Abstract
Interferon (IFN) is effective at inducing complete remissions in patients with chronic myelogenous leukemia (CML), and evidence supports an immune mechanism. Here we show that the type I IFNs (alpha and beta) regulate expression of the IFN consensus sequence-binding protein (ICSBP) in BCR-ABL–transformed cells and as shown previously for ICSBP, induce a vaccine-like immunoprotective effect in a murine model of BCR-ABL–induced leukemia. We identify the chemokines CCL6 and CCL9 as genes prominently induced by the type I IFNs and ICSBP, and demonstrate that these immunomodulators are required for the immunoprotective effect of ICSBP expression. Insights into the role of these chemokines in the antileukemic response of IFNs suggest new strategies for immunotherapy of CML.
Introduction
Chronic myelogenous leukemia (CML) results from a chromosomal translocation in a hematopoietic stem cell that generates the BCR-ABL oncogene. CML initially manifests as a chronic myeloproliferative disease with anemia, extreme granulocytosis, basophilia, splenomegaly, and often thrombocytosis. If left untreated, the disease progresses to acute leukemia (“blast crisis”) and a fatal outcome. Although CML accounts for less than one-fifth of all cases of leukemia, it has frequently served as a paradigm in oncology, both for understanding the molecular basis of cancer and for developing novel approaches to therapy. Several types of therapeutic modalities now commonly used to treat cancer such as bone marrow transplantation, interferons (IFNs), and molecularly targeted protein kinase inhibitors were first introduced as treatments for CML.
The BCR-ABL oncogene encodes the BCR-ABL fusion oncoprotein, a deregulated tyrosine kinase. BCR-ABL phosphorylates tyrosines in various substrates and activates multiple intracellular signaling pathways involving RAS, nuclear factor κB (NFKB), and phosphatidylinositol 3-kinase (PI3K).1–5 Despite the indisputable success of small molecule inhibitors of BCR-ABL in prolonging the survival of patients with CML, these agents are rarely curative and treated patients can relapse with drug-resistant disease. Thus, there is a recognized need for additional approaches to complement BCR-ABL inhibitors in the treatment of CML.
Biologic therapies are one alternative strategy with proven success in treating CML. IFN alpha, until recently considered first-line therapy for CML, induces long-term survival and complete cytogenetic responses that are maintained for many years, even off therapy, in a subset of patients. Although some argue that IFN alpha can be curative,6–8 most “disease-free” patients retain BCR-ABL–positive cells.9,10 Therefore, rather than eradicating the disease, IFN alpha appears to induce a long-term state of “tumor dormancy.”11 Alternatively, IFN alpha might reduce the number of CML clones below a certain threshold so that they have a low probability of regrowing. The immunomodulatory properties of IFN alpha play a pivotal role in its activity as an anticancer agent for CML,12 and there is a strong correlation between the presence of cytotoxic T lymphocytes (CTLs) specific for certain tumor antigens, such as proteinase 3, and clinical responses to IFN alpha.13,14 The downstream molecules through which IFN alpha induces its antileukemic effects and how these connect to pathways deregulated in CML remain poorly understood.
In addition to the central role of BCR-ABL in CML pathogenesis, there is substantial evidence that the IFN consensus sequence-binding protein (ICSBP; also known as IFN regulatory factor 8 [IRF8]) is a tumor suppressor in CML.15–20 Mice lacking ICSBP die from a malignancy that is remarkably similar to CML.15 Patients with CML have low levels of ICSBP in BCR-ABL–expressing cells, and successful CML therapy is associated with a restoration of ICSBP levels.21 Lastly, the expression of ICSBP in BCR-ABL–transformed cells abolishes their capacity to cause leukemia in a syngeneic mouse leukemia model by inducing a long-lasting, T cell–mediated response against antigens preferentially expressed on BCR-ABL–transformed cells.17
Much is known about the normal physiologic role of ICSBP in controlling macrophage and dendritic cell differentiation.22–25 However, how ICSBP is regulated in CML cells and the downstream pathways through which it induces an antileukemic response remain unclear. Here, we show that INF alpha up-regulates ICSBP expression in BCR-ABL–transformed cells and can substitute for ICSBP overexpression in protecting mice from leukemia. We show that ICSBP can act in a non–cell-autonomous fashion by up-regulating the expression of the chemokines CCL6 and CCL9, which we demonstrate are key mediators of the antileukemic effects of ICSBP.
Methods
Mice and analysis of murine leukemia
Female Balb/c- and Rag 2–deficient mice (6-9 weeks old; The Jackson Laboratory, Bar Harbor, ME) were injected intravenously with 105 to 106 parental BaF3 cells or BaF3 cells expressing different genes (see “Cell lines, plasmids, and retroviral and lentiviral constructs”). Mice were closely monitored and killed if moribund and peripheral blood was harvested for both complete blood count (CBC) and flow cytometric analysis before being killed. Peripheral blood smears were prepared and stained with eosin solution azure A and methylene blue. Spleen size and weight was determined in mice found dead. Some spleens harvested from killed animals were used for RNA and DNA isolation and to obtain single-cell suspensions of splenocytes for flow cytometry analysis. Research with mice was approved by the institutional review board of Children's Hospital Boston.
Cell lines, plasmids, and retroviral and lentiviral constructs
BaF3 cells, a murine pro–B-cell line26 and the murine myeloid precursor line 32Dcl3 (hereafter referred to as 32D) were maintained in complete RPM 1640 supplemented with 10% conditioned medium from WEHI-3B cells, as a source of IL-3. The human CML cell lines K562, Lama 84, and AR 230 were maintained in complete RPMI medium. Peripheral blood mononuclear cells from healthy volunteers were purchased from AllCells (Emeryville, CA) and maintained in complete RPMI medium. Murine and human cells were treated with IFN alpha 2A or IFN beta (R&D Systems, Minneapolis, MN), as described in the figure legends.
