Abstract
Cholesterol-rich plasma membrane microdomains are important for entry of many viruses, including retro-viruses. Depletion of cholesterol with 2-hydroxypropyl-β-cyclodextrin inhibits entry of human T cell leukemia virus type I (HTLV-1) and HTLV-I envelope pseudotyped lentivirus particles. Using a soluble fusion protein of the HTLV-I surface envelope protein with the immunoglobulin Fc domain, the HTLV-I receptor was found to colocalize with a raft-associated marker and to cluster in specific plasma membrane microdomains. Depletion of cholesterol did not alter receptor binding activity, suggesting a requirement for cholesterol in a postbinding virus entry step.
INTRODUCTION
The human T cell leukemia virus type I (HTLV-1) infects approximately 10 million people worldwide and causes adult T cell leukemia lymphoma (ATLL) in 1–10% of infected individuals.1,2 HTLV-I primarily infects CD4+ T cells in vivo, and in a poorly understood mechanism, causes ATLL, a CD4+ lymphoproliferative malignancy.3 The identification of the HTLV-I receptor would help in the design of a faithful animal model system that closely resembles the disease manifested in humans, as well as identification of a potential target for drugs that block HTLV-I infection. The HTLV-I receptor is widely expressed on a variety of cells, particularly activated T cells, and includes a protein component.4–6
The identity of the receptor is unclear, although a recent study suggests that GLUT-1, a glucose transporter known to mediate uptake of glucose in a stress-induced manner, binds the HTLV-I and -II envelope receptor binding domain (RBD) and mediates uptake of HTLV-II pseudotyped particles.7 However, since these studies utilized cell lines that bind high levels of HTLV envelope, this work did not address whether GLUT-1 allows HTLV-I uptake in nonsusceptible target cells. Thus, it is unclear whether GLUT-1 is functioning as a receptor, a coreceptor, or an attachment factor for HTLV-I and -II. The role of GLUT-1 in infection by HTLV-I virions has not yet been examined.
Regardless of the identity of the HTLV-I receptor, little is known about where or how the HTLV-I receptor mediates HTLV-I infection. For example, are there cofactors that modulate HTLV-I infection independent of RBD binding, or conditionally with RBD binding to the HTLV-I receptor? Is the HTLV-I receptor found in discrete microdomains on the plasma membrane, such as lipid rafts, or is it evenly distributed on the plasma membrane? Niyogi and Hildreth showed that antibodies that bind lipid raft-associated proteins inhibit HTLV-I syncytium formation and that disruption of lipid rafts disrupts syncytium formation.8 It has also been shown that disruption of lipid rafts decreases the binding of HTLV-I virions to a T cell line.9 In addition, biochemical fractionation studies and fluorescence microscopy revealed that GLUT-1 is raft associated, suggesting that there is spatial interference between these antibodies and virus access to the receptor.10
Lipid rafts are microdomains of the plasma membrane that contain high concentrations of cholesterol and glycosphingolipids, resulting in insolubility in ice-cold detergent.11 In addition, lipid rafts contain many different kinds of proteins, particularly those involved in cell signaling or structural proteins that may help concentrate signaling proteins, including caveolins, flotillins, glycosylphosphatidylinositol (GPI)-linked proteins, low-molecular-weight and heterotrimeric G proteins, src family kinases, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), endothelin receptors, the phosphotyrosine phosphatase syp, Grb2, Shc, mitogen-activated protein (MAP) kinase, protein kinase C, and the p85 subunit of PI 3-kinase.12 It is important to note that not all lipid rafts are identical, and there is heterogeneity in protein and lipid composition of lipid rafts from different sources.12
In this study, we examined the significance of plasma membrane cholesterol in HTLV-I infection and determined whether or not the HTLV-I receptor associates with lipid rafts. We found that HTLV-I infection is cholesterol dependent and it is raft associated as assessed by fluorescent microscopy.
MATERIALS AND METHODS
Reagents
2-Hydroxypropyl-β-cyclodextrin (2-HPβCD), sodium butyrate, biotin-labeled cholera toxin, goat antibiotin horseradish peroxidase (HRP) label, murine antibiotin Cy3 conjugate, and goat anti-rabbit Fc IgG were from Sigma (St. Louis, MO). Murine anti-human transferrin receptor PE conjugate was from Calbiochem (La Jolla, CA). Rabbit polyclonal anti-caveolin I was from Santa Cruz (Santa Cruz, CA). Donkey anti-rabbit HRP and sheep anti-mouse HRP were from Amersham Life Sciences (Arlington Heights, IL). All tissue culture media were from Gibco-BRL (Bethesda, MD) or Washington University (St. Louis, MO). Plasmids pNL4-3SV40LUC+E−, pHCMV-G, pHXADA, pACH-1, and pHTE-1 have been described previously.6 The plasmids encoding the HTLV-I and avian leukemia virus subtype A envelope immunoadhesins (HTSU-IgG and SUA-IgG) were previously described.4 3,3′-Dithiobis(sulfosuccinimidylpropionate) (DTSSP) was from Pierce (Rockford, IL).
Cell cultures
HOS, 293T, B5, and MAGI-5 cell lines were maintained in Dulbecco’s modified Eagle’s medium, 10% heat-inactivated fetal calf serum (HI-FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 1 mM L-glutamine. Peripheral blood mononuclear cells (PBMCs) were maintained in RPMI 1640 medium, 10% HI-FCS, 1 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 U/ml interleukin (IL)-2.
