Abstract
Carotenoids, vitamin A and tocopherols serve important roles in many key body functions. However, availability of these compounds may be decreased in patients with short bowel syndrome (SBS) due to decreased oral intake of fruits and vegetables and/or decreased intestinal absorption. Little information is available on serum concentrations of carotenoids, vitamin A and tocopherols during chronic parenteral nutrition (PN) or during PN weaning. The aim of this study was to prospectively examine serum concentrations of a wide variety of carotenoids, vitamin A and tocopherols in SBS patients undergoing an intensive 12-week intestinal rehabilitation program. Twenty-one PN-dependent adult SBS patients were enrolled in a 12-week intestinal rehabilitation program, which included individualized dietary modification, multivitamin supplementation, and randomization to receive either s.c. placebo (n=9) or human growth hormone (GH, 0.1 mg/kg/day). PN weaning was initiated after week 4 and advanced as tolerated. Serum concentrations of carotenoids, vitamin A and tocopherols were determined at baseline and at weeks 4 and 12. Results showed that a significant % of subjects exhibited low serum concentrations for carotenoids and α-tocopherol at study entry, while a few subjects had low concentrations of retinol (5%). Serum α-tocopherol concentration was negatively associated with PN lipid dose (r = - 0.34, p < 0.008). We conclude that SBS patients are depleted in diet-derived carotenoids despite oral and intravenous multivitamin supplementation and dietary adjustment during intestinal rehabilitation and PN weaning. Reduction of PN lipid infusion may improve serum α-tocopherol concentrations.
Keywords: short bowel syndrome, carotenoids, vitamin A, tocopherols, parenteral nutrition
Introduction
Carotenoids, vitamin A and tocopherols are diet-derived micronutrient compounds that participate in a wide variety of key body functions. Some carotenoids, including α-carotene, β-carotene, β-cryptoxanthin are precursors of vitamin A, whereas other carotenoids, such as lycopene, lutein and zeaxanthin, function independent of vitamin A activity [1]. Carotenoids are primarily derived from fruits and vegetables, while animal liver, eggs and whole or fortified milk are rich food sources of vitamin A [2]. Tocopherols are abundant in vegetable oils and nuts. These micronutrient compounds individually or collectively participate in functions relevant to vision, bone growth, cell proliferation and differentiation, immunity and antioxidant protection, among other functions [1, 3-5].
SBS is characterized by malabsorption, diarrhea, fluid and electrolyte disturbances, and malnutrition due to extensive loss of intestinal absorptive area after bowel removal [6]. In addition to macronutrient deficiency, inadequacy of micronutrient absorption is also a concern. SBS patients typically self-report that fruits and vegetables worsen diarrhea and commonly avoid these food items [7-8]. A low fat diet is also commonly recommended to SBS patients with in-continuity colon because of the decreased lipid absorption from the intestine and the resultant steatorrhea [7-8]. Patients with short bowel syndrome (SBS) may be at increased risk to develop deficiency in carotenoids, vitamin A and/or tocopherols, but little data on status of these compounds have been published in this condition.
Parenteral nutrition (PN) commonly prescribed to SBS patients include conventional multivitamin formulations that provide amounts of essential vitamins believed to meet metabolic needs. Oral multivitamin or multivitamin-mineral supplements are also recommended to SBS patients [particularly on days when PN is not infused], but in our experience these are commonly not prescribed by primary physicians unless they have specific expertise in the care of SBS patients [8]. Moreover, these oral supplementation regimens contain variable types and amounts of carotenoids, vitamin A and tocopherols. Also limited data suggest that the need for α-tocopherol may be increased in patients receiving standard soybean oil-based PN lipid emulsion [9-10].
Adequate provision of carotenoids, vitamin A and tocopherols may be important to promote nutrient absorption and intestinal adaptation in SBS patients. For example, vitamin A deficiency markedly inhibits small intestinal mucosal adaptive growth in rat model of massive small bowel resection [11]. Treatments under investigation for SBS patients include recombinant growth hormone [GH] administration and individualized dietary modification [12]. However, it is unknown whether these treatment regimens alter serum concentrations of carotenoids, vitamin A and tocopherols, particularly in the face of PN weaning. Therefore, this study was designed to prospectively examine serum concentrations of the major carotenoids, vitamin A and tocopherols in SBS patients before and during an intensive intestinal rehabilitation program. We also explored whether the use of conventional, soybean oil-based parenteral lipid emulsion affects serum levels of carotenoids, vitamin A and tocopherols over time.