BaF3 BCR-ABL cell lines were generated by infecting BaF3 cells with different retroviral constructs expressing BCR-ABL. Full-length BCR-ABL cDNA was inserted into the EcoRI site of the pEYK3.1 retroviral vector,27 generating pEYKBA. Similarly, the BCR-ABL cDNA was inserted into the EcoRI site of pBabe-puro and of MSCV-IRES-GFP retroviral vectors. Cells infected with these different constructs were selected with IL-3 independence, 2.5 μg/mL puromycin or sorting for green fluorescence protein (GFP)–high cells, respectively. BaF3 BCR-ABL mutants L248R and T315I were generated as previously described.27 32D cells expressing BCR-ABL were generated by infecting cells with VSVG-pseudotyped pBabe-puro BCR-ABL retrovirus. BaF3 cells expressing ICSBP, IFN alpha, or IFN beta were generated by infecting BaF3 cells with retroviral constructs expressing cDNAs for ICSBP, IFN alpha, or IFN beta. The cDNAs were obtained in our laboratory by reverse transcription–polymerase chain reaction (RT-PCR), verified by sequencing, and subcloned into the EcoRI sites of the pBabe-puro or MSCV-IRES-GFP retroviral vectors. Cells were then selected with 2.5 μg/mL puromycin or by sorting for GFP-high cells, respectively. BaF3 CCL6 and BaF3 CCL9 cell lines were generated by infecting BaF3 cells with retroviral supernatants prepared from pBabe-puro CCL6 or pBabe-puro CCL9 cDNAs for CCL6 and CCL9 were generated from Balb/c cDNA and subcloned into the EcoRI sites of pBabe-puro. Retroviral supernatants were produced as previously shown.28
We used both the Lentilox 3.7 and TRC pLKO.1 lentiviral short hairpin RNA (shRNA) vectors for RNA interference against CCL6, CCL9, ICSBP, luciferase, and LacZ genes. shRNAs for the Lentilox vector were designed as described (http://jura.wi.mit.edu/siRNAext/), while those in the TRC pLKO.1 vector were purchased (Sigma-Aldrich, St Louis, MO). The extents of gene knockdown for the chemokines were calculated by performing a semiquantitative PCR. PCRs were performed on serially diluted samples of RNA isolated from cells infected with control shRNAs or the shRNAs targeting the chemokines. After running the PCRs on agarose gels the intensity of the PCR bands stained with ethidium bromide was compared between the control and experimental samples to determine the level of knockdown. Serial dilutions were chosen so that the PCRs gave band intensities that correlated linearly with the amount of sample in each reaction. Figure 4 shows a representative image for PCRs performed on 1:8 dilutions of the samples.
Figure 4.
CCL6 and CCL9 are necessary for ICSBP-dependent protection against BCR-ABL–induced leukemia. (A) RT-PCR showing down-regulation by indicated lentiviral shRNAs of CCL6 and CCL9 expression in BaF3 cells that overexpress ICSBP and BCR-ABL. Two effective shRNAs were used for each chemokine; the control shRNA targeted luciferase. (B) Reduced expression of CCL6 or CCL9 prevents the antileukemic survival response normally mediated by ICSBP. Survival curves obtained after tail vein injection of 6- to 9-week-old Balb/c mice with: (left panel) 106 BaF3 cells expressing BCR-ABL alone (×) or together with ICSBP (△) (experiment was repeated 4 times and n = 20 to 30 mice per cell line); (middle panel) 106 BaF3 cells expressing ICSBP and BCR-ABL and a control shRNA (shControl, ◇) or either of the 2 shRNAs targeting CCL6 (♦) (experiments were repeated twice with n = 20 mice per condition); (right panel) 106 BaF3 cells expressing ICSBP and BCR-ABL and a control shRNA (shControl, △) or either of the 2 shRNAs targeting CCL9 (♦) (experiments were repeated twice with n = 20 mice per condition). (C) In panel B, transformed cells were marked with GFP. Percentages of GFP+ blast cells in peripheral blood or spleen suspensions from mice injected with the indicated cell types are shown. Experiment was repeated 2 to 3 times with n = 3 mice per condition. (D) Representative images and weights of spleens harvested from a Balb/c control mouse, and from mice injected with BaF3 cells expressing the indicated genes. (E) Representative images of smears of peripheral blood cells from a Balb/c control mouse, and from mice injected with BaF3 cells expressing the indicated genes.
Flow cytometric analysis, cell sorting, and cell blood counts
Mouse peripheral blood was collected by retro-orbital bleeding of the animals through heparin-coated capillary tubes. Peripheral blood counts were performed using the Hemavet 950 (Drew Scientific, Dallas, TX) instrument. Splenocytes were obtained by reducing mouse spleen to single-cell suspensions, using 40 μM filters. Growing BaF3 cells (0.5 × 106 log phase), native or expressing BCR-ABL, ICSBP or ICSBP, and BCR-ABL were also used for flow analysis. All cell types were preincubated with mouse anti-CD16/CD32 antibody to block nonspecific binding via Fc receptors (BD Pharmingen, San Diego, CA) for 30 minutes. Subsequently, the samples were washed and incubated with 0.5% of the following monoclonal antibodies: anti–CD3-allophycocyanin (APC) conjugated and anti–CD8-phycoerythrin (PE) conjugated, anti–CD4-PE conjugated, anti–CD56-PE or anti–IgM-APC conjugated and anti–CD19-PE conjugated or, finally, anti-D11b (Mac1)–APC conjugated and anti–CD86 (B7.2)–PE conjugated (all antibodies were from BD Pharmingen). Isotype controls were run each time. The samples were analyzed using a FACSCalibur cytometer and FlowJo software.