Transfections
Transfections were performed with 293T cells at 50–75% confluency in a T75 culture flask, using 3 μl/μg of DNA of TranIT transfection reagent (Mirus Corporation, Madison, WI). Sixteen hours posttransfection, the DNA was removed, replaced with fresh medium, and 293T cells were maintained for 1–2 days at 37°C, until supernatant (virus particles) or cell lysate (immunoadhesin) was isolated.
Virus preparation
Pseudotyped particles were generated as described previously with some modification.6 293T cells were treated with 20 mM sodium butyrate for 16 hr at 37°C. The medium was removed, and the cells were transfected with 10 μg of pNL4-3SV40LUC+E− plasmid, an HIV envelope-defective mutant that encodes the firefly luciferase gene, and 2.5 μg of pHCMVG plasmid for VSV-G enveloped pseudotypes, 10 μg pHTE-1 plasmid for HTLV-I enveloped pseudotypes, 10 μg pMLV plasmid for murine amphotropic leukemia virus (A-MLV) enveloped pseudotypes, or 10 μg of pHXADA plasmid for HIV enveloped pseudotypes. To generate wild-type HTLV-I particles, 15 μg of pACH-1 plasmid was used for transfection. To generate HTLV-I particles pseudotyped with the VSVg envelope, 12 μg of pACH− Env and 3 μg of pHCMVG were used for transfection. Two to 3 days posttransfection, the supernatant and cells were collected via centrifugation at 900 × g for 5 min at 4°C. The viral supernatant was collected and treated with 0.1 mg/ml DNase I with 10 mM MgSO4 for 1 hr at 37°C. The viral supernatants were passed through 0.22-μm syringe filters, aliquoted, and stored at −80°C.
Infections
Infections were performed after mock or 20 mM 2-HPβCD treatment for 45–60 min at 37°C. Cells were washed with phosphate-buffered saline (PBS) before addition of virus particles. To assess the effect of 2-HPβCD on pseudotyped particle infection, 3 × 104 HOS or MAGI-5 cells per well in 24-well plates were infected in triplicate with 50 μl volumes of HTLV-I, vesicular stomatitis virus (VSV), A-MLV, or HIV-1 glycoprotein pseudotyped particles. The cells were incubated at 37°C for 1 hr, and rocked every 20 min. At 1 hr postinfection, the inoculae were removed, the cells were washed with PBS, and 0.5 ml of culture media was added. Two days postinfection, the culture media were removed, and the cells were solubilized with 250 μl of LUC lysis buffer (100 mM potassium phosphate, pH 7.8, and 0.2% Triton X-100). Of each sample 200 μl was analyzed using a luminometer. The standard deviation associated with each sample was represented with error bars from three independent experiments.
To assess the effect of 2-HPβCD on wild-type HTLV-I particle infection, 106 B5 cells were infected with 1 ml of ACH-1, or VSV-G pseudotyped ACH-1 Env−, or heat-inactivated virus particles. After 1 hr incubation at 37°C, the inoculae were removed, the cells were washed with PBS, 1 ml of media was added, and incubated at 37°C for 3 days. The media were removed, B5 cells were washed in PBS, and genomic DNA was isolated with a QIAamp Blood mini Kit (Qiagen Inc., Valencia, CA). Three micrograms of genomic DNA per sample was used to amplify the 3′ LTR of HTLV-I using HTLV-I primers [5′-ATCCACGCCGGTTGAGTCGC-3′ (forward) and 5′-CACTCAGTCGTGAATGAAAG-3′ (reverse)] in 35 amplification cycles (94°C for 30 sec, 55°C for 20 sec, and 72°C for 30 sec). Genomic DNA (0.3 μg) was used to amplify GAPDH, using GAPDH-specific primers [5′-ACCACAGTCCATGCCATCAC-3′ (forward) and 5′-TCCACCACCCTGTTGCTGTA-3′ (reverse)] in 35 amplification cycles, as described above. GAPDH was used as a loading control to monitor the levels of HTLV-I 3′ LTR between samples. PCR reactions were amplified with REDTAQ (Sigma, St. Louis, MO).
Expression of immunoadhesin proteins
293T cells were transfected with 15 μg HTSU-IgG/pSK100 encoding the HTSU-Fc fusion protein or SUA-IgG encoding the SUA-Fc protein of the avian retrovirus ALSV-A. Two days posttransfection, the media were removed, the cells were washed with PBS, and resuspended in ice cold PBS, and sonicated twice. Proteinase inhibitor cocktail (Sigma, St. Louis, MO) was added and lysates were centrifuged at 12,000 × g for 1 min. The clarified lysates were pooled, aliquoted, and stored at −80°C.
FACS analyses
Cells were resuspended in 200 μl of HTSU-Fc or SUA-Fc, incubated at 20°C for 25 min, collected by centrifugation, and washed with PBS. A 1:25 mixture of FITC-labeled anti-rabbit IgG antibody in PBS (stock concentration 40 μg/ml, Sigma) with 1% HI-FCS was added and incubated at 4°C for 25 min. The cells were washed with PBS, incubated at 20°C for 10 min with 1% paraformaldehyde, and filtered to remove cell clumps.
