HISTORY
A 76-year-old female on chronic supplemental oxygen was seen in the sleep center; she had multiple medical problems, including sarcoidosis with restrictive lung disease resulting in both hypoxemia and hypercapnia, pulmonary and systematic hypertension, type 2 diabetes, congestive heart failure, and obstructive sleep apnea. In the previous 3 weeks, she noted increasing fatigue, chest discomfort, lower extremity edema, and dyspnea with exertion. She had not been seen by a sleep specialist for > 3 years. Regarding her sleep, she noted some difficulty falling asleep and frequent awakenings. She reported feeling unrefreshed upon awakening and complained of excessive daytime sleepiness. She attempted to use her continuous positive airway pressure (CPAP) nightly, [10 cm H2O with supplemental oxygen at 2 liters per minute (LPM)] but after 2-3 hours, she would take off the mask because of pressure intolerance and continued with oxygen alone.
PHYSICAL EXAMINATION
On physical examination, her body mass index (BMI) was 35.8 kg/m2, and her vital signs were unremarkable except for a SpO2 87% on 2 LPM of supplemental oxygen. Bilateral rhonchi were noted, more prominent on the left than the right, with overall decreased breath sounds. Cardiac examination revealed a regular rhythm and a slight increase in the P2 heart sound. She had 2 plus pitting edema in both lower extremities.
CARDIOPULMONARY TEST RESULTS
Recent work-up included: Pulmonary Functions Tests (PFT) - FVC of 1.06 L (41% predicted), FEV1 of 0.78 L (39% predicted), FEV1/FVC of 74.1%; arterial blood gas (on 2 LPM oxygen) - pH 7.41, pCO2 58 mm Hg, and pO2 81 mm Hg; Electrocardiogram (EKG) - right bundle-branch block and left anterior fascicular block; transthoracic echocardiogram - elevated right ventricular systolic pressure, diminished left ventricular ejection fraction (40% to 50%), and increased right ventricular cavity size. Chest radiograph demonstrated moderate cardiomegaly with enlargement of the pulmonary arteries.
SLEEP STUDY
A split-night sleep study was ordered to reevaluate obstructive sleep apnea (OSA) and CPAP level. The patient continued to receive supplemental oxygen at 2 LPM throughout the entire study. The baseline portion of the study showed a Non-Rapid Eye Movement Apnea Hypopnea Index (NREM AHI) of 34 events per hour (increased from 21 events per hour on her initial baseline study). The patient did poorly with CPAP with < 1 hour of total sleep time. The following morning, during scoring, the technician noted the following ECG abnormality.
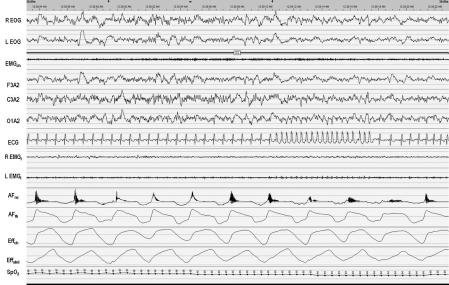
Identify the arrhythmia in Figure 1.
ANSWER: Nonsustained monomorphic wide complex tachycardia
DISCUSSION
R. Mehra and colleagues published a multicenter study reviewing the incidence of serious adverse events occurring during overnight polysomnography (PSG).1 Adverse events were defined as one of the following: (1) an episode identified by the technician as requiring medical attention by the medical director or emergency room at the time of the sleep study, or (2) an episode identified by the scoring team as requiring specific attention by the physician. After reviewing over 16,000 PSGs, an overall adverse event rate of 0.35% was reported, with a mortality rate of 0.006%. Most of the 56 documented adverse events were arrhythmias, and half were ventricular in origin. Unlike this case, the arrhythmias observed in this series were often associated with sleep disordered breathing events.