BaF3 cells infected with MSCV-ICSBP-Ires-GFP or MSCV-BCR-ABL-Ires-GFP retroviruses or BaF3 cells infected with lentilox-3.7 GFP for the expression of hairpins against CCL6, CCL9, and Lac Z were filtered through a 70 μm filter and sorted for GFP high on a MoFlo instrument (Dako, Carpinteria, CA) at the Massachusetts Institute of Technology (MIT) Cancer Center and at the Dana-Farber Cancer Institute Sorting Facilities.
Qualitative and quantitative RT-PCR
mRNA was isolated from cell lines or spleens preserved in RNA later (QIAGEN, Valencia, CA), using the RN-easy kit (QIAGEN) and subjected to on-column DNAse digestion (QIAGEN). cDNA was obtained by reverse transcription of 100 ng to 2 μg RNA, after DNAse digestion (QIAGEN), using the Superscript II enzyme (Invitrogen, Carlsbad, CA). Taq polymerase (CLP) was used for the qualitative RT-PCR (see Table S1 for primer sequences, available on the Blood website; see the Supplemental Materials link at the top of the online article). For real-time PCR, the Brilliant Q-PCR SYBR green master mix (Stratagene, La Jolla, CA) and the Stratagene and Bio-Rad instruments were used and primers were purchased from Realtime primers (Elkins Park, PA) and Superarrays (Frederick, MD). RT controls were run in parallel with the correspondent samples. Data were analyzed with the cycle threshold (Ct) comparative method using actin as a housekeeping gene. Other investigators studying ICSBP function in the context of CML have also used actin as a control gene.12,18
Microarray analysis
Total RNA (15 μg) was isolated from parental BaF3 cells, or BaF3 cells expressing either ICSBP or BCR-ABL, or ICSBP and BCR-ABL. Affymetrix mouse whole genome arrays 430_2 were used for the analysis. Probe labeling, chip hybridization, and chip scanning were performed at the Biopolymer Facility at Harvard Medical School. Each chip was normalized to the median expression on the chip. A threshold value of 50 was set for all genes and the list of genes filtered to include only those that had at least one present flag (“P” flag) in 1 of the 4 conditions. For each gene, the ratio of its expression in a particular condition and its expression in parental BaF3 cells was determined. Only genes that had at least a 3-fold up or down change in expression were considered, leaving a set of 1431 genes for further analysis. K-means clustering with Gene cluster 3.0 was used to group these 1431 genes into 15 clusters and JavaTree was used to visualize the results. The microarray data can be found in the public database Gene Expression Omnibus (GEO) under accession number GSE14416.
Statistics
We expressed data as arithmetic means with standard deviation (SD) and performed statistical analysis as indicated in the figure legends. All data are obtained from independent measurements.
Results
Type I IFNs induce ICSBP expression in mouse and human BCR-ABL–transformed cells
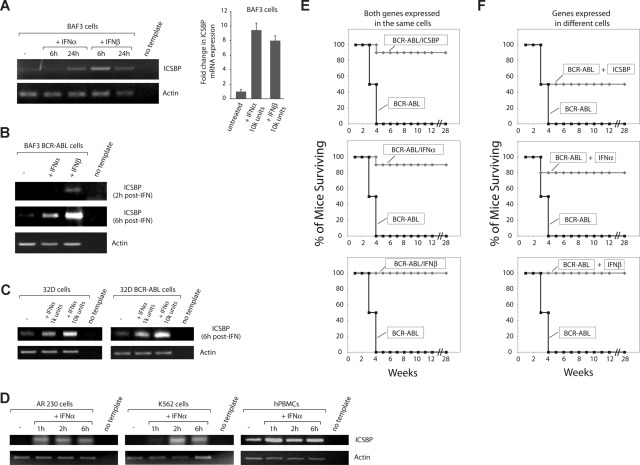
The ICSBP transcription factor is exclusively expressed in immune system cells and was first identified as a gene regulated by IFN gamma, a type II IFN (IFN).29 Treatment of patients with CML with IFN alpha results in increased levels of ICSBP expression in peripheral blood cells,21 but it remains unclear whether type I IFNs directly up-regulate ICSBP expression. We explored the relationship between IFN alpha and ICSBP expression in murine pro–B-lymphoid BaF3 cells, which have been used extensively as a syngeneic mouse model of BCR-ABL–induced leukemia,30,31 and in human primary cells and cell lines from patients with CML. In BaF3 cells, addition of murine IFN alpha or beta to the cell media greatly increased ICSBP levels in the parental and BCR-ABL–transformed cells (Figure 1A,B). A similar up-regulation was seen in the murine myeloid cell line 32D (Figure 1C), in peripheral blood mononuclear cells from a healthy human volunteer, and in both the K562 and AR230 human CML cell lines (Figure 1D). These results establish that type I IFNs can activate ICSBP expression in both normal and CML cells from mouse and human.
Figure 1.
Type I IFNs induce ICSBP expression and rescue mice from a BCR-ABL–induced leukemia. RT-PCR and Q-RT-PCR showing the expression of ICSBP before and at different time points after IFN alpha or IFN beta treatment (104 U/mL unless otherwise specified) in (A) parental BaF3 cells (n = 3 ± standard deviation); (B) BaF3 cells transformed with BCR-ABL; (C) parental and BCR-ABL transformed 32D cells; and (D) healthy human peripheral blood mononuclear cells (hPBMCs) and human CML cell lines AR230 and K562. Similar results were obtained from hPBMCs from 3 different healthy donors. (E) Cell-autonomous protection against leukemia conferred by ICSBP and type I IFNs. Survival curves of 6- to 9-week-old Balb/c mice injected with 106 BaF3 cells expressing the indicated cDNAs. At least 2 experiments per condition were performed, each containing 5 to 10 mice for each cell type. (F) Non–cell-autonomous protection against leukemia conferred by ICSBP and type I IFNs. Survival curves for 6- to 9-week-old Balb/c mice injected with 0.5 × 106 BaF3 cells separately expressing indicated cDNAs. At least 2 experiments per condition were performed, each containing 5 to 10 mice for each cell type.
