Abstract
Age-related declines in human cognition are well known, and there are correlative changes in the function of neocortical and hippocampal neurons. Similarly, age-related declines in learning have been observed in rodents, including deficits in a hippocampal-dependent learning paradigm, the Morris water maze. Furthermore, there are correlative deficits in specific signaling pathways, including protein kinase C (PKC) pathways, in cerebellar, hippocampal, or neocortical neurons. PKC pathways are strong candidates for mediating the molecular changes that underlie spatial learning, as they play critical roles in neurotransmitter release and synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD), and deletion of specific PKC genes results in deficits in learning. Conversely, genetic activation of PKC pathways in small groups of hippocampal or cortical neurons enhances learning in specific paradigms. In this study, we delivered a constitutively active PKC into small groups of hippocampal dentate granule neurons in aged rats (using a Herpes Simplex Virus-1 vector). Aged two-year old rats that received the constitutively active PKC displayed improved performance in the Morris water maze relative to controls in three different measures. These results indicate that PKC pathways play an important role in mediating spatial learning in aged rats. Additionally, these results represent a system for studying the neural mechanisms underlying aging-related learning deficits, and potentially developing gene therapies for cognitive and age-related deficits.
Keywords: spatial discrimination, aged rats, protein kinase C, dentate granule neurons, Herpes Simplex Virus vector
INTRODUCTION
Age-related declines in human cognition, particularly in learning and memory, are well documented. During aging, there is strong correlative evidence of changes in the function of neocortical and hippocampal neurons (reviews (Della-Maggiore et al., 2002; West et al., 2002; Wu et al., 2002)). Similar age-related declines in cognitive functions have been modeled in rodents. In particular, changes in hippocampal-dependent learning tasks during aging have been characterized, including deficits in spatial learning, as tested in the Morris water maze (review (Rosenzweig and Barnes, 2003)). In aged rodents, the numbers of hippocampal neurons are unaltered, but there are decreases in the numbers of synapses, synaptic responses, and synaptic plasticity, including changes in long-term potentiation (LTP) and long-term depression (LTD) (Rosenzweig and Barnes, 2003).
Protein kinase C (PKC) signaling pathways are strong candidates for mediating the molecular changes that underlie learning and aging-related learning deficits. Specific PKC substrates play critical roles in neuronal physiology (Tanaka and Nishizuka, 1994), and activation of PKC increases release of specific classical neurotransmitters from neurons in the hippocampus and throughout the nervous system (Nichols et al., 1987; Waters and Smith, 2000). PKC inhibitors block LTP (Malenka and Nicoll, 1999); a constitutively active form of PKCζ, transcribed from an internal promoter (Hernandez et al., 2003), is necessary for LTP (Ling et al., 2002); and LTD requires PKC-mediated phosphorylation of specific AMPA receptor subunits (Chung et al., 2003; Seidenman et al., 2003).
There is accumulating evidence that PKC pathways have critical roles learning. For example, PKC is activated in hippocampal cells during associative or visual learning (Olds et al., 1989; Olds et al., 1990). PKCγ knockout mice display defective regulation of LTP and mild deficits in some learning paradigms, but the absence of PKCγ from every cell, and defects in cerebellar development and motor coordination, complicate the interpretation (Abeliovich et al., 1993a; Abeliovich et al., 1993b; Chen et al., 1995; Kano et al., 1995). PKCγ knockout mice display deficits in fear conditioning (Weeber et al., 2000). Conversely, a constitutively active PKCζ is necessary for specific types of memory maintenance (Pastalkova et al., 2006; Shema et al., 2007). Furthermore, delivery of a constitutively active PKC (using a virus vector) into small groups of postrhinal cortex neurons enhanced visual image discrimination learning, but delivery of this PKC into an irrelevant brain area (primary somatosensory cortex) did not affect this learning (Zhang et al., 2005). Also, delivery of this PKC into hippocampal dentate granule neurons enhanced auditory discrimination reversal learning (Neill et al., 2001). The capability of localized gene transfer of a constitutively active PKC to enhance learning is consistent with learning theories that hypothesize that neurons in a particular circuit use specific signaling pathways to modify synaptic strengths, thereby mediating learning (Hebb, 1949; Kandel and Schwartz, 1982).
Aged rodents display deficits in PKC signaling and spatial learning. Aged rats with spatial learning deficits have reduced PKC activity in the hippocampus (Colombo and Gallagher, 2002; Colombo et al., 1997), cerebellum, and neocortex (Battaini et al., 1995). Similarly, PKC activity correlates with spatial learning in aged mice, and between strains of mice (Fordyce and Wehner, 1993). Aged rabbits exhibit reduced hippocampal PKC activity (Van der Zee et al., 2004). One possible cause of this altered PKC signaling is related to the receptor for activated C kinase (RACK1), which anchors activated PKC to the plasma membrane (Ron et al., 1994; Schechtman and Mochly-Rosen, 2001). RACK1 levels are reduced in aged rat cortex (Battaini et al., 1999; Battaini et al., 1997; Pascale et al., 1996) and in aged rabbit hippocampus and cortex (Van der Zee et al., 2004). Another possible cause of this altered PKC signaling, in aged rats, are deficits in metabotropic glutamate receptor-mediated phosphoinositide turnover, and reduced levels of phospholipaseCß-1, which activates PKC (Nicolle et al., 1999).
Taken together, these previous results suggest that restoring PKC activity in specific groups of forebrain neurons of aged rats might correct specific deficits in learning. To support such studies, we previously isolated a constitutively active, catalytic domain of rat PKCßII (PkcΔ), and a point mutation (PkcΔGG) that lacks enzyme activity (Song et al., 1998). PkcΔ increased phosphorylation of specific PKC substrates that play critical roles in neuronal physiology; in postrhinal cortex neurons, PkcΔ increased phosphorylation of specific subunits of AMPA or NMDA receptors, MARCKS, GAP-43, and dynamin (Zhang et al., 2005); and in striatal neurons, PkcΔ increased phosphorylation of a specific AMPA receptor subunit (Oh et al., 2003). Consistent with increased phosphorylation of two proteins that have critical roles in neurotransmitter release, GAP-43, and dynamin, PkcΔ increased release of dopamine and norepinephrine from cultured sympathetic neurons (Song et al., 1998), and glutamate and GABA from cultured temporal cortex cells (Zhang et al., 2005). In support of this approach, constitutively active PKCs are produced naturally by calpain (Kishimoto et al., 1989), and levels are increased during LTP (Powell et al., 1994). Also, a constitutively active form of PKCζ is necessary for LTP and specific types of memory maintenance (Ling et al., 2002; Pastalkova et al., 2006; Shema et al., 2007). Thus, these results suggest that aged rats that receive PkcΔ might learn a cognitive task better than control groups.
In this study, we delivered PkcΔ into small groups of hippocampal dentate granule neurons in two year-old rats (using a virus vector). These rats displayed improved learning by three different measures of Morris water maze performance. The results have direct implications for studying the mechanisms underlying aging-induced learning deficits, and benefit development of gene therapies for specific cognitive deficits.
METHODS
Materials
Dulbecco’s modified minimal essential medium (DMEM), fetal bovine serum (FBS), glutamine, G418, lipofectamine, and OPTI-MEM I were obtained from Invitrogen. Aged (24 months old) male hybrid F344/Brown Norway F1 (F344/BN F1) rats were obtained from the NIA/Harlan (Indianapolis, IN). A rat water maze (2 m diameter) and platform (10 cm square) were obtained from San Diego Instruments (San Diego CA); videocamera and software were obtained from Accuscan Inc. (Columbus OH). 5-bromo-4-chloro-3-indoyl-ß-D-galactopyranoside (X-Gal) was obtained from Sigma (St. Louis MO). Rabbit anti-ß-galactosidase (ß-gal) and mouse monoclonal anti-NeuN were obtained from Chemicon (Temecula,CA), and fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin (Ig) G and rhodamine isothiocyanate-conjugated goat anti-mouse IgG were obtained from Sigma.
