Abstract
Unrelated developmental neurotoxicants can elicit similar functional outcomes, whereas agents in the same class may differ. We compared two organophosphate insecticides (chlorpyrifos, diazinon) with an organochlorine (dieldrin) and a metal (Ni2+) for similarities and differences in their effects on gene expression encoding subtypes of protein kinase C and their modulators, a cell signaling cascade that integrates the actions of neurotrophic factors involved in brain development. We conducted evaluations in PC12 cells, a model for neuronal development, with each agent introduced at 30 μM for 24 or 72 hr, treatments devoid of cytotoxicity. Chlorpyrifos evoked by far the largest effect, with widespread upregulation of multiple genes; the effects were greater during neurodifferentiation than when cells were exposed prior to differentiation. Diazinon had smaller and less widespread effects, consistent with its lesser long-term impact on synaptic function and behavior noted for in vivo exposures in developing rats. Surprisingly, the effects of diazinon, dieldrin and Ni2+ showed basic similarities despite the fact that all three come from different classes of toxicants. Our findings provide some of the first evidence for a specific mechanistic cascade contributing to the cholinesterase-independent developmental neurotoxicant actions of chlorpyrifos and its differences from diazinon, while at the same time identifying mechanistic convergence between otherwise unrelated toxicants that provide predictions about common neurodevelopmental outcomes. These results further show how combined use of cell cultures and microarray technology can guide future in vivo work on diverse developmental neurotoxicants.
Keywords: Organophosphate pesticides, Organochlorine pesticides, Metal neurotoxicity, Microarrays, PC12 cells, Protein kinase C
INTRODUCTION
Of the tens of thousands of chemicals in active production that are thought to be developmental neurotoxicants, only a small percentage have ever been evaluated for such activity (Boyes, 2001; Grandjean and Landrigan, 2006). The combined effects of these agents are likely contributors to what has been called a “silent pandemic” of neurobehavioral dysfunction in children (Grandjean and Landrigan, 2006), including learning disabilities, cognitive impairment and autism spectrum disorders (Eriksson, 1997; Grandjean and Landrigan, 2006; Landrigan et al., 1994, 1999; Szpir, 2006a, b; Weiss et al., 2004). Although basic research tends to focus on specific mechanisms of action of individual agents or classes of neurotoxicants, there are surprising similarities in outcomes among apparently unrelated agents, as well as disparities between chemicals within the same class (Barone et al., 2000; Monnet-Tschudi et al., 2007; Slotkin, 2004; Slotkin et al., 2006, 2007c, 2008a, b, 2009; Slotkin and Seidler, 2007, 2008; Szpir, 2006a; Yanai et al., 2002, 2004). In a series of recent studies, we examined how chlorpyrifos and diazinon, two organophosphates, show similarities but also major differences in their impact on the expression of neurotrophic factors that coordinate neuronal cell differentiation and brain assembly (Slotkin et al., 2007c, 2008c, 2009). The disparities are likely to explain divergent outcomes in terms of synaptic function of specific neurotransmitter pathways and their dependent behaviors (Aldridge et al., 2004, 2005a; Levin et al., 2001; Roegge et al., 2008; Slotkin et al., 2001, 2008a, b; Slotkin and Seidler, 2005; Timofeeva et al., 2008).
At the same time, we found surprising similarities in some of the effects of unrelated agents, such as diazinon and the organochlorine insecticide, dieldrin, as well as a metal, divalent nickel (Dotan et al., 2009; Slotkin et al., 2007b, 2009; Slotkin and Seidler, 2009), suggesting that a wide variety of agents may all converge on common sets of pathways that govern neurodevelopment. Several studies point to cell signaling cascades that transduce the actions of numerous neurotransmitters and hormones as likely points of crosstalk for the integration of diverse trophic signals toward common events in neurodifferentiation, notably the inputs converging on protein kinases such as PKA, PKC and tyrosine kinases (Aldridge et al., 2005b; Kapfhammer, 2004; Meyer et al., 2004, 2005; Nakagawara, 2001; Reuss and von Bohlen und Halbach, 2003; Slikker et al., 2005; Slotkin et al., 2003, 2008c, 2009; Slotkin and Seidler, 2007; Yanai et al., 2002, 2004, 2006). The proof of the importance of these pathways has been reinforced by demonstrating amelioration or reversal of neurotoxicant effects by agents that offset the actions at the level of PKA or PKC (Beer et al., 2005; Slotkin et al., 2007a; Steingart et al., 2000; Yanai et al., 2006).
Administration of developmental neurotoxicants in vivo elicits actions that reflect both the inherent neurotoxicity of the agent as well as indirect effects mediated through actions on the maternal-fetal or maternal-neonatal unit. In the current study, we wanted to compare and contrast the direct effects of chlorpyrifos, diazinon, dieldrin and Ni2+ on the expression of PKC isoforms and PKC regulators, especially given the key role of PKC in neuritic outgrowth and synaptic connectivity (Kapfhammer, 2004), effects that are known targets for the organophosphates (Slotkin, 1999, 2004, 2005). Furthermore, PKC is a collecting point for expression of the genes encoding the neurotrophic factors of the fgf family (Reuss and von Bohlen und Halbach, 2003) and the wnt/fzd pathway (Li et al., 2005), both of which are targeted by chlorpyrifos, diazinon, dieldrin, and Ni2+ (Slotkin et al., 2007c, 2008c, 2009). PKC is also mediates neurotoxic effects of metals, environmental tobacco smoke, pesticides and neuroactive drugs in the developing brain (Hasan et al., 2001; Haykal-Coates et al., 1998; Hilliard et al., 1999; Yanai et al., 2002), and therefore represents a likely point where disparate agents may lead to convergent neurodevelopmental outcomes.
