Abstract
OBJECTIVE
We have previously reported a highly diabetogenic CD8 T-cell clone, G9C8, in the nonobese diabetic (NOD) mouse, specific to low-avidity insulin peptide B15-23, and cells responsive to this antigen are among the earliest islet infiltrates. We aimed to study the selection, activation, and development of the diabetogenic capacity of these insulin-reactive T-cells.
RESEARCH DESIGN AND METHODS
We generated a T-cell receptor (TCR) transgenic mouse expressing the cloned TCR Vα18/Vβ6 receptor of the G9C8 insulin-reactive CD8 T-cell clone. The mice were crossed to TCRCα−/− mice so that the majority of the T-cells expressed the clonotypic TCR, and the phenotype and function of the cells was investigated.
RESULTS
There was good selection of CD8 T-cells with a predominance of CD8 single-positive thymocytes, in spite of thymic insulin expression. Peripheral lymph node T-cells had a naïve phenotype (CD44lo, CD62Lhi) and proliferated to insulin B15-23 peptide and to insulin. These cells produced interferon-γ and tumor necrosis factor-α in response to insulin peptide and were cytotoxic to insulin peptide–coated targets. In vivo, the TCR transgenic mice developed insulitis but not spontaneous diabetes. However, the mice developed diabetes on immunization, and the activated transgenic T-cells were able to transfer diabetes to immunodeficient NOD.scid mice.
CONCLUSIONS
Autoimmune CD8 T-cells responding to a low-affinity insulin B-chain peptide escape from thymic negative selection and require activation in vivo to cause diabetes.
Type 1 diabetes is a complex, multifactorial disease in which genetic factors interact with environmental modifiers to give rise to immune abnormalities leading to pancreatic β-cell damage and destruction. Both CD4 and CD8 T-cells have a major role in pathogenesis of type 1 diabetes. There are a number of autoantigens that are recognized by CD8 T-cells, including proinsulin (PI) (1), islet-specific glucose-6-phosphatase catalytic subunit–related protein (IGRP) (2), dystrophia myotonica kinase (3), and glutamic acid decarboxylase (4) in the nonobese diabetic (NOD) animal model of diabetes. In HLA-A2 transgenic mice, both PI and IGRP have also been shown to be important targets (5,6). Furthermore, emerging evidence suggests that CD8 T-cells in humans also recognize these antigens (7–9), although their role in pathogenesis is currently unknown.
In humans, CD8 T-cells are present in islet infiltrates at the time diabetes develops (10). CD8 T-cells were predominant in the islet infiltrate when diabetes recurred within 6 weeks after transplantation of a hemipancreas from normal, identical nondiabetic twins into their diabetic cotwins (11). In NOD mouse studies, it is well established that CD8 T-cells play an important role both in early events leading to insulitis and diabetes (12,13) as well as in the final effector stage (14) of diabetes development.
PI is an important autoantigen in diabetes, and it has been suggested that it is the “prime” autoantigen (15) recognized by CD8 T-cells, CD4 T-cells, and autoantibodies. There is clear evidence that PI is expressed in the thymus (16) in humans and in mice (17), which influences the expression of autoreactive T-cells to PI/insulin. In humans, although the major histocompatibility complex (MHC) is the most important genetic susceptibility factor, the second most important genetic region (IDDM2) is the insulin 5′VNTR region. This controls expression of PI in the thymus and the pancreas (18). Mice have two types of insulin, produced as proinsulin 1 (PI1), mainly in the pancreas, and proinsulin 2 (PI2) in the thymus and pancreas. Studies using individual proinsulin PI1−/− and PI2−/− knockout mice, when backcrossed to the NOD background, showed that PI1−/− mice have a reduced incidence of diabetes (19), whereas in PI2−/− mice, diabetes is accelerated with 100% developing diabetes (19,20). In addition, insulin autoantibody production is increased in PI2−/− mice, and spleen cells from young PI2−/− animals have an increased ability to transfer diabetes (20). Furthermore, when PI2 was overexpressed in the thymus (and on peripheral antigen-presenting cells [APCs]) on the MHC class II promoter (PI2tg), the incidence of diabetes was decreased (21,22). This suggests that PI2 is important for both central and peripheral tolerance for islet β-cells.