Subjects and Methods
Study Subjects
The data derived for this study were obtained from a randomized, double-blind, placebo-controlled study primarily designed to determine the effect of an individualized modified oral diet, with or without GH, on nutrient absorption and PN weaning in adults with severe SBS. Twenty-one adult, PN-dependent SBS patients from the main trial had serum available for the current study. The etiology of SBS in these patients included abdominal injury and/or multiple surgery (n=10), Crohn's disease (n=5) and mesenteric ischemia (n=6) (Table 1). Eligible adult SBS subjects were clinically stable and received chronic PN more than 2 days per week. All subjects had an intact duodenum with or without partial or intact residual colon. Exclusion criteria include (1) history of previous stomach or esophageal resection, (2) remnant small bowel was less than 20 cm, (3) history of Type I diabetes or uncontrolled Type II diabetes, (4) significant renal impairment (defined as serum creatinine concentration > 1.8 mg/dL) or hepatic dysfunction (defined as serum total bilirubin concentration > 1.5 mg/dL), (5) history of colonic polyps or colonic adenomas, (6) history of malignancy within the previous 5 years, and (7) other severe chronic illnesses.
Table 1. Short bowel syndrome patient characteristics.
| treatment group | Total | ||
|---|---|---|---|
| Characteristic | Placebo (n=9) |
GH (n=12) |
|
| Age (years) 1 | 51 ± 5 1 | 47 ± 4 | 48 ± 3 |
| Gender 1 | 7F / 2 M | 7F / 5M | 14F / 7M |
| BMI (kg/m2) 1 | 25.0 ± 0.9 | 22.3 ± 0.6 | 23.5 ± 0.6 |
| Diagnoses (n) 2 | |||
| Abdominal Injury or multiple surgery | 4 | 6 | 10 |
| Crohn's disease | 1 | 4 | 5 |
| Mesenteric ischemia | 4 | 2 | 6 |
| Length of residual small bowel (cm) 1 | 97 ± 36 | 123 ± 31 | 112 ± 23 |
| Presence of colon (n) 2 | |||
| No colon | 3 | 4 | 7 |
| Half colon | 3 | 6 | 9 |
| Entire colon | 3 | 2 | 5 |
| PN regimen (days/wk) 1 | 6.3 ± 0.5 | 4.7 ± 0.6 | 5.4 ± 0.4 |
Data were reported as mean ± SEM or number of patients in group (n).
There were no significant differences between groups by 1 Student t-test or 2 Chi-square test.
This study was conducted at the General Clinical Research Center (GCRC) at Emory University Hospital, Atlanta, GA after approval by the Institutional Review Board (IRB) of Emory University and the GCRC Scientific Advisory Committee. Informed consent was obtained from all subjects.
Study procedures
The detailed study procedures were as presented previously [13]. Briefly, eligible subjects were initially admitted to the GCRC for 4 weeks and again at week 12 for metabolic and absorptive studies. During the baseline week (week 1), subjects received their usual medication and PN/hydration prescription, which incorporated Intralipid® (Kabi) a soybean oil-based lipid emulsion. All subjects also received a multivitamin solution in their PN as their routine PN regimen, which contained 1 mg vitamin A [retinol] and 10 mg vitamin E [as dl-α-tocopheryl acetate] per bag. An oral meal plan designed to replicate their habitual home diet was provided by the metabolic research kitchen nutritionist. At the end of the baseline week, a 4-day nutrient absorption study was conducted and % of dietary fat absorption determined, as previously described in detail [13]. Following the baseline week, a modified, individualized SBS oral diet was provided to all subjects, which principally provided complex carbohydrate, high protein, and low fat intake [8]. The meal plan was adjusted daily according to patient's preference, subjective tolerance, and daily clinical/metabolic responses (e.g. stool volume and consistency, urine output, physical examination findings, blood chemistries). Oral anti-diarrheal medication regimens were prescribed and adjusted as indicated. Current Recommended Dietary Allowances (RDAs) for vitamin A are 900 μg/d for males and 700 μg/d for females. The RDA for vitamin E is 15 mg/d for males and females [16]. A conventional high-potency oral multivitamin-mineral supplement (Centrum Silver® Wyeth, Richmond, VA) was administered twice daily. This regimen provided ∼1700 μg (7000 IU) vitamin A (71% from retinyl acetate and 29% from β-carotene), 90 mg vitamin E (as dl-α-tocopheryl acetate), 600 μg lycopene, 500 μg lutein and other micronutrients per day. Separate oral supplements of calcium, vitamin D, iron, zinc, potassium and magnesium were also started and adjusted as indicated based on weekly serum measurement and the need for PN.
In addition to modified diet, all subjects were randomized in a double-blind manner to receive daily subcutaneous infection of either sc saline as the placebo control (n=9) or GH (n=12; Serostim®, Serono, Inc., Rockland, MA) for 21 consecutive days after the baseline week. The GH dose was given at 0.1 mg/kg to a maximum dose of 6 mg/day. During the initial 28-day inpatient period, no attempt was made to wean PN but the PN/fluid volume was decreased in some subjects due to decreased diarrhea while consuming the SBS diet. At the end of study week 4, the 4-day nutrient absorption study was repeated. The subjects were then discharged home. For the subsequent 8 weeks, blinded study drug dosing was reduced to three times weekly (M, W, F). Weaning from PN was initiated and PN was decreased by the investigators using a standardized algorithm [13]. The home oral diet was followed and adjusted by the GCRC nutritionist and the investigators as indicated. At week 12, all subjects returned to the GCRC for a 7-day inpatient period and nutrient absorption study was repeated. The mean daily amount of parenteral lipid emulsion during the 4-day absorption study periods was recorded.