Our evaluations were conducted with a widely-used in vitro model for neuronal development, PC12 cells (Teng and Greene, 1994). This model reproduces the mechanisms and outcomes of in vivo organophosphate exposures of developing rats (Bagchi et al., 1995, 1996; Crumpton et al., 2000a, b; Das and Barone, 1999; Flaskos et al., 1994; Jameson et al., 2006, 2007; Li and Casida, 1998; Nagata et al., 1997; Qiao et al., 2001, 2005; Slotkin et al., 2007a, b;, 2008c, 2009; Song et al., 1998; Tuler et al., 1989), and has already been characterized for comparative effects of the four agents on differentiation outcomes and on expression of the trophic factors encoded by the fgf and wnt/fzd gene families (Jameson et al., 2006; Slotkin et al., 2007b, 2008c, 2009; Slotkin and Seidler, 2008, 2009). In the PC12 model, nerve growth factor (NGF) triggers differentiation into neurotransmitter phenotypes, with formation of neuritic projections and acquisition of electrical excitability (Fujita et al., 1989; Song et al., 1998; Teng and Greene, 1994). For chlorpyrifos, we contrasted the effects in the undifferentiated vs. differentiating state, and then we compared the effects during differentiation for all four agents. Gene expression profiles were assessed with microarrays, using a “planned comparisons” approach (Slotkin and Seidler, 2007, 2009; Slotkin et al., 2007c, 2008c, 2009).
RESULTS
The microarrays detected 11 PKC isoforms (prkca, prkcb1, prkcc, prkcd, prkce, prkch, prkci, prkcm, prkcn, prkcq, prkcz) and 3 regulatory binding proteins (prkcbp1, prkcabp, prkcdbp) that passed the quality control procedures. Because only one agent (chlorpyrifos) was tested in both undifferentiated and differentiating cells, we conducted two sets of global statistical tests. For chlorpyrifos, the ANOVA factors were treatment, differentiation state, time and gene, and we found a main treatment effect (p < 0.02) as well as interactions of treatment × gene (p < 0.0002), treatment × differentiation state × time (p < 0.02), treatment × time × gene (p < 0.05) and treatment × state × gene (p < 0.05). Accordingly, we subdivided the results according to differentiation state and then evaluated main treatment effects and treatment × time interactions for each gene. Chlorpyrifos exposure evoked significant changes in the expression of 10 of the total of 14 genes, as compared to an expected false positive rate of only <1 gene (p < 0.0007), and the same was true for the separate analyses of undifferentiated cells (7 out of 14 genes, p < 0.02) and differentiating cells (9 out of 14 genes, p < 0.003). Diazinon, dieldrin and Ni2+ were studied only in differentiating cells, so the ANOVA factors for these agents were treatment, gene and time. The global test identified significant interactions of treatment × gene (p < 0.0001) and treatment × gene × time (p < 0.003), so we subdivided the data into the individual treatments and then evaluated each gene for main treatment effects and the interaction of treatment × time. Of the 14 total genes, we identified significant differences for 8 (p < 0.007 vs. the false positive rate of <1 gene).
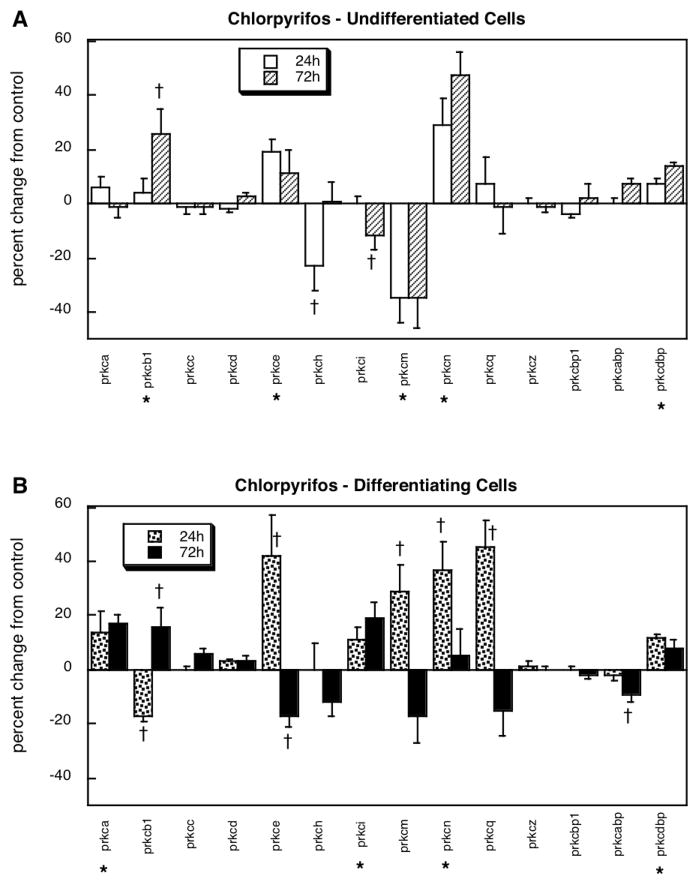
In undifferentiated cells, chlorpyrifos exposure evoked significant upregulation of three PKC isoforms, prkcb1, prkce and prkcn, as well as one of the regulatory binding proteins, prkcdbp (Figure 1A). Two other genes showed significant decrements (prkci, prkcm) and one (prkch) showed a transient decrease at 24h that resolved by 72h. Differentiating cells showed a significant net upregulation of PKC gene expression in response to chlorpyrifos (p < 0.02 for the main treatment effect) as well as much more widespread changes (Figure 1B). Although only three genes showed persistent upregulation (prkca, prkci, prkcdbp), four others showed a basic pattern of rapid upregulation after 24h or exposure and a subsequent rebound fall to normal or subnormal levels (prkc3, prkcm, prkcn, prkcq), one showed initial suppression followed by rebound elevation (prkcb1) and one showed a small, but significant downregulation (prkcabp).
