Abstract
Increased levels of red cell fetal hemogloblin, whether due to hereditary persistence of expression or from induction with hydroxyurea therapy, effectively ameliorate sickle cell disease (SCD). Therefore, we developed erythroid-specific, γ-globin lentiviral vectors for hematopoietic stem cell (HSC)-targeted gene therapy with the goal of permanently increasing fetal hemoglobin (HbF) production in sickle red cells. We evaluated two different γ-globin lentiviral vectors for therapeutic efficacy in the BERK sickle cell mouse model. The first vector, V5, contained the γ-globin gene driven by 3.1 kb of β-globin regulatory sequences and a 130-bp β-globin promoter. The second vector, V5m3, was identical except that the γ-globin 3′-untranslated region (3′-UTR) was replaced with the β-globin 3′-UTR. Adult erythroid cells have β-globin mRNA 3′-UTR-binding proteins that enhance β-globin mRNA stability and we postulated this design might enhance γ-globin expression. Stem cell gene transfer was efficient and nearly all red cells in transplanted mice expressed human γ-globin. Both vectors demonstrated efficacy in disease correction, with the V5m3 vector producing a higher level of γ-globin mRNA which was associated with high-level correction of anemia and secondary organ pathology. These data support the rationale for a gene therapy approach to SCD by permanently enhancing HbF using a γ-globin lentiviral vector.
Introduction
Sickle cell disease (SCD) is a devastating inherited disorder of hemoglobin that both shortens and reduces the quality of life.1,2 Beginning in early childhood, chronic vaso-occlusive events lead to a central nervous system vasculopathy causing impaired intellectual development and, in some patients, devastating strokes.3,4 Acute vaso-occlusive episodes cause tissue ischemia and excruciating pain, while the resulting chronic multiorgan damage leads to disability and early death.5,6 Hydroxyurea therapy diminishes the frequency of crisis in adults and children with SCD7,8 and may delay the onset of disability and the occurrence of death,9 but curative therapies are desperately needed. Bone marrow (BM) transplantation, an established curative therapy, has benefited only a small number of patients because of the limited availability of appropriate donors.10 Gene therapy holds the promise of addressing a significant gap in the availability of curative therapy for patients with SCD.10,11
SCD results from a single amino acid substitution of valine for glutamic acid at position 6 of β-globin.12 Upon deoxygenation, HbS polymerizes which results in deformation and damage to the red cell causing chronic hemolysis and ultimately enhancing the risk of vaso-occlusion. Substantial evidence indicates that increased fetal hemoglobin (α2γ2; HbF) production mitigates the severity of SCD. Patients with co-inheritance of persistence of fetal hemoglobin expression and elevated fetal hemoglobin (HbF) levels or those with certain β-globin locus haplotypes that are associated with increased HbF have relatively much less clinical severity.13,14,15 A small percentage of patients who have elevated HbF for undefined reasons have a diminished risk of chronic and recurrent acute clinical events.16 Additionally, increased HbF in response to hydroxyurea and butyrate administration has therapeutic benefit.7,17,18 The anti-sickling activity of HbF reflects both the lowering of the HbS concentration as well as the direct inhibition of the red cell sickling process due to γ-globin molecules being incorporated into mixed hemoglobin tetramers that do not participate in intracellular polymer formation.19 Direct experimental evidence indicating a beneficial effect of HbF in SCD mouse models has been shown utilizing genetic crosses. In one sickle cell mouse model, γ-globin transgene mRNA expression in a majority of reticulocytes at 19–24% the level of the endogenous globin genes resulted in corresponding levels of HbF of 16–25%, significantly improving the disease phenotype and lifespan of the animals.20 Using a different SCD model, significant phenotypic correction occurred when ~50% of red cells contained 40% HbF per cell.21
The development of lentiviral vectors over a decade ago22 was a key milestone that made possible the subsequent application of globin lentiviral vectors for hematopoietic stem cell (HSC)-targeted gene transfer in preclinical models of murine and human β-thalassemia.23,24,25,26,27 Lentiviral vectors, unlike previously used γ-retroviral vectors, proved capable of efficiently transmitting complex globin expression cassettes, containing transcriptional regulatory sequences from the β-globin locus control region which are required for high-level expression. Correction of β-thalassemia mouse models has been achieved using both β-globin23,24 and γ-globin vectors.25,27
A similar approach was taken using murine models of SCD.28,29 Overexpresssion of a β-globin polypeptide containing specific point mutations designed to optimize anti-sickling activity led to correction28 or improvement29 of two different SCD models. Here we report the use of a γ-globin lentiviral vector for HSC transduction and high-level, therapeutic expression of HbF in the BERK SCD mouse model.30 The BERK SCD mouse displays the severe hemolytic anemia and much of the pathology of SCD as manifested in humans.30,31 Additionally, these mice appear to most closely model human sickle/β0-thalassemia, a population of patients which may be especially likely to benefit from gene therapy since the required output of the anti-sickling globin for a therapeutic effect will be reduced, due to the diminished level of endogenous βs chains. Here, we report an average improvement in the level of Hb of 4.1 g/dl per vector copy along with correction of the multiorgan damage of SCD using a γ-globin expression cassette optimized for expression in adult red cells. Since SCD patients have significant underlying multiorgan dysfunction, it will be important to develop gene therapy protocols which utilize a reduced or submyeloablative conditioning regimen to minimize regimen-related toxicity.32 Our data are therefore important in demonstrating successful treatment of SCD through the expression of a natural, endogenously expressed protein.