Fluorescence and confocal microscopy
For fluorescence and confocal microscopy of the HTLV-I receptor, HOS cells seeded on coverslips were incubated with 200 μg/ml HTSU-Fc or SUA-Fc at 20°C for 25 min. The lysates were removed, the cells were washed with PBS, a 1:25 mixture of FITC-labeled anti-rabbit IgG was added and incubated at 4°C for 30 min. The cells were washed with PBS, fixed with 4% paraformaldehyde, and the coverslips were adhered to microscope slides with 8 μl of 75% glycerol.
To visualize the HTLV-I receptor and ganglioside marker 1 (GM1), HOS cells were incubated with biotin-labeled cholera toxin (CtB, stock concentration 10 μg/ml, Sigma) at a ratio of 1:150 CtB to HTSU-Fc or SUA-Fc lysate, for 25 min at 20°C. The cells were washed with PBS, treated with 1:100 Cy3-labeled anti-biotin antibody (stock concentration 10 μg/ml, Sigma) and 1:25 FITC-labeled antirabbit IgG for 25 min at 4°C, and processed as described above.
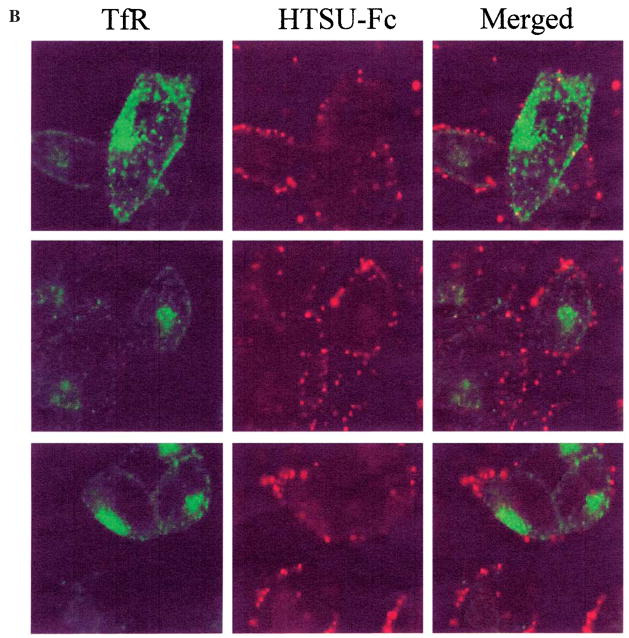
To visualize the HTLV-I and transferrin receptors (TfR), HOS cells were incubated with 200 μg/ml HTSU-Fc lysate for 25 min at 20°C, washed with PBS, then incubated with 1:30 biotin-labeled anti-rabbit IgG antibody (stock concentration 80 μg/ml, Sigma, to bind HTSU-Fc) and 1:15 FITC-labeled anti-TfR antibody (stock concentration 10 μg/ml, Santa Cruz, for 25 min at room temperature. The cells were washed with PBS, treated with 1:100 Cy3-labeled anti-biotin antibody to label HTSU-Fc with Cy3 for 25 min at 4°C, and processed as described above.
RESULTS
Cholesterol is important for HTLV-I envelope-mediated, pseudotyped particle infection
We have previously shown that HTLV-I requires intact lipid raft membranes for viral budding from the host plasma membrane.13 In addition, Niyogi and Hildreth found that treatment of target cells with 2-HPβCD, a drug that binds cholesterol and disrupts lipid rafts, inhibits HTLV-I envelope-mediated syncytium formation.8 Similar findings have been described for HIV as well.14–16
These findings suggested that cell-free HTLV-I infection may also depend on the integrity of cholesterol-rich lipid rafts. To examine this issue, HOS cells were cultured with or without cyclodextrin, followed by infection with HIV particles encoding the firefly luciferase gene, pseudotyped with either HTLV-I, A-MLV, or vesicular stomatitis virus glycoproteins (VSV-G). Several different β-cyclodextrins, including β-cyclodextrin, methyl-β-cyclodextrin, and 2-hydroxypropyl-β-cyclodextrin, (2-HPβCD) were examined. Each reagent resulted in similar effects on pseudotyped particle infection, but the solubility and cell toxicity varied; 2-HPβCD was the most soluble and least toxic agent under the assay conditions. Moreover, 2-HPβCD exhibited a dose–response effect on infection (data not shown).