Figure 1.
Effects of chlorpyrifos exposure in undifferentiated (A) and differentiating (B) PC12 cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which a treatment × time interaction was detected and show the individual times for which treatment effects were present. Multivariate ANOVA (treatment, differentiation state, gene, time) indicates a significant main treatment effect (chlorpyrifos > control, p < 0.02) and interactions of treatment × gene (p < 0.0002), treatment × state × time (p < 0.02), treatment × gene × time (p < 0.05) and treatment × state × gene (p < 0.05).
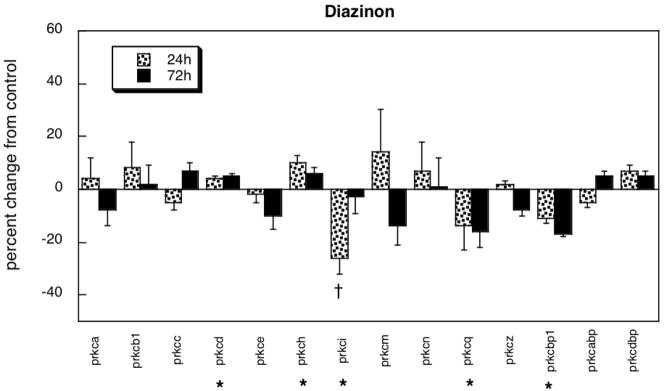
In contrast to the robust effects of chlorpyrifos in differentiating PC12 cells, diazinon affected a more restricted repertoire of genes (Figure 2). Further, diazinon had a smaller absolute magnitude of effect (8% average change as compared to 13% for chlorpyrifos, p < 0.05) and the net direction was a slight decrease rather than an increase. Small but statistically significant upregulation was seen for prkcd and prkch, as well as downregulation for prkcq and prkcbp1; prkci showed a transient decrement that resolved by 72h. Overall, the effects of diazinon were not significantly related to those seen with chlorpyrifos exposure (r=0.12).
Figure 2.
Effects of diazinon in differentiating PC12 cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which a treatment × time interaction was detected and show the individual times for which treatment effects were present. Multivariate ANOVA (treatment, gene, time) indicates a significant treatment × gene interaction (p < 0.02).
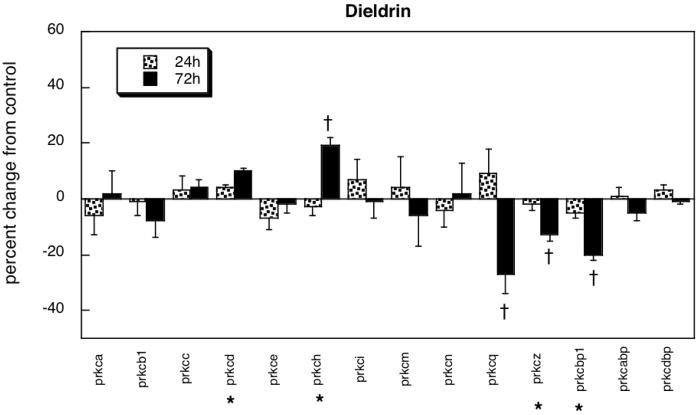
Dieldrin also affected a lesser proportion of genes than did chlorpyrifos (Figure 3). There were small but significant increments in prkcd and prkch, and reductions in prkcq, prkcz and prkcbp1. Again, the absolute magnitude of effect of dieldrin was significantly smaller than for chlorpyrifos (6% vs. 13%, p < 0.03) and showed a net change favoring downregulation instead of upregulation. There was no significant correlation between the effects of dieldrin and chlorpyrifos (r=0.18). The correlation between dieldrin and diazinon was higher (r=0.33) but just failed to meet the significance criterion (p < 0.09).
Figure 3.
Effects of dieldrin in differentiating PC12 cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which a treatment × time interaction was detected and show the individual times for which treatment effects were present. Multivariate ANOVA (treatment, gene, time) indicates a significant treatment × gene × time interaction (p < 0.05).