Results
Efficient γ-globin lentiviral vector gene transfer into steady-state HSCs from BERK SCD mice
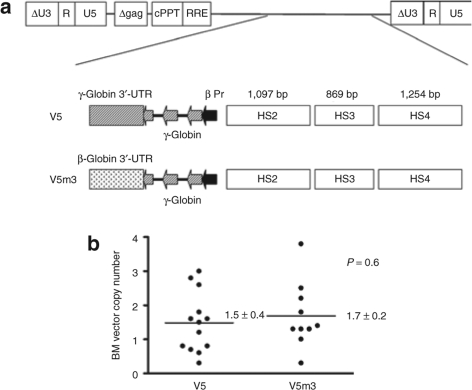
We previously demonstrated correction of murine β-thalassemia with a γ-globin lentiviral vector, termed V5, which contains 3.1 kb of regulatory sequences from the β-globin locus control region, a 130-bp β-globin promoter and the γ-globin genomic sequences (Figure 1a).27 Recent studies indicate that the 3′-UTR of the β-globin mRNA contains a pyrimidine-rich motif that is bound by the mRNA-binding proteins nucleolin and αCP, providing the basis for the hyperstability of β-globin mRNA.33,34 This element, which forms a stem–loop structure, is not present in the γ-globin 3′-UTR. Other studies show that γ-globin transcripts cannot accumulate to high levels in cells containing significant amounts of β-globin mRNA.35,36 Together, these data suggested to us that the β-globin 3′-UTR may have a higher relative affinity, compared to its γ-globin counterpart, for the binding factors in the adult erythroid cell that mediate globin mRNA stability.35 This would be comparable to the known preferential stabilization of α-globin mRNA, relative to ξ-globin mRNA, due to higher binding efficiency of a similar αCP protein complex to the α-globin 3′-UTR.37,38 Therefore, to test the possibility that the β-globin 3′-UTR might enhance HbF expression and improve therapeutic efficacy after lentiviral vector–mediated HSC gene transfer, we replaced the γ-globin 3′-UTR with its β-globin counterpart, resulting in vector V5m3 (Figure 1a). These globin vectors, identical except for the 3′-UTR sequences, were produced with equivalent high titer (3× 106 to 5 × 106 transducing units/ml, unconcentrated) and both transmitted an unrearranged proviral genome of the correct size to target cells in culture (data not shown).
Figure 1.
Self-inactivating (SIN) γ-globin lentiviral vectors demonstrate efficient gene transfer into murine sickle cell hematopoietic stem cells (HSCs). (a) Schematic representation of the integrated γ-globin lentiviral vector proviral form. Shown at top is the vector backbone which contains the central polypurine track (cPPT) and the rev responsive element (RRE) and has a SIN design in which the promoter and enhancer of the HIV U3 region have been deleted. Shown below are the V5 and V5m3 globin expression cassettes which are identical with the exception of the 3′-untranslated region (3′-UTR) (hatched rectangle and speckled rectangle). Both vectors contain the same composite 3.1 kb of transcriptional regulatory sequences from the β-globin locus control region with contributions, as indicated, from HS4, HS3, and HS2 (open rectangles). The filled arrowhead represents the 130-bp β-globin promoter while the γ-globin exons are indicated by hatched arrowheads. (b) Bone marrow (BM) vector copy numbers of individual mice transplanted with sickle cell HSCs transduced with the V5 and V5m3 vectors. Four to five months post-transplant, copy numbers were determined by Southern blot analysis of BM DNA from transplanted animals, relative to a cell line known to contain a single proviral copy.
Lineage-depleted steady-state BM cells from the SCD mice were obtained using immunomagnetic separation and cultured overnight in cytokine-containing media before being exposed to a lentiviral vector encoding green fluorescent protein (GFP) or either of the two γ-globin vectors, V5 and V5m3. Mock-transduced cells and cells transduced with the three lentiviral vectors were then transplanted into groups of lethally irradiated (1125 cGy) C57Bl/6 wild-type mice. To estimate the gene transfer efficiency into primitive hematopoietic cells, we determined the frequency of vector transfer into spleen colony-forming unit (CFU-S) cells for each of the transduced grafts. For the V5 vector, 14 of 25 (64%) CFU-S were positive for the vector DNA by Southern blot analysis, while the V5m3 vector had a similar frequency with 11 of 22 (50%) positive (data not shown). Twenty-four of twenty-eight (86%) CFU-S derived from cells transduced with the GFP vector were positive. These data indicate a high level of gene transfer of all three vectors into primitive hematopoietic cells.
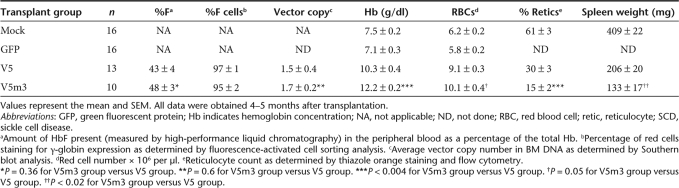
Recipient mice with no residual endogenous hematopoiesis (<1% endogenous wild-type Hb) were identified for long-term analysis, to preclude potential artifactual disease improvement which would confound the assessment of the effect of HbF expression. Animals were analyzed 4–5 months post-transplantation for HSC gene transfer, HbF expression in red cells, complete blood count, renal function, and organ pathology. Southern blot analysis was performed on BM DNA from all the animals in both globin vector groups to determine average vector copy number (VCN). As shown in Figure 1b, comparable HSC gene transfer was obtained in both globin vector groups (V5: 1.5 ± 0.4; V5m3: 1.7 ± 0.2; P = 0.6; Table 1). Additionally, 95–97% of peripheral blood (PB) red blood cells (RBCs) in both sets of mice expressed the γ-globin transgene as judged by immunostaining with an anti-HbF antibody (Table 1 and Supplementary Figure S1). Mice transplanted with cells transduced with the GFP control vector demonstrated GFP expression in 64 ± 6% of PB granulocytes.
Table 1.