Cells
Baby hamster kidney fibroblast (BHK21) cells and 2-2 cells (Smith et al., 1992) were grown in DMEM supplemented with 10 % FBS, 4 mM glutamine, and penicillin/streptomycin, at 37°C in humidified incubators with 5 % CO2. G418 (0.5 mg/ml) was present during growth of 2-2 cells but was removed prior to plating the cells for packaging vectors.
Vectors and Packaging
pkcΔ contains the catalytic domain of rat PKCßII (nucleotide 994 to the 3′ end), with the flag tag fused to the 5′ end (Song et al., 1998). PkcΔGG has a point mutation; a gly replaced an absolutely conserved lys that is required for phosphoryl transfer (Hanks et al., 1988; Song et al., 1998). Following expression in the yeast S. cerevisiae, we used cell extracts and peptide substrates to establish that PkcΔ has a substrate specificity similar to rat brain PKC and that PkcΔGG lacks protein kinase activity (Song et al., 1998).
Gene transfer used a helper virus-free Herpes Simplex Virus (HSV-1) plasmid, or amplicon, vector system (Fraefel et al., 1996; Geller and Breakefield, 1988). The vectors contained a modified neurofilament promoter that supports long-term expression in forebrain neurons (Zhang et al., 2000). An upstream enhancer element from the rat tyrosine hydroxylase promoter (TH; -0.5 kb to -6.8 kb) was placed at the 5′ end of a mouse neurofilament heavy gene promoter (NFH; 0.6 kb); and an insulator (INS; 1.2 kb) from the chicken ß-globin locus was placed at the 5′ end of the TH promoter fragment (Zhang et al., 2000). HSV-1 vectors containing this promoter (INS-TH-NFH promoter) support recombinant gene expression in forebrain neurons for 6 to 14 months, the longest time points examined, and two time courses in the striatum or hippocampus showed similar numbers of X-gal positive cells between 2 weeks and 6 months after gene transfer (Sun et al., 2004; Zhang et al., 2000).
The two transcription units in pINS-TH-NFHpkcΔINS-TH-NFHlac (Wang et al., 2001) express either pkcΔ or Lac Z, to facilitate detection of cells that contain the vector. This vector coexpressed ß-gal and PkcΔ in postrhinal cortex cells (Wang et al., 2001); 96 % of the positive cells contained both flag-immunoreactivity (IR) and ß-gal-IR. In pINS-TH-NFHpkcΔGG\INS-TH-NFHlac, pkcΔGG replaced pkcΔ (Zhang et al., 2005).
Helper virus-free HSV-1 vector packaging was performed as described using a modified procedure that improves the titers (Fraefel et al., 1996; Sun et al., 1999); resulting vector stocks were purified and titered (Wang et al., 2001; Zhang et al., 2000). The titers were 1.0×107 infectious vector particles (IVP)/ml pINS-TH-NFHpkcΔINS-TH-NFHlac and 1.0×107 IVP/ml pINS-TH-NFHpkcΔGG/INS-TH-NFHlac. In each experiment, the titers of the different vector stocks were matched by dilution. No HSV-1 (<10 plaque forming units/ml) was detected in any of these vector stocks.
Water Maze Training and Gene Transfer
These studies were approved by the West Roxbury VA Hospital IACUC. Male hybrid F344/Brown Norway F1 (F344/BN F1) rats were used for this study; the rats were either young adult (250-275 gm) or 24 months old. We chose to use F344/Brown Norway F1 rats because the pigmentation, from the Brown Norway parent, enables these hybrid aged rats to be trained for visual learning (Cook et al., 2004). Although aged F344 rats are known to have deficits in the Morris water maze (reviewed in (Rosenzweig and Barnes, 2003)), F344/Brown Norway F1 rats have not been examined in the water maze to our knowledge. After arrival, the rats were given 3 days to acclimate to the animal facility. The rats were then trained for 5 sessions (1 session per day) in the Morris water maze (Morris et al., 1982) using a hidden platform in the same location for each session. Each session consisted of 4 trials, with each trial’s start point evenly distributed around the circular border of the water maze (Attaway et al., 1999; Bizon et al., 2004; Klein et al., 2000). On the day after the completion of the training, a probe trial was performed, and the number of platform crossings during a 1 minute period was measured (Attaway et al., 1999; Bizon et al., 2004; Klein et al., 2000). A second purpose of this initial training was to teach the rats the water maze procedure, so the training after gene transfer will quantify any differences between the groups in learning a new platform location, rather than differences in acquiring the procedure. All performance was monitored using a videocamera and commercially available software (see materials section).
Following this initial training, all of the 24-month aged rats were randomly assigned to one of three conditions; i) PkcΔ (pINS-TH-NFHpkcΔINS-TH-NFHlac), ii) PkcΔGG (pINS-TH-NFHpkcΔGG\INS-TH-NFHlac), or iii) PBS (phosphate buffered saline). Two to five days later, PkcΔ, or PkcΔGG, or PBS was delivered into the hippocampus, using previously established gene transfer conditions (Neill et al., 2001): Vector stocks were delivered by stereotactic injection into the dentate gyrus {1.8×105 IVP total injected; 6 sites, 3 in each hemisphere; anteroposterior (AP) 3.3, mediolateral (ML) 2.0, dorsoventral (DV) 3.5; AP 4.3, ML 2.5, DV 3.4; AP 5.3, ML 3.25, DV 3.7}. AP is relative to bregma, ML is relative to the sagittal suture, and DV is relative to the bregma-lambda plane (Paxinos and Watson, 1986). Vector stocks were injected using a micropump (model 100, KD Scientific); 3 μl was injected over 8 minutes, the needle remained in place for an additional 5 minutes, and was then slowly withdrawn over approximately 5 minutes. This protocol was designed to ensure that the inoculum diffused into the brain tissue proximal to the needle tip and did not travel up the needle track. One PkcΔ and one PBS rat died during the gene transfer surgery. Gene transfer was followed by a 2 week recovery period which allowed gene expression to reach steady-state levels (Zhang et al., 2000) and the skull to become watertight.
After recovery, seven training sessions (1 session per day) of hidden platform training was conducted, using a new platform position, with all three groups of aged rats. On the following day, a probe test trial was performed, using the same procedures as before gene transfer. This experiment was performed on two independent and sequential sets of rats, with similar results, and the data from the two independent replications were combined for analysis. Subsequent histological analyses (see below) revealed that 2 PkcΔ rats had greatly enlarged needle tracks with large necrotic areas surrounding the needle tracks, and these rats were excluded from the behavioral analysis.
Immunohistochemistry
Rats were perfused with 50 ml phosphate buffered saline (PBS) followed by 200 ml of 4 % paraformaldehyde in PBS. Brains were postfixed using 4 % paraformaldehyde in PBS (4 hr, 4 °C), and then cryoprotected in 25 % sucrose in PBS (2 days, 4 °C). Twenty-five μm coronal sections were cut using a freezing microtome. Enzymatic staining and immunohistochemistry were performed on free-floating sections. X-gal staining was performed as described (Zhang et al., 2000); some of these sections were counterstained with 0.1 % neutral red.
For immunofluorescent visualization, sections were permeabilized by incubation in PBS, 2 % normal goat serum, 0.2 % Triton X-100 (buffer A; 30 min, 37 °C). Sections were then incubated with rabbit anti-ß-gal (1:1,000 dilution) and mouse monoclonal anti-NeuN (1:200 dilution; Chemicon Inc.) in buffer A (overnight, 4 °C; then 1 hr, 37 °C). Next, sections were washed with PBS (3 times, 5 min each) and then incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG and rhodamine isothiocyanate-conjugated goat anti-mouse IgG, (1:150 dilutions) in buffer A (3 hr, room temperature). Lastly, sections were washed (PBS; 3 times, 5 min each), mounted in PBS, and immediately examined under the microscope.
Cell Counts
For quantitative analyses of the numbers of X-gal positive cells, 25 μm coronal brain sections from the hippocampus were examined, every 4th section was analyzed, and ∼24 of these sections contained the positive cells. For immunofluorescent costaining for ß-gal-IR and NeuN-IR, every 4th section from 3 rats was assayed, and randomly chosen sections were examined; >300 ß-gal-IR cells were examined for containing or lacking NeuN-IR, and all the ß-gal-IR cells were scored from the sections that were examined. To quantify either the numbers of X-gal positive cells or the % costaining, digital images of either the X-gal cells or the stained cells were obtained under 60X magnification, and cell counts were performed. To verify the accuracy of the cell counts, each section was counted at least two times independently, and the two values differed by <10 % for each section.