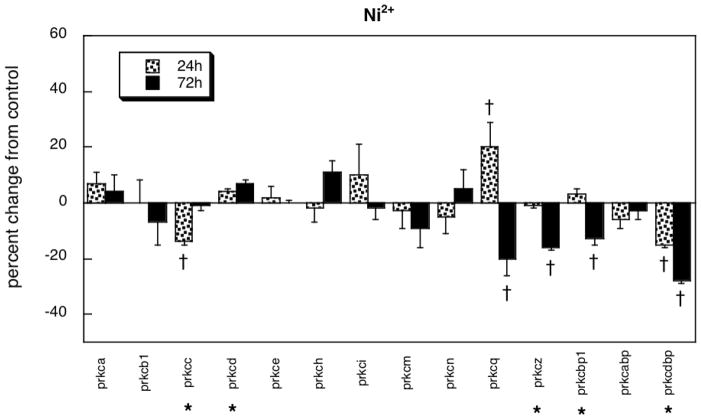
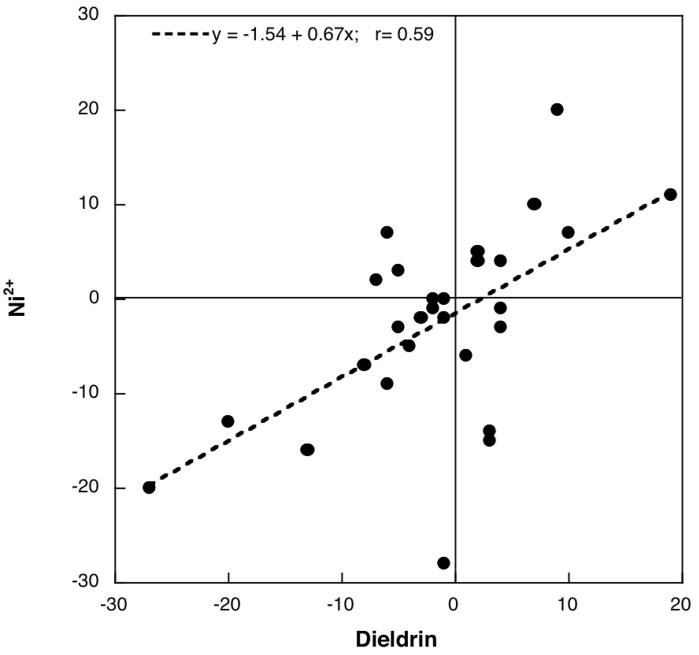
Exposure to Ni2+ evoked decrements in expression of four genes, encoding two PKC isoforms (prkcc, prkcz) and two of the regulatory proteins (prkcbp1, prkcdbp), while causing transient changes (upregulation at 24h, downregulation at 72h) for prkcq (Figure 4). As with diazinon and dieldrin, the absolute magnitude of effect for Ni2+ was significantly smaller than for chlorpyrifos (8% vs. 13%, p < 0.05) and the net direction was downward instead of upward. The pattern of changes in gene expression evoked by Ni2+ exposure was distinctly similar to that of dieldrin, as evidenced by a significant correlation (r=0.59, p < 0.0001; Figure 5).
Figure 4.
Effects of Ni2+ in differentiating PC12 cells. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which a treatment × time interaction was detected and show the individual times for which treatment effects were present. Multivariate ANOVA (treatment, gene, time) indicates significant interactions of treatment × time (p < 0.04), treatment × gene (p < 0.004) and treatment × gene × time (p < 0.02).
Figure 5.
Correlation between changes in gene expression evoked by dieldrin and those caused by Ni2+ exposure.
DISCUSSION
The developmental neurotoxicity evoked by organophosphate insecticides depends strongly on mechanisms other than their shared property as cholinesterase inhibitors (Casida and Quistad, 2004; Colborn, 2006; Pope, 1999; Slotkin, 1999, 2004, 2005). Indeed, recent studies point to cell signaling cascades involved in neurodifferentiation as a major contributor to adverse neurodevelopmental and behavioral outcomes (Meyer et al., 2004; Olivier et al., 2001; Slotkin and Seidler, 2007; Song et al., 1997; Yanai et al., 2002, 2004). In the current study, we identified effects of chlorpyrifos on the expression of genes encoding the PKC isoforms and modulators of PKC activity, reflecting the similar targeting of PKC signaling evoked by chlorpyrifos in vivo in the developing rat brain (Izrael et al., 2004; Slotkin and Seidler, 2007; Yanai et al., 2002, 2004). Since the effects seen here were obtained with an in vitro model, the actions of chlorpyrifos on the PKC cascade are clearly not mediated indirectly via effects on the mother or on the maternal-fetal unit, thus demonstrating direct involvement of this organophosphate with the control of a key cell signaling cascade involved in neurodifferentiation. Further, we found distinct differences between chlorpyrifos and diazinon that are likely to play a role in the disparities in the outcomes of exposure to these two organophosphates seen after in vivo treatments (Aldridge et al., 2004, 2005a; Levin et al., 2001; Roegge et al., 2008; Slotkin et al., 2001, 2008a, b; Slotkin and Seidler, 2005; Timofeeva et al., 2008), as well finding as unexpected similarities between otherwise unrelated neurotoxicants that thus predict convergent outcomes.
Of the four agents tested here, chlorpyrifos had by far the greatest effect on transcription of PKC-related genes. Notably, there was a distinct change in the transcriptional pattern for treatment of undifferentiated vs. differentiating cells. In the differentiating state, chlorpyrifos had a far more widespread effect on gene expression, a greater overall magnitude of effect, a distinct direction of change (upregulation), and a specific temporal pattern of rapid initial induction and an equally rapid subsequent fall. The greater response in the differentiating state recapitulates the critical period in which developing neurons are most sensitive to chlorpyrifos exposure in vivo, which also centers around axonogenesis and the emergence of neurotransmitter phenotypes (Das and Barone, 1999; Jameson et al., 2006; Slotkin, 1999, 2004, 2005). Indeed, the same pattern has been seen for the effects of chlorpyrifos on the expression of neurotrophic factors and their receptors that exert their control of neurodifferentiation via downstream PKC signaling, notably the fgf, wnt and fzd families (Slotkin et al., 2007c, 2008c, 2009).