Therapeutic efficacy of γ-globin lentiviral vectors in SCD
High-level HbF expression ameliorates the anemia of SCD
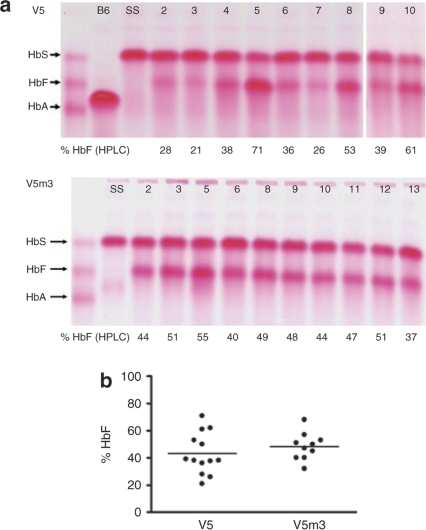
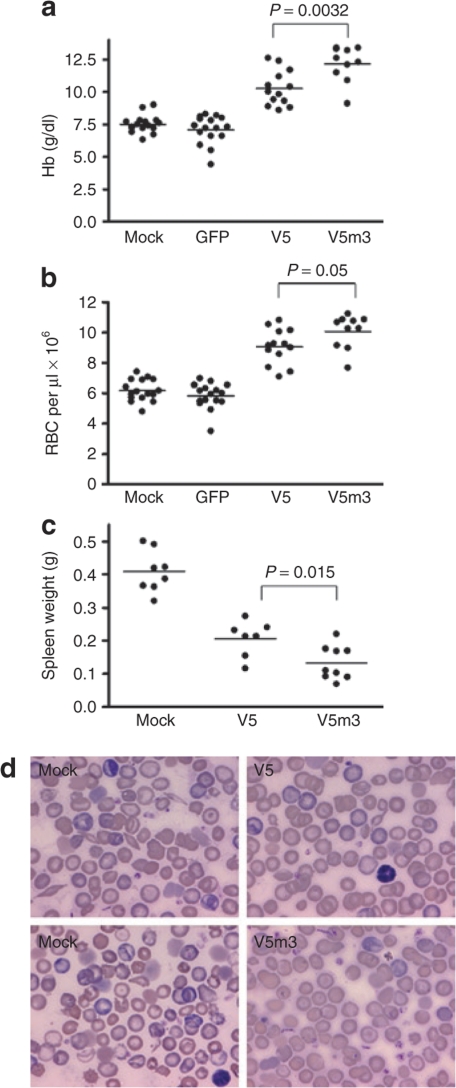
HbF derived from the vector-encoded γ-globin transgene was measured in RBC lysates from recipients 4–5 months after transplantation using cellulose acetate gel electrophoresis and high-performance liquid chromatography. V5 mice had high levels of HbF (Figure 2a,b), ranging from 21 to 71% of the total Hb, with a mean level of 43 ± 4% (Table 1). As shown in Figure 2b, V5m3 mice had a slightly higher mean level of HbF (48 ± 3% of total Hb, Table 1), with a trend toward less variable expression (range 32–68%; P = 0.23), although not statistically significant. Importantly, these high levels of HbF led to significant increases in the Hb and RBCs in the PB of mice receiving transplants of both sets of γ-globin vector-transduced cells, compared to mice that received mock- or GFP-transduced cells (Figure 3a,b). V5m3 animals had the best improvement in anemia, with a Hb level of 12.2 ± 0.2 g/dl, while V5 animals, although significantly improved relative to GFP animals (7.1 ± 0.3 g/dl), had a less robust increase, with a Hb level of 10.3 ± 0.4 g/dl compared to V5m3 (P = 0.0032, Table 1). Using the independent measure of RBC count, a similar difference was also observed between the V5 and V5m3 groups (P = 0.05). Furthermore, although both globin vector groups demonstrated a dramatic reduction in extramedullary hematopoiesis as compared to the mock and GFP groups as reflected by the degree of splenomegaly (Figure 3c), this measure of anemia resolution was more complete in the V5m3 group (P = 0.015).
Figure 2.
High-level expression of HbF in the red blood cells (RBCs) of mice transplanted with γ-globin vector-transduced sickle hematopoietic stem cells (HSCs). (a) Cellulose acetate hemoglobin (Hb) electrophoresis gels were used to separate the different Hb species in RBC lysates from mice transplanted with sickle cell HSCs transduced with the V5 vector (top) or with the V5m3 vector (bottom). The migration of the Hb standards (mixture of HbS, HbF, and HbA) is indicated at left of each panel. B6 = C57Bl/6, SS = sickle. (b) HbF levels, as a % of total Hb, in the individual transplanted mice 4–5 months post-transplant. Mean values for each group are indicated by the horizontal lines. There was no statistically significant difference between the two groups (P = 0.36). HPLC, high-performance liquid chromatography.
Figure 3.
Correction of anemia and compensatory splenic extramedullary hematopoiesis in mice transplanted with γ-globin lentiviral vector-transduced cells. (a) Hemoglobin (Hb) levels of individual mice transplanted with mock-transduced cells or cells transduced with the indicated vectors. The mean Hb level in V5m3 mice was higher than that of V5 mice (P = 0.0032), as well as those of the green fluorescent protein (GFP) and mock groups (P < 0.0001). The mean Hb level of the V5 mice was also statistically higher than those of the GFP and mock groups (P < 0.0001). Data were obtained 4–5 months post-transplantation. (b) red blood cell (RBC) counts of mice transplanted with the indicated mock- or vector-transduced cells. The V5m3 mean RBC count was higher than that of the V5 group (P = 0.05). Both the V5 and V5m3 had mean RBC counts were higher than the mock and GFP groups (P < 0.0001). Data were obtained 4–5 months post-transplantation. (c) Spleen weights of the indicated groups of transplanted mice. Mean values for each group are represented by the horizontal line and the P value for the comparison between V5 and V5m3 is indicated. Mean values for V5 and V5m3 were significantly different from that of the mock group (P < 0.0001). Data were obtained 4–5 months post-transplantation. (d) Wright-Giemsa-stained peripheral blood smears from representative mice transplanted with mock-transduced or γ-globin vector-transduced cells as indicated. Data were obtained 4–5 months post-transplantation.
The presence of sickled cells was greatly reduced on blood smears from the V5 group, while they were rare or absent on smears of the V5m3 animals (Figure 3d). In contrast, sickled cells were abundant on blood smears from the mock-transplanted group (Figure 3d). When the increase in Hb in individual mice, relative to GFP control mice, was normalized to the mean VCN in the BM of each animal, the V5 group displayed a average increase of 2.6 ± 0.3 g/dl/copy, while the V5m3 animals showed a 4.1 ± 1.0 g/dl/copy rise. These values exceed the 1.9–2.1 g/dl/copy increases previously reported with mutant anti-sickling β-globin lentiviral vectors.28,29
We studied several individual mice from both globin vector groups that had VCN ranging from 0.3 to 0.6, a level of transduced HSCs with direct clinical relevance. Significantly, considerable phenotypic improvement was observed in both hematologic parameters (Hb and RBC counts) and renal function in these mice (Table 2). These results suggest that a 30% level of γ-globin vector-transduced HSCs might have a major effect in SCD.
Table 2.
γ-Globin expression and disease improvement in low copy number animals
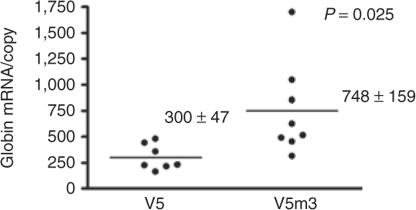
To evaluate whether the β-globin 3′-UTR provided an enhanced level of γ-globin transgene mRNA relative to that of the transgene containing the γ-globin 3′-UTR, we used quantitative reverse transcription, real-time PCR (qRT-PCR) to measure globin transgene mRNA in PB of V5 and V5m3 animals. As shown in Figure 4, the V5m3 vector containing the β-globin 3′-UTR produced more mRNA per vector copy than the V5 vector, which contains the γ-globin 3′-UTR.
Figure 4.