Blood samples were obtained at baseline week, week 4 and week 12 to determine the serum concentrations of carotenoids (α-carotene, trans β-carotene, cis β-carotene, β-cryptoxanthin, lutein & zeaxanthin, and lycopene), vitamin A (retinol, retinyl palmitate and retinyl stearate), and tocopherols [α-tocopherol and γ-tocopherol]. Serum concentrations of these nutrient compounds were measured using a modification of a reversed-phase HPLC method [14] performed at the Centers for Disease Control and Prevention (CDC) Nutrition Laboratory. Normal ranges of these nutrient compounds, reported in μg/dL units, were either 5-95th or 10-90th percentile values based on measurements in subjects enrolled in recent National Health and Nutrition Examination Surveys who were age-matched for this population [15]. Deficiencies of carotenoids, vitamin A and/or tocopherols were determined if serum levels of such nutrient compound were below its reference range. The α-tocopherol results in μg/dL were converted into μmol/L by multiplying by 0.02322 and the γ-tocopherol results in μg/dL were converted into μmol/L by multiplying by 0.02402. The α- and γ-tocopherol concentrations were then corrected by total cholesterol levels in mmol/L units. Statistical analyses in tocopherols were performed using values in μmol:mmol units.
Statistical Analysis
Analyses were performed using the SPSS statistical package (version 14.0, Chicago, IL). Data were reported as means ± SEMs. Student t-tests and Chi-square tests were carried out to compare results between control and GH groups and results between subjects who were weaned from PN and who were not weaned at week 12. Results from baseline to week 4 and baseline to week 12 were examined by paired sample t-test. Spearman's correlation test was used to examine the correlations between serum concentrations of carotenoids, vitamin A and tocopherols and PN lipid dose and intestinal fat absorption rates. P-values < 0.05 were considered statistically significant.
Results
The baseline demographics and clinical characteristics are summarized in Table 1. Patients receiving GH or placebo were similar in age, gender, BMI, diseases leading to SBS, length of residual small bowel, presence of colon and PN regimen at study entry. The median remnant post-duodenal small bowel length in the GH-treated patients was 75 cm (range=15-350cm) and in the control patients 60 cm (range=0-350 cm) (NS).The administration of GH had no effects on serum concentrations of carotenoids, vitamin A and tocopherols (data not shown). In addition, subjects in both control and GH groups received similar amounts of PN lipids (data not shown). Therefore, we present combined data from the entire cohort of 21 SBS subjects.
Serum concentrations of carotenoids over time
At baseline, a majority of these SBS patients exhibited very low carotenoid levels, compared with the reference ranges obtained from the NHANES surveys (Table 2, Fig 1). The serum concentrations of the individual carotenoids and sum of all carotenoids did not change from baseline at weeks 4 and 12 of measurement (Table 2). Similarly, the proportion of subjects with serum carotenoid depletion (essentially 100%) did not change during the 12-week study. The Spearman's correlations between serum concentrations of carotenoids and intestinal fat absorption rates were variable at each time point [data not shown] due to the small number of study subjects; however, when the available data from the three serial measurements of carotenoids and concomitant fat absorption were combined (n=58), significantly positive correlations with % fat absorption were observed for all individual carotenoids (α-carotene: r=0.39, p=0.003; trans β-carotene: r=0.45, p<0.001; cis β-carotene: r=0.27; p=0.04; β-cryptoxanthin: r= 0.32, p=0.014; lutein & zeaxanthin: r=0.31, p=0.02; lycopene: r=0.47, p<0.001; and the sum of carotenoids: r=0.46, p<0.001).
Table 2. serum concentrations of carotenoids, vitamin A and tocopherols In all short bowel syndrome subjects (n=21).
| Baseline | Week 4 | Week 12 | |
|---|---|---|---|
| Carotenoids (μg/dL) | |||
| α-carotene (0.8-10.7) † | 0.15 ± 0.04 | 0.14 ± 0.04 | 0.23 ± 0.07 |
| trans β-carotene (4.2-42.8) † | 0.80 ± 0.31 | 1.50 ± 0.54 | 2.44 ± 1.16 |
| cis β-carotene (N/A) | 0.16 ± 0.10 | 0.04 ± 0.02 | 0.14 ± 0.06 |
| β-cryptoxanthin (3.0-17.4) † | 0.29 ± 0.13 | 0.24 ± 0.10 | 0.27 ± 0.12 |
| lutein & zeaxanthin (7.7-27.3) † | 1.35 ± 0.43 | 1.36 ± 0.38 | 1.44 ± 0.46 |
| lycopene (10.7-38.1) † | 1.38 ± 0.42 | 1.32 ± 0.36 | 1.22 ± 0.34 |
| Sum of carotenoids | 4.13 ± 1.21 | 4.60 ± 1.15 | 5.66 ± 1.95 |
| Vitamin A (μg/dL) | |||
| retinyl palmitate (N/A) | 1.43 ± 0.41 | 1.23 ± 0.31 | 1.13 ± 0.28 |
| retinyl stearate (N/A) | 0.41 ± 0.21 | 0.21 ± 0.11 | 0.45 ± 0.23 |
| retinol (37.1-92.6) †† | 63.23 ± 6.13 | 63.70 ± 4.76 | 62.84 ± 5.86 |
| Tocopherols (μg/dL) | |||
| α-tocopherol (774-2620) †† | 819 ± 71 | 891 ± 99 | 966 ± 133 ‡ |
| γ-tocopherol (60-530) †† | 300 ± 48 | 243 ± 30 ‡ | 218 ± 20 |
| α-tocopherol : total cholesterol (μmol:mmol) | 6.08 ± 0.38 | 6.46 ± 0.47 | 7.22 ± 0.62 § |
| γ-tocopherol : total cholesterol (μmol:mmol) | 97.8 ± 14.8 | 79.8 ± 10.5 § | 76.7 ± 6.9 |
Data were reported as mean ± SEM
reference range (10-90th percentile) from NHANES 2001-02. Compared with 40-59 year olds, including both genders and all race/ethnicities.
