFv
A3-μl aliquot of brain sample was applied to a nitrocellulose membrane and air-dried. The membrane was blocked with 1% PBSM and probed with 0.3 mg/ml scFv for 24 h at 4 °C, followed by incubation for 2 h with a 1:500 dilution of 9E10. The immunoreactivity was detected after a 1-h incubation using a 1:1000 dilution of anti-mouse-HRP.
Statistical Analysis
Data are reported as means ± S.E. Differences were tested for significance using paired Student's t test. Statistical significance was established using one-way analysis of variance followed by Bonferroni's post test. A p value of less than 0.05 denotes statistical significance.
RESULTS
Bio-panning against Human Oligomeric/Fibrillar α-Synuclein—The Tomlinson I and J antibody libraries were used to pan against a sample of aggregated α-synuclein (predominantly oligomeric/fibrillar forms) immobilized on an immunotube surface. After three rounds of panning, 2 of 10 randomly selected clones showed positive binding (the binding values detected in the wells coated with aggregated α-synuclein were at least twice that of wells with no antigen) to α-synuclein sample as indicated by phage ELISA (data not shown). The strongest binding full-length scFv (syn-10H) as determined by ELISA and PCR, respectively, was selected for further study. DNA sequencing revealed the presence of a false stop codon (TGA) in a constant region of the syn-10H, which was corrected and replaced with a tryptophan (TGG).
Expression and Purification of Soluble scFv—We purified soluble scFv from the corrected syn-10H clone for further characterization. Purified scFv showed a single protein band with a molecular mass of 29 kDa, corresponding to expression of a full-length scFv on both SDS-PAGE and Western blot (data not shown).
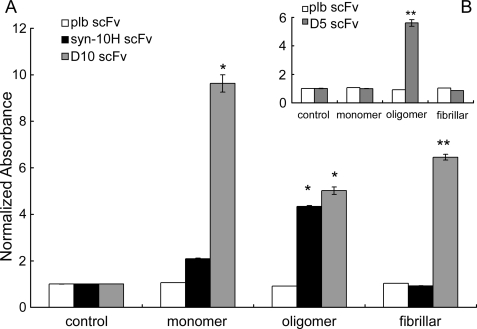
Binding Specificity of syn-10H scFv to Oligomeric α-Synuclein—Purified soluble syn-10H scFv recognized only oligomeric α-synuclein as determined by soluble ELISA using monomeric, oligomeric, and fibrillar forms of α-synuclein. The positive control scFv D10 that reacts with all forms of α-synuclein (36, 49) showed reactivity to all three morphologies, whereas a negative control scFv (phosphorylase b) did not react with any of the morphologies (Fig. 1).
FIGURE 1.
Soluble ELISA showing binding of purified scFv (syn-10H) to three different synuclein morphologies: monomeric, oligomeric, and fibrillar α-synuclein. A, microtiter plate was coated for 2 h at 37 °C with or without 5 μm (final concentration) of three different morphologies of α-synuclein as follows: monomer, oligomer, and fibrillar. The monomer was obtained by dissolving the lyophilized stock of α-synuclein (100 μm) in PBS and filtered through a 0.2-μm filter followed by size exclusion chromatography. The oligomer was obtained by dissolving 50 μm monomeric α-synuclein in Buffer A and incubated at 37 °C for 7 days without shaking. Fibrillar α-synuclein was prepared by incubating 70 μm α-synuclein in Buffer A at 37 °C for 35 days. After blocking, the plate was incubated for 2 h at room temperature with 10μm purified syn-10H scFv, D10 scFv, and control scFv (phosphorylase b). Bound scFv was detected by an anti-c-Myc tag (9E10) HRP conjugate. Activity was determined by subtracting A650 from A450 and comparing absorbance between control well (without immobilized α-synuclein) to the sample well (with α-synuclein) where the control value was normalized to 1. The data represent the means ± S.E. of four separate experiments. A p value of (*, p < 0.05) was considered to be statistically significant. B, ELISA showing binding of purified D5 scFv to three different α-synuclein morphologies as follows: monomeric, oligomeric, and fibrillar reproduced from Emadi et al. (32).
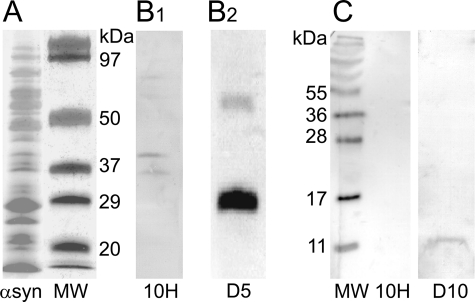
Western blot analysis showed that syn-10H scFv reacts with two aggregated α-synuclein bands of molecular mass of around 42 and 84 kDa corresponding to trimeric (42 kDa) and hexameric (84 kDa) α-synuclein (Fig. 2B1) but not with monomeric α-synuclein (Fig. 2C). A previously characterized anti-oligomeric scFv isolated in our lab (D5) reacted with dimeric and tetrameric α-synuclein bands (Fig. 2B2) (32) indicating that the scFvs recognize different oligomeric forms.
FIGURE 2.
Tris/Tricine SDS-PAGE and Western blot. Lyophilized α-synuclein (α-syn) was dissolved to a final concentration of 100 μm in Buffer A filtered through a 0.2-μm filter. For probing with syn-10H scFv, the sample was incubated at 37 °C for 7 days without shaking. An aliquot (20 μl) of incubated solution was run on 10% polyacrylamide Tris-Tricine SDS-PAGE followed by silver staining (A). The sample was then transferred to nitrocellulose membrane (B1) and probed with 0.3 mg/ml scFv (syn-10H) antibody. An aliquot (50 μm) of monomeric α-synuclein (time 0) was transferred to nitrocellulose membrane and probed with 0.3 mg/ml syn-10H and D10 scFv (C). Bound antibodies were detected after a 24-h incubation at 4 °C, using a 1:500 dilution of 9E10 antibody, followed by a 1-h incubation with 1:1000 dilution of anti-mouse HRP followed by staining with 3,3′-diaminobenzidine tetrachloride (Sigma). Western blot analysis of oligomeric α-synuclein probed with 0.3 mg/ml D5 scFv was reproduced from Emadi et al. (32) (B2). MW, molecular weight.