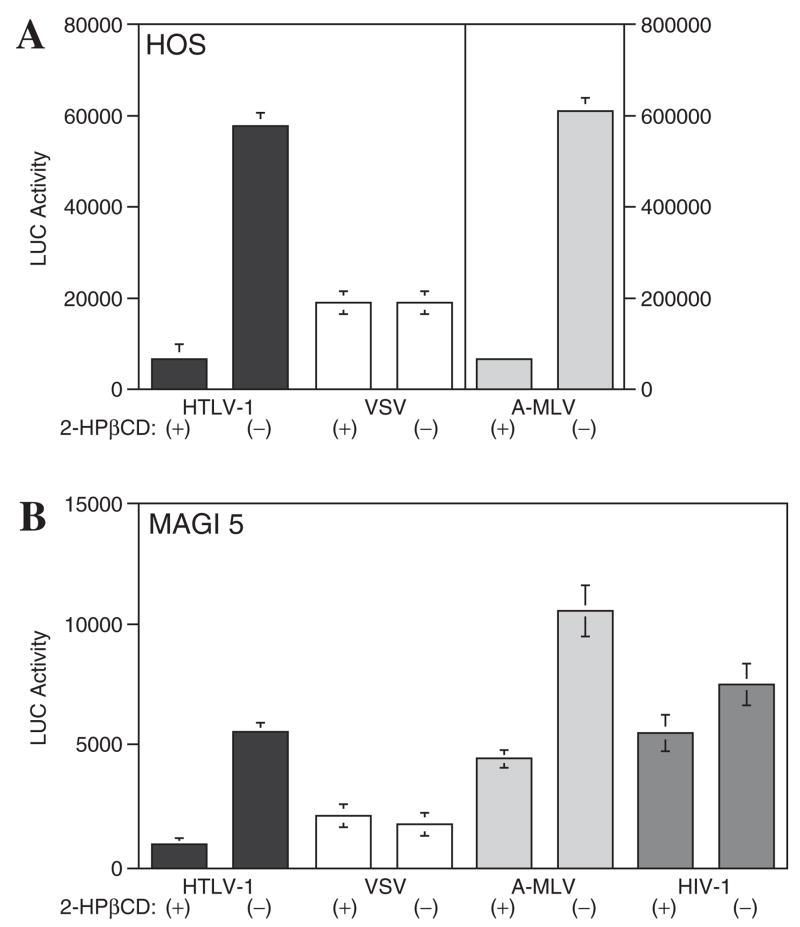
As shown in Figure 1A, 2-HPβCD did not significantly affect VSV-G pseudotyped particle infection in HOS cells. However, both HTLV-I and A-MLV envelope pseudotyped particles were sensitive to 2-HPβCD treatment, and exhibited a 85–90% reduction in luciferase activity. Similar assays were also performed with MAGI-5 cells, a HeLa cell line that expresses CD4, CXCR4, and CCR5, with or without prior 2-HPβCD treatment. In this case, HIV particles pseudotyped with an R5 HIV-1 envelope were used as an additional control. As shown in Figure 1B, the effect of 2-HPβCD on VSV-G pseudo-typed particle infection of MAGI-5 cells was not diminished. In contrast, entry of HTLV-I, A-MLV, and HIV-1 pseudotyped particles was sensitive to 2-HPβCD treatment, and luciferase activity decreased 80%, 60%, and 25%, respectively. These results demonstrate that HTLV-I pseudotyped particle infection depends on cholesterol, and further shows that 2-HPβCD does not negatively affect cell viability since VSV-G particle entry was not adversely affected by cholesterol extraction.
FIG. 1.
2HPβCD inhibits infection of (A) HOS cells and (B) MAGI-5 cells by a lentivirus particles carrying a luciferase gene pseudotyped with HTLV-I Env. Cells were treated with (+) or without (−) 2HPβCD prior to infection with HTLV-I, VSV, A-MLV, or HIV-I glycoprotein pseudotyped particles. After 2 days, luciferase activity was measured.
Cholesterol is important for wild-type HTLV-I particle entry into B5 cells
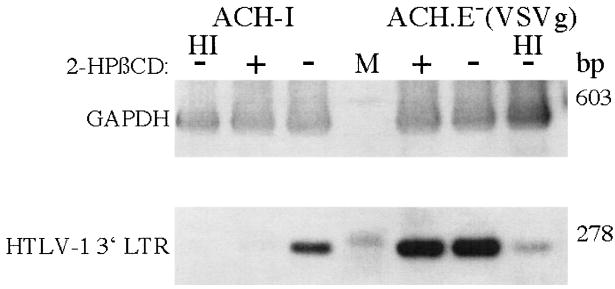
The cholesterol dependence of infection of wild-type HTLV-I particles was also examined. B5 cells, a monkey lung fibroblast cell line, is the most suitable cell line for efficient replication of free HTLV-I particles. These cells were treated with 2-HPβCD, and infected with virions from the ACH-1 molecular clone or an ACH Env− virus pseudotyped with VSV-G as a control.17,18 Viral entry was measured by polymerase chain reaction (PCR) analysis of newly synthesized DNA. Heat-inactivated (HI) virions were used as negative controls. As shown in Figure 2, the entry of wild-type HTLV-I particles into B5 cells was reduced with 2-HPβCD treatment prior to infection, but 2-HPβCD treatment had no effect on the infection of VSV-G pseudotyped HTLV-I envelope negative virus. These results show that wild-type HTLV-I particles require cholesterol for efficient infection.
FIG. 2.
Lipid rafts are important for wild type HTLV-I particle entry into B5 cells. B5 cells were pretreated with (+) or without (−) 2HPβCD, and then infected with HTLV-I particles from ACH or an env mutant of ACH pseudotyped with VSV glycoprotein, ACH.E−-(VSV-G), or heat inactivated particles (HI). After 3 days, DNA was harvested and PCR performed for HTLV-I 3′ LTR or GAPDH sequences. Molecular weight markers were run in the lane designated M, and the size of the amplified DNA fragments indicated to the right.