Earlier work showed that diazinon, even when given in the same developmental period as chlorpyrifos and in pharmacodynamically-equivalent doses, elicits lesser long-term disruption of synaptic function and behavior (Aldridge et al., 2004, 2005a; Levin et al., 2001; Roegge et al., 2008; Slotkin et al., 2001, 2008a, b; Slotkin and Seidler, 2005; Timofeeva et al., 2008). Here, we similarly found far less impact of diazinon on gene expression related to PKC signaling. Again, this resembles findings in vivo for the comparative effects of the two organophosphates on PKC in the developing brain (Izrael et al., 2004; Slotkin and Seidler, 2007; Yanai et al., 2004), as well as their relative actions on the fgf, wnt and fzd families in vivo and vitro (Slotkin et al., 2008c, 2009). Surprisingly, diazinon, dieldrin and Ni2+ displayed a number of basic similarities in their patterns, especially for the latter two, again reflecting shared effects identified earlier for upstream neurotrophic factors (Slotkin et al., 2009) and downstream neurotoxic endpoints (Slotkin et al., 2007b; Slotkin and Seidler, 2008). The implication is inescapable: these results predict that diazinon (an organophosphate), dieldrin (an organochlorine) and Ni2+ (a metal) may in fact produce convergent developmental neurotoxicant outcomes. Clearly, further studies with in vivo models is required to establish this relationship.
Combining an in vitro model with a planned comparisons approach to microarray data has a number of key limitations and advantages that have been detailed earlier (Slotkin and Seidler, 2007, 2008, 2009; Slotkin et al., 2007c, 2008c) but are worth repeating in relation to the present results. The PC12 model is a transformed neuronotypic cell line, which, unlike primary neurons, maintain their ability to divide in culture and differentiates in a coordinated way into specificied phenotypes, important considerations for these neurotoxicants, which target the cell cycle and phenotypic fate. Transformed cells are inherently less sensitive to toxicant injury than are developing neurons in vivo and further, cell culture treatments involve much shorter durations than with environmental exposures extending throughout brain development. Both of these factors were considered in our selection of the 30 μM test concentrations, which nevertheless is below the threshold for cytotoxicity (Slotkin et al., 2007b; Song et al., 1998). This concentration is approximately an order of magnitude higher than that found for organophosphates in newborn babies after nonsymptomatic environmental exposures in agricultural communities (Ostrea et al., 2002). However, the cultures contain high concentrations of serum proteins; accordingly, less than 10% of the nominal concentration is actually available to diffuse into the cells (Qiao et al., 2001). The most important proof of relevance, though is the fact that for chlorpyrifos and diazinon, parallel outcomes have been identified between in vivo exposures and the PC12 model (Bagchi et al., 1995, 1996; Crumpton et al., 2000a, b; Das and Barone, 1999; Flaskos et al., 1994; Jameson et al., 2006, 2007; Li and Casida, 1998; Nagata et al., 1997; Qiao et al., 2001, 2005; Slotkin et al., 2007a, b, 2008c, 2009; Song et al., 1998; Tuler et al., 1989), thus providing validation of the in vitro approach. The second factor in these studies is our use of planned comparisons, as distinct from the global examination of the tens of thousands of genes present on the microarrays. Planned comparisons are based on testing a specific hypothesis that centers around a defined set of genes, and rests on known, validating outcomes from prior work, in this case for the organophosphates. With examination of the entire genome, verification via RT-PCR and other techniques is required because the enormous number of comparisons generates many false positive findings (e.g. the >2000 genes that would be false positives if we had considered all 42,000 probes on the array). For our study, we compared only a handful genes that would generate only 1 false positive, and instead we found alterations in the majority of these genes; for interpretation, we relied on the overall pattern of multiple gene changes for each agent, as well as effects that were repeated across different treatments and/or different times, rather than changes in any one gene. The odds of all those genes being false positives is astronomically small. However, even for individual genes, there were multiple probes and multiple spots on a given array (see Methods), so the changes cannot be “chance.” Unlike many array studies, where a single mRNA set combined from multiple samples might be evaluated, we evaluated up to 8 separate samples for each treatment condition, so again it is inconceivable that one could statistically produce these outcomes by accident. Indeed, one of the key points of this study is to demonstrate that a planned comparisons approach may provide a superior strategy for the use of microarray data, provided that the relevant target pathways are known in advance. Notably, for chlorpyrifos in particular, the parallel effects on PKC protein expression, enzymatic activity and membrane translocation, and their relationship to behavioral deficits, have all been validated with in vivo models (Izrael et al., 2004; Yanai et al., 2002, 2004), so that the comparative transcriptional “fingerprints” for each of the agents tested here can guide future evaluations at these higher levels of integration.
In conclusion, our findings provide some of the first evidence for a specific mechanistic sequence contributing to the cholinesterase-independent developmental neurotoxicant actions of chlorpyrifos, involving a chain of effects mediated through specific neurotrophic factors and their receptors, converging on expression and function of PKC isoforms and their modulators. The effects seen here for chlorpyrifos and diazinon with an in vitro model, bear direct similarities to those obtained in vivo, pointing to a direct effect of organophosphates on these key elements in neurodifferentiation, and thus providing at least one mechanism for adverse developmental actions distinct from cholinesterase inhibition. Thus, through the combined use of an in vitro neurodevelopmental model and transcriptional profiling, we were able to distinguish between the outcomes of two related organophosphates, and by identifying similarities among unrelated agents, to make predictions about their likely, shared impact on brain development, providing guidance for future in vivo studies.