We have previously isolated a highly pathogenic CD8 T-cell clone (G9C8) from the islets of young, pre-diabetic NOD mice that is capable of very rapidly causing diabetes (5–10 days) upon adoptive transfer to young or irradiated nondiabetic NOD mice (23). The autoantigen is an insulin B-chain peptide (amino acids 15–23 [B15-23]) (1). The epitope is common to both mouse insulins (and is also conserved in human insulin) and is restricted by MHC-Kd. The insulin peptide is recognized by a small population of cells present in the very early stages of the islet infiltrate, identified using a Kd–B15-23 tetramer (1) and is found in mice aged <5 weeks (1,24). Subsequently, other specificities such as IGRP become increasingly dominant (24–26), and this insulin-specific population becomes a smaller percentage of the infiltrate as the disease progresses (1). To further study the selection and activation of this early population of CD8 T-cells, we have generated a T-cell receptor (TCR) transgenic mouse in which the T-cells express the receptor of the highly pathogenic G9C8 clone. Unlike other CD8 TCR transgenic mice that recognize higher-affinity MHC binding peptides of IGRP (2,27), G9C8 T-cells have low avidity, and G9 transgenic mice do not develop spontaneous diabetes. However, transgenic G9 cells can be highly diabetogenic after activation both in vitro and in vivo.
RESEARCH DESIGN AND METHODS
Generation of insulin-reactive TCR transgenic mice.
Insulin-specific TCR transgenic mice were generated by isolating TCR genomic DNA from G9C8 cloned T-cells (23), which have previously been shown to have specific reactivity to amino acids 15–23 of the insulin B-chain (1). TCRα (Vα18S1 Jα18 Cα)- and β (Vβ6S1 Dβ1.1 Jβ2.3 Cβ1)-chain DNA was purified and cloned into pTαcass and pTβcass constructs (28), respectively, kindly provided by D. Mathis. The cloned constructs were injected directly into NOD ova to generate independent TCRα and β founder lines in the Yale Diabetes Endocrinology Research Center (DERC) transgenic core (R.A. Flavell, Yale School of Medicine), which were intercrossed to produce αβ TCR transgenic mice (G9NOD). Three independent founder lines each of TCRα (lines 17, 22, and 24) and β (lines 1, 2, and 3) were generated, and α lines 17, 22, and 24 were crossed with each of β lines 2 and 3. Results with each of these lines was similar in terms of selection and functional phenotype (data not shown), and, thus, TCRα line 22 and TCRβ line 2 were chosen for all subsequent studies.
To generate repertoire-restricted αβ TCR transgenic mice, G9Cα−/−.NOD mice were generated by crossing the αβ TCR transgenic mice to NOD.Cα−/− mice. The NOD.Cα−/− mice were generated by backcrossing TCR.Cα−/− mice (B6/129 background; kindly provided by A.C. Hayday) (29) >20 generations to NOD/Caj mice in the laboratory of R.S. Sherwin (Yale University). G9RAG−/−.NOD mice were generated by crossing the αβ TCR transgenic mice to NOD.RAG2−/− mice, generated by backcrossing RAG2−/− mice (kindly provided by D. Schatz, Yale University) to NOD/Caj mice >12 generations. To generate G9Cα−/−.RIP-B7.1 NOD mice, the RIP-B7.1 mice were 10 generations backcrossed to NOD.Cα−/− mice and intercrossed to generate NOD.Cα−/−RIP-B7.1 mice and then crossed with the G9Cα−/−.NOD mice to generate G9Cα−/−.RIP-B7.1 NOD mice. NOD.scid mice were originally obtained from The Jackson Laboratories but were bred for many years at Yale University. The mice were housed in microisolators or scantainers in barrier rooms. All procedures were performed in accordance with the protocols approved by the U.K. Home Office or institutional animal care and use committee at Yale University.
Flow cytometry.