Replacement of the γ-globin 3′-untranslated region with its β-globin counterpart in the γ-globin lentiviral vector leads to increased levels of transgene mRNA in peripheral blood reticulocytes. Relative levels of γ-globin mRNA, normalized to bone marrow vector copy, as determined by quantitative reverse transcription–PCR in individual transplanted mice of the indicated groups. Mean ± SEM and the P value are shown. Data were obtained 4–5 months post-transplantation.
High-level HbF expression protects against the secondary organ damage of SCD
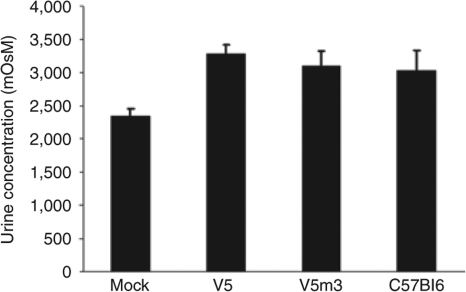
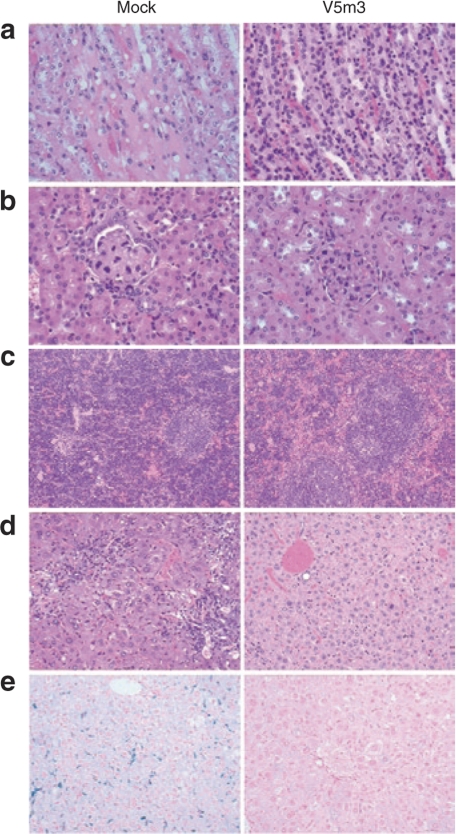
Since many SCD patients develop a significant nephropathy, sometimes leading to chronic renal failure,39 we investigated whether γ-globin gene therapy preserved renal tubular function in the transplanted mice. Both SCD patients and SCD mice have a defect in urine concentrating ability, we therefore measured the urine osmolality after water deprivation of the different groups of transplanted mice. The V5 and V5m3 groups showed normal urine concentrating ability, similar to that of wild-type C57Bl/6 mice, while a failure to concentrate urine was observed in the group transplanted with mock-transduced cells (Figure 5). Consistent with this functional data, the mock group showed significantly pathology throughout the kidney, while the kidneys in the V5 and V5m3 groups showed no visible pathology. The mock group of animals displayed a highly attenuated and sometimes sloughed renal tubular epithelium with intracytoplasmic deposition of hemosiderin (Figure 6a). Prussian blue staining confirmed that cells were heavily laden with iron. In both the cortex and medulla, residual tubules often contained proteinaceous casts. The mock group also demonstrated severe glomerular pathology evidenced by glomerular capillary segmental wall thickening with dilation and adhesion to Bowman's capsule. Marked glomerular vascular congestion and multifocal glomerular obsolescence was also observed in the mock group (Figure 6b). Renal glomeruli were normal by morphologic examination in recipients that received γ-globin vector-transduced cells.
Figure 5.
Normalization of renal tubular urine concentrating ability in mice transplanted with γ-globin lentiviral vector-transduced cells. Urine osmolality is shown for each of the transplanted groups (at least seven mice in each group) as well as for wild-type C57Bl/6 mice (n = 5). There was a statistically significant difference between the mock group and all three of the other groups (P < 0.001). Data were obtained 4–5 months post-transplantation.
Figure 6.
Correction of splenic, renal, and hepatic sickle cell-related pathologic abnormalities in mice receiving transplants of γ-globin vector-transduced hematopoietic stem cells. (a) Representative hematoxylin and eosin (H&E)-stained sections of renal tubular epithelium (×400) from mock and V5m3mice. (b) Examples of renal glomeruli from mock and V5m3 mice (×400). (c) Representative spleen sections from mock and V5m3 mice stained with H&E at ×100 magnification. (d) Representative sections of liver from mock and V5m3 mice (×400). (e) Liver sections from the indicated mice stained for iron content with Prussian blue (×200).
Spleens of untreated mice were markedly expanded by extramedullary erythropoiesis with effacement of lymphoid tissue (Figure 6c). In contrast, both sets of gene therapy mice displayed normal splenic architecture, with only mildly increased erythropoiesis observed in the V5 group. The livers of mock animals showed sinusoids filled by Kupffer cells and macrophages impregnated with hemosiderin. Examples of acute and chronic damage from thrombotic events, with necrosis and fibrosis was readily observed in the mock group while absent in the globin vector groups. The mock group also showed lymphocytic and histiocytic infiltration with prominent siderosis (Figure 6d). Prussian blue staining for intracellular iron showed marked hepatic iron overload in these animals, while the V5 and V5m3 groups showed no increase in liver iron content (Figure 6e). Finally, the lungs of mock-treated animals were characterized by an interstitium that was typically congested, with thickening of alveolar walls by erythrocytes, leukocytes, and occasionally fibrin thrombi. Interstitial fibrosis was also occasionally identified. Throughout the lung parenchyma there was an increase of perivascular lymphocytes and plasma cells. Recipients of γ-globin vector-transduced cells displayed little or no pulmonary pathology (data not shown).
Persistence of γ-globin-mediated phenotypic correction in secondary transplanted animals
At the time of euthanasia, 4–5 months after transplantation, BM cells from six primary V5m3 mice were transplanted into three to five secondary C57Bl/6 recipients to establish that HSCs were transduced and whether high-level γ-globin expression persisted. Five months after secondary transplantation, high levels of HbF expression were observed in recipient mice, at levels similar in most cases to that of the primary donor (Supplementary Figure S2a). Similarly, secondary mice displayed sustained correction of anemia, compared to secondary recipients transplanted with BM from the primary mock group (Supplementary Figure S2b).