HTLV-I receptor abundance at the cell surface is not reduced by 2-HPβCD
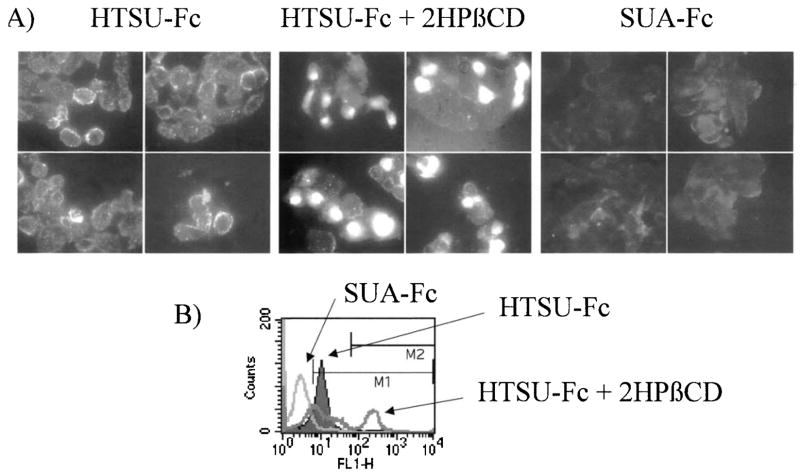
Since 2-HPβCD decreases the ability of HTLV-I particles to infect susceptible cell lines, we wanted to determine if this effect was due to a decreased abundance of the HTLV-I receptor on the surface of cells. Therefore, fluorescent microscopy and FACS analyses were employed using the HTSU-Fc fusion protein.4 The HTSU-Fc fusion protein contains the surface protein of the HTLV-I envelope (SU; gp46) fused to the Fc domain of rabbit IgG. This fusion protein has been used to test for the presence of the HTLV-I receptor on many different cell lines.4,5 In addition, the SU portion of ALV, fused to the rabbit Fc domain of IgG (SUA-Fc), was used as a negative control to demonstrate the specificity of HTSU-Fc binding. As shown in Figure 3A, HOS cells incubated with HTSU-Fc, but not SUA-Fc, were fluorescently labeled with an FITC-conjugated antirabbit antibody. The binding of HTSU-Fc to the surface of HOS cells exhibited a speckled pattern, suggesting that the HTLV-I receptor is located in discrete domains on the surface of HOS cells.
FIG. 3.
2-HPβCD does not reduce the cell surface expression of the HTLV-I receptor. (A) Fluorescent microscopy was performed and four representative fields each are shown for HOS cells treated with HTSU-Fc, HTSU-Fc following 2HPβCD treatment, or SU-FcA. (B) FACS analysis was performed for 293T cells after treatment with HTSU-Fc (solid curve), HTSU-Fc after 2HPβCD treatment (dark line), or SU-FcA (light line).
When HOS cells were pretreated with 2-HPβCD and then incubated with HTSU-Fc, cell staining for the HTLV-I receptor did not diminish, but in fact appeared to increase on a subpopulation of cells (Fig. 3A). Most of the HOS cells treated with 2-HPβCD maintained the speckled staining pattern, but 5–20% of the cells exhibit increased fluorescence, and HTLV-I receptor staining in these cells appeared diffusely distributed over the cell surface. Similar results were observed with 293T cells, by FACS analyses (Fig. 3B). 293T cells that were not treated with drug, but incubated with HTSU-Fc, exhibited fluorescent staining, compared to those cells that were treated with the SUA-Fc negative control. However, this shift was even more pronounced in a subpopulation of 15% of the cells when 293T cells were treated with 2-HPβCD before HTSU-Fc incubation.
The HTLV-I receptor colocalizes with lipid rafts markers
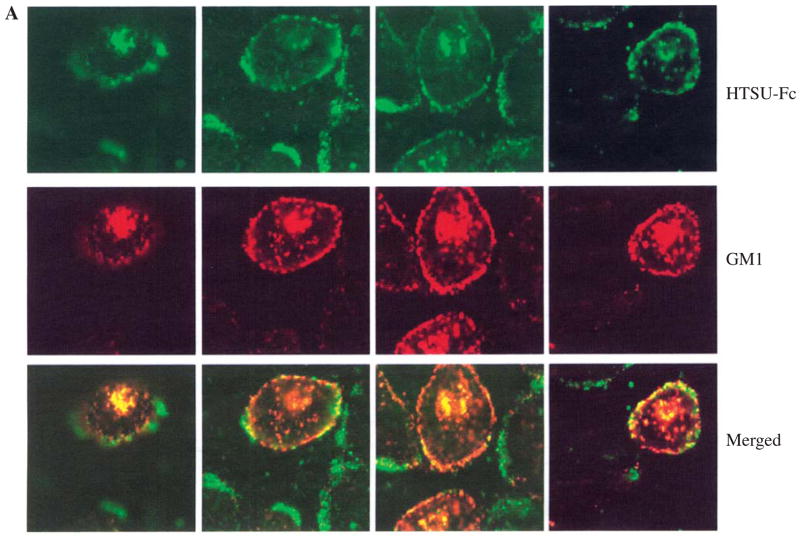
Since antibodies to raft-associated proteins inhibit HTLV-I envelope-mediated syncytium formation,14 it was logical to determine if the HTLV-I receptor exhibited a distribution similar to that of lipid raft marker ganglioside marker 1 (GM1). As shown in Figure 4, the HTLV-I receptor colocalized with GM1, as assessed by biotin-labeled cholera toxin binding and Cy3-labeled antibiotin antibody. In contrast, the localization of the HTLV-I receptor and the transferrin receptor (TfR), a nonlipid raft marker, was distinct and did not overlap. These results indicate that a significant population of the HTLV-I receptor resides in GM1-containing lipid rafts.