Type I IFNs are as effective as ICSBP in conferring immune-mediated antileukemia protection
We have shown previously that ICSBP overexpression in BCR-ABL–transformed BaF3 cells elicits a vaccine-like antileukemic immune response.17 As type I IFNs induce ICSBP expression in BCR-ABL–transformed cells, we reasoned that IFN alpha or beta expression might also protect mice from leukemia. To test this, we expressed ICSBP, IFN alpha, or IFN beta in BaF3 cells transformed by BCR-ABL. In tissue culture, the expression of these proteins did not affect the proliferation rate of the BaF3 BCR-ABL cells (data not shown). We then injected these cells into syngeneic Balb/c mice. As expected, mice injected with cells expressing only BCR-ABL developed leukemia and died 2 to 4 weeks after injection, while those receiving cells coexpressing ICSBP and BCR-ABL remained disease-free for at least 28 weeks after injection (Figure 1E and data not shown). When expressed in BCR-ABL–transformed cells, both IFN alpha and beta were as effective as ICSBP in preventing leukemia in the mice (Figure 1E and data not shown). Expression of reverse orientation cDNAs for IFN alpha did not provide antileukemic protection (Figure S1B), and, as shown for ICSBP,17 the protection conferred by the IFNs required intact immunity, as it was not observed in immunodeficient Rag 2 knockout mice (Figure S1A). Patients with CML treated with imatinib and newer generation BCR-ABL inhibitors can develop resistance to these drugs by acquiring point mutations in the kinase domain of BCR-ABL. Expression of ICSBP and the type I IFNs rescued mice from leukemias caused by imatinib-resistant BCR-ABL mutants commonly found in patients with CML, including the infamous T315I BCR-ABL mutant (Figure S2A,B).
IFNs and ICSBP act in a non–cell-autonomous manner
Given that IFNs are secreted proteins, we asked whether they could exert a vaccine-like effect if delivered in nonleukemic parental BaF3 cells. Indeed, the IFNs protected nearly 100% of the mice even when expressed in different cells than BCR-ABL (Figure 1F). Unexpectedly, even ICSBP, an intracellular regulator of transcription, gave a substantial but incomplete degree of antileukemic protection to mice when it was expressed in separate cells (Figure 1F). A potential explanation for these results is that the expression of ICSBP in nonleukemogenic cells triggers the production of a secreted or cell-surface molecule that mediates protection against the leukemia induced by transformed BCR-ABL cells.
Identification of candidate genes for mediating the antileukemic effects of ICSBP
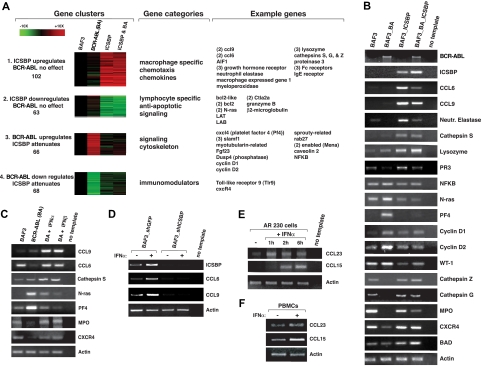
To explore how ICSBP induces protection against the BCR-ABL–induced leukemia, we performed transcriptional profiling to identify candidate genes that mediate its non–cell autonomous effects. We reasoned that such genes should be induced by ICSBP in parental and BCR-ABL–transformed BaF3 cells. We used microarrays covering all mouse genes to profile parental BaF3 cells as well as BaF3 cells expressing BCR-ABL, ICSBP, or both BCR-ABL and ICSBP. Using k-means clustering, we organized genes into 15 different clusters that reflected their varying expression patterns across the 4 cell lines (“Microarray analysis” in “Methods” and Figure S3). Four gene clusters were particularly interesting. Clusters 1 and 2 contain genes whose expression was increased or decreased by ICSBP, respectively, but not affected by BCR-ABL. Clusters 3 and 4 contain genes whose expression was increased or decreased by BCR-ABL, respectively, and normalized by coexpression with ICSBP. Consistent with known results, cluster 3 contains cyclin D1 and NFKBP, genes whose expression is induced by BCR-ABL and normalized by ICSBP and which may participate in BCR-ABL–driven cell transformation.32,33
Cluster 1 is heavily enriched for genes involved in macrophage function, such as Fc receptors and cathepsins, suggesting that ICSBP expression may promote the differentiation of the cells into macrophages or antigen-presenting cells. However, extensive FACS analysis of macrophage cell-surface markers failed to support this hypothesis for BaF3 cells (“Flow cytometric analysis, cell sorting, and cell blood counts” in “Methods” and data not shown). As we were particularly interested in genes that could mediate the non–cell-autonomous effects of ICSBP, we focused on genes in cluster 1 that encode secreted proteins that engage the immune system. The genes most highly up-regulated by ICSBP in cluster 1 were the related CCL6 and CCL9 chemokines, which were increased 10- to 20-fold (Figure 2A,B). The functions of these chemokines are not well understood, but they are thought to act as chemoattractants for CD4+ and CD8+ T lymphocytes as well as monocytes.34 Using RT-PCR, we verified the transcriptional profiling results for CCL6 and CCL9 as well as for many other highly regulated genes (Figure 2B). Consistent with type I IFNs inducing ICSBP, murine IFN alpha and beta also induce the expression of CCL6 and CCL9 in parental and BCR-ABL–transformed BaF3 cells (Figure 2C). The effects of IFN alpha are mediated by ICSBP, because in cells where ICSBP expression is reduced using RNA interference (RNAi), IFN alpha no longer induces CCL6 and CCL9 expression (Figure 2D). Furthermore, in human AR230 CML cells and normal PBMCs, human IFN alpha induces the expression of CCL15 and CCL23, the human orthologues of CCL6 and CCL9, respectively (Figure 2E,F). Our transcriptional profiling data demonstrate that enforced expression of ICSBP can counteract some of the deregulation in gene expression caused by BCR-ABL. More importantly, we identify the CCL6 and CCL9 chemokines as genes strongly induced by ICSBP and IFN alpha in mouse and human CML cells.