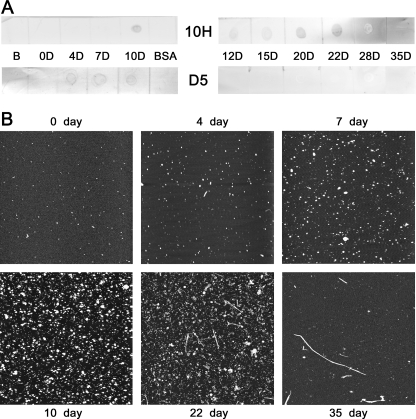
A time course dot blot and AFM analysis revealed that although the D5 scFv recognizes α-synuclein aggregates occurring during early (4–10 day) time points, the syn-10H scFv recognizes later stage α-synuclein aggregates (10–22 day) (Fig. 3A). No reactivity was observed with either scFv with the 0-day sample, which is predominantly monomeric, or the 28- and 35-day samples, which are mostly fibrillar (Fig. 3, A and B), or with a control protein (BSA). Size distribution analysis of the aggregates in the AFM images showed substantial differences in sizes of the α-synuclein aggregates recognized by the two different scFvs (Table 1, top). The earlier time point samples recognized by D5 contained aggregate sizes predominantly between 0.4 and 1 nm in height, whereas the later time point samples recognized by syn-10H contained predominantly larger sizes, generally greater than 4 nm. Both D5 and syn-10H show binding to the 10-day sample, which contains both small and large aggregates.
FIGURE 3.
Binding specificity of syn-10H scFv by time course immunoreactivity assay. A, lyophilized α-synuclein (70 μm) was incubated at 37 °C without stirring for 35 days. Aliquots at indicated time points (days (D)) were removed, applied to a nitrocellulose membrane, and probed with 0.3 mg/ml syn-10H (10H) and D5 scFv. Immunoreactivity was detected after a 24-h incubation using a 1:500 dilution of 9E10 antibody, followed by a 1-h incubation with 1:1000 dilution of anti-mouse-HRP followed by staining with 3,3′-diaminobenzidine tetrachloride (Sigma). B represents a blank control. B, AFM analysis of incubated sample after 0, 4, 7, 10, 22, and 35 days. Images were acquired in air using a tapping mode AFM. AFM image was a 5 × 5 μm scan.
TABLE 1.
Particle height distribution of α-synuclein
α-Synuclein (70 μm) was incubated with or without syn-10H (15 μm) at 37 °C for 28 days. Data represent the height distribution analysis of the aggregates observed in the AFM images.
| Time | 0-0.4 nm | 0.4-0.8 nm | 0.8-1 nm | 1-2 nm | 2-3 nm | 3-4 nm | > 4 nm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Synuclein alone | ||||||||||||||
| 0 h | 0.3 | 41.3 | 40.7 | 17.7 | 0.1 | 0.0 | 0.0 | |||||||
| 4 days | 3.9 | 70.0 | 19.6 | 5.4 | 0.8 | 0.3 | 0.1 | |||||||
| 7 days | 0.0 | 39.2 | 48.7 | 9.3 | 1.1 | 0.5 | 1.2 | |||||||
| 10 days | 0.1 | 0.8 | 4.2 | 65.5 | 11.5 | 7.5 | 10.4 | |||||||
| 12 days | 0.0 | 0.0 | 0.0 | 0.9 | 15.4 | 21.8 | 61.9 | |||||||
| 15 days | 0.0 | 0.0 | 0.0 | 1.4 | 26.1 | 20.3 | 52.2 | |||||||
| 20 days | 0.0 | 0.0 | 0.0 | 1.8 | 14.3 | 14.3 | 68.5 | |||||||
| 22 days | 0.0 | 0.0 | 0.1 | 17.9 | 54.6 | 5.0 | 22.4 | |||||||
| 28 days | 0.0 | 0.4 | 0.9 | 54.7 | 17.8 | 4.5 | 21.7 | |||||||
| α-Synuclein + syn-10H scFv | ||||||||||||||
| 0 h | 22.2 | 74.6 | 2.2 | 0.7 | 0.2 | 0.1 | 0.0 | |||||||
| 4 days | 7.4 | 80.0 | 5.3 | 3.5 | 1.4 | 0.8 | 1.6 | |||||||
| 7 days | 0.5 | 26.2 | 35.4 | 37.7 | 0.2 | 0.0 | 0.0 | |||||||
| 10 days | 6.9 | 76.0 | 13.7 | 3.4 | 0.1 | 0.0 | 0.0 | |||||||
| 12 days | 0.1 | 18.9 | 41.7 | 37.1 | 1.2 | 0.4 | 0.6 | |||||||
| 15 days | 0.1 | 1.6 | 5.1 | 76.7 | 5.8 | 2.2 | 8.6 | |||||||
| 20 days | 0.0 | 0.9 | 5.7 | 71.4 | 8.7 | 5.4 | 7.8 | |||||||
| 22 days | 0.0 | 0.0 | 0.1 | 8.8 | 31.0 | 14.2 | 45.9 | |||||||
| 28 days | 0.0 | 0.0 | 0.0 | 2.7 | 13.6 | 20.0 | 63.7 | |||||||
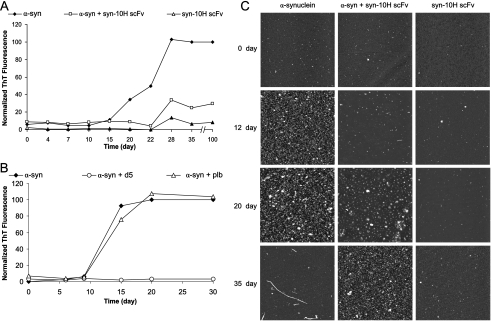
Syn-10H Inhibits α-Synuclein Aggregation—We tested whether syn-10H could block α-synuclein aggregation by co-incubating α-synuclein (70 μm) with scFv (15 μm). Incubation of α-synuclein alone shows a typical time-dependent increase in ThT fluorescence staining, with a characteristic lag phase as the α-synuclein begins to aggregate, and reaching a plateau after 28 days when fibrils are formed. Co-incubation of α-synuclein with syn-10H scFv significantly decreases the aggregation rate (Fig. 4A), whereas syn-10H scFv alone does not increase ThT levels. Furthermore, co-incubation of α-synuclein with syn-10H scFv for up to 100 days did not show any significant increase in ThT fluorescence intensity (Fig. 4A). Similarly, co-incubation of α-synuclein with D5 scFv, which recognizes smaller oligomeric aggregates, also significantly reduces the aggregation rate (Fig. 4B). In contrast co-incubation of α-synuclein with a nonspecific control scFv (phosphorylase b) did not alter aggregation kinetics as determined by ThT fluorescence (Fig. 4B). Inhibition of α-synuclein aggregation is confirmed by AFM images. After 12 days, small amorphous aggregates are present in the sample containing only α-synuclein, whereas the sample co-incubated with syn-10H has substantially fewer amorphous aggregates. After 35 days, the α-synuclein alone sample shows extensive fibril formation, whereas the sample incubated with scFv does not contain any fibrillar structures (Fig. 4C). Height distribution analyses of the AFM images indicate that substantially smaller aggregate sizes are present in the α-synuclein samples co-incubated with scFv compared with samples without scFv. For example, for α-synuclein alone, the 12–20-day samples indicate substantial aggregation with most aggregate particles having heights greater than 2 nm (28.6–46.4% between 2 and 4 nm and over 50% greater than 4 nm) (Table 1, top), whereas the corresponding samples co-incubated with scFv contain smaller particles with most aggregate particles having heights less than 2 nm (78–97.8%) (Table 1, bottom).