EXPERIMENTAL PROCEDURE
Cell cultures
Because of the clonal instability of the PC12 cell line (Fujita et al., 1989), the experiments were performed on cells that had undergone fewer than five passages. As described previously (Qiao et al., 2003; Song et al., 1998), PC12 cells (American Type Culture Collection, 1721-CRL, obtained from the Duke Comprehensive Cancer Center, Durham, NC) were seeded onto poly-D-lysine-coated plates in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% inactivated horse serum (Sigma Chemical Co., St. Louis, MO), 5% inactivated fetal bovine serum (Sigma), and 50 μg/ml penicillin streptomycin (Invitrogen). Incubations were carried out with 7.5% CO2 at 37°C, standard conditions for PC12 cells. To initiate neurodifferentiation (Jameson et al., 2006; Slotkin et al., 2007b; Teng and Greene, 1994) twenty-four hours after seeding, the medium was changed to include 50 ng/ml of 2.5 S murine NGF (Invitrogen). Along with the NGF, we added 30 μM of each of the test agents: chlorpyrifos (Chem Service, West Chester, PA), diazinon (Chem Service), dieldrin (Chem Service) or NiCl2 (Sigma). The concentration was chosen from earlier studies that demonstrated adverse effects on differentiation of PC12 cells without outright cytotoxicity (Jameson et al., 2007; Qiao et al., 2001; Slotkin et al., 2007b, 2008c). Because of the limited water solubility of the three insecticides, these agents were dissolved in dimethylsulfoxide (final concentration 0.1%), which was also added to the control cultures and to cultures containing NiCl2; this concentration of dimethylsulfoxide has no effect on PC12 cell growth or differentiation (Qiao et al., 2001, 2003; Song et al., 1998). Cultures were examined 24 and 72 hr after commencing exposure, with 5–8 independent cultures evaluated for each treatment at each time point. We used two time points so as to be able to evaluate changes in gene expression regardless of whether the mRNA for a given gene has a rapid turnover (and hence can rise rapidly) or a slower turnover that would require a longer period to show corresponding increases or decreases.
Microarray determinations
Our earlier studies detailed the techniques for mRNA isolation, preparation of cDNA, conversion to cRNA incorporating cyanine-3 (reference RNA) or cyanine-5 (sample RNA), verification of RNA purity and quality, hybridization to the microarrays, washing and scanning (Slotkin and Seidler, 2007; Slotkin et al., 2007c, 2008c). These all involve commercial kits and procedures, and since the current studies were done identically, the techniques will not be described here. The mRNA used for the reference standard was created by pooling aliquots from each of the samples in the study. Similarly, array normalizations and error detection were carried out by procedures described previously (Slotkin and Seidler, 2007; Slotkin et al., 2007c, 2008c). We used Agilent Whole Rat Genome Arrays (Agilent Technologies, Palo Alto, CA), type G4131A for the studies of chlorpyrifos in undifferentiated and differentiating cells, and type G4131F for the studies of diazinon, dieldrin and Ni2+ in differentiating cells. The two chips contain exactly the same sequences but the latter has a lower detection threshold; however, all the genes reported here passed the quality control filters with both arrays.
For many of the genes, the arrays contain multiple probes for the same gene and/or replicates of the same probe in different locations on the chip, and these were used to verify the reliability of values and the validity of the measures on the chip. To avoid artificially inflating the number of positive findings, we limited each gene to a single set of values, selecting those obtained for the probe showing the smallest intragroup variance. The other values for that gene were used only to corroborate direction and magnitude of change. We also validated the readings on the arrays through the use of duplicate arrays for selected samples (Slotkin and Seidler, 2007; Slotkin et al., 2007c).
Statistical procedures
Because of the requirement to normalize the data across arrays and within each gene, the absolute values for a given gene are meaningless, so only the relative differences between treatments can be compared. Accordingly, results are presented as means and standard errors of the percentage change from control values to allow for visual comparison of the effects across families of genes. However, statistical comparisons were based on the actual ratios (log-transformed, since the data are in the form of ratios) rather than the percent change.
Our design involved planned comparisons of four agents at two time points, as well as the effects of one agent (chlorpyrifos) in undifferentiated vs. differentiating states. It was therefore important to consider the false positive rate and to protect against the increased probability of type 1 errors engendered by repeated testing of the same data base. Accordingly, before looking at effects on individual genes, we performed a global ANOVA incorporating all the variables in a single comparison: treatment, time, and all genes. Lower-order ANOVAs on subdivisions of the data set were then carried out as permitted by the interactions of treatment with the other variables. Differences for individual treatments for a specified gene at a single time point were evaluated with Fisher’s Protected Least Significant Difference. However, for a given gene where there was no interaction of treatment with other variables (time, differentiation state), only the main treatment effect was reported without subtesting of effects at a single time point. Treatment effects were considered significant at p < 0.05 (two-tailed, since we were interested in both increases and decreases in gene expression). Finally, concordance of patterns of effects between different agents was evaluated by linear regression analysis.
In addition to these parametric tests of the direction and magnitude of changes in gene expression, we evaluated the incidence of significant differences as compared to the predicted false positive rate, using Fisher’s Exact Test, applying a one-tailed criterion of p < 0.05, since only an increase above the false positive rate would be predicted; at the criterion of p < 0.05, one gene out of every 20 tested can be expected to show a difference at random. Finding a significant decrease in the incidence of detected differences relative to the false positive rate would be biologically implausible and statistically meaningless.
Acknowledgments
Research was supported by NIH ES10356. The authors state that they have no competing financial interests but have provided expert witness testimony on behalf of government agencies, corporations and/or individuals.