reference range (5-95th percentile) from NHANES 1999-2002. Compared with 40-59 year olds, including both genders and all race/ethnicities.
N/A: reference ranges are not available.
p < 0.05 compared with baseline values by paired-sample t-test.
p < 0.01 compared with baseline values by paired-sample t-test.
Figure 1.
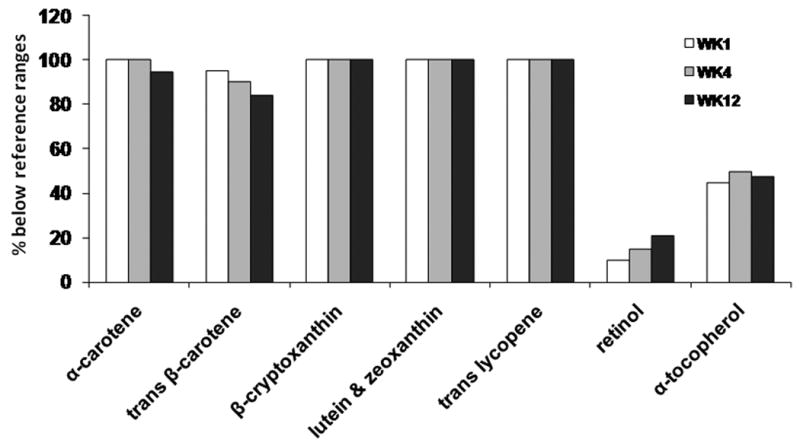
A high percentage of SBS patients exhibited below normal serum concentrations of carotenoids, vitamin A and tocopherols. SBS patient data were compared with 5-95th or 10-90th percentile reference values from subjects enrolled in NHANES surveys between 1999 and 2002. SBS patients underwent an intestinal rehabilitation protocol during which serial serum samples were collected at baseline and at weeks 4 and 12 for analyte concentration determinations.
At week 12, five subjects were successfully weaned from PN. The mean remnant post-duodenal small intestinal length was 208±52 cm (median=180 cm, range=75-350 cm) in the weaned subjects and 82±21 cm (median=65 cm, range=0-350cm) in the unweaned subjects (n=16). Although subjects who were weaned from PN at week 12 demonstrated higher concentrations of lutein & zeaxanthin at weeks 4 and 12, and higher trans-lycopene concentrations at baseline, week 4 and week 12, their values were still less than the lower limit of the reference range (Table 3). There were no differences in serum concentrations of α-carotene, trans β-carotene, cis β-carotene and β-cryptoxanthin between patients who were remained on PN by week 12 and patients who were weaned at any of the three measurements.
Table 3. Serum concentrations of carotenoids, vitamin A and tocopherols in short bowel syndrome patients during the treatment period.