Statistical Analyses
Statistical analyses of water maze performance used between groups or repeated measures ANOVAs (Sigmastat; SPSS Inc.; Chicago, IL).
RESULTS
Aged, 2-year old, Hybrid F344/Brown Norway F1 Rats have Deficits in Spatial Learning
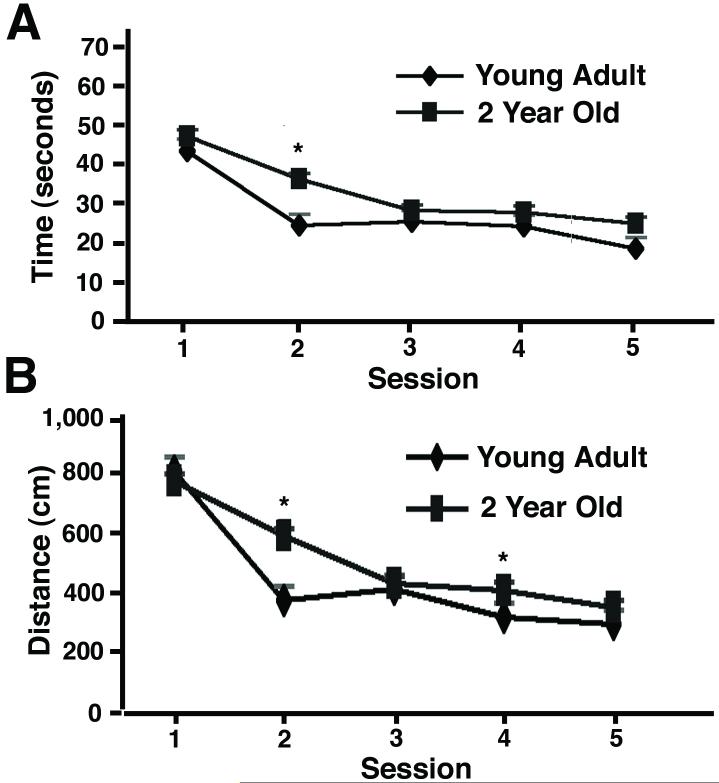
Overall, we found that the young adult rats learned the task faster than the aged rats. The young group of rats found the platform faster (Fig. 1A) and took a shorter route to it (Fig. 1B) than the aged group of rats (all data in this paper are mean±s.e.m.). A two-way ANOVA conducted on the time to locate the platform for each group confirmed the significant effect of group (p<0.001), and session (p<0.001), as the young group learned faster than the aged group, but both groups did improve over the five sessions of training. To localize these differences to specific sessions, one-way ANOVAs were performed on the data from each session; the differences between the groups was significant for session 2 (p<0.01), approached significance for session 5 (p=0.08) and were not significant for sessions 1, 3, or 4 (p>0.05). Additionally, a two-way ANOVA conducted on the distance traveled for each group confirmed the significant effect of group (p<0.01), and session (p<0.001), for this measure too. There was also a significant interaction between group and session (p<0.05). To localize these differences to specific sessions, one-way ANOVAs were performed on the data from each session; the differences between the groups were significant for sessions 2 or 4 (p<0.05), but were not significant for sessions 1, 3, or 5 (p>0.05). These differences appeared relatively modest, likely because improvements in performance are usually more modest in magnitude than deficits (see discussion). The superior learning of the young group was further confirmed by the probe test, performed on the day after session 5. In this probe test, the young adult rats spent significantly more time in the quadrant that had formerly contained the platform than did the aged group (Fig. 2A; p<0.05). The young adult rats also entered the quadrant that had contained the platform and crossed the platform position more times than did the aged group (Fig. 2B; p<0.01). Thus, these two-year old hybrid F344/Brown Norway F1 rats exhibit deficits in the water maze compared to young adult rats, equivalent to results obtained using other specific strains of rats (Rosenzweig and Barnes, 2003).
Figure 1.
Performance in the Morris water maze by young adult rats or 2-year old rats. The rats were trained daily in the water maze for 5 days. (A) The time to find the platform (mean±s.e.m.; young adult rats n=12 rats, 2-year old rats n=46 rats). (B) The distance traveled to the platform (mean±s.e.m.).
Figure 2.
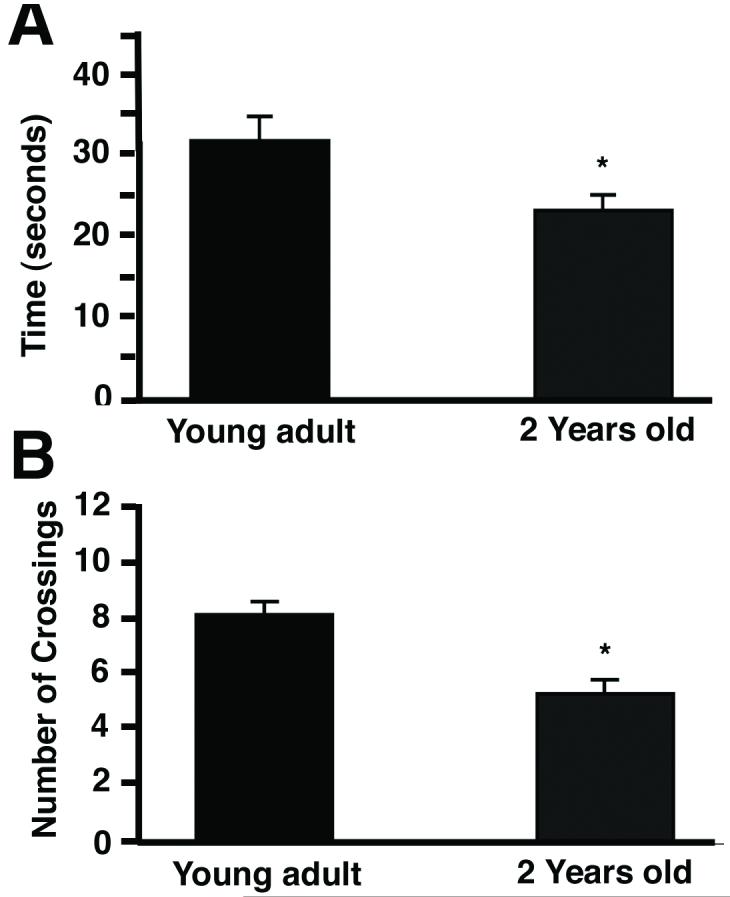
The time in the quadrant containing the platform, and the number of quadrant & platform crossings, in the probe trial, by young adult rats or 2-year old rats. On the day after the last test to find the platform (session 5 in Fig. 1), the rats were given a probe test. In the probe test, the platform was removed, and the rats were allowed to swim in the tank for 1 minute. (A) The amount of time the rats spent in the quadrant that had contained the platform (mean±s.e.m.). (B) The number of times the rats crossed the position of the platform, plus the number of times the rats entered the quadrant that had contained the platform (mean±s.e.m.).