Single-cell suspensions of 0.1–1 × 106 cells were stained for various cell surface markers (rat anti-murine CD8; CD4, CD62L, and CD44). FcRs were blocked with anti-CD16 (2.4G2; provided by the late C.A. Janeway), and cells were stained for surface markers. Intracellular staining was performed on cells cultured for 15 h with differing concentrations of peptide, for interferon (IFN)-γ and tumor necrosis factor (TNF)-α, after cell surface staining with anti-CD8 following the BD Bioscience protocol. They were analyzed by flow cytometry using a FACSCalibur, and results were analyzed using CellQuest (BD Bioscience) or FlowJo (Tree Star) software. All antibodies were purchased from BD Bioscience, except anti-murine CD8-allophycocyanin (Caltag Laboratories).
Cells were stained with Kd-B15-23-PE tetramer or control Kd-listeriolysin (LLO) 91-99-PE tetramer (obtained from the National Institutes of Health Tetramer Facility) for 1 h at 4°C. Anti–CD8–fluorescein isothiocyanate was added for the last half hour. The cells were then washed and analyzed by flow cytometry.
Enriched CD8 T-cells from splenocytes of G9Cα−/−.NOD mice were labeled with 5 μmol/l carboxyfluorescein succinimidyl ester (CFSE) for 10 min at 37°C and washed with ice-cold RPMI-1640 with 5% FCS and then with 0.9% normal saline. Ten million cells were resuspended in 0.9% normal saline and transferred intravenously to 4- or 6-week-old NOD mice. The spleen, pancreatic, axillary, and mesenteric lymph nodes were removed 6–10 days posttransfer, and CFSE dilution was analyzed by flow cytometry after staining with anti–CD8-allophycocyanin and anti–CD69-phycoerythrin.
Proliferation and cytotoxicity assays: proliferation.
Insulin-reactive CD8 T-cells from 5- to 8-week-old G9Cα−/−.NOD mice were cocultured with bone marrow–derived dendritic cells and B15-23 peptide or influenza hemagglutinin peptide IYSTVASSL (control) for peptide responses in RPMI complete medium. Similarly, soluble insulin or keyhole limpet hemocyanin (control) was used as antigen for protein cross-presentation responses. CD8 T-cells were purified from splenocytes of G9Cα−/−.NOD mice using positive selection beads (Miltenyi Biotec) with >95% purity. After 3 days of incubation, cells were pulsed with [3H]thymidine for 14 h to determine proliferation.
Cytotoxicity.
CD8 T-cells purified from 5- to 8-week-old G9Cα−/−.NOD mice, used directly ex vivo or prestimulated for 24 h with 1 μg/ml insulin B15-23 peptide, were harvested and further cocultured with 104 51Cr-sodium chromate (Amersham)-labeled P815 cells together with B15-23 insulin peptide at an effector-to-target (E:T) ratio of 10:1 for 16 h. Specific lysis was calculated as [(cytotoxic release − min)/(max − min)] × 100%, where the minimal release (min) corresponded to the spontaneous lysis, and the maximal lysis corresponded to lysis induced by addition of Triton-X100 (max).
In vivo immunization.
G9Cα−/−.NOD mice were immunized intraperitoneally with 50 μg B15-23 peptide together with different concentrations of CpG 1826 oligonucleotides (Coley pharmaceuticals). Nine to twenty-one days later, a further injection of the same dose was given. Controls were injected with either 50 μg B15-23 peptide or the different doses of CpG alone.
Diagnosis of diabetes.
Diabetes was detected by initially testing for glycosuria and confirmed by blood glucose measurements >13.9 mmol/l/l.
Adoptive transfer of diabetes.
A total of 5–7 × 106 purified CD8 T-cells from G9Cα−/−.NOD mice, either taken directly ex vivo or preactivated by CpG-matured bone marrow–derived dendritic cells and insulin B15-23 peptide, were washed, resuspended in a volume of 200 μl of normal saline, and transferred intravenously to 6-week-old NOD.scid mice, 3-week-old NOD mice, and 6-week-old NOD mice. CD4 T-cells purified either from young 6-week-old mice (107) or from mice that had become diabetic (4–10 × 106) were transferred intravenously to 3- to 4-week-old G9Cα−/−.NOD mice.