Discussion
HSC transfer and permanent high-level erythroid expression of a γ-globin gene is an attractive approach for gene therapy of SCD.11,40 This is the first report to establish that this approach can successfully treat an exceptionally severe murine model of SCD.30 Although it is not known what degree of myeloablative conditioning in humans will be needed to obtain sufficient engraftment of genetically modified HSCs for a therapeutic effect, a subablative regimen would be preferable since many SCD patients have pre-existing multiorgan damage. One advantage of using γ-globin, rather than a mutant β-globin molecule, is that additional chemotherapy or irradiation to achieve immunoablation would not be required since γ-globin is endogenously expressed in SCD patients. Numerous examples of self immune responses to cells expressing novel, mutated endogenous antigens have been previously documented,41,42,43 raising the possibility that expression of an altered version of β-globin in developing BM erythroid precursors could be problematic. The potential need for immunoablation in the conditioning regimen with this approach would add significant risk to the gene therapy procedure for patients with SCD.
We observed an increase in Hb per vector copy of 2.6 g/dl with the V5 vector, similar to what we found in a previous gene therapy study involving β-thalassemia mice.27 The V5m3 vector, containing the β-globin 3′-UTR in lieu of its γ-globin counterpart, produced a more robust improvement in Hb, with a rise of 4.1 g/dl per vector copy. These values exceed those observed by others (1.9–2.1 g/dl/copy) using a mutant β-globin vector in the same or similar SCD mouse models.28,29 Mice receiving transplants of cells transduced with the V5m3 vector, relative to the V5 vector, had a significantly better correction of their anemia as evidenced by their higher Hb and RBC counts. Extramedullary hematopoiesis, as judged by spleen size and pathology, was also more completely resolved in the V5m3 group than the V5 group. This improved performance of the V5m3 vector correlated with the higher levels of γ-globin mRNA per vector copy, relative to that observed in animals transplanted with V5-transduced cells. Recent studies indicate that, in addition to the normal developmental transcriptional down-regulation of γ-globin, there is also a post-transcriptional silencing mechanism affecting γ-globin mRNA stability in adult erythroid cells.36 Russell has proposed a model based on data in transgenic mice wherein limiting quantities of α-CP bind preferentially to the 3′-UTR of “adult” globin mRNAs, leaving stage inappropriate globin mRNAs such as γ-globin destabilized. Consistent with this model, others previously showed that accumulation of the native γ-globin transcript occurs most optimally in the absence of or with reduced β-globin expression.35 Our data are also in line with these results as we observed that removal of the γ-globin 3′-UTR from the V5 vector and replacement with its β-globin counterpart led to a significant 2.5-fold increase in the relative amount of steady-state, γ-globin mRNA per vector copy. It appears that the native γ-globin transcript, which does not contain the unique β-globin 3′-UTR stem–loop motif that mediates the specific protein binding necessary for β-globin transcript stability,34 is not optimal for high-level γ-globin protein expression in the adult erythroid environment. In addition to potentially enhancing γ-globin transgene mRNA stability, it is also possible that the 545 bp of β-globin 3′ sequences that extend beyond the 3′-UTR and are included in the V5m3 vector might play a role in enhancing transgene mRNA levels. For instance, these sequences might enhance pre-mRNA processing, including polyadenylation. Further studies may allow delineation of the mechanism(s) that facilitate higher globin mRNA production by the V5m3 vector.
Despite the higher levels of γ-globin transgene mRNA in the PB of V5m3 mice compared to V5 mice, which correlated with a more complete correction of all aspects of the SCD phenotype that we measured, the differences in the mean and variance of HbF level of the two groups did not achieve statistical significance. This is most likely due to the limited number of animals analyzed. However, we cannot rule out that there is a steep response curve, as a function of HbF level, for achieving increasingly more complete phenotypic correction in this model.
Transplantation of BM from individual V5m3 primary recipients into secondary recipients showed continued, high levels of γ-globin expression, similar to those observed in the primary donors. This not only suggests effective HSC gene transfer but also is consistent with a lack of significant vector silencing. Despite this, we did observe evidence of position effect variegation of vector transgene expression. The correlation between BM VCN and the amount of HbF was not copy number dependent (r2 = 0.37 and data not shown). In some mice, lower VCN gave equivalent HbF levels as those observed in some mice with higher VCN (data not shown). These results are consistent with our previous work and that of others highlighting the impact of position effect on globin lentiviral vector expression.25,28,44 This reflects the variability of expression of an integrated transgene at ectopic genomic locations that do not reproduce the transcriptional environment of the native locus. A recent study indicates that detrimental position effects can be reduced by use of the 1.2-kb insulator element from the chicken β-globin locus.45 However, inclusion of the 1.2-kb fragment in lentiviral vectors, particularly globin vectors, can significantly reduce titer46 (P.W.H., H.H. Hanawa, and D.A.P., unpublished results). One potential solution would be to utilize a 400-bp subfragment that seems to retain significant protection against position effects.46 Further studies will be required to identify the optimal insulator element which retains functional activity while having minimal impact on globin vector titer.
In addition to their sickle cell phenotype, the BERK mouse model used in these studies exhibits a mild β-thalassemic phenotype, with an α/β-globin chain ratio of 0.79–0.82.21,30 The relative deficiency of βS chains in this model closely represents the situation of humans with sickle/β0-thalassemia. Given our data and previous studies showing that SCD transgenic mice with a relative deficiency of βS chains have a reduction in the HbF level therapeutic threshold,21 patients with sickle/β0-thalassemia may be an ideal population for initial γ-globin gene therapy clinical trials. Similarly, incorporation of an short hairpin RNA targeting βS-globin into the γ-globin vector, as proposed by Sadelain and colleagues, may be beneficial to improve the therapeutic efficacy for SCD patients with balanced globin chain synthesis.47
Therapeutic protein production was obtained in the majority of the mice in this study with an average VCN ranging from 0.3 to 1.8 copies, less than in previous studies.28,29 One of the goals in HSC-targeted gene therapy is to obtain functional cell correction with the lowest number of vector copies per cell since the risk of genotoxicity likely increases with increasing copy number.48 Although this work was not a safety study, we observed no evidence of myelodysplasia or leukemia in the different groups of primary and secondary transplant recipients. This is consistent with our previous work that showed that although globin vector integrations can lead to cellular gene dysregulation in erythroid cells, even at great distances, no functional consequences in terms of clonal dominance or leukemia occurred.49 Using a primate autologous transplant model, we have recently found that lentiviral vectors have a more favorable vector insertion profile than that associated with γ-retroviral vectors (D.A.P. and C.E. Dunbar, manuscript submitted). We therefore believe that use of a γ-globin lentiviral vector for gene therapy of human SCD will have an acceptable benefit-to-risk ratio. With continued focused effort, successful gene therapy for SCD may become a reality in the near future.