FIGURE 4.
Effect of syn-10H scFv on aggregation of α-synuclein. A, kinetics of α-synuclein (a-syn) fibril formation was monitored by ThT fluorescence in the presence and absence of scFv. α-Synuclein (70 μm) was incubated with purified syn-10H scFv (15 μm) at 37 °C without stirring, and 10-μl aliquots were added to 2 ml of 5 μm ThT. Fluorescence intensity was measured at an excitation wavelength of 450 nm and emission wavelength of 482 nm. The data represent one experiment with each experimental value being repeated in triplicate. B, thioflavin T fluorescence in the presence and absence of 15 μm of D5 and control scFv (phosphorylase b (plb)). C, AFM imaging of α-synuclein aggregation with or without scFv. AFM analysis of α-synuclein aggregates at 0, 12, 20, and 35 days when incubated alone (70 μm) or with 15 μm syn-10H scFv. Images were acquired in air using a tapping mode AFM. AFM image was a 5 × 5 μm scan.
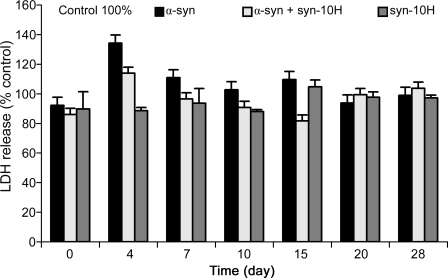
scFv Blocks Cytotoxicity of Aggregated α-Synuclein—The cytotoxicity of aggregated α-synuclein samples in the human neuroblastoma cell line (SH-SY5Y) was studied over a 28-day period (Fig. 5). Significant cytotoxicity was observed with the 4–15-day aggregated α-synuclein alone samples with the greatest toxicity with the 4-day sample (34%). When co-incubated with the syn-10H scFv, the α-synuclein aggregated samples are less toxic at 4 days compared with α-synuclein alone, and no toxicity is observed with the 7–15-day aggregated samples. Addition of syn-10H scFv alone to the cells also showed a decrease in toxicity compared with the control cells (Fig. 5).
FIGURE 5.
Effect of scFv on α-synuclein-induced cytotoxicity. Aliquots of α-synuclein (70 μm) preincubated at indicated times with or without scFv (15 μm) were added to wells coated with SH-SY5Y cells. After an additional 2-day incubation of cells and samples, the viability of the cells was measured using an LDH toxicity assay. The final concentrations of α-synuclein and scFv were 3 and 0.3 μm, respectively. Data shown are expressed as percentage of control values from three independent experiments with each experimental value being repeated in triplicate.
Because soluble oligomeric aggregates have been identified as the primary toxic species of α-synuclein in numerous studies (16, 18, 20, 32), we further examined the effects of the larger, later stage α-synuclein aggregates and the syn-10H scFv on neuronal cells. We studied the cytotoxicity of the 7-day preaggregated α-synuclein samples with and without scFv toward differentiated and undifferentiated human neuroblastoma cell line SH-SY5Y using both LDH and trypan blue assays. When incubated alone, the pre-aggregated 7-day α-synuclein sample showed increased cytotoxicity toward differentiated compared with undifferentiated SH-SY5Y cells by both LDH (38 versus 20%) and trypan blue assays (20 versus 15% cell death) (Tables 2 and 3). Addition of syn-10H scFv to the α-synuclein sample shows complete protection from toxic effects in the 7-day sample as indicated by both assays in both differentiated and undifferentiated SH-SY5Y cells (Tables 2 and 3). Addition of D5 scFv to the α-synuclein sample also shows complete protection from toxic effects in the 7-day sample (Table 2). In contrast, co-incubation of α-synuclein with a nonspecific control scFv (phosphorylase b) did not alter the toxicity of α-synuclein when added to cells, nor did it alter LDH activity when added alone to cells (Table 2). Addition of scFv samples alone to the cells showed LDH activity comparable with that of the control buffer sample (data not shown).
TABLE 2.
Effect of syn-10H scFv on toxicity induced by aggregated α-synuclein on differentiated and undifferentiated human neuroblastoma cell line (SH-SY5Y)
α-Synuclein (70 μm) was incubated in Buffer A for 7 days at 37 °C without shaking. Samples were preincubated with or without scFv for 2 h at room temperature and then assayed for cytotoxicity. Cell viability was measured using LDH toxicity assay after an additional 2 days of incubation of cells and aggregate samples. The data are expressed as percentage of control values from three different experiments repeated in triplicate. Statistical significance was established using one-way analysis of variance followed by Bonferroni's post test. p values ranged between <0.001 and <0.05.
| LDH release of differentiated cell | LDH release of undifferentiated cell | |
|---|---|---|
| % | % | |
| Control | 100 ± 3 | 100 ± 4 |
| α-Synuclein alone (5 μm) | 138 ± 7a,b | 120 ± 5a |
| α-Synuclein (5 μm) + Syn-10H (1 μm) | 100 ± 8c | 103 ± 3c |
| α-Synuclein (5 μm) + D5 scFv (1 μm) | 94 ± 8c | 90 ± 10c |
| α-Synuclein (5 μm) + phospholipase b (1 μm) | 140 ± 1 | 123 ± 3 |
α-Synuclein alone was compared with control for both differentiated and undifferentiated cells.
α-Synuclein toxicity was compared between differentiated and undifferentiated cell with same treatment.
α-Synuclein was incubated with syn-10H scFv and D5 scFv compared with α-synuclein alone for differentiated and undifferentiated cells.
TABLE 3.