Histology.
Pancreata were harvested and fixed, and immunohistochemistry was performed as previously described (30). Insulitis was measured in 6-, 9-, 12-, 15-, 20-, and 25-week-old mice (n = 3–4 each). Sections were stained with hematoxylin and eosin (Vector Laboratories) and scored for insulitis.
RESULTS
TCR transgenic cells are selected in the thymus.
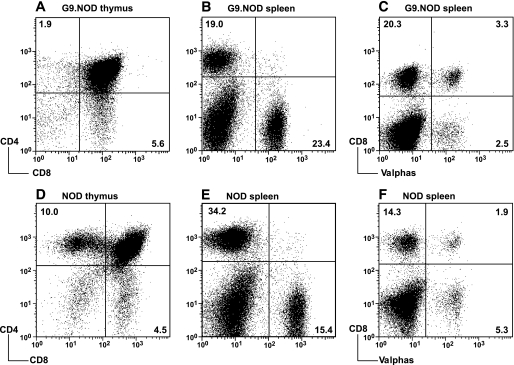
The transgenic TCR in G9NOD mice is expressed in both thymus and the periphery, and there is an increased proportion of CD8 T-cells and reduced CD4 T-cells compared with nontransgenic NOD mice (Fig. 1A–E). The allelic exclusion at the TCRα chain locus is poor (Fig. 1C and F), and other TCRα chains can be selected as shown by a high frequency of expression of the TCRα chains 2, 3, 8, and 11, which were identified by flow cytometry. Similar results were obtained from different TCRαβ transgenic lines (combination of mice from three different TCRα founder lines with two different TCRβ founder lines; data not shown). Thus, to study the transgenic TCRαβ of the original G9C8 T-cell clone, we selected one TCRαβ transgenic line and crossed the G9NOD mice with TCRCα−/− and RAG2−/− mice in order to study the characteristics of the repertoire-restricted TCRαβ transgenic cells.
FIG. 1.
Comparison of thymocytes and splenocytes from G9NOD transgenic mice and nontransgenic NOD mice. A–C: Cells from G9NOD transgenic mice. D–F: Cells from nontransgenic NOD mice. A and D: Thymocytes single-positive CD4 and anti-CD8. B and E: Splenocytes stained with anti-CD4 and anti-CD8. C and F: Splenocytes stained with anti-CD8 and a pool of anti-Vα2, -3, -8, and -11. The results are representative of 15 transgenic mice in more than five independent experiments. The mean (SD) values for the cell populations are as follows: A: CD4 3.46 (1.71); CD8 5.89 (2.28). B: CD4 17.38 (4.91); CD8 20.35 (3.77). C: CD8Vα2, -3, -8, -11 negative 20.0 (4.1); CD8Vα2, -3, -8, -11 positive 3.8 (1.9). D: CD4 10.2 (1.9); CD8 4.7 (0.7). E: CD4 40.63 (5.6); CD8 17.07(1.9). F: CD8Vα2, -3, -8, -11 negative 15.36 (1.03); CD8Vα2, -3, -8, -11 positive 1.85 (0.08).
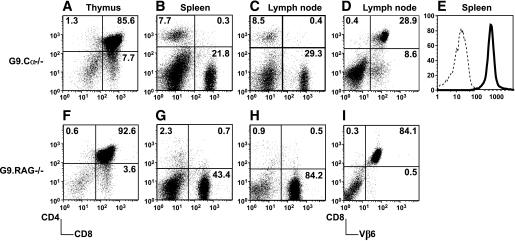
Despite thymic expression of preproinsulin 2 (31), the G9Cα−/− and G9RAG−/− transgenic cells are clearly selected in the thymus (Fig. 2A and F). There is peripheral expansion of the T-cells in the spleen and lymph nodes (illustrated in Fig. 2B and C and Fig. 2G and H, respectively), and, as expected, the cells express TCRVβ6 (shown in Fig. 2D and I), and the majority of cells expressing CD8 are also positive for Kd-insB15-23 tetramer staining (Fig. 2E).