FIG. 4.
The HTLV-I receptor localizes in GM1-containing lipid rafts. HOS cells were treated with HTSU-Fc and biotin-labeled cholera toxin and visualized with (A) FITC-labeled anti-rabbit IgG and Cy3-labeled anti-biotin antibody, or (B) biotin-labeled anti-rabbit IgG and Cy3-labeled antibiotin antibody and FITC-labeled anti-transferrin receptor (TfR) antibody. Representative fields are shown using filters allowing visualization of only (A) FITC-labeled HTLV-I receptor (top panels), Cy3 labeled GM1 (middle panels), or both (bottom panels), and (B) Cy3 labeled HTLV receptor (left panels), FITC-labeled TfR (middle panels), or both (right panels).
DISCUSSION
Lipid rafts, which are enriched in cholesterol and sphingolipids, play a critical role in the lateral organization and mobility of plasma membrane constituents.11,19,20 Rafts are important in cell signaling, including activation of tyrosine kinases, NFκB activation, and interleukin expression.21–24 Several viruses use lipid rafts in one or more aspects of their replication cycle,25–28 including virus assembly,14,29–35 budding36,37 fusion,38 and virus entry.39–44 Receptors for HIV, murine leukemia virus, herpes simplex virus, pseudorabies virus, and coxsackie viruses are raft associated.16,45–47 Moreover, viral docking proteins, such as DC-SIGN, are also associated with rafts.48
The current studies demonstrate that rafts are important for entry of HTLV-I and HTLV-I envelope pseudotyped particles (Figs. 1 and 2). Moreover, the HTLV-I receptor is associated with rafts (Fig. 4). However, envelope protein binding to the receptor is not disrupted by cholesterol depletion (Fig. 3). One explanation for these results is that receptor binding does not require rafts, but once bound, lateral mobility into rafts is required for subsequent entry events.
Several potential explanations can be proposed for these findings. (1) The HTLV-I receptor may require a cholesterol-rich domain to allow appropriate conformational changes of the envelope protein to occur to become fusion competent. This explanation was offered to explain the role of cholesterol in HIV-1 entry to permit the HIV-1 gp41 transmembrane protein to form “stalk intermediates” required for fusion.49 (2) Alternatively, an HTLV-I coreceptor, required for virus entry, may be raft associated. (3) A third possibility is that raft-association may be required to exclude inhibitors of virus entry. (4) Another possibility is that raft-associated components may allow virus entry to avoid the degradative endocytic pathways, and direct virus uptake to a recycling endosomes low in protease activity, as described for avian leukemia virus subtype A entry.50,51 (5) Another consideration is that virus targeting to rafts during entry may be required for a signaling event necessary for the virus uptake. One example of this phenomenon is that cholesterol depletion blocks the ability of HIV-1 coreceptors CCR5 and CXCR4 to mediate activation of G proteins, as well as gp120 surface envelope protein activation of MAPK.52,53
GLUT-1 and neuropilin-1 have been proposed as HTLV receptors, and both proteins have also been reported to be raft associated.7,9,54 However, further work is required to define the role of each of these proteins in HTLV entry. Infection by free HTLV-I virions is not efficient. Recently, new observations have identified specialized contacts between infected and un-infected cells, designated viral synapses. Viral synapses have been shown to be important in facilitating HTLV-I transmission.55 Based on their similarity to immunological synapses, these domains are likely enriched in rafts. The current work provides new information regarding the role of rafts that is relevant to understanding the mechanism of HTLV entry.
Acknowledgments
We would like to thank Nancy Campbell and Xuan Feng for experimental expertise and use of some antibody reagents, and Michael Belshan and Suzanne Pontow for critiques of the manuscript.