Materials and Methods
Lentiviral vector design and production. The γ-globin lentiviral vector termed V5 (previously termed mLARV5) and the control GFP vector were previously described.27 To construct the V5m3 plasmid, plasmid pMD-G a gift from Dr. Richard Mulligan (Harvard, Boston, MA) was used as a template to PCR amplify the last 36 bp of the γ-globin open reading frame (provided in the 5′ oligo PCR primer, which contains a BtsI site) fused with 680 bp of DNA sequence immediately downstream of the human β-globin stop codon, which contains the 135 bp β-globin 3′-UTR. This fragment was then used to replace the 471-bp BtsI/SbfI fragment of plasmid V5 which contains the 87 bp γ-globin 3′-UTR and 384 bp of additional 3′ downstream γ-globin genomic sequences. Vesicular stomatitis virus glycoprotein pseudotyped lentiviral vector particles were prepared and titered by Southern blot analysis for genome transfer as previously described.27
Mice. BERK SCD mice were originally obtained from Dr. Mohandas Narla (then at the Lawrence Berkeley National Laboratory, Berkeley, CA). They express exclusively human α- and sickle β-globin and were originally bred by selective mating and exist on a mixed genetic background (strains FVB/N, 129, DBA/2, C57BL/6, and Black Swiss), with histocompatibility with C57Bl/6 mice. Exclusive expression of HbS in sickle mice was confirmed by differential hemoglobin electrophoresis using the Helena Titan III system (Helena Laboratories, Beaumont, TX). All experimental animal protocols were approved by the St. Jude Children's Research Hospital Institutional Animal Care and Use Committee.
Isolation, transduction, and transplantation of BM cells. BM cells were isolated from 2- to 5-month-old SCD mice. Depletion of lineage positive cells from pooled whole BM preparations was performed using the Lineage Cell Depletion Kit (cat. no. 130-090-858; Miltenyi Biotec, Auburn, CA) and the autoMACS separation system (Miltenyi Biotec, Auburn, CA). Lineage negative (Lin−) cell purities were always >80%. Cells at a concentration 1 × 106/ml were cultured for 24 hours in StemSpan serum-free medium (StemCell Technologies, Vancouver, British Columbia, Canada) supplemented with 10 µg/ml heparin (Sigma-Aldrich, St. Louis, MO), 50 U/ml penicillin, 50 µg/ml streptomycin, 2 mmol/l glutamine and cytokines: 10 ng/ml mouse stem cell factor, 50 ng/ml mouse thrombopoietin, 20 ng/ml mouse Insulin-like growth factor 2 (all from Peprotech, Rocky Hill, NJ) and 10 ng/ml human fibroblast growth factor 1 (R&D Systems, Minneapolis, MN). For transduction, cells were transferred to RetroNectin-coated 24-well plates (Takara, Shiga, Japan) at a concentration of 7× 106/ml to 11 × 106/ml in the above media and incubated overnight with viral vector particles at a multiplicity of infection of 25–55 for the γ-globin vectors and of 7–11 for the GFP control vector. A second viral vector exposure was performed the following day for an additional 6–8 hours. Cells were subsequently harvested and 0.7–1 × 106 cells were injected by tail-vein into C57Bl/6 (Jackson Laboratories, Bar Harbor, ME) recipients after 1125 cGy of total body irradiation given as a single dose.
Hematologic and HbF protein analysis. Complete blood and reticulocyte counts were determined using an automated blood cell analyzer and fluorescence-activated cell sorting analysis as previously described.25 Hb cellulose acetate gel electrophoresis and fluorescence-activated cell sorting analysis of red cells for expression of human γ-globin were also performed as previously described.25 Hb standards for cellulose acetate electrophoresis were purchased from Helena Laboratories (Beaumont, TX). Fractionation and quantitation of HbS and HbF was performed by high-performance liquid chromatography using a cation-exchange column (Ultra2 Variant Resolution Analyzer; Primus Diagnostics, Kansas City, MO).
Pathology analysis. At necropsy, mice were humanely euthanized and organ weights were obtained on heart, liver, spleen, and kidneys of all animals. Tissues collected for microscopic evaluation were fixed overnight in 10% neutral buffered formalin. Following fixation, tissues were processed routinely, embedded in paraffin, sectioned at 4 µm and stained with hematoxylin and eosin. Histochemical staining by Prussian blue to evaluate iron deposition and Trichrome to evaluate fibrosis was also performed. Pathology analysis was performed by an experienced veterinary pathologist (K.M.B.).
Determination of BM VCN by Southern blot analysis. BM DNA samples were digested with BglII, which cuts at the ends of the provirus and liberates a near unit length provirus. A radiolabeled HIV-1 rev responsive element DNA probe was hybridized with the blot and the signal intensity of the hybridizing band for each DNA sample was compared to that of the DNA from a K562 clone harboring a single vector copy using a Molecular Dynamic Storm 860 Phosphorimager (Molecular Dynamics, Sunnyvale, CA) and its accompanying software. Equivalent loading of lanes was confirmed by ethidium bromide staining of gels before DNA transfer to the nylon filters.
Spleen colony-forming unit assay. Transduced Lin– cells (5× 103 to 8 × 103) were transplanted into normal C57Bl/6 mice conditioned with 950 cGy. Thirteen days following transplantation, mice were killed and well-separated, discrete splenic colonies were carefully dissected and genomic DNA prepared.
Urine osmolality determination. Following a 16-hour food and water deprivation period, urine was collected from transplanted mice and C57Bl/6 controls. Osmolality was determined using a freezing point depression osmometer (model 3300; Advanced Instruments, Norwood, MA). Samples were diluted tenfold and results corrected for the dilution factor.
Measurement of γ-globin transcript levels using qRT-PCR. qRT-PCR measurements of γ-globin and α-globin mRNA transcript levels were performed in triplicate for each sample using primer/probe set combinations (γ-globin: Hs00361131_g1; α-globin: Hs00361191_g1) obtained from the Assay-on-Demand catalog from Applied Biosystems (Foster City, CA), according to the manufacturer's recommendations. Reactions were performed with the ABI StepOnePlus Real-Time PCR System (Foster City, CA). Its accompanying software was used to calculate relative γ-globin mRNA expression levels by the ΔΔCT method, using α-globin mRNA for normalization. The variability in the α-globin level from mouse to mouse was <1%, as was the case for each set of replicate samples.
Statistical analysis. Student's two-tailed t-test and F-test were used to determine statistically significant differences between mean values and variances of different data sets using GraphPad Prism Software (GraphPad, San Diego, CA).