Figure 2.
ICSBP and the type I IFNs regulate expression of the CCL6 and CCL9 chemokines. (A) Cluster analysis of the genes that changed at least 3-fold in expression among pairwise comparisons of the 4 indicated cell types: parental BaF3 cells, or BaF3 cells expressing BCR-ABL (BaF3 BA), ICSBP, or ICSBP and Bcr-Abl (ICSBP BA). Expression was normalized to that in parental BaF3 cells. Four clusters containing genes that reflect ICSBP-regulated expression are illustrated. The colors signify the magnitude and direction of the changes in gene expression. To the right of the clusters are the gene functional categories enriched in each and examples of genes present in each category. The numbers in parentheses indicate the number of times each gene appeared in a cluster. (B) RT-PCR validation of gene expression for several of the genes detected by gene expression profiling within the indicated cell types. (C) Confirmation by RT-PCR that genes regulated by ICSBP are also regulated by treatment with 104 U/mL IFN alpha or beta for 6 hours in the indicated cell types. In some cases the IFNs restore expression of a gene that is otherwise down-regulated by BCR-ABL. (D) Requirement of ICSBP for IFN alpha to induce CCL6 and CCL9 expression. RT-PCR was used to detect expression of indicated genes in BaF3 parental cells expressing a control shRNA or an shRNA that decreases the expression of ICSBP. Cells were analyzed before and after treatment with IFN alpha (104 U/mL for 6 hours). (E) RT-PCR showing CCL23 and CCL15 expression in the human AR230 CML cell line upon treatment with 104 U/mL IFN alpha. (F) RT-PCR showing CCL23 and CCL15 expression in normal human PBMCs upon treatment with 104 U/mL IFN alpha for 1 hour.
CCL6 and CCL9 are necessary but not sufficient to induce the antileukemic immune protection mediated by ICSBP
We explored the possibility that the CCL6 and CCL9 chemokines might participate in the antileukemic immune protection caused by the expression of ICSBP in BCR-ABL–transformed cells. We tested the role of CCL6 and CCL9 by overexpressing each chemokine in parental BaF3 cells and coinjecting them with BCR-ABL–expressing leukemogenic cells into syngeneic mice (Figure 3A). While the expression of the 2 chemokines alone (data not shown) or in combination could not fully rescue the mice from developing leukemia, it consistently and significantly delayed disease progression by several weeks (Figure 3B,C). Protection from leukemia progression was mediated by the immune system, as it did not occur when the same cells were injected in immunocompromised mice (Figure 3C). These data suggest that CCL6 and CCL9 contribute to, but are not themselves sufficient to confer, an antileukemic immune response.
Figure 3.
CCL6 and CCL9 are not sufficient to induce full protection against a BCR-ABL–induced leukemia. (A) RT-PCR showing BCR-ABL, CCL9, and CCL6 expression in the BaF3 cells injected into mice. (B) CCL6 and CCL9 cannot substitute for ICSBP in conferring immune protection from leukemia. Survival curves for 6- to 9-week-old Balb/c mice injected with 0.3 × 106 BaF3 cells expressing BCR-ABL alone (×), or together with 0.15 × 106 BaF3 cells expressing CCL6 and 0.15 × 106 BaF3 cells expressing CCL9 (△). Experiment was repeated 3 times, with n = 15 for each condition. Unpaired t test shows significance at P < .003 for differences in survival curves. (C) CCL6 and CCL9 promote survival only in immunocompetent mice. Survival curves obtained for 9- to 12-week-old Rag 2−/− mice injected with 3 × 105 BaF3 cells expressing BCR-ABL alone (×) or together with 3 × 105 BaF3 cells expressing CCL6 and 3 × 105 BaF3 cells expressing CCL9 (△). N = 10 for each condition.