FIG. 2.
Phenotype of G9Cα−/−.NOD mice and G9RAG−/−.NOD mice. G9Cα−/−.NOD thymocytes (A), splenocytes (B), and PLN cells (C) were stained with anti-CD4 and -CD8. D: PLN cells were stained with anti-Vβ6 and CD8. E: Gated CD8 T-cells were stained with Kd-B15-23 (bold line) and control Kd-LLO 91–99 (dotted line) tetramer, respectively. G9RAG−/−.NOD thymocytes (F), splenocytes (G), and PLN cells (H) were stained with anti-CD4 and -CD8. I: PLN cells were stained with anti-Vβ6 and CD8. The results represent three independent experiments. The mean (SD) values for the distribution of cell populations are as follows: A: CD4 1.43 (0.42); CD8 5.1 (1.6). B: CD4 8.28 (1.35); CD8 21.14 (4.62). C: CD4 6.28 (0.57); CD8 52.18 (9.26). F: CD4 0.85 (0.44); CD8 2.93 (1.22). G: CD4 2.21 (2.16); CD8 33.42 (6.94). H: CD4 1.18 (0.66); CD8 68.7 (13.32).
G9Cα−/− TCR transgenic cells respond to antigen by proliferation, cytokine production, and cytotoxicity.
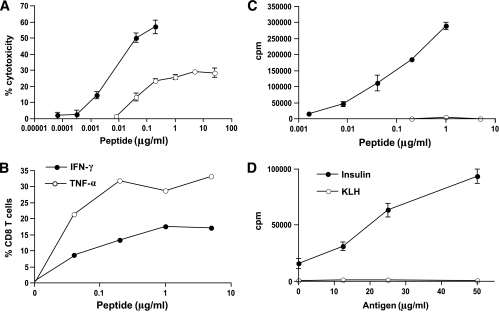
The T-cells demonstrate antigen-specific cytotoxicity directly ex vivo (shown in Fig. 3A), but their ability to kill in vitro targets is enhanced by nearly two logs after culturing the cells with antigen, indicating that the cells are relatively naïve when derived directly ex vivo from the transgenic mouse (Fig. 3A). They produce IFN-γ and TNF-α in response to antigenic peptide (Fig. 3B). As expected, they proliferate to insulin peptide (Fig. 3C) and also respond to insulin protein cross-presented by bone marrow–derived dendritic cells (shown in Fig. 3D), indicating that the peptide is naturally processed and presented. Thus, the transgenic G9 cells maintain the immunological function of their parental G9C8 clone.
FIG. 3.
Functional responses of purified CD8 T-cells from G9Cα−/−.NOD mice. A: Cytotoxic assay of insulin-reactive CD8 T-cells directly ex vivo (○) from G9Cα−/−.NOD mice compared with precultured cells (●) in response to increasing B15-23 peptide concentrations at an E:T ratio of 10:1. B: Intracellular IFN-γ and TNF-α production in response to increasing concentrations of insulin B15-23 peptide after a 40-h culture. C: 3H-Thymidine incorporation proliferation assay of splenocytes from G9Cα−/−.NOD mice in response to B15-23 peptide and to influenza hemagglutinin (flu HA) control peptide. ●, Ins B15-23; ○, flu HA. D: 3H-Thymidine incorporation proliferation assay of splenocytes from G9Cα−/−.NOD mice in response to cross-presented insulin protein or control KLH. The results were from one of four independent experiments.
TCR transgenic cells cause insulitis but not diabetes.
In view of the highly pathogenic nature of the original G9C8 T-cell clone, we tested whether the mice developed insulitis. We found that in the G9Cα−/− TCR transgenic mice, a few islets have a large infiltration by CD8 T-cells but also by B-cells and CD4 T-cells (online appendix Fig. 1 [available at http://diabetes.diabetesjournals.org/cgi/content/full/db08-0800/DC1]). However, infiltration is only seen after 15 weeks of age, and even then most of the islets do not become infiltrated (islets examined from mice at 6, 9, 12, 15, 20, and 25 weeks of age) (insulitis scores shown in online appendix Table 1). Neither the G9Cα−/− TCR mice (30 mice observed until 35 weeks of age) nor the G9RAG−/− TCR mice developed spontaneous diabetes, similar to the three lines of G9NOD mice observed that were not on the Cα−/− background.