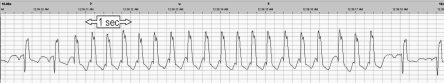
The American Academy of Sleep Medicine (AASM) recommends a single modified EKG lead II using torso electrode placement aligned in parallel to the right shoulder and left hip.2 Per the AASM guidelines, sinus tachycardia is defined as > 90 beats per minute (bpm), and wide complex tachycardia should be scored for rhythms lasting ≥ 3 consecutive beats at a rate of > 100 bpm with QRS duration ≥ 120 msec. Narrow complex tachycardia should be scored for rhythms lasting ≥ 3 consecutive beats at a rate > 100 bpm with a QRS duration < 120 msec. “Nonsustained” tachycardia label requires the duration to be < 30 seconds.3 The event shown in the present case qualifies as a wide complex tachycardia, as the QRS is > 120 msec and the rate is 162 bpm (Figure 1 and Figure 2).
Figure 1.
This is a 30-second epoch; SpO2 during this epoch ranges from 94% to 97%. R EOG: right electrooculogram; L EOG: left electrooculogram; EMGch: chin electromyogram; F3A2: frontal electroencephalogram; C3A2: central electroencephalogram; O1A2: occipital electroencephalogram; ECG: lead II electrocardiogram; R EMGl: Right leg electromyogram; L EMGl: Left leg electromyogram; AFnc: nasal cannula pressure transducer airflow; AFth: thermistor airflow; Effch: chest effort; Effabd: Abdominal effort; SpO2: Pulse oxygen saturation
Figure 2.
This represents a 10-second window view of the EKG abnormality depicted in Figure 1.
The approach to nonsustained ventricular tachycardia (NSVT) hinges upon whether underlying structural heart disease is present. Most studies would suggest that in the absence of underlying structural heart disease (typically ruled out by exercise testing, echocardiography, and/or coronary arteriography), the likelihood of a subsequent adverse event is low. However, the prognosis is significantly different if underlying heart disease is present. Structural heart disease includes coronary heart disease, hypertrophic cardiomyopathies, dilated cardiomyopathies, and valvular heart disease. NSVT in patients with known heart disease is clearly associated with an increased risk for sudden death and warrants a prompt referral to a cardiologist. Our patient had significant structural heart disease, with pulmonary hypertension, a reduced ejection fraction, and possible cardiac sarcoidosis. Clinical evidence of myocardial involvement is present in approximately 5% of patients with sarcoidosis, although autopsy studies suggest that subclinical cardiac involvement is present in up to 30% of cases.4 She was referred for an electrophysiology (EP) evaluation and underwent a cardiac MRI, which revealed a delayed enhancement at the right ventricular insertion site concerning for cardiac sarcoid involvement. Given the high incidence of sudden death in the setting of cardiac sarcoidosis, and given the patient's known left ventricular dysfunction and documented 19-beat run of VT, the EP team recommended implantable cardioverter-defibrillator (ICD) implantation.
Although serious adverse events such as ventricular tachycardia rarely occur during a polysomnogram, sleep studies are commonly sought in individuals with co- morbid conditions such as hypertension, interstitial lung disease, diabetes, coronary artery disease, and stroke. Therefore, it is important for all members of the sleep center technical and medical staff to demonstrate proficiency in identification of common cardiac arrhythmias. Although there is no consensus, when such an arrhythmia is noted during the study, the patient should be awakened and queried about associated chest pain or shortness of breath and, at a minimum, the physician on-call should be notified. This case also underscores the fact that management of cardiac arrhythmias, whether identified during a sleep study or during a cardiac work-up, are often individualized to consider the particular clinical background and conditions of the patient.5–19
PEARLS
Cardiac arrhythmias are among the most common serious adverse events encountered during a PSG.
The AASM recommends that significant arrhythmias should be reported if the quality of the single lead is sufficient for accurate scoring (AASM manual).
- According to AASM guidelines, tachycardia is classified as either narrow complex or wide complex based on the following:
-
3a.Narrow complex tachycardia is characterized by ≥ 3 consecutive beats occurring at a rate of > 100 bpm, with a QRS interval < 120 ms (narrow complex).