To determine whether CCL6 and CCL9 are necessary for ICSBP to mediate its antileukemic action, we used RNAi to decrease CCL6 or CCL9 expression in BaF3 cells that coexpress BCR-ABL and ICSBP (and thus do not cause leukemia when injected into mice). To implement RNAi, we targeted each chemokine with 2 independent shRNA-expressing lentiviruses.35,36 Compared with cells expressing either of 2 control shRNAs (targeting luciferase or LacZ), cells expressing the chemokine-specific shRNAs caused a greater than 80% reduction (“Cell lines, plasmids, and retroviral and lentiviral constructs” in “Methods”) in the expression of their respective targets (Figure 4A). As before, all control mice injected with BaF3 cells expressing BCR-ABL died within 5 weeks, while all those injected with BaF3 cells coexpressing BCR-ABL and ICSBP survived for the course of the experiment. The expression of the control shRNAs did not prevent the antileukemic effects of ICSBP. In contrast, mice injected with cells coexpressing BaF3, ICSBP, and shRNAs that reduce CCL6 or CCL9 expression died within 4 to 7 weeks after injection (Figure 4B). The leukemia observed in these mice was indistinguishable from that caused by the injection of BaF3 cells expressing only BCR-ABL. The mice had massive splenomegaly, anemia, and leukocytosis with relative lymphopenia (Figure S4). Moreover, by exploiting the fact that the BCR-ABL–expressing BaF3 cells also coexpress GFP, we determined that the diseased mice have high BCR-ABL–positive blast counts in the peripheral blood and spleen (Figure 4C-E). We verified that a knockdown in expression of CCL6 or CCL9 does not also lead to a change in the expression of the other chemokine (data not shown). In experiments using shRNAs that did not give effective chemokine knockdown, the vaccine-like effect of ICSBP expression persisted (data not shown). Taken together, our data indicate that both the CCL6 and CCL9 chemokines are necessary, although alone not sufficient, for ICSBP to confer immune protection against a BCR-ABL–induced leukemia in mice.
Discussion
Several lines of evidence suggest that immunotherapy can play a major role in the treatment of CML. Clinical experience shows that a graft-versus-leukemia (GVL) effect is highly relevant to the long-term remissions achieved after bone marrow transplantation37,38 and that adoptive immunotherapy through donor lymphocyte infusions (DLIs) can cure CML in some relapsed patients.39–41 Similar results have been observed in mouse models of the disease, where donor T cells transferred during bone marrow transplantation can effectively control the leukemic cells.42 A fraction of patients with CML treated with IFN alpha develop cytotoxic T lymphocytes that target leukemic cells,12,14 suggesting that IFNs can mediate an antileukemic immune response.
Expression of ICSBP in BCR-ABL–transformed cells prevents their capacity to cause leukemia in a syngeneic mouse model of leukemia by inducing a T lymphocyte–mediated immune response against the leukemic cells.17 In this study, we examined the relationship between ICSBP and type I IFNs, and found that IFN alpha induces ICSBP expression in nontransformed and in BCR-ABL–expressing cells, and that IFN alpha and ICSBP are equally effective at inducing the antileukemic immune response in our model. Because we found that ICSBP can act in a non–cell-autonomous fashion, we searched for secreted factors that participate in its antileukemic activity and identified CCL6 and CCL9 as related chemokines produced by cells after ectopic expression of ICSBP or treatment with type I IFNs. CCL6 and CCL9 are proinflammatory chemokines that serve as chemoattractants for CD11b+, IFN-producing dendritic cells, NK cells, and CD4+ T cells.43,44 Cells transformed by BCR-ABL down-regulate CCL6 and CCL9 expression. Both chemokines are necessary for the antileukemic immune response mediated by ICSBP, and while their expression cannot substitute for ICSBP, it does significantly delay leukemia progression and death in the murine model of CML, suggesting that CCL6 and CCL9 are necessary, but not sufficient to mediate the immunoprotective effects of ICSBP in our model. Although it is possible that ICSBP might directly transactivate the CCL6 and CCL9 genes, the mechanism of chemokine up-regulation is not known.
Previously, it was shown that CCL9 expression is reduced in murine 32D cells transformed by BCR-ABL and restored to normal levels by Gleevec treatment. Furthermore, overexpression of CCL9 in the transformed cells reduced their leukemic capacity, likely by modulating a T-cell response against the leukemic cells.45 Down-regulation of CCL6 and CCL9 chemokines by BCR-ABL could be one mechanism adopted by the leukemic cells to evade the immune system. Our work indicates that CCL6 and CCL9 are induced in normal cells and reactivated in leukemic cells by IFN alpha treatment. IFN is known to normalize some activities of the immune system that are otherwise impaired in patients with CML. For example, IFN alpha restores NK and T-cell function in patients with CML46,47 and induces CML mononuclear cells to differentiate toward antigen-presenting dendritic cells.46 Our findings, together with the prior clinical observation that ICSBP levels are restored in patients with CML who respond to IFN alpha, suggest a key role for ICSBP and CCL15 and CCL23, the human orthologues of CCL6 and CCL9, respectively, in mediating the therapeutic effects of IFN alpha in CML. In preliminary studies, we have observed higher expression of CCL23 in patients with CML who respond to treatment with IFN alpha, relative to nonresponders (V.N., F. Castagnetti, M. Amabile, G. Rosti, G. Martinelli, unpublished data, March 2008), and have observed increased expression of the CCL15 and 23 chemokines after exposure to IFN alpha of diverse malignant tumor cells, including multiple myeloma, melanoma, renal cell carcinoma, and mycosis fungoides. Coadministration of CCL15 and CCL23 might be a valuable adjunctive therapy to the peptide vaccines that are currently in clinical development as CML therapies,48 and may have wider therapeutic applications in diverse tumor types.
Supplementary Material
Acknowledgments
We acknowledge David M. Sabatini for helpful discussion and for comments on the manuscript.
This work was supported by grants from the Leukemia & Lymphoma Society of America (White Plains, NY), the Burroughs Wellcome Fund (Research Triangle Park, NC), the National Institutes of Health (NIH; Bethesda, MD), the NIH Director's Pioneer Award of the NIH Roadmap for Medical Research, and by a training grant from the NIH (T32-HL 66987-07).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.N. designed, performed, and analyzed results and wrote the manuscript; O.N. and M.A. helped perform the experiments with mice; and G.Q.D. helped design the research and planned and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George Q. Daley, Children's Hospital Boston and Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, New Research Building, 7th Fl, 300 Longwood Ave, Boston, MA 02115; e-mail: george.daley@childrens.harvard.edu.
