Abstract
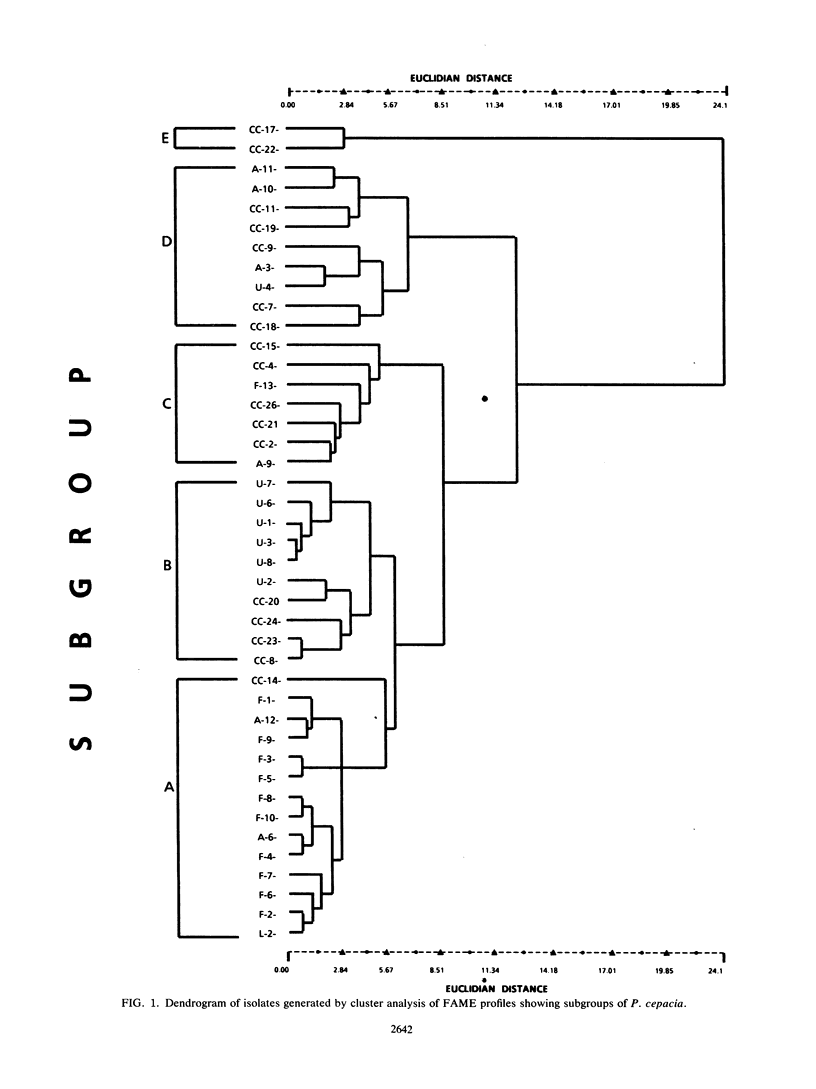
The cellular fatty acid compositions were determined for 42 strains of Pseudomonas cepacia from five cystic fibrosis centers in North America. All isolates contained significant (20%) amounts of hexadecanoic (C16:0), and cis-9 hexadecenoic (C16:1 cis9) acids and an isomer of octadecenoic acid (C18:1). None had hydroxy acids containing fewer than 14 carbon atoms. The quantitative data from the fatty acid analysis were highly reproducible and provided a basis for numerical analysis. Five subgroups comprising all the strains were obtained by cluster analysis and further characterized by principal-component analysis. With minor exceptions, the predominant subgroup identified in each center was different from that identified in other centers and accounted for one-half of the isolates within each center. Cellular fatty acid composition is a useful adjunct to biochemical characterization for the identification of P. cepacia isolated from cystic fibrosis patients. Numerical analysis of the fatty acid data can separate P. cepacia into subgroups, which may provide useful epidemiologic information or a basis for further analysis by more complex techniques such as DNA probe analysis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff S. C., Klinger J. D. Comparison of cefpiramide (HR-810) and four anti-pseudomonal beta-lactam agents against pseudomonas isolates from children with cystic fibrosis. J Antimicrob Chemother. 1985 May;15(5):545–549. doi: 10.1093/jac/15.5.545. [DOI] [PubMed] [Google Scholar]
- Aronoff S. C., Labrozzi P. H. Differences in drug susceptibility between isolates of Pseudomonas cepacia recovered from patients with cystic fibrosis and other sources and its relationship to beta-lactamase focusing pattern. Pediatr Pulmonol. 1986 Nov-Dec;2(6):368–372. doi: 10.1002/ppul.1950020609. [DOI] [PubMed] [Google Scholar]
- Bøe B., Gjerde J. Fatty acid patterns in the classification of some representatives of the families Enterobacteriaceae and Vibrionaceae. J Gen Microbiol. 1980 Jan;116(1):41–49. doi: 10.1099/00221287-116-1-41. [DOI] [PubMed] [Google Scholar]
- Christenson J. C., Welch D. F., Mukwaya G., Muszynski M. J., Weaver R. E., Brenner D. J. Recovery of Pseudomonas gladioli from respiratory tract specimens of patients with cystic fibrosis. J Clin Microbiol. 1989 Feb;27(2):270–273. doi: 10.1128/jcm.27.2.270-273.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees S. B., Hollis D. G., Weaver R. E., Moss C. W. Cellular fatty acid composition of Pseudomonas marginata and closely associated bacteria. J Clin Microbiol. 1983 Nov;18(5):1073–1078. doi: 10.1128/jcm.18.5.1073-1078.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees S. B., Powell J., Moss C. W., Hollis D. G., Weaver R. E. Cellular fatty acid composition of organisms frequently associated with human infections resulting from dog bites: Pasteurella multocida and groups of EF-4, IIj, M-5, and DF-2. J Clin Microbiol. 1981 Dec;14(6):612–616. doi: 10.1128/jcm.14.6.612-616.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerola E., Lehtonen O. P. Optimal data processing procedure for automatic bacterial identification by gas-liquid chromatography of cellular fatty acids. J Clin Microbiol. 1988 Sep;26(9):1745–1753. doi: 10.1128/jcm.26.9.1745-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass S., Govan J. R. Pseudomonas cepacia--fatal pulmonary infection in a patient with cystic fibrosis. J Infect. 1986 Sep;13(2):157–158. doi: 10.1016/s0163-4453(86)92953-1. [DOI] [PubMed] [Google Scholar]