Abbreviations
- ANOVA
analysis of variance
- fgf
fibroblast growth factor gene family
- fzd
frizzled gene family
- NGF
nerve growth factor
- PKA
protein kinase A
- PKC
protein kinase C
- wnt
wingless gene family
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005a;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol Appl Pharmacol. 2005b;203:134–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Bhattacharya G, Stohs SJ. In vitro and in vivo induction of heat shock (stress) protein (Hsp) gene expression by selected pesticides. Toxicology. 1996;112:57–68. doi: 10.1016/0300-483x(96)03350-1. [DOI] [PubMed] [Google Scholar]
- Barone S, Das KP, Lassiter TL, White LD. Vulnerable processes of nervous system development: a review of markers and methods. Neurotoxicology. 2000;21:15–36. [PubMed] [Google Scholar]
- Beer A, Slotkin TA, Seidler FJ, Aldridge JE, Yanai J. Nicotine therapy in adulthood reverses the synaptic and behavioral deficits elicited by prenatal exposure to phenobarbital. Neuropsychopharmacology. 2005;30:156–165. doi: 10.1038/sj.npp.1300582. [DOI] [PubMed] [Google Scholar]
- Boyes WK. Neurotoxicology and behavior. In: Bingham E, Cohrseen B, Powell CH, editors. Patty’s Toxicology. 5. John Wiley & Sons; New York: 2001. pp. 55–121. [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Colborn T. A case for revisiting the safety of pesticides: a closer look at neurodevelopment. Environ Health Perspect. 2006;114:10–17. doi: 10.1289/ehp.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev Brain Res. 2000a;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: effects on nuclear transcription factor involved in cell replication and differentiation. Brain Res. 2000b;857:87–98. doi: 10.1016/s0006-8993(99)02357-4. [DOI] [PubMed] [Google Scholar]
- Das KP, Barone S. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol Appl Pharmacol. 1999;160:217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- Dotan S, Goldstein RS, Slotkin TA, Yanai J. An avian model for neural stem cell transplantation. J Neuropathol Exp Neurol. 2009 submitted. [Google Scholar]
- Eriksson P. Developmental neurotoxicity of environmental agents in the neonate. Neurotoxicology. 1997;18:719–726. [PubMed] [Google Scholar]
- Flaskos J, McLean WG, Hargreaves AJ. The toxicity of organophosphate compounds towards cultured PC12 cells. Toxicol Lett. 1994;70:71–76. doi: 10.1016/0378-4274(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Hasan SU, Simakajornboon N, MacKinnon Y, Gozal D. Prenatal cigarette smoke exposure selectively alters protein kinase C and nitric oxide synthase expression within the neonatal rat brainstem. Neurosci Lett. 2001;301:135–138. doi: 10.1016/s0304-3940(01)01624-x. [DOI] [PubMed] [Google Scholar]
- Haykal-Coates N, Shafer TJ, Mundy WR, Barone S. Effects of gestational methylmercury exposure on immunoreactivity of specific isoforms of PKC and enzyme activity during post-natal development of the rat brain. Dev Brain Res. 1998;109:33–49. doi: 10.1016/s0165-3806(98)00039-x. [DOI] [PubMed] [Google Scholar]
- Hilliard A, Ramesh A, Zawia NH. Correlation between lead-induced changes in cerebral ornithine decarboxylase and protein kinase C activities during development and in cultured PC12 cells. Intl J Dev Neurosci. 1999;17:777–785. doi: 10.1016/s0736-5748(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Izrael M, Van der Zee EA, Slotkin TA, Yanai J. Cholinergic synaptic signaling mechanisms underlying behavioral teratogenicity: effects of nicotine, chlorpyrifos and heroin converge on PKC translocation in the IMHV and on imprinting behavior in an avian model. J Neurosci Res. 2004;78:499–507. doi: 10.1002/jnr.20287. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: critical stages targeting differentiation in PC12 cells. Environ Health Perspect. 2006;114:667–672. doi: 10.1289/ehp.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Slotkin TA. Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: chlorpyrifos, chlorpyrifos oxon and diazinon. Environ Health Perspect. 2007;115:65–70. doi: 10.1289/ehp.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhammer JP. Cellular and molecular control of dendritic growth and development of cerebellar Purkinje cells. Prog Histochem Cytochem. 2004;39:131–182. doi: 10.1016/j.proghi.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Graham DG, Thomas RD. Environmental neurotoxic illness: research for prevention. Environ Health Perspect. 1994;102(Suppl 2):117–120. doi: 10.1289/ehp.94102117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, Wetmur JG, Matte TD, Gore AC, Godbold JH, Wolff MS. Pesticides and inner-city children: exposures, risks, and prevention. Environ Health Perspect. 1999;107(suppl 3):431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Vital elements of the Wnt-Frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2:331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Casida JE. Organophosphorus neuropathy target esterase inhibitors selectively block outgrowth of neurite-like and cell processes in cultured cells. Toxicol Lett. 1998;98:139–146. doi: 10.1016/s0378-4274(98)00116-7. [DOI] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Tate CA, Cousins MM, Slotkin TA. Critical periods for chlorpyrifos-induced developmental neurotoxicity: alterations in adenylyl cyclase signaling in adult rat brain regions after gestational or neonatal exposure. Environ Health Perspect. 2004;112:295–301. doi: 10.1289/ehp.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Slotkin TA. Developmental exposure to terbutaline alters cell signaling in mature rat brain regions and augments the effects of subsequent neonatal exposure to the organophosphorus insecticide, chlorpyrifos. Toxicol Appl Pharmacol. 2005;203:154–166. doi: 10.1016/j.taap.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich MG, Honegger P. Neurotoxicant-induced inflammatory response in three-dimensional brain cell cultures. Human Exp Toxicol. 2007;26:339–346. doi: 10.1177/0960327107074589. [DOI] [PubMed] [Google Scholar]