Effect of syn-10H scFv on toxicity induced by aggregated α-synuclein on differentiated and undifferentiated human neuroblastoma cell line (SH-SY5Y)
α-Synuclein (70 μm) was incubated in Buffer A for 7 days at 37 °C without shaking. Samples were preincubated with or without scFv for 2 h at room temperature and then assayed for cytotoxicity. Cell viability was measured using trypan blue after an additional 2 days of incubation of cells and aggregate samples. The data are expressed as percentage of control values from three different experiments repeated in triplicate. Statistical significance was established using one-way analysis of variance followed by Bonferroni's post test. p values ranged between <0.001 and <0.05.
| % dead cells and differentiated cells | % of dead cells and undifferentiated cells | |
|---|---|---|
| Control | 4.4 ± 0.9 | 3.1 ± 0.8 |
| α-Synuclein alone (5 μm) | 20.0 ± 0.3a,b | 15.1 ± 1.5a |
| Syn-10H scFv (1 μm) | 2.9 ± 1.0 | 3.2 ± 0.7 |
| α-Synuclein (5 μm) + syn-10H (1 μm) | 3.4 ± 1.6 | 3.5 ± 0.8 |
α-Synuclein alone was compared with control for both differentiated and undifferentiated cells.
α-Synuclein toxicity was compared between differentiated and undifferentiated cell with same treatment.
To study whether the syn-10H scFv would cross-react with oligomeric aggregates of other proteins, we determined the cytotoxicity of pre-aggregated Aβ samples with and without scFv toward differentiated and undifferentiated human neuroblastoma cell line SH-SY5Y. When incubated alone, the pre-aggregated 5-day Aβ sample showed increased cytotoxicity, again more so toward differentiated compared with undifferentiated SH-SY5Y cells (LDH increase of 24 versus 4%) (Table 4). Addition of syn-10H scFv to the pre-aggregated sample of Aβ did not inhibit toxicity in any samples. Addition of syn-10H scFv alone shows similar LDH activity to that of control sample (data not shown).
TABLE 4.
Effect of Aβ and syn-10H scFv on toxicity induced by aggregated Aβ on differentiated and undifferentiated human neuroblastoma cell line (SH-SY5Y)
Aβ (20 μm) was incubated in Buffer A for 5 days at 37 °C without shaking. Samples were preincubated with or without scFv for 2 h at room temperature and then assayed for cytotoxicity. Cell viability was measured using LDH toxicity assay after an additional 2-day incubation of cells and aggregate samples. The data are expressed as percentage of control values from three different experiments repeated in triplicate. Statistical significance was established as p < 0.001 using one-way analysis of variance followed by Bonferroni's post test.
| % LDH release differentiated cell | % LDH release undifferentiated cell | |
|---|---|---|
| Control | 100 ± 3 | 100 ± 4 |
| Aβ alone (2 μm) | 124 ± 4a,b | 104 ± 1 |
| Aβ (2 μm) + syn-10H scFv (1 μm) | 121 ± 5 | 101 ± 1 |
α-Synuclein alone compared with control for both differentiated and undifferentiated cells.
α-Synuclein toxicity compared between differentiated and undifferentiated cells with the same treatment.
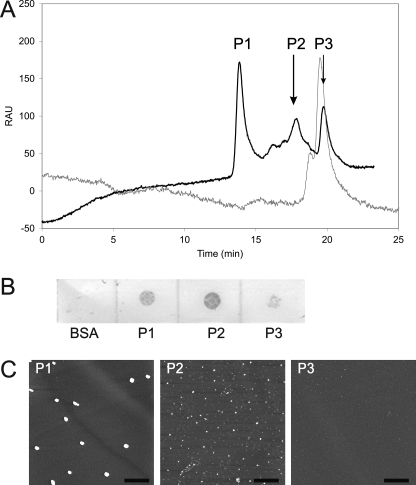
To further characterize the aggregated morphologies of α-synuclein recognized by syn-10H scFv, we separated different aggregated species in the 7-day pre-aggregated α-synuclein sample by size exclusion chromatography and obtained three distinct peaks (P1, P2, and P3) (Fig. 6A). Aliquots of the three peak fractions were tested for reactivity with syn-10H scFv, and morphologies of each peak were imaged by AFM. Height distribution analysis of the AFM images of each peak fraction showed substantial differences in particle sizes, where peak 1 contained large oligomers with heights greater than 4 nm (99.6%), peak 2 contained smaller 2–4-nm oligomer particles (87.4%) along with a small percentage (10.6%) of 1–2-nm particles, and peak 3 contained predominantly monomeric species with more than 96% of the particles having size of <1 nm (Table 5). The syn-10H scFv showed reactivity to the P1 and P2 fractions containing two different oligomeric α-synuclein size ranges but not to P3 which is predominantly monomeric (<1 nm) or to a control protein (Fig. 6B and Table 5), verifying specificity for oligomeric forms of α-synuclein.
FIGURE 6.
Characterization of α-synuclein oligomeric species. A, elution profile of aggregated α-synuclein (in black) and monomeric α-synuclein (in gray) by size exclusion chromatography monitored at 220 nm. Absorption is measured in relative absorbance units (RAU). An aliquot of purified α-synuclein (100 μm) was incubated in Buffer A at 37 °C for 7 days and was loaded onto a BioSep-SEC S-2000 size exclusion column. Column was pre-equilibrated in PBS running buffer, pH 7.4, and run at a flow rate 0.8 ml/min. B, dot blot of eluted α-synuclein fractions from SEC. Aliquots of eluted peaks (3 μl) were applied to nitrocellulose membranes (Bio-Rad). The membrane was blocked with 0.5% PBSM and probed with 0.3 mg/ml syn-10H scFv. The immunoreactivity was detected as described under “Experimental Procedures.” C, AFM images of eluted α-synuclein fractions. 10 μl of eluted α-synuclein sample was deposited on freshly cleaved mica for 10 min followed by water wash and drying under nitrogen gas. Images were acquired in air using a tapping mode AFM. The scale bars represent 1 μm.
TABLE 5.
Size distribution analysis of the peak fractions from size exclusion chromatography
An aliquot (10 μl) of the peak fractions obtained from size exclusion chromatography was deposited onto a mica surface, incubated for 10 min, and dried under nitrogen gas. Morphology of α-synuclein was determined by AFM. Size distribution analysis was done using scanning probe image processor software. Data shown are distribution of particle height expressed as a percentage of the total sample frequency.
| <1 nm | 1-2 nm | 2-4 nm | >4 nm | |
|---|---|---|---|---|
| P1 | 0.00 | 0.1 | 0.3 | 99.6 |
| P2 | 0.00 | 10.6 | 87.4 | 2.0 |
| P3 | 96.0 | 2.9 | 0.9 | 0.2 |
Surface Expression of Oligomeric α-Synuclein—The ability of syn-10H scFv to detect the presence of oligomeric α-synuclein on the surface of undifferentiated SH-SY5Y cells was probed by incubating freshly grown cells with syn-10H scFv and detecting bound scFv by flow cytometry. The MFI of the cells incubated with syn-10H scFv increased by 15 and the number of labeled cells increased by 4% compared with control cells.