Activation of PKC Pathways in Dentate Granule Neurons Corrects Deficits in Spatial Learning in Aged Rats
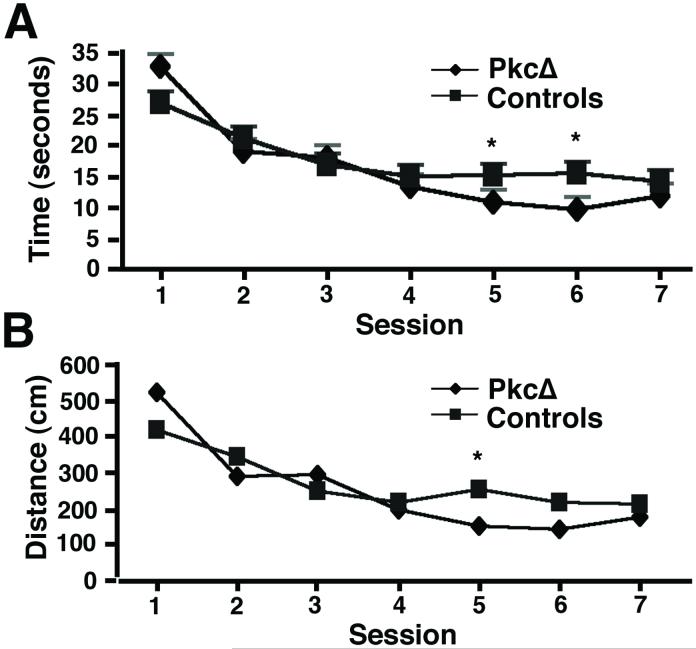
Following this training, the aged rats were divided into three groups and received gene transfer of vectors expressing either PkcΔ or PkcΔGG, or injections of PBS, into the hippocampus. After a two-week recovery period to allow the skull to become watertight, the rats were trained in the water maze, using a new, different position for the platform. During this new training, we found no significant differences in either time or distance to the platform between the PkcΔGG and PBS control groups (not shown). Two-way ANOVAs showed no effect of group for either time or distance to the platform (p>0.05) or its interaction with session (p>0.05). As both groups improved with training, there were significant effects of session for both time and distance traveled to the platform (p<0.02). Because there were no differences between the PkcΔGG and PBS groups of aged rats, these data were subsequently pooled into one combined control group. The improvement in time or distance to the platform during the training for this combined aged control group is shown in Fig. 3. This control group showed modestly better learning than the 2-year old rat group before gene transfer (compare Fig. 1, 2-year old rats to Fig. 3, control group); the 2-year old rat group contained all the aged rats before gene transfer, and a randomly chosen subset of these rats comprised the control group after gene transfer (see methods). The differences in performance between these tests likely reflects that these rats learned the water maze procedure during the first set of sessions before gene transfer, and thus performed better during the second set of sessions after gene transfer. All the aged rats received the same training before gene transfer, and were then randomly assigned to one of the gene transfer groups; thus, any effects on performance due to learning the water maze procedure before gene transfer were controlled for across the groups.
Figure 3.
Performance in the Morris water maze by the rats that received either PkcΔ or a control treatment. After gene transfer, the rats were given 2 weeks to recover and for the skull to become watertight. The rats were then trained daily in the water maze for 7 days. (A) The time to find the platform (mean±s.e.m.; PkcΔ n=17 rats, controls n=18 rats (PkcΔGG n=9 rats, PBS n=9 rats)). (B) The distance traveled to the platform (mean±s.e.m.).
Next, we compared the performance of the PkcΔ group with these control animals and found important and significant behavioral differences in performance. The PkcΔ group found the new location of the platform faster (Fig. 3A) and took a shorter route (Fig. 3B) than the control group. A two-way ANOVA conducted on time to find the new platform revealed a significant effect of group (p<0.05), a significant effect of session (p<0.001), and a significant interaction between group and session (p<0.02). This interaction appeared to be because the PKC group found the platform faster over the latter sessions of training than the aged controls. To better localize these differences, one-way ANOVAs were performed on the data from each session. These revealed significant group differences for sessions 1, 5, or 6 (p<0.05). The two-way ANOVA conducted on distance traveled for the two groups in finding the new platform revealed a significant effect of session (p<0.001), and a significant interaction between group and session (p<0.05). This interaction, too, appeared to be because the PKC group found the platform more efficiently over the latter sessions of training than the aged controls. One-way ANOVAs performed on the data from each session showed significant differences between the PkcΔ and control group for sessions 1 and 5 (p<0.05), and a difference that approached significance for session 6 (p=0.097). Thus, despite the PkcΔ group performing worse than the aged control group during the first session (Fig. 3A and B), by session 5 and afterwards the performance of the PkcΔ group had improved to be significantly better than the aged control by both measures. Similar to Fig. 1, these differences appeared relatively modest in magnitude, and improvements in performance are typically more modest than deficits (see discussion). This superior learning was further confirmed by the probe test, performed on the day after session 7. In this test, the PkcΔ group spent significantly more time in the quadrant that had contained the platform than the control group (Fig. 4A; p<0.05). The PkcΔ group also entered the quadrant that had contained the platform, and crossed the platform position, more times than the control group (Fig. 4B; p<0.001). Thus, these results indicate that the PkcΔ group had a stronger representation of the platform’s spatial location than did the aged control group.
Figure 4.
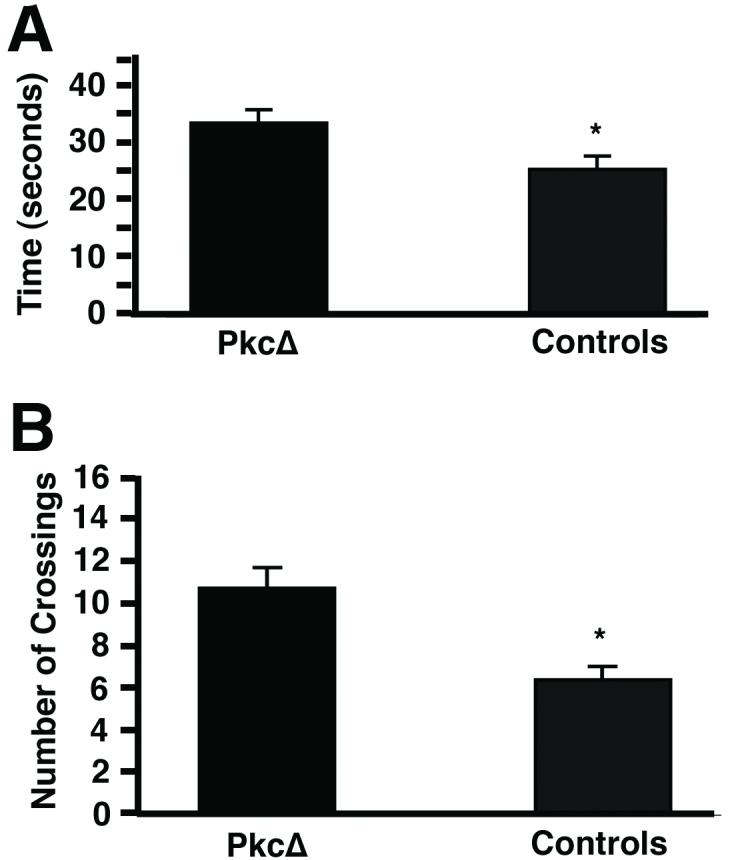
The time in the quadrant containing the platform, and the number of quadrant & platform crossings, in the probe trial, by rats that received either PkcΔ or a control treatment. On the day after that the last test to find the platform (session 7 in Fig. 3), the rats were given a probe test. In the probe test, the platform was removed, and the rats were allowed to swim in the tank for 1 minute. (A) The amount of time the rats spent in the quadrant that had contained the platform (mean±s.e.m.). (B) The number of times the rats crossed the position of the platform, plus the number of times the rats entered the quadrant that had contained the platform (mean±s.e.m.).
The rats that received PkcΔ exhibited apparently normal motor behavior in their home cages. Consistent with this normal motor behavior, gene transfer was specifically into the hippocampus (detailed in next section), a cognitive area, and gene transfer was not into a locomotion control area, such as primary motor cortex. Thus, it is unlikely that the improved performance in the water maze reported here was due to nonspecific changes in locomotion.