References
- 1.Ratner L, Poiesz BJ. Leukemias associated with human T-cell lymphotropic virus type I in a non-endemic region. Medicine. 1988;67:401–422. doi: 10.1097/00005792-198811000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hollsberg P, Hafler DA. Human T-Cell Lymphotropic Virus Type I. John Wiley & Sons; New York: 1996. [Google Scholar]
- 3.Richardson J, Edwards A, Cruicksank J, Rudge P, Dalgeish A. In vivo cellular tropism of human T-cell leukemia virus type 1. J Virol. 1990;64:5682–5687. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones KS, Nath M, Petrow-Sadowski C, Baines AC, Dambach M, Huang Y, Ruscetti FW. Similar regulation of cell surface human T-cell leukemia virus type 1 (HTLV-1) surface binding proteins in cells highly and poorly transduced by HTLV-1-pseudo-typed virions. J Virol. 2002;76:12723–12734. doi: 10.1128/JVI.76.24.12723-12734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nath MD, Ruscetti FW, Petrow-Sadowski C, Jones KS. Regulation of the cell-surface expression of an HTLV-1 binding protein in human T cells during immune activation. Blood. 2003;101:1–8. doi: 10.1182/blood-2002-07-2277. [DOI] [PubMed] [Google Scholar]
- 6.Trejo SR, Ratner L. The HTLV receptor is a widely expressed protein. Virology. 2000;268:41–48. doi: 10.1006/viro.2000.0143. [DOI] [PubMed] [Google Scholar]
- 7.Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini J-L. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell. 2003;115:449–459. doi: 10.1016/s0092-8674(03)00881-x. [DOI] [PubMed] [Google Scholar]
- 8.Niyogi K, Hildreth JEK. Characterization of new syncytium-inhibiting monoclonal antibodies implicates lipid rafts in human T-cell leukemia virus type 1 syncytium formation. J Virol. 2001;75:7351–7361. doi: 10.1128/JVI.75.16.7351-7361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hague BF, Zhao TM, Kindt TJ. Binding of HTLV-1 virions to T cell occurs by a temperature and calcium-dependent process and is blocked by certain type 2 adenosine receptor antagonists. Virus Res. 2003;93:31–39. doi: 10.1016/s0168-1702(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 10.Barnes K, Ingram JC, Bennett MD, Stewart GW, Baldwin SA. Methyl-β-cyclodextrin stimulates glucose uptake in clone 9 cells: A possible role for lipid rafts. Biochem J. 2004;378(Pt 2):343–351. doi: 10.1042/BJ20031186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown DA, London E. Structure and function of sphingolipid and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 12.Pike LJ. Lipid rafts: Bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Feng X, Vander Heyden N, Ratner L. Alpha interferon inhibits human T-cell leukemia virus type 1 assembly by preventing Gag interaction with rafts. J Virol. 2003;77:13389–13395. doi: 10.1128/JVI.77.24.13389-13395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Z, Cimakasky LM, Hampton LM, Nguyen R, Hildreth JEK. Lipid rafts and HIV pathogenesis: Host membrane cholesterol is required for infection by HIV type 1. AIDS Res Hum Retro-viruses. 2001;17:1009–1019. doi: 10.1089/088922201300343690. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen DH, Hildreth JEK. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popik W, Alce TM, Au W-C. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J Virol. 2002;76:4709–4722. doi: 10.1128/JVI.76.10.4709-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimata JT, Wong FH, Wang JJ, Ratner L. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology. 1994;204:656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- 18.Tsukahara T, Wielgosz MM, Ratner L. Characterization of envelope glycoprotein mutants for human T-cell leukemia virus type 1 infectivity and immortalization. J Virol. 2001;75:9553–9559. doi: 10.1128/JVI.75.19.9553-9559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Norkin LC. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp Cell Res. 1999;246:83–90. doi: 10.1006/excr.1998.4301. [DOI] [PubMed] [Google Scholar]
- 22.Dykstra ML, Cherukuri A, Pierce SK. Floating the raft hypothesis for immune receptors: Access to rafts controls receptor signaling and trafficking. Traffic. 2001;2:160–166. doi: 10.1034/j.1600-0854.2001.020302.x. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi M, Izumi KM, Kieff E. Epstein-Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: Protein 1 binds to the cytoskeleton through tumor necrosis factor receptor cytoplasmic factors. Proc Natl Acad Sci USA. 2001;98:4675–4680. doi: 10.1073/pnas.081075298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidalain PO, Azocar O, Servet-Delprat C, Rabourdin-Combe C, Gerlier D, Manie S. CD40 signaling in human dendritic cells is initiated within membrane rafts. EMBO J. 2000;19:3304–3313. doi: 10.1093/emboj/19.13.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell SM, Crowe SM, Mak J. Lipid rafts and HIV-1: From viral entry to assembly of progeny virions. J Clin Virol. 2001;22:217–227. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
- 26.Nayak DP, Barman S. Role of lipid rafts in virus assembly and budding. Adv Virus Res. 2002;58:1–28. doi: 10.1016/s0065-3527(02)58001-5. [DOI] [PubMed] [Google Scholar]
- 27.Shin JS, Abraham SN. Caveolae—not just craters in the cellular landscape. Science. 2001;293:1447–1448. doi: 10.1126/science.1061079. [DOI] [PubMed] [Google Scholar]
- 28.van der Goot FG, Harder T. Raft membrane domains: From a liquid-ordered membrane phase to a site of pathogen attack. Semin Immunol. 2001;13:89–97. doi: 10.1006/smim.2000.0300. [DOI] [PubMed] [Google Scholar]