Supplementary MaterialFigure S1. Pancellular expression of γ-globin in the RBCs of representative mice transplanted with sickle HSCs transduced with the indicated γ-globin lentiviral vectors.Figure S2. Sustained, high level expression of HbF and amelioration of anemia after secondary transplantation.
Supplementary Material
Pancellular expression of γ-globin in the RBCs of representative mice transplanted with sickle HSCs transduced with the indicated γ-globin lentiviral vectors.
Sustained, high level expression of HbF and amelioration of anemia after secondary transplantation.
Acknowledgments
This investigation was supported in part by the National Heart, Lung, and Blood Institute (NHLBI) Comprehensive Sickle Cell Center Grant U54 HL070590, NHLBI Program Project PO1HL053749, NHLBI Basic and Translational Research Program for Sickle Cell Disease Grant U54 HL070590-06, St. Jude Children's Research Hospital (SJCRH) Cancer Center Support (Core) Grant CA-21765, and the American Lebanese Syrian Associated Charities. We thank Brian Sorrentino and Russell Ware (SJCRH) for critical reading of the manuscript and useful discussion. Special appreciation goes to Arthur Nienhuis (SJCRH) for ongoing input into the project and for critical reading of the manuscript. We also thank the Flow Cytometry Core Facility at SJCRH and the staff of the Animal Resource Center of SJCRH for animal care and experimental analysis. The authors have no conflicts of interest and have nothing to disclose.
REFERENCES
- Dover GT, Platt OS, Nathan DE, Orkin SH, Look AT., and , Ginsberg D. Nathan DG., and , Orkin SH. Nathan and Oski's Hematology of Infancy and Childhood. 6th edn. WB Saunders: Philadelphia, PA; 2004. Sickle cell disease; pp. 790–841. [Google Scholar]
- Madigan C., and , Malik P. Pathophysiology and therapy for haemoglobinopathies. Part I: sickle cell disease. Expert Rev Mol Med. 2006;8:1–23. doi: 10.1017/S1462399406010659. [DOI] [PubMed] [Google Scholar]
- Adams RJ, Ohene-Frempong K., and , Wang W. Sickle cell and the brain. Hematology Am Soc Hematol Educ Program. 2001. pp. 31–46. [DOI] [PubMed]
- Steen RG, Miles MA, Helton KJ, Strawn S, Wang W, Xiong X, et al. Cognitive impairment in children with hemoglobin SS sickle cell disease: relationship to MR imaging findings and hematocrit. AJNR Am J Neuroradiol. 2003;24:382–389. [PMC free article] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Wierenga KJ, Hambleton IR., and , Lewis NA. Survival estimates for patients with homozygous sickle-cell disease in Jamaica: a clinic-based population study. Lancet. 2001;357:680–683. doi: 10.1016/s0140-6736(00)04132-5. [DOI] [PubMed] [Google Scholar]
- Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- Hankins JS, Ware RE, Rogers ZR, Wynn LW, Lane PA, Scott JP, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: the HUSOFT extension study. Blood. 2005;106:2269–2275. doi: 10.1182/blood-2004-12-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- Walters MC. Stem cell therapy for sickle cell disease: transplantation and gene therapy. Hematology Am Soc Hematol Educ Program. 2005. pp. 66–73. [DOI] [PubMed]
- Persons DA., and , Tisdale JF. Gene therapy for the hemoglobin disorders. Semin Hematol. 2004;41:279–286. doi: 10.1053/j.seminhematol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Pauling L, Itano HA, Singer SJ., and , Wells IC. Sickle cell anemia, a molecular disease. Science. 1949;110:543. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- Perrine RP, Pembrey ME, John P, Perrine S., and , Shoup F. Natural history of sickle cell anemia in Saudi Arabs. A study of 270 subjects. Ann Intern Med. 1978;88:1–6. doi: 10.7326/0003-4819-88-1-1. [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos G, Wood WG, Papayannopoulou T., and , Nute PE. A new form of hereditary persistence of fetal hemoglobin in blacks and its association with sickle cell trait. Blood. 1975;46:683–692. [PubMed] [Google Scholar]
- Serjeant GR, Serjeant BE., and , Mason K. Heterocellular hereditary persistence of fetal haemoglobin and homozygous sickle-cell disease. Lancet. 1977;1:795–796. doi: 10.1016/s0140-6736(77)92976-2. [DOI] [PubMed] [Google Scholar]
- Powars DR, Weiss JN, Chan LS., and , Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia. Blood. 1984;63:921–926. [PubMed] [Google Scholar]
- Atweh GF, Sutton M, Nassif I, Boosalis V, Dover GJ, Wallenstein S, et al. Sustained induction of fetal hemoglobin by pulse butyrate therapy in sickle cell disease. Blood. 1999;93:1790–1797. [PMC free article] [PubMed] [Google Scholar]
- Hankins JS, Helton KJ, McCarville MB, Li CS, Wang WC., and , Ware RE. Preservation of spleen and brain function in children with sickle cell anemia treated with hydroxyurea. Pediatr Blood Cancer. 2008;50:293–297. doi: 10.1002/pbc.21271. [DOI] [PubMed] [Google Scholar]
- Goldberg MA, Husson MA., and , Bunn HF. Participation of hemoglobins A and F in polymerization of sickle hemoglobin. J Biol Chem. 1977;252:3414–3421. [PubMed] [Google Scholar]
- Blouin MJ, Beauchemin H, Wright A, De Paepe M, Sorette M, Bleau AM, et al. Genetic correction of sickle cell disease: insights using transgenic mouse models. Nat Med. 2000;6:177–182. doi: 10.1038/72279. [DOI] [PubMed] [Google Scholar]
- Fabry ME, Suzuka SM, Weinberg RS, Lawrence C, Factor SM, Gilman JG, et al. Second generation knockout sickle mice: the effect of HbF. Blood. 2001;97:410–418. doi: 10.1182/blood.v97.2.410. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L, et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406:82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- Imren S, Payen E, Westerman KA, Pawliuk R, Fabry ME, Eaves CJ, et al. Permanent and panerythroid correction of murine beta thalassemia by multiple lentiviral integration in hematopoietic stem cells. Proc Natl Acad Sci USA. 2002;99:14380–14385. doi: 10.1073/pnas.212507099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons DA, Hargrove PW, Allay ER, Hanawa H., and , Nienhuis AW. The degree of phenotypic correction of murine beta -thalassemia intermedia following lentiviral-mediated transfer of a human gamma-globin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003;101:2175–2183. doi: 10.1182/blood-2002-07-2211. [DOI] [PubMed] [Google Scholar]