References
- 1.Hoover RR, Gerlach MJ, Koh EY, Daley GQ. Cooperative and redundant effects of STAT5 and Ras signaling in BCR/ABL transformed hematopoietic cells. Oncogene. 2001;20:5826–5835. doi: 10.1038/sj.onc.1204549. [DOI] [PubMed] [Google Scholar]
- 2.Skorski T, Kanakaraj P, Nieborowska-Skorska M, et al. Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood. 1995;86:726–736. [PubMed] [Google Scholar]
- 3.Sattler M, Griffin JD. Mechanisms of transformation by the BCR/ABL oncogene. Int J Hematol. 2001;73:278–291. doi: 10.1007/BF02981952. [DOI] [PubMed] [Google Scholar]
- 4.Ren R. The molecular mechanism of chronic myelogenous leukemia and its therapeutic implications: studies in a murine model. Oncogene. 2002;21:8629–8642. doi: 10.1038/sj.onc.1206090. [DOI] [PubMed] [Google Scholar]
- 5.Reuther JY, Reuther GW, Cortez D, Pendergast AM, Baldwin AS., Jr A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev. 1998;12:968–981. doi: 10.1101/gad.12.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarjian HM, O'Brien S, Cortes JE, et al. Complete cytogenetic and molecular responses to interferon-alpha-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer. 2003;97:1033–1041. doi: 10.1002/cncr.11223. [DOI] [PubMed] [Google Scholar]
- 7.Bonifazi F, de Vivo A, Rosti G, et al. Chronic myeloid leukemia and interferon-alpha: a study of complete cytogenetic responders. Blood. 2001;98:3074–3081. doi: 10.1182/blood.v98.10.3074. [DOI] [PubMed] [Google Scholar]
- 8.Mahon FX, Delbrel X, Cony-Makhoul P, et al. Follow-up of complete cytogenetic remission in patients with chronic myeloid leukemia after cessation of interferon alfa. J Clin Oncol. 2002;20:214–220. doi: 10.1200/JCO.2002.20.1.214. [DOI] [PubMed] [Google Scholar]
- 9.Lee MS, Kantarjian H, Talpaz M, et al. Detection of minimal residual disease by polymerase chain reaction in Philadelphia chromosome-positive chronic myelogenous leukemia following interferon therapy. Blood. 1992;79:1920–1923. [PubMed] [Google Scholar]
- 10.Hochhaus A, Lin F, Reiter A, et al. Variable numbers of BCR-ABL transcripts persist in CML patients who achieve complete cytogenetic remission with interferon-alpha. Br J Haematol. 1995;91:126–131. doi: 10.1111/j.1365-2141.1995.tb05257.x. [DOI] [PubMed] [Google Scholar]
- 11.Kujawski LA, Talpaz M. The role of interferon-alpha in the treatment of chronic myeloid leukemia. Cytokine Growth Factor Rev. 2007;18:459–471. doi: 10.1016/j.cytogfr.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Burchert A, Wolfl S, Schmidt M, et al. Interferon-alpha, but not the ABL-kinase inhibitor imatinib (STI571), induces expression of myeloblastin and a specific T-cell response in chronic myeloid leukemia. Blood. 2003;101:259–264. doi: 10.1182/blood-2002-02-0659. [DOI] [PubMed] [Google Scholar]
- 13.Molldrem JJ, Clave E, Jiang YZ, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997;90:2529–2534. [PubMed] [Google Scholar]
- 14.Molldrem JJ, Lee PP, Wang C, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 15.Holtschke T, Lohler J, Kanno Y, et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 16.Hao SX, Ren R. Expression of interferon consensus sequence binding protein (ICSBP) is downregulated in Bcr-Abl-induced murine chronic myelogenous leukemia-like disease, and forced coexpression of ICSBP inhibits Bcr-Abl-induced myeloproliferative disorder. Mol Cell Biol. 2000;20:1149–1161. doi: 10.1128/mcb.20.4.1149-1161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng M, Daley GQ. Expression of interferon consensus sequence binding protein induces potent immunity against BCR/ABL-induced leukemia. Blood. 2001;97:3491–3497. doi: 10.1182/blood.v97.11.3491. [DOI] [PubMed] [Google Scholar]
- 18.Burchert A, Cai D, Hofbauer LC, et al. Interferon consensus sequence binding protein (ICSBP; IRF-8) antagonizes BCR/ABL and down-regulates bcl-2. Blood. 2004;103:3480–3489. doi: 10.1182/blood-2003-08-2970. [DOI] [PubMed] [Google Scholar]
- 19.Tamura T, Kong HJ, Tunyaplin C, Tsujimura H, Calame K, Ozato K. ICSBP/IRF-8 inhibits mitogenic activity of p210 Bcr/Abl in differentiating myeloid progenitor cells. Blood. 2003;102:4547–4554. doi: 10.1182/blood-2003-01-0291. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M, Bies J, Tamura T, Ozato K, Wolff L. The interferon regulatory factor ICSBP/IRF-8 in combination with PU.1 up-regulates expression of tumor suppressor p15(Ink4b) in murine myeloid cells. Blood. 2004;103:4142–4149. doi: 10.1182/blood-2003-01-0285. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt M, Nagel S, Proba J, et al. Lack of interferon consensus sequence binding protein (ICSBP) transcripts in human myeloid leukemias. Blood. 1998;91:22–29. [PubMed] [Google Scholar]
- 22.Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 2000;13:155–165. doi: 10.1016/s1074-7613(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 23.Tsujimura H, Nagamura-Inoue T, Tamura T, Ozato K. IFN consensus sequence binding protein/IFN regulatory factor-8 guides bone marrow progenitor cells toward the macrophage lineage. J Immunol. 2002;169:1261–1269. doi: 10.4049/jimmunol.169.3.1261. [DOI] [PubMed] [Google Scholar]