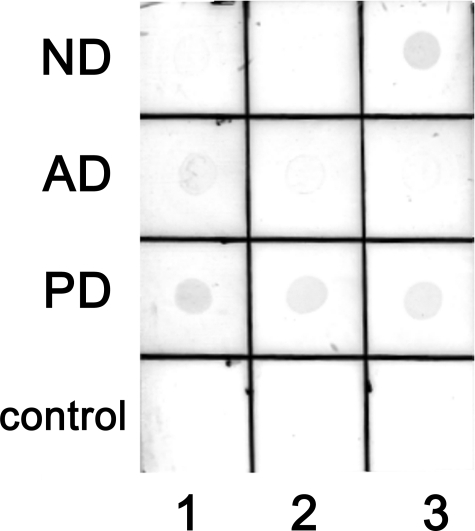
Syn-10H scFv Specifically Reacts with α-Synuclein Aggregates in Parkinson Brain Tissue—We tested whether the syn-10H scFv could recognize naturally occurring α-synuclein aggregates in human brain tissue by incubating the scFv with postmortem brain samples taken by autopsy from healthy brain tissue (ND) and patients with PD and AD (based on clinical diagnosis and postmortem histopathology). Three different cases of each sample pool were utilized. The syn-10H scFv showed strong reactivity with α-synuclein aggregates present in all the PD brain samples and one ND sample, showed weak reactivity with one of the AD samples, and did not react with the other AD or ND brain samples (Fig. 7). Equal protein loading in each dot blot was confirmed using anti-glyceraldehyde-3-phosphate dehydrogenase and anti-β-actin antibodies as well as an Amido Black stain of the total protein (data not shown).
FIGURE 7.
syn-10H scFv binds to the brain extract derived from patient with PD. An aliquot containing 6 μg of total protein of brain extracts derived from ND, AD, and PD patients along with control protein (phosphorylase b and BSA) samples were applied to a nitrocellulose membrane and air-dried. The membrane was probed with 0.3 mg/ml syn-10H scFv followed by incubation with anti-c-Myc tag (9E10) mouse monoclonal antibody (1:500 dilutions) for 24 h at 4 °C. The immunoreactivity was detected as described under “Experimental Procedures.”
DISCUSSION
Misfolding and aggregation of α-synuclein has been strongly implicated in the pathogenesis of PD (5, 50) and other related neurodegenerative disorders (6, 7). Aggregation of α-synuclein is a complex process that involves oligomeric intermediates of various sizes and morphologies, rather than a simple two state transition from monomer to fibrils (51, 52). A growing body of evidence implicates the oligomeric intermediates rather than the mature fibril form of α-synuclein as the toxic species responsible for neurodegeneration and neuronal cell death (16, 17, 19, 52–54). A heterogeneous population of oligomeric α-synuclein is generated depending on physiological conditions, and different aggregate preparations have different biophysical properties and cellular effects (55, 56). Several different oligomeric forms of α-synuclein such as spherical, annular, pore-like, and dopamine-stabilized structures have been identified in vitro (18, 19, 21, 52, 57, 58).
Despite these studies, it is not known which oligomeric forms of α-synuclein occur in vivo and which are involved in the progression of PD. Therefore, reagents that can specifically recognize individual aggregate forms would be important tools to help understand the progression of the disease and to develop effective therapeutic and diagnostic strategies. We have previously identified an scFv (D5) that specifically binds oligomeric α-synuclein and inhibits both aggregation and cytotoxicity of α-synuclein in vitro (32). Here we show that a second scFv (syn-10H) specifically recognizes an oligomeric morphology of α-synuclein that is different from the previously reported scFv. Although D5 scFv binds SDS-stable α-synuclein aggregates with molecular masses of about 29 and 56 kDa corresponding to dimeric and tetrameric forms (32), syn-10H scFv binds α-synuclein aggregates with molecular masses of 42 and ∼80 kDa corresponding to trimeric and hexameric forms (Fig. 2B). A time course dot blot indicates that the syn-10H scFv binds later stage α-synuclein oligomer aggregates (10–22 days) with particle heights >2 nm (Fig. 3 and Table 1, top) compared with the D5 scFv, which recognizes earlier stage α-synuclein aggregate forms (4–10 days) with particle heights of <2 nm (Fig. 3 and Table 1, top). Both anti-oligomeric scFvs are specific to oligomeric morphologies as neither scFv reacted with the 0-day sample that contains predominantly monomeric α-synuclein or with 28–35-day samples that contain mostly fibrillar α-synuclein as indicated by AFM images (Fig. 3B).
Several studies have shown that aggregated α-synuclein added extracellularly to the culture medium is cytotoxic (26–32). Our results provide additional evidence for the toxicity of aggregated α-synuclein showing that smaller aggregates corresponding to earlier time points of aggregation are more toxic to SH-SY5Y cells than larger aggregates found at later time points. These results are consistent with other studies that identified the prefibrillar oligomers rather than mature fibril as the toxic species (14, 17, 20, 59). Our results also suggest that oligomeric species of α-synuclein may be present on the cell surface causing some intrinsic toxicity, which could be ameliorated by the addition of syn10H scFv. This was further confirmed using flow cytometry, where syn-10H scFv was able to detect a small but significant percentage of α-synuclein oligomers on the surface of SH-SY5Y cells (75 ± 2 MFI, 5 ± 2%) compared with control cells without syn-10H scFv (60 ± 2 MFI, 1 ± 1%).
To further study the effects of oligomeric α-synuclein on neuronal cells, we examined whether any differences in cytotoxicity of the oligomeric α-synuclein could be observed in differentiated and undifferentiated neuroblastoma cells. Oligomeric α-synuclein was significantly more toxic toward differentiated SH-SY5Y cells compared with undifferentiated cells (Tables 2 and 3). Co-incubation of oligomeric α-synuclein with syn-10H completely protected differentiated and undifferentiated cells from any toxic effects (Tables 2 and 3), even providing protection in both cases compared with the control cells. Next, we studied whether the syn-10H scFv could cross-react with other oligomeric species by testing whether it could protect against toxicity induced by oligomeric aggregates of Aβ. Aβ aggregates also showed an increased toxicity toward the differentiated compared with the undifferentiated neuronal cell lines; however, addition of syn-10H scFv did not protect against Aβ-induced toxicity in either cell line.