- Malik P, Arumugam PI, Yee JK., and , Puthenveetil G. Successful correction of the human Cooley's anemia beta-thalassemia major phenotype using a lentiviral vector flanked by the chicken hypersensitive site 4 chromatin insulator. Ann NY Acad Sci. 2005;1054:238–249. doi: 10.1196/annals.1345.030. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Hargrove PW, Kepes S, Srivastava DK, Nienhuis AW., and , Persons DA. Extended beta-globin locus control region elements promote consistent therapeutic expression of a gamma-globin lentiviral vector in murine beta-thalassemia. Blood. 2004;104:2281–2290. doi: 10.1182/blood-2004-03-0863. [DOI] [PubMed] [Google Scholar]
- Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- Levasseur DN, Ryan TM, Pawlik KM., and , Townes TM. Correction of a mouse model of sickle cell disease: lentiviral/antisickling beta-globin gene transduction of unmobilized, purified hematopoietic stem cells. Blood. 2003;102:4312–4319. doi: 10.1182/blood-2003-04-1251. [DOI] [PubMed] [Google Scholar]
- Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA., and , Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood. 2006;107:1651–1658. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone R, Casella JF, Fuchs EJ, Chen AR, Jones RJ, Woolfrey A, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant. 2003;9:519–528. doi: 10.1016/s1083-8791(03)00192-7. [DOI] [PubMed] [Google Scholar]
- Yu J., and , Russell JE. Structural and functional analysis of an mRNP complex that mediates the high stability of human beta-globin mRNA. Mol Cell Biol. 2001;21:5879–5888. doi: 10.1128/MCB.21.17.5879-5888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Xu XS., and , Russell JE. A nucleolin-binding 3′ untranslated region element stabilizes beta-globin mRNA in vivo. Mol Cell Biol. 2006;26:2419–2429. doi: 10.1128/MCB.26.6.2419-2429.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakalova L, Osborne CS, Dai YF, Goyenechea B, Metaxotou-Mavromati A, Kattamis A, et al. The Corfu deltabeta thalassemia deletion disrupts gamma-globin gene silencing and reveals post-transcriptional regulation of HbF expression. Blood. 2005;105:2154–2160. doi: 10.1182/blood-2003-11-4069. [DOI] [PubMed] [Google Scholar]
- Russell JE. A post-transcriptional process contributes to efficient gamma-globin gene silencing in definitive erythroid cells. Eur J Haematol. 2007;79:516–525. doi: 10.1111/j.1600-0609.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- Russell JE, Morales J, Makeyev AV., and , Liebhaber SA. Sequence divergence in the 3′ untranslated regions of human zeta- and alpha-globin mRNAs mediates a difference in their stabilities and contributes to efficient alpha-to-zeta gene development switching. Mol Cell Biol. 1998;18:2173–2183. doi: 10.1128/mcb.18.4.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chkheidze AN, Lyakhov DL, Makeyev AV, Morales J, Kong J., and , Liebhaber SA. Assembly of the alpha-globin mRNA stability complex reflects binary interaction between the pyrimidine-rich 3′ untranslated region determinant and poly(C) binding protein alphaCP. Mol Cell Biol. 1999;19:4572–4581. doi: 10.1128/mcb.19.7.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinman JI. Sickle cell disease and the kidney. Semin Nephrol. 2003;23:66–76. doi: 10.1053/snep.2003.50006. [DOI] [PubMed] [Google Scholar]
- Sabatino DE, Seidel NE, Aviles-Mendoza GJ, Cline AP, Anderson SM, Gallagher PG, et al. Long-term expression of gamma-globin mRNA in mouse erythrocytes from retrovirus vectors containing the human gamma-globin gene fused to the ankyrin-1 promoter. Proc Natl Acad Sci USA. 2000;97:13294–13299. doi: 10.1073/pnas.230453097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, El-Gamil M, Li YF, Kawakami Y, Loftus D, Appella E, et al. A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J Exp Med. 1996;183:1185–1192. doi: 10.1084/jem.183.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiari R, Foury F, De Plaen E, Baurain JF, Thonnard J., and , Coulie PG. Two antigens recognized by autologous cytolytic T lymphocytes on a melanoma result from a single point mutation in an essential housekeeping gene. Cancer Res. 1999;59:5785–5792. [PubMed] [Google Scholar]
- Lennerz V, Fatho M, Gentilini C, Frye RA, Lifke A, Ferel D, et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci USA. 2005;102:16013–16018. doi: 10.1073/pnas.0500090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivella S, May C, Chadburn A, Riviere I., and , Sadelain M. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta-globin gene transfer. Blood. 2003;101:2932–2939. doi: 10.1182/blood-2002-10-3305. [DOI] [PubMed] [Google Scholar]
- Arumugam PI, Scholes J, Perelman N, Xia P, Yee JK., and , Malik P. Improved human beta-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator element. Mol Ther. 2007;15:1863–1871. doi: 10.1038/sj.mt.6300259. [DOI] [PubMed] [Google Scholar]
- Aker M, Tubb J, Groth AC, Bukovsky AA, Bell AC, Felsenfeld G, et al. Extended core sequences from the cHS4 insulator are necessary for protecting retroviral vectors from silencing position effects. Hum Gene Ther. 2007;18:333–343. doi: 10.1089/hum.2007.021. [DOI] [PubMed] [Google Scholar]
- Samakoglu S, Lisowski L, Budak-Alpdogan T, Usachenko Y, Acuto S, Di Marzo R, et al. A genetic strategy to treat sickle cell anemia by coregulating globin transgene expression and RNA interference. Nat Biotechnol. 2006;24:89–94. doi: 10.1038/nbt1176. [DOI] [PubMed] [Google Scholar]
- Fehse B, Kustikova OS, Bubenheim M., and , Baum C. Pois(s)on—it's a question of dose. Gene Ther. 2004;11:879–881. doi: 10.1038/sj.gt.3302270. [DOI] [PubMed] [Google Scholar]
- Hargrove PW, Kepes S, Hanawa H, Obenauer JC, Pei D, Cheng C, et al. Globin lentiviral vector insertions can perturb the expression of endogenous genes in beta-thalassemic hematopoietic cells. Mol Ther. 2008;16:525–533. doi: 10.1038/sj.mt.6300394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pancellular expression of γ-globin in the RBCs of representative mice transplanted with sickle HSCs transduced with the indicated γ-globin lentiviral vectors.
Sustained, high level expression of HbF and amelioration of anemia after secondary transplantation.