- 24.Schiavoni G, Mattei F, Sestili P, et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med. 2002;196:1415–1425. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aliberti J, Schulz O, Pennington DJ, et al. Essential role for ICSBP in the in vivo development of murine CD8alpha+ dendritic cells. Blood. 2003;101:305–310. doi: 10.1182/blood-2002-04-1088. [DOI] [PubMed] [Google Scholar]
- 26.Palacios R, Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 27.Azam M, Latek RR, Daley GQ. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–843. doi: 10.1016/s0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 28.Azam M, Raz T, Nardi V, Opitz SL, Daley GQ. A screen to identify drug resistant variants to target-directed anti-cancer agents. Biol Proced Online. 2003;5:204–210. doi: 10.1251/bpo63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driggers PH, Ennist DL, Gleason SL, et al. An interferon gamma-regulated protein that binds the interferon-inducible enhancer element of major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1990;87:3743–3747. doi: 10.1073/pnas.87.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilaria RL, Jr, Van Etten RA. The SH2 domain of P210BCR/ABL is not required for the transformation of hematopoietic factor-dependent cells. Blood. 1995;86:3897–3904. [PubMed] [Google Scholar]
- 31.Peters DG, Hoover RR, Gerlach MJ, et al. Activity of the farnesyl protein transferase inhibitor SCH66336 against BCR/ABL-induced murine leukemia and primary cells from patients with chronic myeloid leukemia. Blood. 2001;97:1404–1412. doi: 10.1182/blood.v97.5.1404. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez de Mattos S, Essafi A, Soeiro I, et al. FoxO3a and BCR-ABL regulate cyclin D2 transcription through a STAT5/BCL6-dependent mechanism. Mol Cell Biol. 2004;24:10058–10071. doi: 10.1128/MCB.24.22.10058-10071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cilloni D, Messa F, Arruga F, et al. The NF-kappaB pathway blockade by the IKK inhibitor PS1145 can overcome imatinib resistance. Leukemia. 2006;20:61–67. doi: 10.1038/sj.leu.2403998. [DOI] [PubMed] [Google Scholar]
- 34.Hara T, Bacon KB, Cho LC, et al. Molecular cloning and functional characterization of a novel member of the C-C chemokine family. J Immunol. 1995;155:5352–5358. [PubMed] [Google Scholar]
- 35.Moffat J, Grueneberg DA, Yang X, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Rubinson DA, Dillon CP, Kwiatkowski AV, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 37.Porter DL, Antin JH. The graft-versus-leukemia effects of allogeneic cell therapy. Annu Rev Med. 1999;50:369–386. doi: 10.1146/annurev.med.50.1.369. [DOI] [PubMed] [Google Scholar]
- 38.Leis J, Porter DL. Unrelated donor leukocyte infusions to treat relapse after unrelated donor bone marrow transplantation. Leuk Lymphoma. 2002;43:9–17. doi: 10.1080/10428190210202. [DOI] [PubMed] [Google Scholar]
- 39.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 40.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 41.Jiang YZ, Barrett AJ, Goldman JM, Mavroudis DA. Association of natural killer cell immune recovery with a graft-versus-leukemia effect independent of graft-versus-host disease following allogeneic bone marrow transplantation. Ann Hematol. 1997;74:1–6. doi: 10.1007/s002770050246. [DOI] [PubMed] [Google Scholar]
- 42.Krause DS, Van Etten RA. Adoptive immunotherapy of BCR-ABL-induced chronic myeloid leukemia-like myeloproliferative disease in a murine model. Blood. 2004;104:4236–4244. doi: 10.1182/blood-2004-06-2229. [DOI] [PubMed] [Google Scholar]
- 43.Zhao X, Sato A, Dela Cruz CS, et al. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer's patch CD11b+ dendritic cells. J Immunol. 2003;171:2797–2803. doi: 10.4049/jimmunol.171.6.2797. [DOI] [PubMed] [Google Scholar]
- 44.Coelho AL, Schaller MA, Benjamim CF, Orlofsky AZ, Hogaboam CM, Kunkel SL. The chemokine CCL6 promotes innate immunity via immune cell activation and recruitment. J Immunol. 2007;179:5474–5482. doi: 10.4049/jimmunol.179.8.5474. [DOI] [PubMed] [Google Scholar]
- 45.Iotti G, Ferrari-Amorotti G, Rosafio C, et al. Expression of CCL9/MIP-1gamma is repressed by BCR/ABL and its restoration suppresses in vivo leukemogenesis of 32D-BCR/ABL cells. Oncogene. 2007;26:3482–3491. doi: 10.1038/sj.onc.1210146. [DOI] [PubMed] [Google Scholar]
- 46.Pawelec G, Da Silva P, Max H, et al. Relative roles of natural killer- and T cell-mediated anti-leukemia effects in chronic myelogenous leukemia patients treated with interferon-alpha. Leuk Lymphoma. 1995;18:471–478. doi: 10.3109/10428199509059647. [DOI] [PubMed] [Google Scholar]
- 47.de Castro FA, Palma PV, Morais FR, et al. Immunological effects of interferon-alpha on chronic myelogenous leukemia. Leuk Lymphoma. 2003;44:2061–2067. doi: 10.1080/1042819031000110973. [DOI] [PubMed] [Google Scholar]
- 48.Heslop HE, Stevenson FK, Molldrem JJ. Immunotherapy of hematologic malignancy. Hematology Am Soc Hematol Educ Program. 2003:331–349. doi: 10.1182/asheducation-2003.1.331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.