-
3b.Wide complex tachycardia is characterized by ≥ 3 consecutive beats occurring at a rate of > 100 bpm, with a QRS interval > 120 ms (wide complex).
-
3a.
NSVT always requires further investigation, because of the risk of sudden death in patients with structural cardiac disease.
Sleep technicians and sleep specialists should be versed in proper policy and procedures when a patient demonstrates a cardiac arrhythmia on a PSG.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Mehra R, Strohl KP. Incidence of serious adverse events during nocturnal polysomnography. Sleep. 2004;27:1379–83. doi: 10.1093/sleep/27.7.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. p. 39. [Google Scholar]
- 3.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. New Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 4.Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. 1994;11:26–31. [PubMed] [Google Scholar]
- 5.Koehler U, Schafer H. Is obstructive sleep apnea (OSA) a risk factor for myocardial infarction and cardiac arrhythmias in patients with coronary heart disease (CHD)? Sleep. 1996;19:283–6. [PubMed] [Google Scholar]
- 6.Liston R, Deegan PC, McCreery C, McNicholas WT. Role of respiratory sleep disorders in the pathogenesis of nocturnal angina and arrhythmias. Postgrad Med J. 1994;70:275–80. doi: 10.1136/pgmj.70.822.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepard JW., Jr. Hypertension, cardiac arrhythmias, myocardial infarction, and stroke in relation to obstructive sleep apnea. Clin Chest Med. 1992;13:437–58. [PubMed] [Google Scholar]
- 8.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–94. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 9.Hoffstein V, Mateika S. Cardiac arrhythmias, snoring, and sleep apnea. Chest. 1994;106:466–71. doi: 10.1378/chest.106.2.466. [DOI] [PubMed] [Google Scholar]
- 10.Miller WP. Cardiac arrhythmias and conduction disturbances in the sleep apnea syndrome. Prevalence and significance. Am J Med. 1982;73:317–21. doi: 10.1016/0002-9343(82)90716-1. [DOI] [PubMed] [Google Scholar]
- 11.Randazo DN, Winters SL, Schweitzer P. Obstructive sleep apnea-induced supraventricular tachycardia. J Electrocardiol. 1996;29:65–7. doi: 10.1016/s0022-0736(96)80115-4. [DOI] [PubMed] [Google Scholar]
- 12.Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Sleep-induced apnea syndrome. Prevalence of cardiac arrhythmias and their reversal after tracheostomy. Am J Med. 1977;63:348–58. doi: 10.1016/0002-9343(77)90272-8. [DOI] [PubMed] [Google Scholar]
- 13.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–16. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harbison J, O'Reilly P, McNicholas WT. Cardiac rhythm disturbances in the obstructive sleep apnea syndrome: effects of nasal continuous positive airway pressure therapy. Chest. 2000;118:591–5. doi: 10.1378/chest.118.3.591. [DOI] [PubMed] [Google Scholar]
- 15.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–7. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 16.Shepard JW, Jr, Garrison MW, Grither DA, Dolan GF. Relationship of ventricular ectopy to oxyhemoglobin desaturation in patients with obstructive sleep apnea. Chest. 1985;88:335–40. doi: 10.1378/chest.88.3.335. [DOI] [PubMed] [Google Scholar]
- 17.Ryan CM, Juvet S, Leung R, Bradley TD. Timing of nocturnal ventricular ectopy in heart failure patients with sleep apnea. Chest. 2008;133:934–40. doi: 10.1378/chest.07-2595. [DOI] [PubMed] [Google Scholar]
- 18.Fischter J, Bauer D, Arampatis S, Fries R, Heisel A, Sybrecht GW. Sleep-related breathing disorders are associated with ventricular arrhythmias in patients with an implantable cardioverter-defibrillator. Chest. 2002;122:558–61. doi: 10.1378/chest.122.2.558. [DOI] [PubMed] [Google Scholar]
- 19.Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60:781–5. doi: 10.1136/thx.2005.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]