Recombinant Gene Expression
Rats were sacrificed following completion of the behavioral training, ∼3.5 weeks after gene transfer. These vectors contain both pkcΔ (or pkcΔGG) and the Lac Z gene; we previously showed that 96 % of the cells that contained recombinant gene products coexpressed PkcΔ and ß-gal (Wang et al., 2001), and we obtained similar results in another study (Zhang et al., 2005). Thus, we used the sensitive assays for ß-gal to detect cells that contained recombinant gene products. ß-gal was assayed using X-gal staining (Zhang et al., 2000), and subregions within the hippocampus were revealed by counterstaining with neutral red. The injection sites were located in the dentate gyrus. Low power views showed that the dentate granule cell layer contained X-gal positive cells (Fig. 5A). Additionally, in some rats, some X-gal positive cells were located within the dentate gyrus, but dorsal to the granule cell layer (Fig. 5A). Most of the positive cells were located in groups near the injection sites. This section was chosen to show positive cells both within, and dorsal to, the granule cell layer. This one section contained a modestly thinner molecular layer proximal to the transduced cells; other sections showed no change in the thickness of the molecular layer proximal to the transduced cells. The modestly thinner molecular layer in this one section likely reflects variations in hippocampal morphology, sectioning, and section mounting; it is unlikely to be due to neurotoxicity, because this helper virus-free HSV-1 vector system causes minimal cell damage and immune response (Bowers et al., 2003; Fraefel et al., 1996; Olschowka et al., 2003; Zhang and Geller, 2002). High power views revealed X-gal positive, large cell bodies in the granule cell layer, suggestive of neuronal morphology (Fig. 5B), and neuronal identity is demonstrated below by costaining for a neuronal marker. Rats that received PBS lacked X-gal positive hippocampal cells, although some rats contained a low level of faint staining in brain vascular endothelium (not shown).
Figure 5.
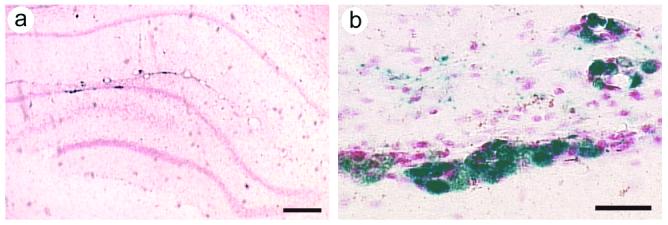
Recombinant gene expression in the dentate gyrus. After the water maze training was completed, 3-4 weeks after gene transfer, the rats were sacrificed, and ß-gal was detected with X-gal staining. These vectors coexpress PkcΔ (or PkcΔGG) and ß-gal. (A) A low power view from a rat that had received PkcΔ, shows X-gal positive cells in the dentate granule cell layer. The section was counterstained with neutral red. (B) A high power view showis X-gal positive cells with neuronal morphology in the dentate granule cell layer; some X-gal positive cells are also present outside of the granule cell layer. Scale bars: (A) 300 μm; (B) 30 μm.
To determine the numbers of cells that contained recombinant gene products, three hippocampii, from three different rats that received PkcΔ, were examined. X-gal staining was performed on every 4th section, along the entire longitudinal length of the hippocampus. Cell counts revealed 4,879±429 (mean ± s.e.m.) X-gal cells per hippocampus, and these cells were present in ∼2.4 mm in the anteroposterior direction. Although recombinant gene expression was in only ≤0.49 % of the ∼990,000 dentate granule neurons (Boss et al., 1985), the transduced cells were grouped together spatially, and the local density of transduced cells was greater than 1 %, accounting for the changes in learning (see discussion).
HSV-1 particles can be transported retrogradely through axons to distant sites. We examined the sections from the 3 hippocampii for X-gal cells outside of the dentate gyrus. Entorhinal cortex, a major projection area to the dentate gyrus, contained few positive cells, <1 % of the number of X-gal cells as the dentate gyrus (not shown; 45±6 X-gal cells). Also, the CA1-3 fields of the hippocampus contained <3 % of the number of X-gal cells as the dentate gyrus (97±14 X-gal cells).
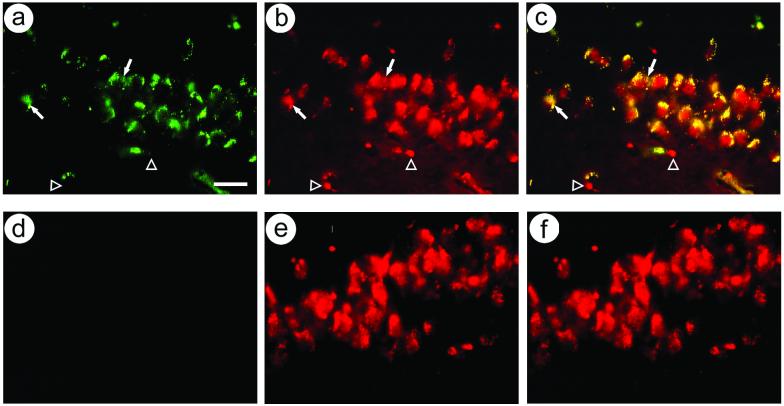
We confirmed that recombinant gene expression was targeted to neurons, as this vector contained a modified neurofilament promoter (Wang et al., 2001; Zhang et al., 2000). Double staining was performed using antibodies against either E. coli ß-gal or a neuronal marker, NeuN. Rats sacrificed after behavioral training contained numerous ß-gal-IR cells, and most of these cells contained NeuN-IR (Fig. 6A-C). Rats that did not receive gene transfer lacked ß-gal-IR cells, but contained NeuN-IR cells (Fig. 6D-F). Cell counts showed 98 % neuronal-specific expression (331 costained cells, 6 ß-gal-IR only cells). Similarly, other studies that used this modified neurofilament promoter reported ∼90 % neuronal-specific expression in the hippocampus (Zhang et al., 2000), postrhinal cortex (Zhang et al., 2005), or striatum (Sun et al., 2004; Sun et al., 2005; Sun et al., 2003; Zhang et al., 2000).
Figure 6.
Immunofluorescent colocalization of ß-gal-IR and NeuN-IR in cells in the hippocampal dentate granule cell layer. Rats were sacrificed after behavioral training. ß-gal-IR was visualized using a fluorescein-conjugated secondary antibody, and NeuN-IR was visualized using a rhodamine-conjugated secondary antibody. (A-C) Rat received PkcΔ; (A) ß-gal-IR, (B) NeuN-IR, and (C) merge. Filled arrows, costained cells; open arrowheads, cells contain NeuN-IR only. (D-F) Rat received PBS; (D) ß-gal-IR, (E) NeuN-IR, and (F) merge. Scale bar: 30 μm.
DISCUSSION
PkcΔ-Tranduced Hippocampal Dentate Granule Neurons Improved Spatial Learning
Overall, these findings importantly show that the PkcΔ condition improved spatial learning in aged, 2-year old, rats, compared to aged control conditions. The aged control rats received either PkcΔGG, a control vector lacking enzyme activity, or PBS. PkcΔ supported improved learning in the water maze as evidenced by learning the task faster, swimming to a new platform location more quickly and more directly, and showing stronger persistence in searching for this learned location after learning. Similar to previous studies on improved visual discrimination learning supported by PkcΔ (Zhang et al., 2005), the differences in learning were not present immediately upon the beginning of training, but emerged after several sessions. In fact, the PkcΔ group performed more poorly than the control group on session 1. The explanation for this latter effect is not known, but might be because the stronger representation of the new learning eventually produced by PkcΔ is formed modestly slower. If wt rats stored the post-gene transfer platform location in the same circuit as the pre-gene-transfer platform location; but the PkcΔ-transduced rats stored the post-gene-transfer platform location in a different circuit than the pre-gene-transfer platform location, specifically the circuit containing the transduced neurons; then the wt rats may support faster initial learning because they do not have to set up a new circuit for water maze learning. It seems unlikely that PkcΔ altered retrieval of the pre-gene-transfer platform location, because only ∼1 % of the dentate granule neurons contained PkcΔ (see below).