- Nagata K, Huang CS, Song JH, Narahashi T. Direct actions of anticholinesterases on the neuronal nicotinic acetylcholine receptor channels. Brain Res. 1997;769:211–218. doi: 10.1016/s0006-8993(97)00707-5. [DOI] [PubMed] [Google Scholar]
- Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett. 2001;169:107–114. doi: 10.1016/s0304-3835(01)00530-4. [DOI] [PubMed] [Google Scholar]
- Olivier K, Liu J, Pope C. Inhibition of forskolin-stimulated cAMP formation in vitro by paraoxon and chlorpyrifos oxon in cortical slices from neonatal, juvenile, and adult rats. J Biochem Mol Toxicol. 2001;15:263–269. doi: 10.1002/jbt.10002. [DOI] [PubMed] [Google Scholar]
- Ostrea EM, Morales V, Ngoumgna E, Prescilla R, Tan E, Hernandez E, Ramirez GB, Cifra HL, Manlapaz ML. Prevalence of fetal exposure to environmental toxins as determined by meconium analysis. Neurotoxicology. 2002;23:329–339. doi: 10.1016/s0161-813x(02)00077-3. [DOI] [PubMed] [Google Scholar]
- Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ Health Perspect. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev Brain Res. 2003;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol Appl Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction — developmental neurotoxicity of nicotine. Crit Rev Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Auman JT, Seidler FJ. Ontogenesis of β-adrenoceptor signaling: implications for perinatal physiology and for fetal effects of tocolytic drugs. J Pharmacol Exp Ther. 2003;306:1–7. doi: 10.1124/jpet.102.048421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ Health Perspect. 2006;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Seidler FJ. Ameliorating the developmental neurotoxicity of chlorpyrifos: a mechanisms-based approach in PC12 cells. Environ Health Perspect. 2007a;115:1306–1313. doi: 10.1289/ehp.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ Health Perspect. 2007b;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environ Health Perspect. 2007c;115:909–916. doi: 10.1289/ehp.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect. 2008a;116:340–348. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Levin ED, Seidler FJ. Developmental neurotoxicity of low-dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res Bull. 2008b;75:640–647. doi: 10.1016/j.brainresbull.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol Appl Pharmacol. 2008;233:211–219. doi: 10.1016/j.taap.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Targeting of neurotrophic factors, their receptors, and signaling pathways in the developmental neurotoxicity of organophosphates in vivo and in vitro. Brain Res Bull. 2008c;76:424–438. doi: 10.1016/j.brainresbull.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Transcriptional profiles reveal similarities and differences in the effects of developmental neurotoxicants on differentiation into neurotransmitter phenotypes in PC12 cells. Brain Res Bull. 2009;78:211–225. doi: 10.1016/j.brainresbull.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Unrelated developmental neurotoxicants elicit similar transcriptional profiles for effects on neurotrophic factors and their receptors in an in vitro model. Neurotoxicol Teratol. 2009 doi: 10.1016/j.ntt.2008.11.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Steingart RA, Silverman WF, Barron S, Slotkin TA, Awad Y, Yanai J. Neural grafting reverses prenatal drug-induced alterations in hippocampal PKC and related behavioral deficits. Dev Brain Res. 2000;125:9–19. doi: 10.1016/s0165-3806(00)00123-1. [DOI] [PubMed] [Google Scholar]
- Szpir M. Tracing the origins of autism: a spectrum of new studies. Environ Health Perspect. 2006a;114:A412–A417. doi: 10.1289/ehp.114-a412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpir M. New thinking on neurodevelopment. Environ Health Perspect. 2006b;114:A101–A107. doi: 10.1289/ehp.114-a100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng KK, Greene LA. Cultured PC12 cells: a model for neuronal function and differentiation. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. Academic Press; San Diego: 1994. pp. 218–224. [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol Teratol. 2008;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuler SM, Hazen AA, Bowen JM. Release and metabolism of dopamine in a clonal line of pheochromocytoma (PC12) cells exposed to fenthion. Fund Appl Toxicol. 1989;13:484–492. doi: 10.1016/0272-0590(89)90284-4. [DOI] [PubMed] [Google Scholar]
- Weiss B, Amler S, Amler RW. Pesticides. Pediatrics. 2004;113:1030–1036. [PubMed] [Google Scholar]
- Yanai J, Vatury O, Slotkin TA. Cell signaling as a target and underlying mechanism for neurobehavioral teratogenesis. Ann NY Acad Sci. 2002;965:473–478. doi: 10.1111/j.1749-6632.2002.tb04188.x. [DOI] [PubMed] [Google Scholar]
- Yanai J, Beer A, Huleihel R, Izrael M, Katz S, Levi Y, Rozenboim I, Yaniv SP, Slotkin TA. Convergent effects on cell signaling mechanisms mediate the actions of different neurobehavioral teratogens: alterations in cholinergic regulation of PKC in chick and avian models. Ann NY Acad Sci. 2004;1025:595–601. doi: 10.1196/annals.1316.074. [DOI] [PubMed] [Google Scholar]
- Yanai J, Ben-Shaanan TL, Haimovitch H, Katz S, Kazma M. Mechanism-based approaches for the reversal of drug neurobehavioral teratogenicity. Annals NY Acad Sci. 2006;1074:659–671. doi: 10.1196/annals.1369.066. [DOI] [PubMed] [Google Scholar]