| Week 1 | Week 4 | Week 12 | ||||
|---|---|---|---|---|---|---|
| (μg/dL) | Unweaned (n=15) |
Weaned (n=5) |
Unweaned (n=15) |
Weaned (n=5) |
Unweaned (n=14) |
Weaned (n=5) |
| α-carotene | 0.11 ± 0.05 | 0.26 ± 0.09 | 0.13 ± 0.06 | 0.18 ± 0.05 | 0.12 ± 0.04 | 0.32 ± 0.14 |
| trans β-carotene | 0.35 ± 0.11 | 2.16 ± 1.03 | 1.45 ± 0.71 | 1.64 ± 0.51 | 1.05 ± 0.39 | 6.20 ± 4.10 |
| cis β-carotene | 0.05 ± 0.02 | 0.50 ± 0.38 | 0.04 ± 0.03 | 0.04 ± 0.04 | 0.06 ± 0.03 | 0.38 ± 0.19 |
| β-cryptoxanthin | 0.10 ± 0.08 | 0.86 ± 0.36 | 0.09 ± 0.05 | 0.70 ± 0.33 | 0.12 ± 0.08 | 0.70 ± 0.35 |
| lutein & zeaxanthin | 0.62 ± 0.17 | 3.52 ± 1.27 | 0.66 ± 0.23 | 3.46 ± 0.83‡ | 0.59 ± 0.19 | 3.84 ± 1.14‡ |
| lycopene | 0.59 ± 0.24 | 3.76 ± 0.92‡ | 0.83 ± 0.37 | 2.76 ± 0.59‡ | 0.72 ± 0.27 | 2.62 ± 0.80‡ |
| retinyl palmitate | 1.38 ± 0.46 | 1.56 ± 0.96 | 1.22 ± 0.40 | 1.26 ± 0.37 | 1.02 ± 0.36 | 1.42 ± 0.40 |
| retinyl stearate | 0.34 ± 0.20 | 0.62 ± 0.62 | 0.25 ± 0.14 | 0.10 ± 0.10 | 0.52 ± 0.31 | 0.26 ± 0.16 |
| retinol | 54.71 ± 4.97 | 88.78 ± 15.40‡ | 58.43 ± 5.26 | 79.50 ± 7.52 | 53.79 ± 5.02 | 88.18 ± 11.84‡ |
| α-tocopherol | 740 ± 71 | 1057 ± 149‡ | 817 ± 122 | 1113 ± 121 | 768 ± 117 | 1520 ± 266‡‡ |
| γ-tocopherol | 296 ± 63 | 312 ± 43 | 241 ± 38 | 249 ± 47 | 215 ± 26 | 227 ± 33 |
| α-tocopherol: total cholesterol (μmol/mmol) | 5.87 ± 0.38 | 6.69 ± 1.05 | 6.08 ± 0.55 | 7.60 ± 0.78 | 6.45 ± 0.54 | 9.38 ± 1.55 § |
| γ-tocopherol: total cholesterol (μmol/mmol) | 103 ± 19.4 | 83.5 ± 10.5 | 82.6 ± 13.8 | 71.4 ± 8.4 | 82.2 ± 8.5 | 31.4 ± 8.9 |
Data were reported as mean ± SEM
p < 0.05 comparing unweaned and weaned subjects by Student t-test.
p=0.058.
p = 0.034
Serum concentrations of vitamin A over time
Serum concentrations of retinyl palmitate, retinyl stearate and retinol were not changed from baseline to weeks 4 and 12 in the 21 subjects (Table 2). Compared to the reference ranges, few subjects had low retinol concentrations during the 12-week study period (Fig 1). In addition, the retinyl palmitate and retinyl stearate concentrations were similar between weaned and unweaned subjects (Table 3). The weaned subjects demonstrated 36-64% higher retinol concentrations at baseline (p=0.012), week 4 (p=0.052) and week 12 (p=0.006) versus unweaned subjects. There was no correlation between serum concentrations of vitamin A and % intestinal fat absorption (data not shown).
Serum concentrations of tocopherols over time
Nine subjects (45%) exhibited low serum α-tocopherol concentrations at baseline (Fig 1). In all subjects, serum α-tocopherol levels (mol:mol ratio to total cholesterol concentrations) were significantly improved from the baseline to week 12 (p=0.007) whereas γ-tocopherol concentrations were reciprocally decreased at week 4 (p=0.006) and showed a trend to decrease further at week 12 (p=0.197) (Table 2).
Given the previous findings that parenteral lipid administration may contribute to depletion of serum α-tocopherol level in both hospital and non-hospital patients [9, 10], we examined the correlation between the daily dose of parenteral lipid emulsion administered at the time of blood sampling and the serum α-tocopherol and γ-tocopherol concentrations. The γ-tocopherol concentrations (mol:mol ratio to total cholesterol concentrations) did not change over time in weaned and unweaned patients and there was no relation between levels of this metabolite and PN lipid dose (Table 3). In contrast, we observed a significant, negative correlation between PN lipid dose and concomitant serum α-tocopherol concentrations (r= - 0.34, p=0.008, Fig 2) when combined values from the three serial measurements and lipid infusion doses at these times were analyzed (baseline, week 4 and week 12). In addition, the weaned subjects showed higher concentrations of α-tocopherol at week 12 (p=0.034) than values in unweaned subjects (Table 3). The increase in serum α-tocopherol levels (change from baseline to week 12) in weaned subjects (Δ= 2.69 μmol/mmol cholesterol, n=5) was statistical significant (p=0.04), whereas the serum α-tocopherol concentrations were unchanged in patients who remained on PN at week 12.
Figure 2.