- Goldmann D. A., Klinger J. D. Pseudomonas cepacia: biology, mechanisms of virulence, epidemiology. J Pediatr. 1986 May;108(5 Pt 2):806–812. doi: 10.1016/s0022-3476(86)80749-1. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Harris G. Typing of Pseudomonas cepacia by bacteriocin susceptibility and production. J Clin Microbiol. 1985 Oct;22(4):490–494. doi: 10.1128/jcm.22.4.490-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K. A., McGowan K. L., Fisher M. C., Schidlow D. V. Pseudomonas cepacia in the hospital setting: lack of transmission between cystic fibrosis patients. J Pediatr. 1986 Jul;109(1):51–54. doi: 10.1016/s0022-3476(86)80571-6. [DOI] [PubMed] [Google Scholar]
- Heidt A., Monteil H., Richard C. O and H serotyping of Pseudomonas cepacia. J Clin Microbiol. 1983 Sep;18(3):738–740. doi: 10.1128/jcm.18.3.738-740.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P. R., Gaston M. A. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988 Nov;26(11):2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984 Feb;104(2):206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- Lambert M. A., Patton C. M., Barrett T. J., Moss C. W. Differentiation of Campylobacter and Campylobacter-like organisms by cellular fatty acid composition. J Clin Microbiol. 1987 Apr;25(4):706–713. doi: 10.1128/jcm.25.4.706-713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiPuma J. J., Mortensen J. E., Dasen S. E., Edlind T. D., Schidlow D. V., Burns J. L., Stull T. L. Ribotype analysis of Pseudomonas cepacia from cystic fibrosis treatment centers. J Pediatr. 1988 Nov;113(5):859–862. doi: 10.1016/s0022-3476(88)80018-0. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Dees S. B. Cellular fatty acids and metabolic products of Pseudomonas species obtained from clinical specimens. J Clin Microbiol. 1976 Dec;4(6):492–502. doi: 10.1128/jcm.4.6.492-502.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Hyodo S., Chonan E., Shigeta S., Yabuuchi E. Serological classification of Pseudomonas cepacia by somatic antigen. J Clin Microbiol. 1986 Jul;24(1):152–154. doi: 10.1128/jcm.24.1.152-154.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell A. G., Nahaie M. R., Goodfellow M., Minnikin D. E., Hájek V. Numerical analysis of fatty acid profiles in the identification of staphylococci. J Gen Microbiol. 1985 Aug;131(8):2023–2033. doi: 10.1099/00221287-131-8-2023. [DOI] [PubMed] [Google Scholar]
- Ogle J. W., Janda J. M., Woods D. E., Vasil M. L. Characterization and use of a DNA probe as an epidemiological marker for Pseudomonas aeruginosa. J Infect Dis. 1987 Jan;155(1):119–126. doi: 10.1093/infdis/155.1.119. [DOI] [PubMed] [Google Scholar]
- Tablan O. C., Chorba T. L., Schidlow D. V., White J. W., Hardy K. A., Gilligan P. H., Morgan W. M., Carson L. A., Martone W. J., Jason J. M. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr. 1985 Sep;107(3):382–387. doi: 10.1016/s0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A., Doershuk C. F., Stern R. C., Klinger J. D. Pseudomonas cepacia: decrease in colonization in patients with cystic fibrosis. Am Rev Respir Dis. 1986 Oct;134(4):669–671. doi: 10.1164/arrd.1986.134.4.669. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A., Klinger J. D., Stern R. C. Pseudomonas cepacia colonization among patients with cystic fibrosis. A new opportunist. Am Rev Respir Dis. 1985 May;131(5):791–796. doi: 10.1164/arrd.1985.131.5.791. [DOI] [PubMed] [Google Scholar]
- Welch D. F., Muszynski M. J., Pai C. H., Marcon M. J., Hribar M. M., Gilligan P. H., Matsen J. M., Ahlin P. A., Hilman B. C., Chartrand S. A. Selective and differential medium for recovery of Pseudomonas cepacia from the respiratory tracts of patients with cystic fibrosis. J Clin Microbiol. 1987 Sep;25(9):1730–1734. doi: 10.1128/jcm.25.9.1730-1734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