- 29.Barman S, Nayak DP. Analysis of the transmembrane domain of influenza virus neuraminidase, a type II transmembrane glycoprotein, for apical sorting and raft association. J Virol. 2000;74:6538–6545. doi: 10.1128/jvi.74.14.6538-6545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson G, Murray J, Yeo RP. Sorting of the respiratory syncytial virus matrix protein into detergent-resistant structures is dependent on cell-surface expression of the glycoproteins. Virology. 2002;300:244–254. doi: 10.1006/viro.2002.1540. [DOI] [PubMed] [Google Scholar]
- 31.Lindwasser OW, Resh MD. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J Virol. 2001;75:7913–7924. doi: 10.1128/JVI.75.17.7913-7924.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manie SN, Debreyne S, Vincent S, Gerlier D. Measles virus structural components are enriched into lipid raft microdomains: A potential cellular location for virus assembly. J Virol. 2000;74:305–311. doi: 10.1128/jvi.74.1.305-311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sapin C, Colard O, Delmas O, Tessier C, Breton M, Enouf V, Chwetzoff S, Ouanich J, Cohen J, Wolf C, Trugnan G. Rafts promote assembly and atypical targeting of a nonenveloped virus, rotavirus, in Caco-2 cells. J Virol. 2002;76:4591–4602. doi: 10.1128/JVI.76.9.4591-4602.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheiffele P, Roth MG, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Pekosz A, Lamb RA. Influenza virus assembly and lipid raft microdomains: A role for the cytoplasmic tails of the spike glycoproteins. J Virol. 2000;74:4634–4644. doi: 10.1128/jvi.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen DH, Hildreth JE. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 38.Ahn A, Gibbons DL, Kielian M. The fusion peptide of Semliki Forest virus associates with sterol-rich membrane domains. J Virol. 2002;76:3267–3275. doi: 10.1128/JVI.76.7.3267-3275.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson HA, Chen Y, Norkin LC. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bavari S, Bosio CM, Wiegand E, Ruthel G, Will AB, Geisbert TW, Hevey M, Schmaljohn C, Schmaljohn A, Aman MJ. Lipid raft microdomains: A gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med. 2002;195:593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu X, Silver J. Ecotropic murine leukemia virus receptor is physically associated with caveolin and membrane rafts. Virology. 2000;276:251–258. doi: 10.1006/viro.2000.0555. [DOI] [PubMed] [Google Scholar]
- 42.Manes S, del Real G, Lacalle RA, Lucas P, Gomez-Mouton, Sanchez-Palomino S, Delgado R, Alcami J, Mira E, Martinez AC. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000;1:190–196. doi: 10.1093/embo-reports/kvd025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 44.Stuart AD, Eustace HE, McKee TA, Brown TD. A novel cell entry pathway for a DAF-with human enterovirus is dependent on lipid rafts. J Virol. 2002;76:9307–9322. doi: 10.1128/JVI.76.18.9307-9322.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Favoreel HW, Mettenleiter TC, Nauwynck HJ. Copatching and lipid raft association of different viral glycoproteins expressed on the surfaces of pseudorabies virus-infected cells. J Virol. 2004;78:5279–5287. doi: 10.1128/JVI.78.10.5279-5287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bender FC, Whitbeck JC, Ponce de Leon M, Lou H, Eisenberg RJ, Cohen GH. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J Virol. 2003;77:9542–9552. doi: 10.1128/JVI.77.17.9542-9552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Triantafilou K, Triantafilou M. Lipid raft microdomains: Key sites for Coxsackievirus A9 infectious cycle. Virology. 2003;317:128–135. doi: 10.1016/j.virol.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 48.Cambi A, De Lange F, Van Maarseveen NM, Nijhuis M, Joosten B, Van Dijk EM, De Bakker BI, Fransen JA, Bovee-Geurts PH, Van Leeuwen FN, Van Hulst NF, Figdor CG. Microdomains of the C-type lectin DC-SIGN are portals for virus entry into dendritic cells. J Cell Biol. 2004;164:145–155. doi: 10.1083/jcb.200306112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Percherancier Y, Lagane B, Planchenault T, Staropoli I, Altmeyer R, Virelizier J-L, Arenzana-Seisdedos F, Hoessli DC, Bachelerie F. HIV-1 entry into T-cells is not dependent on CD4 and CCR5 localization to sphingolipid-enriched, detergent-resistant, raft membrane domains. J Biol Chem. 2003;278:3153–3161. doi: 10.1074/jbc.M207371200. [DOI] [PubMed] [Google Scholar]
- 50.Narayan S, Barnard RJO, Young JAT. Two retroviral entry pathways distinguished by lipid raft association of the viral receptor and differences in viral infectivity. J Virol. 2003;77:1977–1983. doi: 10.1128/JVI.77.3.1977-1983.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gagescu R, Demaurex N, Parton RG, Hunziker W, Huber LA, Gruenberg J. The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol Biol Cell. 2000;11:2775–2791. doi: 10.1091/mbc.11.8.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lagane B, Gaibelet G, Meilhoc E, Masson J-M, Cezanne L, Lopez A. Role of sterols in modulating the human mu-opioid receptor function in Saccharomyces cerevisiae. J Biol Chem. 2000;275:33197–33200. doi: 10.1074/jbc.C000576200. [DOI] [PubMed] [Google Scholar]
- 53.Kinet S, Bernard F, Mongellaz C, Perreau M, Goldman FD, Taylor N. gp120-mediated induction of the MAPK cascade is dependent on the activation state of CD4(+) lymphocytes. Blood. 2002;100:2546–2553. doi: 10.1182/blood-2002-03-0819. [DOI] [PubMed] [Google Scholar]
- 54.Ghez D, Lepelletier Y, et al. Identification of a cell surface protein behaving as a cellular receptor for HTLV-1 and HTLV-2. AIDS Res Hum Retroviruses. 2003;19:S15. [Google Scholar]
- 55.Igakura T, Stinchcombe JC, Goon PKC, Taylor GP, Weber JN, Griffiths TM, Tanaka Y, Osame M, Bangham CRM. Spread of HTLV-1 between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]