To verify whether the syn-10H scFv can recognize naturally occurring α-synuclein aggregate forms in human PD brain tissue, we performed dot blot analyses using extracts from human brain tissue from healthy (ND), PD, and AD brains. The syn-10H scFv strongly labeled α-synuclein aggregates present in each of the PD samples, but it only weakly labeled one of three AD samples and one of three healthy brain samples (Fig. 7). The labeling of selected AD and ND samples may be due to presymptomatic PD pathology in these selected cases, and it suggests the importance of morphology-specific antibodies such as syn-10H as important tools for diagnosing early stages of the disease. The previously reported anti-oligomeric scFv (D5) also showed binding to α-synuclein aggregates present in the PD brain sample but not in the healthy control brain or the AD brain sample (data not shown). Furthermore, an anti-oligomeric scFv against Aβ isolated in our laboratory reacted only with brain extract from patient with AD but not to the PD or healthy brain control (40). Although previous anti-oligomer antibody studies described antibodies that recognized an oligomeric aggregate form shared by a variety of different proteins, including α-synuclein and Aβ (18), the antibodies we describe here and previously (32) react specifically with PD brain sample without cross-reacting with similarly treated samples taken from AD or healthy brain sample. Further evidence for this protein specificity is also seen in the toxicity studies where the syn-10H scFv protected against extracellular α-synuclein-induced toxicity but not Aβ. These morphology-specific scFvs therefore have potential value in diagnosing protein misfolding diseases such as PD.
Depending on the conditions utilized, α-synuclein can aggregate into various oligomeric forms (18, 56, 58, 60) that can damage the cell directly or indirectly through different pathways. One form of oligomeric α-synuclein was shown to directly enter the cells resulting in increased α-synuclein aggregation, whereas another form induced cell death by disruption of cellular ion homeostasis presumably by forming pores in the membrane (55). The syn-10H scFv described here and the D5 scFv described previously recognize two different α-synuclein oligomeric species that have distinct conformations and may potentially have different toxic mechanisms. Aggregation of an amyloidogenic protein, such as α-synuclein, is therefore a complex process that can form a variety of morphologically distinct intermediate oligomeric species, resulting in multiple different cytotoxic mechanisms. The morphology-specific scFvs described here and elsewhere can be useful diagnostic tools to study these different cytotoxic mechanisms.
We show that both α-synuclein and Aβ oligomeric forms are more toxic when exposed to differentiated compared with undifferentiated neuronal cells. The anti-oligomeric α-synuclein scFvs isolated in our laboratory do not show reactivity with oligomeric Aβ, and the anti-oligomeric Aβ scFv we reported does not react with oligomeric α-synuclein (49), and therefore the various oligomeric aggregate species formed by different proteins each have at least some unique epitopes. These structural differences may account for the specific vulnerability of different types of neurons in the brain to specific protein aggregate species. Because the scFvs recognize the various aggregate species in PD or AD brain tissue, these morphology- and protein-specific scFvs can be useful to help study why, for example, dopaminergic neurons seem to be targeted in PD and cholinergic neurons in AD.
In summary, we demonstrate that phage display technology can be used to isolate scFvs against specific oligomeric morphologies of proteins. Several different strategies have been studied to control aggregation and toxicity of α-synuclein, including addition of magnesium, hsp70, truncated forms of α-synuclein, small peptides, and vaccination (37, 61–65). The current approach of using scFvs that only target oligomeric morphologies of α-synuclein will not interfere with any necessary functions of the monomeric protein form. In addition, these scFvs can be affinity-matured as described previously (66) to increase targeting specificity for potential diagnostic and therapeutic applications.
scFvs that can detect specific oligomeric morphologies of a target protein, such as α-synuclein, can serve as powerful tools to probe the roles of the various oligomeric species in cell function and disease progression. The scFvs can also be very useful as diagnostic reagents to identify the presence of specific oligomeric forms in PD tissue and fluid samples. Finally, the scFvs can be used either extracellularly or intracellularly expressed (intrabodies) as potential therapeutics for PD in vivo. Because α-synuclein aggregation is still primarily considered to be an intracellular phenomenon, an effective therapeutic for PD will likely need to target α-synuclein intracellularly. Such intracellular use of scFvs (termed intrabodies) to neutralize toxic effects of different pathogenic agents, including Huntingtin protein (33–36) and α-synuclein, has been demonstrated (33, 34, 36), so there is sufficient precedent for developing an intrabody-based therapeutic to specifically target toxic protein aggregate forms.
Acknowledgments
We thank Shanta Boddapati for assistance with statistical analyses.
This work was supported by grants from the Michael J. Fox Foundation, the Arizona Department of Health Services for the Arizona Alzheimer's Consortium, and the Arizona Biomedical Research Commission. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PD, Parkinson disease; scFv, single chain antibody fragment; AFM, atomic force microscope; ThT, Thioflavin T; AD, Alzheimer disease; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine; HRP, horseradish peroxidase; ELISA, enzyme-linked immunosorbent assay; LDH, lactic acid dehydrogenase; BSA, bovine serum albumin; ND, no disease; MFI, mean fluorescence intensity.
