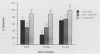Abstract
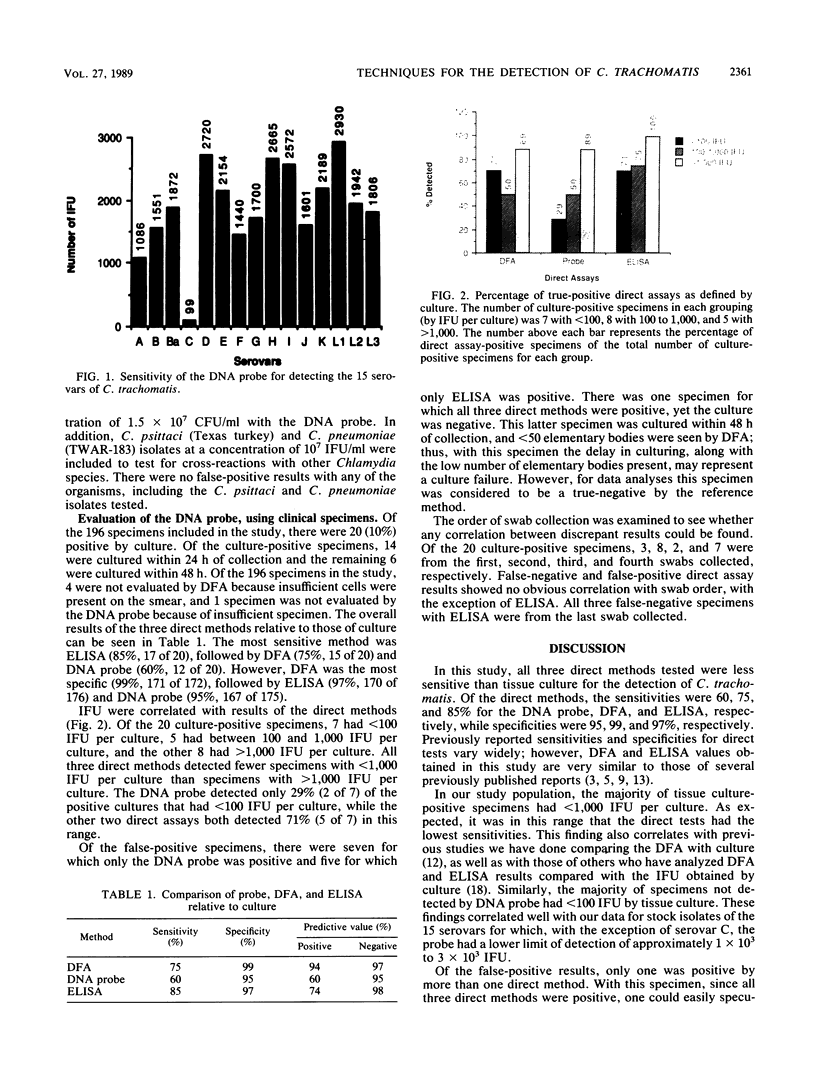
A DNA probe assay (PACE; Gen-Probe, San Diego, Calif.) was compared with a culture reference method for the detection of Chlamydia trachomatis. Using stock isolates of each of the 15 serovars (A to K, Ba, L1, L2, and L3) of C. trachomatis, the lower limit of sensitivity for the DNA probe ranged between 1,086 inclusion-forming units (IFU) for serovar E (Bour) to 2,930 IFU for serovar L1 (440), with the only exception being serovar C (TW-3), with which 99 IFU was detected. There was no cross-reactivity with Chlamydia psittaci (Texas turkey) and Chlamydia pneumoniae (TWAR-183). Bacterial and fungal isolates representing 14 species of normal vaginal flora as well as Neisseria gonorrhoeae gave negative results with the DNA probe when tested at a level of 1.5 X 10(7) CFU/ml. In addition, the DNA probe, a direct fluorescent-antibody stain (DFA) (MicroTrak; Syva Corp., Palo Alto, Calif.), and an enzyme-linked immunosorbent assay (Chlamydiazyme; Abbott Laboratories, North Chicago, Ill.) were compared with culture for the detection of C. trachomatis, using 196 clinical cervical samples. Of the 196 samples, 20 (10%) were culture positive. Of the 176 culture-negative samples, 1 was not evaluated by DNA probe and 4, because of a lack of cellular material, were not evaluated by DFA. The sensitivities of the DNA probe, DFA, and enzyme-linked immunosorbent assay were 60, 75, and 85%, respectively, and specificities were 95, 99, and 97%, respectively. Of the false-positive direct results, there was only one specimen with which more than one direct method was positive, and with this specimen all three direct methods were positive. The majority of false-negative results by the direct methods were from specimens which by the culture method gave <100 IFU per culture.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarnaes S. L., Peterson E. M., De La Maza L. M. The effect of media and temperature on the storage of Chlamydia trachomatis. Am J Clin Pathol. 1984 Feb;81(2):237–239. doi: 10.1093/ajcp/81.2.237. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Maclean I. W., Binns B., Peeling R. W. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985 Dec;152(6):1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- Chernesky M. A., Mahony J. B., Castriciano S., Mores M., Stewart I. O., Landis S. J., Seidelman W., Sargeant E. J., Leman C. Detection of Chlamydia trachomatis antigens by enzyme immunoassay and immunofluorescence in genital specimens from symptomatic and asymptomatic men and women. J Infect Dis. 1986 Jul;154(1):141–148. doi: 10.1093/infdis/154.1.141. [DOI] [PubMed] [Google Scholar]
- Hernandez T. J., Noller K. L., Smith T. F. Detection of Chlamydia trachomatis using consecutive endocervical swabs. Prevalence in asymptomatic female adolescents and women attending a sexually transmitted disease clinic. J Reprod Med. 1986 Jun;31(6):497–500. [PubMed] [Google Scholar]