The magnitude of the improved spatial learning in these aged rats appears modest, especially in comparison to the decreases in performance produced by lesions, for example. This is because meaningful improvements in learning are limited by the maximum possible level of performance for a task. For example, our control rats traveled ∼250 cm to reach the platform by session 6; a lesion that reduced performance back to the initial levels observed in session 1 (>400 cm) would provide space for a large change of ∼150 cm. In contrast, a similar sized improvement in performance at the end of training would require finding the platform in 100 cm, a result that would be most surprising given the established baseline performance in the water maze. Thus, studies that look for improvements are likely by their nature to only detect and support modest increases in performance measures, such as time, distance, or accuracy (Dudai, 1989; Rumelhart et al., 1986). Also consistent with this thinking, pharmacological interventions that enhanced learning or memory typically produced only modest increases in performance; for example, using a radial arm maze, systemic opiate agonists produced modest enhancements in working memory (Canli et al., 1990). Importantly, it is easier to increase performance early in the training than after learning is almost complete. Thus, the largest increases in performance were observed between sessions 1 and 2, and young rats showed a larger improvement than aged rats, and aged rats that received PkcΔ showed a larger improvement than the controls. Overall, the young rats performed better than the aged rats, and the aged rats that received PkcΔ performed better than the controls, as shown by the two-way ANOVAs. However, the differences between the experimental and control groups on the later sessions were modest, and were significant only for some specific sessions, as shown by the one-way ANOVAs. Moreover, learning processes probably map nonlinearly onto dependent variables such as time or distance to the platform. Thus, although the performance measures of time or distance showed only modest differences, larger differences were measured on the probe test, which is designed to more directly measure the cognitive representation of the task. Specifically, either ∼25 % or ∼40 % differences were observed on the probe test for either time or numbers of platform position crossings, respectively, for both the comparison between young and aged rats and the comparison between aged rats that received PkcΔ and the controls. Similar to these results, only modest increases in performance have been observed in other genetic studies that were designed to improve performance. Using young adult rats, delivery of PkcΔ into postrhinal cortex neurons supported a modest enhancement in accuracy on a visual image discrimination test (Zhang et al., 2005), and delivery of PkcΔ into hippocampal dentate granule neurons supported a modest improvement in the time required for auditory reversal learning (Neill et al., 2001). Also, transgenic mice that overexpress NMDA receptor NR2B subunit displayed only modest increases in performance on several paradigms (Tang et al., 1999). In summary, the magnitude of the enhanced learning reported here is both typical for the literature and consistent with learning theory.
Most of the transduced cells were dentate granule neurons. The vast majority of the transduced cells were in the dentate gyrus, and very few transduced cells were observed in entorhinal cortex or in the CA1-3 subfields of the hippocampus. Similarly, previous studies using this helper virus-free HSV-1 vector system found that the vast majority of the transduced cells were located proximal to the injection sites in the hippocampal dentate gyrus (Neill et al., 2001), or postrhinal cortex (Zhang et al., 2005), or striatum (Sun et al., 2004; Sun et al., 2005; Sun et al., 2003; Zhang et al., 2000). Within the dentate gyrus, over 95 % of the transduced cells were neurons, and most of these neurons were located in the granule cell layer. Granule layer neurons are glutamatergic, and the other neurons in the dentate gyrus are GABAergic; consistent with these results, we previously showed that this vector supports expression in both glutamatergic and GABAergic neurons in postrhinal cortex (Zhang et al., 2005). Thus, the transduced dentate gyrus neurons, and likely the transduced granule neurons, supported the improved learning.
As gene transfer was not exclusively to dentate granule neurons, recombinant gene expression in ≤0.49 % of the ∼990,000 dentate granule neurons (Boss et al., 1985) was sufficient to improve spatial learning in these 2-year old rats. Similarly, in young adult rats, activation of PKC pathways in <1 % of the dentate granule neurons was sufficient to enhance auditory discrimination reversal learning (Neill et al., 2001), and activation of PKC pathways in <1 % of postrhinal cortex neurons was sufficient to enhance visual image discrimination learning (Zhang et al., 2005). Because the transduced cells were grouped together spatially, the local density of transduced cells was greater than 1 %. Our results are consistent with the postulate from neural network theory that activating a small fraction of the neurons in a circuit can potentiate that circuit (Rumelhart et al., 1986), and our results support a central hypothesis of learning theory, that specific circuits support learning of specific tasks (Dudai, 1989).
The gain-of-function strategy used in this study localizes learning to a specific circuit because it primes that circuit for learning in the presence of multiple circuits that could support the learning. In contrast, ablation/loss-of-function strategies must remove/inactivate all the circuits that can support the learning before deficits are observed. If multiple circuits within a forebrain area can support the learning, then all these circuits must be removed/inactivated before a learning deficit is observed. To obtain a deficit, most of a forebrain area must likely be affected by an ablation/loss-of-function intervention; specifically, the entire dorsal quarter of the hippocampus must be ablated before deficits in spatial learning are observed (Moser et al., 1995).
Dominant or Recessive Mutations in PKCß, and Expression of Mutated PkcßII in Different Types of Neurons, Support Different Changes in Learning
PkcΔ was obtained from the rat PKCßII isoform (Song et al., 1998), and PKCß knockout mice exhibit deficits in fear conditioning (Weeber et al., 2000). Following delivery of PkcΔ into hippocampal neurons, young adult rats exhibited enhanced auditory reversal learning (Neill et al., 2001), and aged rats exhibited improved spatial learning. Following delivery of PkcΔ into postrhinal cortex neurons, young adult rats exhibited enhanced visual image discrimination learning (Zhang et al., 2005). It is not surprising that following these different genetic manipulations, the rats exhibited different changes in learning, because these three genetically modified rodents differ in three critical aspects: First, the types of cells that contain or lack specific PKCs. PKCß knockout mice lack PKCß in cells throughout the brain. In contrast, PkcΔ was placed under the control of a heterologous promoter (the modified neurofilament promoter (Zhang et al., 2000)) in a virus vector, and PkcΔ was delivered into small groups of either hippocampal or postrhinal cortex neurons. Second, PkcΔ lacks most of the regulatory domain that supports intracellular targeting to specific compartments (Tanaka and Nishizuka, 1994); PkcΔ is localized to different subcellular compartments than either PKCß isoform (PKCßI or PKCßII). Third, PkcΔ enzyme activity is regulated differently than either PKCß isoform; PkcΔ is constitutively active whereas the PKCß isoforms contain the regulatory domain that controls their activity. Of note, PkcΔ has activity for each of the eight PKC substrates we have examined to date (Song et al., 1998; Zhang et al., 2005), and the catalytic domains of all the PKC isoforms contain extensive sequence homology (Tanaka and Nishizuka, 1994). Thus, in the paradigm examined here, replacing PkcΔ with a catalytic domain from a different PKC isoform might yield similar results.
Gene Therapy Strategies to Improve Learning in Aged Animals
Many previous gene therapy approaches to correct aging-induced cognitive deficits have focused on the cholinergic basal forebrain system. Either fibroblast cells or neural progenitor cells genetically engineered to produce nerve growth factor (NGF), and transplanted into specific basal forebrain nuclei, improved spatial learning in aged rats (Chen and Gage, 1995; Martinez-Serrano et al., 1996), and protected cholinergic basal forebrain neurons in lesioned monkeys (Tuszynski et al., 1996). Similarly, delivery of adeno-associated virus (AAV) vectors, lentivirus vectors, or adenovirus vectors that express NGF into specific basal forebrain nuclei protects cholinergic neurons from degeneration in aged rats, or following transection of the fimbria-fornix, and improved spatial learning (Blesch et al., 2005; Klein et al., 2000; Mandel et al., 1999; Wu et al., 2005; Wu et al., 2004; Zou et al., 2002). In another approach, also using either aged rats or transection of the fimbria-fornix, fibroblasts genetically engineered to express choline acetyltransferase, and transplanted into the hippocampus or neocortex, supported improved spatial learning (Dickinson-Anson et al., 1998; Dickinson-Anson et al., 2003). Strategies using either a neurotrophic factor or acetylcholine are designed to modulate the activity of hippocampal or cortical neurons.
In contrast, the strategy developed here alters a specific signal transduction pathway within these neurons, the PKC pathway. PKC signaling is impaired in aged rodents (Battaini et al., 1995; Battaini et al., 1999; Battaini et al., 1997; Colombo and Gallagher, 2002; Colombo et al., 1997; Fordyce and Wehner, 1993; Nicolle et al., 1999; Pascale et al., 1996; Van der Zee et al., 2004). Thus, gene transfer of PkcΔ may provide an approach for studying aging-induced changes in PKC signaling. Moreover, gene transfer of PkcΔ represents a potential gene therapy treatment for aging-induced cognitive deficits.