References
- 1.Price, D. L. (1999) Nature 399, A3–A5 [DOI] [PubMed] [Google Scholar]
- 2.Schapira, A. H. (1997) Baillieres Clin. Neurol. 6, 15–36 [PubMed] [Google Scholar]
- 3.Gomez-Tortosa, E., Newell, K., Irizarry, M. C., Albert, M., Growdon, J. H., and Hyman, B. T. (1999) Neurology 53, 1284–1291 [DOI] [PubMed] [Google Scholar]
- 4.Spillantini, M. G., Crowther, R. A., Jakes, R., Cairns, N. J., Lantos, P. L., and Goedert, M. (1998) Neurosci. Lett. 251, 205–208 [DOI] [PubMed] [Google Scholar]
- 5.Baba, M., Nakajo, S., Tu, P. H., Tomita, T., Nakaya, K., Lee, V. M., Trojanowski, J. Q., and Iwatsubo, T. (1998) Am. J. Pathol. 152, 879–884 [PMC free article] [PubMed] [Google Scholar]
- 6.Wakabayashi, K., Hayashi, S., Kakita, A., Yamada, M., Toyoshima, Y., Yoshimoto, M., and Takahashi, H. (1998) Acta Neuropathol. 96, 445–452 [DOI] [PubMed] [Google Scholar]
- 7.Tu, P. H., Galvin, J. E., Baba, M., Giasson, B., Tomita, T., Leight, S., Nakajo, S., Iwatsubo, T., Trojanowski, J. Q., and Lee, V. M. (1998) Ann. Neurol. 44, 415–422 [DOI] [PubMed] [Google Scholar]
- 8.Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., Stenroos, E. S., Chandrasekharappa, S., Athanassiadou, A., Papapetropoulos, T., Johnson, W. G., Lazzarini, A. M., Duvoisin, R. C., Di Iorio, G., Golbe, L. I., and Nussbaum, R. L. (1997) Science 276, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 9.Zarranz, J. J., Alegre, J., Gomez-Esteban, J. C., Lezcano, E., Ros, R., Ampuero, I., Vidal, L., Hoenicka, J., Rodriguez, O., Atares, B., Llorens, V., Gomez Tortosa, E., del Ser, T., Munoz, D. G., and de Yebenes, J. G. (2004) Ann. Neurol. 55, 164–173 [DOI] [PubMed] [Google Scholar]
- 10.Singleton, A. B., Farrer, M., Johnson, J., Singleton, A., Hague, S., Kachergus, J., Hulihan, M., Peuralinna, T., Dutra, A., Nussbaum, R., Lincoln, S., Crawley, A., Hanson, M., Maraganore, D., Adler, C., Cookson, M. R., Muenter, M., Baptista, M., Miller, D., Blancato, J., Hardy, J., and Gwinn-Hardy, K. (2003) Science 302, 841. [DOI] [PubMed] [Google Scholar]
- 11.Feany, M. B., and Bender, W. W. (2000) Nature 404, 394–398 [DOI] [PubMed] [Google Scholar]
- 12.Masliah, E., Rockenstein, E., Veinbergs, I., Mallory, M., Hashimoto, M., Takeda, A., Sagara, Y., Sisk, A., and Mucke, L. (2000) Science 287, 1265–1269 [DOI] [PubMed] [Google Scholar]
- 13.Iwai, A., Masliah, E., Yoshimoto, M., Ge, N., Flanagan, L., de Silva, H. A., Kittel, A., and Saitoh, T. (1995) Neuron 14, 467–475 [DOI] [PubMed] [Google Scholar]
- 14.Conway, K. A., Harper, J. D., and Lansbury, P. T. (1998) Nat. Med. 4, 1318–1320 [DOI] [PubMed] [Google Scholar]
- 15.Serpell, L. C., Berriman, J., Jakes, R., Goedert, M., and Crowther, R. A. (2000) Proc. Natl. Acad. Sci. U. S. A. 97, 4897–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volles, M. J., Lee, S. J., Rochet, J. C., Shtilerman, M. D., Ding, T. T., Kessler, J. C., and Lansbury, P. T., Jr. (2001) Biochemistry 40, 7812–7819 [DOI] [PubMed] [Google Scholar]
- 17.Volles, M. J., and Lansbury, P. T., Jr. (2003) Biochemistry 42, 7871–7878 [DOI] [PubMed] [Google Scholar]
- 18.Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., and Glabe, C. G. (2003) Science 300, 486–489 [DOI] [PubMed] [Google Scholar]
- 19.Ding, T. T., Lee, S. J., Rochet, J. C., and Lansbury, P. T., Jr. (2002) Biochemistry 41, 10209–10217 [DOI] [PubMed] [Google Scholar]
- 20.Lashuel, H. A., Hartley, D., Petre, B. M., Walz, T., and Lansbury, P. T., Jr. (2002) Nature 418(6895): 291. [DOI] [PubMed] [Google Scholar]
- 21.Conway, K. A., Rochet, J. C., Bieganski, R. M., and Lansbury, P. T., Jr. (2001) Science 294, 1346–1349 [DOI] [PubMed] [Google Scholar]
- 22.Borghi, R., Marchese, R., Negro, A., Marinelli, L., Forloni, G., Zaccheo, D., Abbruzzese, G., and Tabaton, M. (2000) Neurosci. Lett. 287, 65–67 [DOI] [PubMed] [Google Scholar]
- 23.El-Agnaf, O. M., Salem, S. A., Paleologou, K. E., Curran, M. D., Gibson, M. J., Court, J. A., Schlossmacher, M. G., and Allsop, D. (2006) FASEB J. 20, 419–425 [DOI] [PubMed] [Google Scholar]
- 24.Lee, P. H., Lee, G., Park, H. J., Bang, O. Y., Joo, I. S., and Huh, K. (2006) J. Neural Transm. 113, 1435–1439 [DOI] [PubMed] [Google Scholar]
- 25.Tokuda, T., Salem, S. A., Allsop, D., Mizuno, T., Nakagawa, M., Qureshi, M. M., Locascio, J. J., Schlossmacher, M. G., and El-Agnaf, O. M. (2006) Biochem. Biophys. Res. Commun. 349, 162–166 [DOI] [PubMed] [Google Scholar]
- 26.Bodles, A. M., Guthrie, D. J., Harriott, P., Campbell, P., and Irvine, G. B. (2000) Eur. J. Biochem. 267, 2186–2194 [DOI] [PubMed] [Google Scholar]
- 27.Du, H. N., Tang, L., Luo, X. Y., Li, H. T., Hu, J., Zhou, J. W., and Hu, H. Y. (2003) Biochemistry 42, 8870–8878 [DOI] [PubMed] [Google Scholar]
- 28.El-Agnaf, O. M., Jakes, R., Curran, M. D., Middleton, D., Ingenito, R., Bianchi, E., Pessi, A., Neill, D., and Wallace, A. (1998) FEBS Lett. 440, 71–75 [DOI] [PubMed] [Google Scholar]
- 29.Lee, E. N., Cho, H. J., Lee, C. H., Lee, D., Chung, K. C., and Paik, S. R. (2004) Biochemistry 43, 3704–3715 [DOI] [PubMed] [Google Scholar]
- 30.Seo, J. H., Rah, J. C., Choi, S. H., Shin, J. K., Min, K., Kim, H. S., Park, C. H., Kim, S., Kim, E. M., Lee, S. H., Lee, S., Suh, S. W., and Suh, Y. H. (2002) FASEB J. 16, 1826–1828 [DOI] [PubMed] [Google Scholar]
- 31.Sung, J. Y., Kim, J., Paik, S. R., Park, J. H., Ahn, Y. S., and Chung, K. C. (2001) J. Biol. Chem. 276, 27441–27448 [DOI] [PubMed] [Google Scholar]