- Hipp S. S., Han Y., Murphy D. Assessment of enzyme immunoassay and immunofluorescence tests for detection of Chlamydia trachomatis. J Clin Microbiol. 1987 Oct;25(10):1938–1943. doi: 10.1128/jcm.25.10.1938-1943.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. E., Quinn T., Hammer M., Palmer L., Falkow S. Use of nucleic acid probes for the detection of sexually transmitted infectious agents. Diagn Microbiol Infect Dis. 1986 Mar;4(3 Suppl):101S–109S. doi: 10.1016/s0732-8893(86)80048-7. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Jalava A., Larsen S. H., Terho P., Hukkanen V. Detection of Chlamydia trachomatis in clinical specimens by nucleic acid spot hybridization. J Gen Microbiol. 1985 Apr;131(4):975–978. doi: 10.1099/00221287-131-4-975. [DOI] [PubMed] [Google Scholar]
- Lipkin E. S., Moncada J. V., Shafer M. A., Wilson T. E., Schachter J. Comparison of monoclonal antibody staining and culture in diagnosing cervical chlamydial infection. J Clin Microbiol. 1986 Jan;23(1):114–117. doi: 10.1128/jcm.23.1.114-117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony J. B., Chernesky M. A. Effect of swab type and storage temperature on the isolation of Chlamydia trachomatis from clinical specimens. J Clin Microbiol. 1985 Nov;22(5):865–867. doi: 10.1128/jcm.22.5.865-867.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. C., Lin S. S., Yang T. E., Soong Y. K., Lee P. S., Lin J. Y. Deoxyribonucleic acid hybridization analysis for the detection of urogenital Chlamydia trachomatis infections in women. Am J Obstet Gynecol. 1987 Jan;156(1):195–199. doi: 10.1016/0002-9378(87)90237-7. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Alexander R., Aarnaes S. L., King S., Greenwood J. R., de la Maza L. M. Evaluation of the Cellmatics and direct monoclonal fluorescence antibody staining for detection of genital chlamydial infections. Diagn Microbiol Infect Dis. 1987 Feb;6(2):139–143. doi: 10.1016/0732-8893(87)90098-8. [DOI] [PubMed] [Google Scholar]
- Phillips R. S., Hanff P. A., Kauffman R. S., Aronson M. D. Use of a direct fluorescent antibody test for detecting Chlamydia trachomatis cervical infection in women seeking routine gynecologic care. J Infect Dis. 1987 Oct;156(4):575–581. doi: 10.1093/infdis/156.4.575. [DOI] [PubMed] [Google Scholar]
- Ransohoff D. F., Feinstein A. R. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978 Oct 26;299(17):926–930. doi: 10.1056/NEJM197810262991705. [DOI] [PubMed] [Google Scholar]
- Schachter J. Immunodiagnosis of sexually transmitted disease. Yale J Biol Med. 1985 Sep-Oct;58(5):443–452. [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Moncada J., Dawson C. R., Sheppard J., Courtright P., Said M. E., Zaki S., Hafez S. F., Lorincz A. Nonculture methods for diagnosing chlamydial infection in patients with trachoma: a clue to the pathogenesis of the disease? J Infect Dis. 1988 Dec;158(6):1347–1352. doi: 10.1093/infdis/158.6.1347. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Rogers R. E., Katz B. P., Brickler J. F., Lineback P. L., Van der Pol B., Jones R. B. Diagnosis of chlamydial infection in women attending antenatal and gynecologic clinics. J Clin Microbiol. 1987 May;25(5):868–872. doi: 10.1128/jcm.25.5.868-872.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdea M. S., Running J. A., Horn T., Clyne J., Ku L. L., Warner B. D. A novel method for the rapid detection of specific nucleotide sequences in crude biological samples without blotting or radioactivity; application to the analysis of hepatitis B virus in human serum. Gene. 1987;61(3):253–264. doi: 10.1016/0378-1119(87)90189-2. [DOI] [PubMed] [Google Scholar]