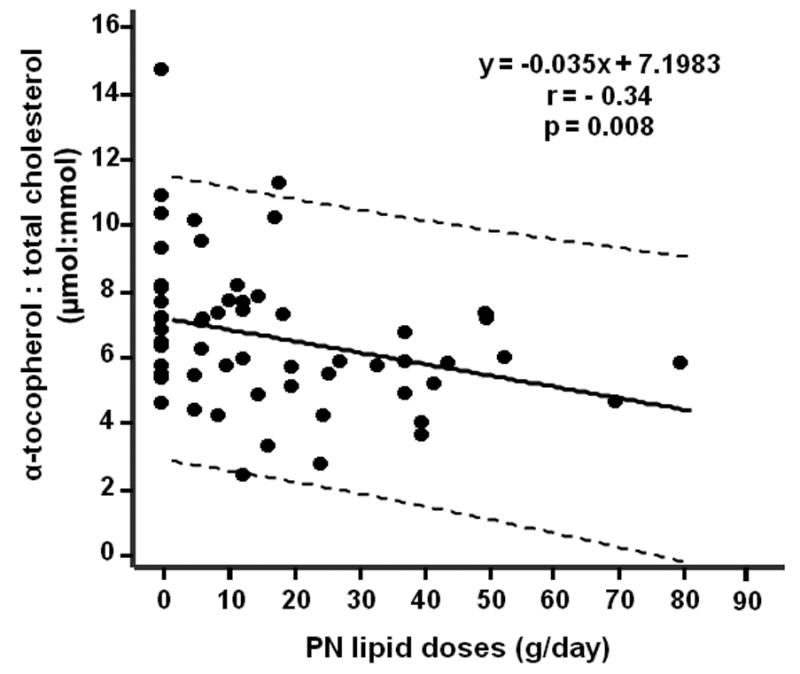
Inverse correlation between daily PN lipid dose and serum α-tocopherol concentrations. The relationship between mean daily dose of PN soybean oil-based lipid emulsion on the day of serial blood sampling was analyzed by regression analysis.
Discussion
Our study prospectively examined a comprehensive serum panel of carotenoids, vitamin A and tocopherols in SBS patients who underwent an intensive intestinal rehabilitation program. SBS patients are characterized by macro and micronutrient malabsorption and typically limit fruit and vegetable intake, which are the likely explanations for the low nutrient and phytochemical values we observed. We previously reported that in nineteen SBS patients from the current study, a large percentage had low habitual dietary intake of vitamin A (47%) and vitamin E (79%) compared with the RDA [8]. By examining the relationship between dietary pattern and antioxidant status in vegetarians and omnivores, Haldar et al found that plasma concentrations of lutein, α-cryptoxanthin, α-carotene and β-carotene were positively associated with vegetable intake, and β-cryptoxanthin level was an indicator of fruit intake [2]. Therefore, the low dietary intake of fruits and vegetables may partially explain the low serum carotenoid concentrations in this SBS patient cohort. However, we did not quantify the intake of amounts of specific dietary fruits or vegetables during the 12 week study period. The average intake of fiber (14 ± 1.7 g/day) in this population at the baseline week was much lower than the recommended amounts for general population [8]. All subjects received oral supplementation of β-carotene (493 μg/day), lycopene (600 μg/day) and lutein (500 μg/day) from the oral multivitamin after the baseline week. Despite this level of supplementation, the serum concentrations of the corresponding carotenoid compounds were unchanged from the baseline and were remained significantly below the lower limits of reference values over the 12 week study period (Figure 1, Table 2). In addition, although subjects who were weaned from PN at week 12 showed higher serum concentrations of lutein & zeaxanthin and lycopene compared with patients who remained on PN, possibly indicative of improved gut mucosal absorptive capacity versus individuals unable to be weaned, values in these patients were also below the reference ranges (Table 3). This may be attributed to low intestinal absorption rates of carotenoids because the digestion and absorption of carotenoids is dependent upon fat absorption [17]. As reported in our preliminary data from these subjects, the intestinal fat absorption rate remained low at baseline and weeks 4 and 12 (∼30% of intake) [18]. These results suggest that oral supplementation of carotenoids to improve serum concentrations in patients with significant malabsorption of fat will require considerably higher doses than are provided in available high-potency multivitamin-mineral supplements.
Retinol is the major circulating form of vitamin A in the blood whereas retinyl palmitate and retinyl stearate are vitamin A storage forms (retinyl esters) with highest concentrations in the liver [19]. These stored metabolites are released from the liver as retinol binding protein-bound-retinol into the circulation as needed to maintain vitamin A homeostasis [19]. The adequacy of vitamin A is important in growth of intestinal epithelial cells [6, 20]. Although SBS may have increased risk of vitamin A deficiency due to decreased intestinal fat absorption [21], almost all SBS subjects in this study had normal serum retinol concentrations from the baseline to week 12, suggesting the effectiveness of current intravenous and/or oral retinol supplementation. The mechanisms leading to higher serum retinol concentrations in weaned subjects may be associated with better intestinal absorption capacity in this subset [20]. However, it would be necessary to continue a vitamin A supplementation regimen with serial monitoring of levels after the PN weaning because of elimination of vitamin A given as a component of complete standard PN solutions.