Acknowledgements
We thank Drs. J. Knopf for the rat PKCßII cDNA, A. Davison for HSV-1 cosmid set C, R. Sandri-Goldin for 2-2 cells, K. O’Malley for the TH promoter, W. Schlaepfer for the NF-H promoter, G. Felsenfeld for the chicken ß-globin insulator, and Ms. Caroline Savage for assistance with some water maze training.
Grant sponsor (G.Z.): NIH; Grant number: AG025894. Grant sponsor (R.C.): NSF. Grant sponsor (A.G.): NIH; Grant numbers: AG021193, NS043107, NS045855, and NS057558.
REFERENCES
- Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993a;75(7):1253–62. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKC gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993b;75(7):1263–71. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- Attaway CM, Compton DM, Turner MD. The effects of nicotine on learning and memory: a neuropsychological assessment in young and senescent Fischer 344 rats. Physiol Behav. 1999;67(3):421–31. doi: 10.1016/s0031-9384(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Battaini F, Elkabes S, Bergamaschi S, Ladisa V, Lucchi L, De Graan PN, Schuurman T, Wetsel WC, Trabucchi M, Govoni S. Protein kinase C activity, translocation, and conventional isoforms in aging rat brain. Neurobiol. Aging. 1995;16(2):137–48. doi: 10.1016/0197-4580(94)00154-5. [DOI] [PubMed] [Google Scholar]
- Battaini F, Pascale A, Lucchi L, Pasinetti GM, Govoni S. Protein kinase C anchoring deficit in postmortem brains of Alzheimer’s disease patients. Exp. Neurol. 1999;159(2):559–64. doi: 10.1006/exnr.1999.7151. [DOI] [PubMed] [Google Scholar]
- Battaini F, Pascale A, Paoletti R, Govoni S. The role of anchoring protein RACK1 in PKC activation in the ageing rat brain. Trends Neurosci. 1997;20(9):410–5. doi: 10.1016/s0166-2236(97)01084-9. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3(4):227–34. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Blesch A, Conner J, Pfeifer A, Gasmi M, Ramirez A, Britton W, Alfa R, Verma I, Tuszynski MH. Regulated lentiviral NGF gene transfer controls rescue of medial septal cholinergic neurons. Mol Ther. 2005;11(6):916–25. doi: 10.1016/j.ymthe.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Boss BD, Peterson GM, Cowan WM. On the number of neurons in the dentate gyrus of the rat. Brain Res. 1985;338(1):144–50. doi: 10.1016/0006-8993(85)90257-4. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, Olschowka JA, Federoff HJ. Immune responses to replication-defective HSV-1 type vectors within the CNS: implications for gene therapy. Gene Ther. 2003;10(11):941–5. doi: 10.1038/sj.gt.3302047. [DOI] [PubMed] [Google Scholar]
- Canli T, Cook RG, Miczek KA. Opiate antagonists enhance the working memory of rats in the radial maze. Pharmacol. Biochem. Behav. 1990;36(3):521–5. doi: 10.1016/0091-3057(90)90250-l. [DOI] [PubMed] [Google Scholar]
- Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim JJ, Hashimoto K, Thompson RF, Tonegawa S. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKC gamma mutant mice. Cell. 1995;83(7):1233–42. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Chen KS, Gage FH. Somatic gene transfer of NGF to the aged brain: behavioral and morphological amelioration. J Neurosci. 1995;15(4):2819–25. doi: 10.1523/JNEUROSCI.15-04-02819.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300(5626):1751–5. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Gallagher M. Individual differences in spatial memory among aged rats are related to hippocampal PKCgamma immunoreactivity. Hippocampus. 2002;12(2):285–9. doi: 10.1002/hipo.10016. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Wetsel WC, Gallagher M. Spatial memory is related to hippocampal subcellular concentrations of calcium-dependent protein kinase C isoforms in young and aged rats. Proc. Natl. Acad. Sci. U.S.A. 1997;94(25):14195–9. doi: 10.1073/pnas.94.25.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RG, Geller AI, Zhang G, Gowda R. Touchscreen enhanced visual learning in rats. Behav. Res. Methods Instrum. Comput. 2004;36:101–6. doi: 10.3758/bf03195555. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V, Grady CL, McIntosh AR. Dissecting the effect of aging on the neural substrates of memory: deterioration, preservation or functional reorganization? Rev. Neurosci. 2002;13(2):167–81. doi: 10.1515/revneuro.2002.13.2.167. [DOI] [PubMed] [Google Scholar]
- Dickinson-Anson H, Aubert I, Gage FH, Fisher LJ. Hippocampal grafts of acetylcholine-producing cells are sufficient to improve behavioural performance following a unilateral fimbria-fornix lesion. Neuroscience. 1998;84(3):771–81. doi: 10.1016/s0306-4522(97)00543-5. [DOI] [PubMed] [Google Scholar]
- Dickinson-Anson H, Winkler J, Fisher LJ, Song HJ, Poo M, Gage FH. Acetylcholine-secreting cells improve age-induced memory deficits. Mol Ther. 2003;8(1):51–61. doi: 10.1016/s1525-0016(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The Neurobiology of Memory. Oxford Univ. Press; Oxford, England: 1989. [Google Scholar]
- Fordyce DE, Wehner JM. Effects of aging on spatial learning and hippocampal protein kinase C in mice. Neurobiol. Aging. 1993;14(4):309–17. doi: 10.1016/0197-4580(93)90116-s. [DOI] [PubMed] [Google Scholar]
- Fraefel C, Song S, Lim F, Lang P, Yu L, Wang Y, Wild P, Geller AI. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J. Virol. 1996;70(10):7190–7. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AI, Breakefield XO. A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science. 1988;241(4873):1667–9. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hebb D. The organization of behavior. Wiley; New York: 1949. [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC. Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J. Biol. Chem. 2003;278(41):40305–16. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218(4571):433–43. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Chen C, Abeliovich A, Aiba A, Kurihara H, Watanabe M, Inoue Y, Tonegawa S. Impaired synapse elimination during cerebellar development in PKC gamma mutant mice. Cell. 1995;83(7):1223–31. doi: 10.1016/0092-8674(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain) J. Biol. Chem. 1989;264(7):4088–92. [PubMed] [Google Scholar]
- Klein RL, Hirko AC, Meyers CA, Grimes JR, Muzyczka N, Meyer EM. NGF gene transfer to intrinsic basal forebrain neurons increases cholinergic cell size and protects from age-related, spatial memory deficits in middle-aged rats. Brain Res. 2000;875(1-2):144–51. doi: 10.1016/s0006-8993(00)02634-2. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat. Neurosci. 2002;5(4):295–6. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285(5435):1870–4. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Gage FH, Clevenger DG, Spratt SK, Snyder RO, Leff SE. Nerve growth factor expressed in the medial septum following in vivo gene delivery using a recombinant adeno-associated viral vector protects cholinergic neurons from fimbria-fornix lesion-induced degeneration. Exp Neurol. 1999;155(1):59–64. doi: 10.1006/exnr.1998.6961. [DOI] [PubMed] [Google Scholar]
- Martinez-Serrano A, Fischer W, Soderstrom S, Ebendal T, Bjorklund A. Long-term functional recovery from age-induced spatial memory impairments by nerve growth factor gene transfer to the rat basal forebrain. Proc. Natl. Acad. Sci. U. S. A. 1996;93(13):6355–60. doi: 10.1073/pnas.93.13.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc. Natl. Acad. Sci. U.S.A. 1995;92(21):9697–701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JC, Sarkisian MR, Wang Y, Liu Z, Yu L, Tandon P, Zhang G, Holmes GL, Geller AI. Enhanced auditory reversal learning by genetic activation of protein kinase C in small groups of rat hippocampal neurons. Molec. Brain Res. 2001;93:127–36. doi: 10.1016/s0165-3806(01)00204-8. [DOI] [PubMed] [Google Scholar]