- 32.Emadi, S., Barkhordarian, H., Wang, M. S., Schulz, P., and Sierks, M. R. (2007) J. Mol. Biol. 368, 1132–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler, Y. Y., Chen, S. Y., and Sane, D. C. (2003) Mol. Ther. 8, 355–366 [DOI] [PubMed] [Google Scholar]
- 34.Murphy, R. C., and Messer, A. (2004) Brain Res. Mol. Brain Res. 121, 141–145 [DOI] [PubMed] [Google Scholar]
- 35.Lecerf, J. M., Shirley, T. L., Zhu, Q., Kazantsev, A., Amersdorfer, P., Housman, D. E., Messer, A., and Huston, J. S. (2001) Proc. Natl. Acad. Sci. U. S. A. 98, 4764–4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou, C., Emadi, S., Sierks, M. R., and Messer, A. (2004) Mol. Ther. 10, 1023–1031 [DOI] [PubMed] [Google Scholar]
- 37.Masliah, E., Rockenstein, E., Adame, A., Alford, M., Crews, L., Hashimoto, M., Seubert, P., Lee, M., Goldstein, J., Chilcote, T., Games, D., and Schenk, D. (2005) Neuron 46, 857–868 [DOI] [PubMed] [Google Scholar]
- 38.Barkhordarian, H., Emadi, S., Schulz, P., and Sierks, M. R. (2006) Protein Eng. Des. Sel. 19, 497–502 [DOI] [PubMed] [Google Scholar]
- 39.Volles, M. J., and Lansbury, P. T., Jr. (2007) J. Mol. Biol. 366, 1510–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zameer, A., Kasturirangan, S., Emadi, S., Nimmagadda, S. V., and Sierks, M. R. (2008) J. Mol. Biol. 384, 917–928 [DOI] [PubMed] [Google Scholar]
- 41.Marks, J. D., Hoogenboom, H. R., Bonnert, T. P., McCafferty, J., Griffiths, A. D., and Winter, G. (1991) J. Mol. Biol. 222, 581–597 [DOI] [PubMed] [Google Scholar]
- 42.Carter, P., Bedouelle, H., and Winter, G. (1985) Nucleic Acids Res. 13, 4431–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoogenboom, H. R., and Winter, G. (1992) J. Mol. Biol. 227, 381–388 [DOI] [PubMed] [Google Scholar]
- 44.Marcus, W. D., Wang, H., Lohr, D., Sierks, M. R., and Lindsay, S. M. (2006) Biochem. Biophys. Res. Commun. 342, 1123–1129 [DOI] [PubMed] [Google Scholar]
- 45.Schagger, H., and von Jagow, G. (1987) Anal. Biochem. 166, 368–379 [DOI] [PubMed] [Google Scholar]
- 46.Liu, R., Yuan, B., Emadi, S., Zameer, A., Schulz, P., McAllister, C., Lyubchenko, Y., Goud, G., and Sierks, M. R. (2004) Biochemistry 43, 6959–6967 [DOI] [PubMed] [Google Scholar]
- 47.Pahlman, S., Hoehner, J. C., Nanberg, E., Hedborg, F., Fagerstrom, S., Gestblom, C., Johansson, I., Larsson, U., Lavenius, E., Ortoft, E., and Söderholm, H. (1995) Eur. J. Cancer 31A, 453–458 [DOI] [PubMed] [Google Scholar]
- 48.Presgraves, S. P., Ahmed, T., Borwege, S., and Joyce, J. N. (2004) Neurotox. Res. 5, 579–598 [DOI] [PubMed] [Google Scholar]
- 49.Wang, M., Zameer, A., Emadi, S., and Sierks, M. (2009) Langmuir 25, 912–918 [DOI] [PubMed] [Google Scholar]
- 50.Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M., and Goedert, M. (1998) Proc. Natl. Acad. Sci. U. S. A. 95, 6469–6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood, S. J., Wypych, J., Steavenson, S., Louis, J. C., Citron, M., and Biere, A. L. (1999) J. Biol. Chem. 274, 19509–19512 [DOI] [PubMed] [Google Scholar]
- 52.Conway, K. A., Lee, S. J., Rochet, J. C., Ding, T. T., Harper, J. D., Williamson, R. E., and Lansbury, P. T., Jr. (2000) Ann. N. Y. Acad. Sci. 920, 42–45 [DOI] [PubMed] [Google Scholar]
- 53.Goldberg, M. S., and Lansbury, P. T., Jr. (2000) Nat. Cell Biol. 2, E115–E119 [DOI] [PubMed] [Google Scholar]
- 54.Gosavi, N., Lee, H. J., Lee, J. S., Patel, S., and Lee, S. J. (2002) J. Biol. Chem. 277, 48984–48992 [DOI] [PubMed] [Google Scholar]
- 55.Danzer, K. M., Haasen, D., Karow, A. R., Moussaud, S., Habeck, M., Giese, A., Kretzschmar, H., Hengerer, B., and Kostka, M. (2007) J. Neurosci. 27, 9220–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Apetri, M. M., Maiti, N. C., Zagorski, M. G., Carey, P. R., and Anderson, V. E. (2006) J. Mol. Biol. 355, 63–71 [DOI] [PubMed] [Google Scholar]
- 57.Lashuel, H. A., Petre, B. M., Wall, J., Simon, M., Nowak, R. J., Walz, T., and Lansbury, P. T., Jr. (2002) J. Mol. Biol. 322, 1089–1102 [DOI] [PubMed] [Google Scholar]
- 58.Lashuel, H. A., and Grillo-Bosch, D. (2005) Methods Mol. Biol. 299, 19–33 [DOI] [PubMed] [Google Scholar]
- 59.Outeiro, T. F., Putcha, P., Tetzlaff, J. E., Spoelgen, R., Koker, M., Carvalho, F., Hyman, B. T., and McLean, P. J. (2008) PLoS ONE 3, e1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kayed, R., and Glabe, C. G. (2006) Methods Enzymol. 413, 326–344 [DOI] [PubMed] [Google Scholar]
- 61.Golts, N., Snyder, H., Frasier, M., Theisler, C., Choi, P., and Wolozin, B. (2002) J. Biol. Chem. 277, 16116–16123 [DOI] [PubMed] [Google Scholar]
- 62.Crowther, R. A., Jakes, R., Spillantini, M. G., and Goedert, M. (1998) FEBS Lett. 436, 309–312 [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto, M., Rockenstein, E., Mante, M., Mallory, M., and Masliah, E. (2001) Neuron 32, 213–223 [DOI] [PubMed] [Google Scholar]
- 64.El-Agnaf, O. M., Paleologou, K. E., Greer, B., Abogrein, A. M., King, J. E., Salem, S. A., Fullwood, N. J., Benson, F. E., Hewitt, R., Ford, K. J., Martin, F. L., Harriott, P., Cookson, M. R., and Allsop, D. (2004) FASEB J. 18, 1315–1317 [DOI] [PubMed] [Google Scholar]
- 65.Klucken, J., Shin, Y., Masliah, E., Hyman, B. T., and McLean, P. J. (2004) J. Biol. Chem. 279, 25497–25502 [DOI] [PubMed] [Google Scholar]
- 66.Boder, E. T., and Wittrup, K. D. (2000) Methods Enzymol. 328, 430–444 [DOI] [PubMed] [Google Scholar]