About half of SBS patients in this study demonstrated low α-tocopherol concentrations at the baseline week and remained low during the 12-week study period compared with serum α-tocopherol concentrations from the NHANES surveys (Table 2). Unlike the carotenoid and retinoid responses over time, serum α-tocopherol levels (corrected by total cholesterol concentrations) gradually increased at week 4 (p=0.26) and week 12 (p=0.007) compared with baseline values in the cohort (Table 2). This improvement in α-tocopherol concentrations could be attributed, in part, to the decrease in PN dose of lipid emulsion, and/or improved absorption of this nutrient. Limited data suggests that soybean oil-based PN lipid emulsions may increase the α-tocopherol requirements in home PN patients [9] and in patients after high dose chemotherapy and irradiation receiving bone marrow transplantation [10], possibly due to utilization to neutralize peroxidized lipids [22, 23]. Our data are consistent with these earlier findings and showed that higher PN lipid doses were linearly associated with lower serum α-tocopherol concentrations (Fig 2). In addition, we showed that compared with subjects who were weaned from PN (and thus received no intravenous α-tocopherol or lipid emulsion), those who remained on PN appeared to have lower concentrations of serum α-tocopherol over time (Table 3).
It has been suggested soybean oil-based lipid emulsions may have potentially pro-inflammatory or pro-oxidant properties due to infusion of predominately ω-6 fatty acids [24]. These effects may be associated with depletion of α-tocopherol and decreased antioxidant capacity in the body [9, 23]. Therefore, the dose of lipid administration should be carefully determined when lipid is needed to provide adequate calories in PN-requiring SBS patients. Additional studies on the potential anti-inflammatory and redox effects of supplementation of higher α-tocopherol doses in PN in patients with SBS may be indicated; vitamin C and glutamine [a precursor of glutathione] may be needed in these patients based on the limited available research [22].
Limitations of our study include the small sample size, the lack of quantitative information on dietary intake of specific fruit, vegetable, or dietary fiber items in individual patients, lack of absorption studies for the specific nutrient compounds in response to test meals, and the relatively short 12-week timeframe during which these nutrient compounds were measured serially. Longer-term studies of carotenoid, vitamin A and tocopherol status and absorption in both adult and pediatric SBS patients, including response to specific supplementation during PN weaning, would be of interest.
In summary, we examined prospectively whether a rigorous intestinal rehabilitation program that incorporates dietary modification with or without GH administration improves serum concentrations of carotenoids, vitamin A and tocopherols in SBS patients, who appear to be at high risk of deficiency of these nutrients. This small study suggests that carotenoid and tocopherol deficiency, and to a lesser extent, vitamin A depletion, were common in our SBS patients. This did not improve with conventional intestinal rehabilitation dietary approaches, use of standard multivitamin-mineral preparations or GH administration. Serum α-tocopherol concentrations also increased concomitant with the reduction of PN lipid administration. Use of multivitamin preparations containing water-solubilized vitamin A and E, individual supplementation of solubilized vitamin A and E based on serial plasma values, increased fruit and vegetable consumption as tolerated, and aggressive dietary and other measures to diminish diarrhea and malabsorption are potential approaches that could be tested to improve carotenoid, vitamin A and tocopherol levels in SBS patients. Specific nutrient absorption studies would help guide recommendations in SBS patients. Serial blood levels of vitamin A, α-tocopherol and possibly carotenoids should be determined serially in SBS patients, particularly during intestinal rehabilitation when there is increased reliance on oral diet to maintain nutrition. Studies to define consequences of depletion and methods to restore serum levels of these important nutrient compounds are needed in SBS.
Acknowledgments
The study was supported by the National Institutes of Health grants R01 DK55850 (to TRZ) and General Clinical Research Center grant M01 RR00039. TRZ, JRG, and LML designed the study. JRG and TRZ enrolled subjects. ML, CFE and NB carried out the clinical protocol and collected samples. ML, CFE and TRZ analyzed the data and prepared the manuscript, which was reviewed by all authors. RLS provided HPLC data for the study.