- Nichols RA, Haycock JW, Wang JK, Greengard P. Phorbol ester enhancement of neurotransmitter release from rat brain synaptosomes. J. Neurochem. 1987;48(2):615–21. doi: 10.1111/j.1471-4159.1987.tb04137.x. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Colombo PJ, Gallagher M, McKinney M. Metabotropic glutamate receptor-mediated hippocampal phosphoinositide turnover is blunted in spatial learning-impaired aged rats. J. Neurosci. 1999;19(21):9604–10. doi: 10.1523/JNEUROSCI.19-21-09604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JD, Geller AI, Zhang G, Chase TN. Gene transfer of constitutively active protein kinase C into striatal neurons accelerates onset of levodopa-induced motor response alterations in parkinsonian rats. Brain Res. 2003;971:18–30. doi: 10.1016/S0006-8993(03)02348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds JL, Anderson ML, McPhie DL, Staten LD, Alkon DL. Imaging of memory-specific changes in the distribution of protein kinase C in the hippocampus. Science. 1989;245(4920):866–9. doi: 10.1126/science.2772638. [DOI] [PubMed] [Google Scholar]
- Olds JL, Golski S, McPhie DL, Olton D, Mishkin M, Alkon DL. Discrimination learning alters the distribution of protein kinase C in the hippocampus of rats. J. Neurosci. 1990;10(11):3707–13. doi: 10.1523/JNEUROSCI.10-11-03707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschowka JA, Bowers WJ, Hurley SD, Mastrangelo MA, Federoff HJ. Helper-free HSV-1 amplicons elicit a markedly less robust innate immune response in the CNS. Mol. Ther. 2003;7(2):218–27. doi: 10.1016/s1525-0016(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Pascale A, Fortino I, Govoni S, Trabucchi M, Wetsel WC, Battaini F. Functional impairment in protein kinase C by RACK1 (receptor for activated C kinase 1) deficiency in aged rat brain cortex. J. Neurochem. 1996;67(6):2471–7. doi: 10.1046/j.1471-4159.1996.67062471.x. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313(5790):1141–4. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sidney: 1986. [DOI] [PubMed] [Google Scholar]
- Powell CM, Johnston D, Sweatt JD. Autonomously active protein kinase C in the maintenance phase of N-methyl-D-aspartate receptor-independent long term potentiation. J. Biol. Chem. 1994;269(45):27958–63. [PMC free article] [PubMed] [Google Scholar]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci U S A. 1994;91(3):839–43. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003;69(3):143–79. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rumelhart DE, McClelland JL, Group PR. Parallel Distributed Processing. MIT Press; Cambridge MA: 1986. [Google Scholar]
- Schechtman D, Mochly-Rosen D. Adaptor proteins in protein kinase C-mediated signal transduction. Oncogene. 2001;20(44):6339–47. doi: 10.1038/sj.onc.1204778. [DOI] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J. Neurosci. 2003;23(27):9220–8. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317(5840):951–3. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Smith IL, Hardwicke MA, Sandri-Goldin RM. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186(1):74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- Song S, Wang Y, Bak SY, During MJ, Bryan J, Ashe O, Ullrey DB, Trask LE, Grant FD, O’Malley KL. Modulation of rat rotational behavior by direct gene transfer of constitutively active protein kinase C into nigrostriatal neurons. J. Neurosci. 1998;18(11):4119–32. doi: 10.1523/JNEUROSCI.18-11-04119.1998. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Kong L, Wang X, Holmes C, Gao Q, Zhang W, Pfeilschifter J, Goldstein DS, Geller AI. Coexpression of Tyrosine Hydroxylase, GTP Cyclohydrolase I, Aromatic Amino Acid Decarboxylase, and Vesicular Monoamine Transporter-2 from a Helper Virus-Free HSV-1 Vector Supports High-Level, Long-Term Biochemical and Behavioral Correction of a Rat Model of Parkinson’s Disease. Hum. Gene Ther. 2004;15:1177–1196. doi: 10.1089/hum.2004.15.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Kong L, Wang X, Lu X, Gao Q, Geller AI. Comparison of protection of nigrostriatal neurons by expression of GDNF, BDNF, or both neurotrophic factors. Brain Res. 2005;1052:119–29. doi: 10.1016/j.brainres.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Zhang G, Kong L, Holmes C, Wang X, Zhang W, Goldstein DS, Geller AI. Correction of a rat model of Parkinson’s disease by coexpression of tyrosine hydroxylase and aromatic amino acid decarboxylase from a helper virus-free herpes simplex virus type 1 vector. Hum. Gene Ther. 2003;14:415–24. doi: 10.1089/104303403321467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Zhang GR, Yang T, Yu L, Geller AI. Improved titers for helper virus-free herpes simplex virus type 1 plasmid vectors by optimization of the packaging protocol and addition of noninfectious herpes simplex virus-related particles (previral DNA replication enveloped particles) to the packaging procedure. Hum. Gene Ther. 1999;10(12):2005–11. doi: 10.1089/10430349950017365. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Ann. Rev. Neurosci. 1994;17:551–67. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401(6748):63–9. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Roberts J, Senut MC, U HS, Gage FH. Gene therapy in the adult primate brain: intraparenchymal grafts of cells genetically modified to produce nerve growth factor prevent cholinergic neuronal degeneration. Gene Ther. 1996;3(4):305–14. [PubMed] [Google Scholar]
- Van der Zee EA, Palm IF, O’Connor M, Maizels ET, Hunzicker-Dunn M, Disterhoft JF. Aging-related alterations in the distribution of Ca(2+)-dependent PKC isoforms in rabbit hippocampus. Hippocampus. 2004;14(7):849–60. doi: 10.1002/hipo.20000. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang G, Sun M, Geller AI. General strategy for constructing large HSV-1 plasmid vectors that co-express multiple genes. BioTechniques. 2001;31:204–12. doi: 10.2144/01311dd05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Smith SJ. Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms. J. Neurosci. 2000;20(21):7863–70. doi: 10.1523/JNEUROSCI.20-21-07863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, Sweatt JD. A role for the beta isoform of protein kinase C in fear conditioning. J. Neurosci. 2000;20(16):5906–14. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Jakubek K, Wymbs N. Age-related declines in prospective memory: behavioral and electrophysiological evidence. Neurosci. Biobehav. Rev. 2002;26(7):827–33. doi: 10.1016/s0149-7634(02)00069-6. [DOI] [PubMed] [Google Scholar]
- Wu K, Meyer EM, Bennett JA, Meyers CA, Hughes JA, King MA. AAV2/5-mediated NGF gene delivery protects septal cholinergic neurons following axotomy. Brain Res. 2005;1061(2):107–13. doi: 10.1016/j.brainres.2005.08.056. [DOI] [PubMed] [Google Scholar]
- Wu K, Meyers CA, Guerra NK, King MA, Meyer EM. The effects of rAAV2-mediated NGF gene delivery in adult and aged rats. Mol. Ther. 2004;9(2):262–9. doi: 10.1016/j.ymthe.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Wu WW, Oh MM, Disterhoft JF. Age-related biophysical alterations of hippocampal pyramidal neurons: implications for learning and memory. Ageing Res. Rev. 2002;1(2):181–207. doi: 10.1016/s1568-1637(01)00009-5. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wang X, Kong L, Lu X, Lee B, Liu M, Sun M, Franklin C, Cook RG, Geller AI. Genetic enhancement of visual learning by activation of protein kinase C pathways in small groups of rat cortical neurons. Journal of Neuroscience. 2005;25:8468–81. doi: 10.1523/JNEUROSCI.2271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Wang X, Yang T, Sun M, Zhang W, Wang Y, Geller AI. A tyrosine hydroxylase--neurofilament chimeric promoter enhances long-term expression in rat forebrain neurons from helper virus-free HSV-1 vectors. Molec. Brain. Res. 2000;84:17–31. doi: 10.1016/s0169-328x(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Zhang W, Geller A. In Chinese: Comparison of the immune response to gene transfer into the rat brain with helper or helper virus-free HSV-1 vectors. Hua Xi Yi Ke Da Xue Xue Bao. 2002;33(2):175–8. [PubMed] [Google Scholar]
- Zou L, Yuan X, Long Y, Shine HD, Yang K. Improvement of spatial learning and memory after adenovirus-mediated transfer of the nerve growth factor gene to aged rat brain. Hum Gene Ther. 2002;13(18):2173–84. doi: 10.1089/104303402320987860. [DOI] [PubMed] [Google Scholar]