Footnotes
None of the authors had a financial or personal conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55:207–16. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Haldar S, Rowland IR, Barnett YA, Bradbury I, Robson PJ, Powell J, et al. Influence of habitual diet on antioxidant status: a study in a population of vegetarians and omnivores. Eur J Clin Nutr. 2007;61:1011–22. doi: 10.1038/sj.ejcn.1602615. [DOI] [PubMed] [Google Scholar]
- 3.Tapiero H, Townsend DM, Tew KD. The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother. 2004;58:100–10. doi: 10.1016/j.biopha.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Mascio P, Murphy ME, Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991;53:194S–200. [PubMed] [Google Scholar]
- 5.Winklhofer-Roob BM, Rock E, Ribalta J, Shmerling DH, Roob JM. Effects of vitamin E and carotenoid status on oxidative stress in health and disease. Evidence obtained from human intervention studies. Molecular aspects of medicine. 2003;24:391–402. doi: 10.1016/s0098-2997(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler TR, Evans ME, Fernandez-Estivariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003;23:229–61. doi: 10.1146/annurev.nutr.23.011702.073036. [DOI] [PubMed] [Google Scholar]
- 7.Matarese LE, O'Keefe SJ, Kandil HM, Bond G, Costa G, Abu-Elmagd K. Short bowel syndrome: clinical guidelines for nutrition management. Nutr Clin Pract. 2005;20:493–502. doi: 10.1177/0115426505020005493. [DOI] [PubMed] [Google Scholar]
- 8.Estívariz CF, Umeakunne K, Bazargan N, Leader LM, Galloway JR, Ziegler TR. Habitual oral nutrient intake in patients with severe short bowel syndrome living in the southeastern United States. Nutrition. 2008;24:330–339. doi: 10.1016/j.nut.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pironi L, Ruggeri E, Zolezzi C, Savarino L, Incasa E, Belluzzi A, et al. Lipid peroxidation and antioxidant status in adults receiving lipid-based home parenteral nutrition. Am J Clin Nutr. 1998;68:888–93. doi: 10.1093/ajcn/68.4.888. [DOI] [PubMed] [Google Scholar]
- 10.Jonas CR, Puckett AB, Jones DP, Griffith DP, Szeszycki EE, Bergman GF, et al. Plasma antioxidant status after high-dose chemotherapy: a randomized trial of parenteral nutrition in bone marrow transplantation patients. Am J Clin Nutr. 2000;72:181–9. doi: 10.1093/ajcn/72.1.181. [DOI] [PubMed] [Google Scholar]
- 11.Swartz-Basile DA, Rubin DC, Levin MS. Vitamin A status modulates intestinal adaptation after partial small bowel resection. JPEN J Parenter Enteral Nutr. 2000;24:81–8. doi: 10.1177/014860710002400281. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler TR, Leader LM. Parenteral nutrition: transient or permanent therapy in intestinal failure? Gastroenterology. 2006;130:S37–42. doi: 10.1053/j.gastro.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 13.Luo M, Fernandez-Estivariz C, Manatunga AK, Bazargan N, Gu LH, Jones DP, et al. Are plasma citrulline and glutamine biomarkers of intestinal absorptive function in patients with short bowel syndrome? JPEN J Parenter Enteral Nutr. 2007;31:1–7. doi: 10.1177/014860710703100101. [DOI] [PubMed] [Google Scholar]
- 14.Sowell AL, Huff DL, Yeager PR, Caudill SP, Gunter EW. Retinol, a-tocopherol, b-cryptoxanthin, lycopene, a-carotene, trans-b-carotene, and four retinyl esters in serum determined simultaneously by reversed-phase HPLC with multi-wavelength detection. Clin Chem. 1994;40:411–416. [PubMed] [Google Scholar]
- 15. [July 28, 2008]; http://www.cdc.gov/nutritionreport.
- 16.A report of the panel on macronutrients, subcommittees on upper reference levels of nutrients and interpretation and uses of dietary reference intakes, and the standing committee on the scientific evaluation of dietary reference intakes. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients) Washington, D.C: National Academies Press; 2005. [Google Scholar]
- 17.Ribaya-Mercado JD. Influence of dietary fat on beta-carotene absorption and bioconversion into vitamin A. Nutr Rev. 2002;60:104–10. doi: 10.1301/00296640260085831. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Estivariz C, Luo M, Bazargan N, Gu LH, Sitaraman SV, Klapproth JM, et al. Effects of modified oral diet and human growth hormone on nutrient absorption and parenteral nutrition needs in adults with severe short bowel syndrome: results of a randomized, double-blind, placebo-controlled clinical trial [abstract] Gastroenterology. 2006;130:A68. [Google Scholar]
- 19.Biesalski HK, Nohr D. New aspects in vitamin A metabolism: the role of retinyl esters as systemic and local sources for retinol in mucous epithelia. J Nutr. 2004;134:3453S–7. doi: 10.1093/jn/134.12.3453S. [DOI] [PubMed] [Google Scholar]
- 20.Swartz-Basile DA, Rubin DC, Levin MS. Vitamin A status modulates intestinal adaptation after partial small bowel resection. JPEN J Parenter Enteral Nutr. 2000;24:81–8. doi: 10.1177/014860710002400281. [DOI] [PubMed] [Google Scholar]
- 21.Chae T, Foroozan R. Vitamin A deficiency in patients with a remote history of intestinal surgery. Br J Ophthalmol. 2006;90:955–6. doi: 10.1136/bjo.2006.092502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manuel-y-Keenoy B, Nonneman L, De Bosscher H, Vertommen J, Schrans S, Klutsch K, et al. Effects of intravenous supplementation with alpha-tocopherol in patients receiving total parenteral nutrition containing medium- and long-chain triglycerides. Eur J Clin Nutr. 2002;56:121–8. doi: 10.1038/sj.ejcn.1601294. [DOI] [PubMed] [Google Scholar]
- 23.Jonas CR, Ziegler TR. Nutrition support and antioxidant defenses: a cause for concern? Am J Clin Nutr. 1998;68:765–7. doi: 10.1093/ajcn/68.4.765. [DOI] [PubMed] [Google Scholar]
- 24.Krogh-Madsen R, Plomgaard P, Akerstrom T, Møller K, Schmitz O, Pedersen BK. Effect of short-term intralipid infusion on the immune response during low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab. 2008;294:E371–379. doi: 10.1152/ajpendo.00507.2007. [DOI] [PubMed] [Google Scholar]