Role of CD4 T-cells.
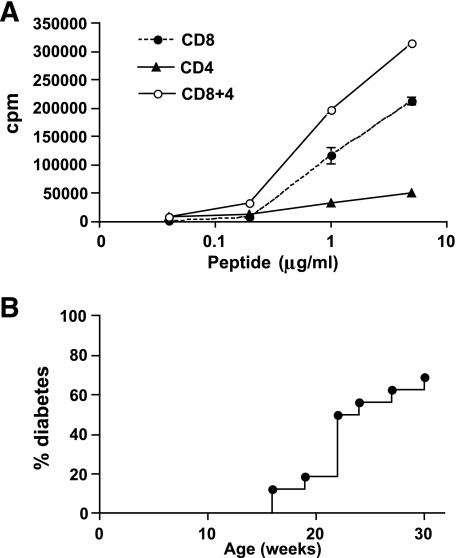
As can be seen from Fig. 2, although there are fewer CD4 T-cells in the G9Cα−/−.NOD mice compared with the G9NOD mice (shown in Fig. 1), the question arises as to whether these CD4 T-cells contain a regulatory T-cell population that prevented the G9Cα−/−.NOD mice from developing diabetes. To examine whether the CD4 T-cell population inhibited the CD8 T-cells in vitro, proliferation assays were performed that showed that G9Cα−/−.NOD CD4 T-cells can proliferate to the insulin B15-23 peptide but at a lower level. Furthermore, they do not inhibit the CD8 T-cells from proliferating when they are added to CD8 T-cells in the proliferation assay but rather enhance the proliferation (shown in Fig. 4A). However, to test further whether these cells were having an inhibitory effect in vivo, we depleted both total CD4 T-cells (n = 6) using anti-CD4 antibody and CD4+CD25+ T-cells (n = 3) using anti-CD25 antibody injected four times over a period of 6 weeks starting at 4 weeks of age. The experiments were controlled by injection of rat isotype control antibody (n = 3). Efficacy of depletion was shown by testing peripheral blood just before the next injection, and at each time point in mice injected with anti-CD4 or anti-CD25, the CD4 or CD4+CD25+ T-cells were <1% in the peripheral blood (data not shown). None of the mice in any of the groups, observed until 20 weeks of age, developed diabetes. Thus, the lack of diabetes did not appear to be related to CD4 regulatory T-cells.
FIG. 4.
The role of CD4 T-cells. A: 3H-Thymidine incorporation proliferation assay of CD8, CD4, and mixed CD8 and CD4 splenocytes from G9Cα−/−.NOD mice in response to B15-23 peptide G9Cα−/−. B: Adoptive transfer of purified CD4 T-cells of splenocytes from diabetic NOD intravenously into 4-week-old G9Cα−/−.NOD mice (n = 16). No diabetes occurred in mice adoptively transferred with CD4 T-cells purified from young NOD mice (n = 5). P < 0.018 when both groups were compared. The mice were tested weekly for glycosuria and diabetes confirmed by blood glucose of >13.9mmol/l.
To further examine whether CD4 T-cell help was required, G9Cα−/−.NOD mice were infused with either purified CD4 T-cells from young NOD mice or with CD4 T-cells from diabetic NOD mice. None of the G9Cα−/−.NOD mice given CD4 cells from young NOD mice developed diabetes, whereas diabetes occurred in G9Cα−/−.NOD mice transferred with CD4 T-cells from the diabetic NOD spleens (shown in Fig. 4B). Thus, activated but not nonactivated polyclonal CD4 T-cells are able to promote diabetes in the G9Cα−/−.NOD mice.
TCR transgenic cells become highly diabetogenic after activation in vitro or in vivo.
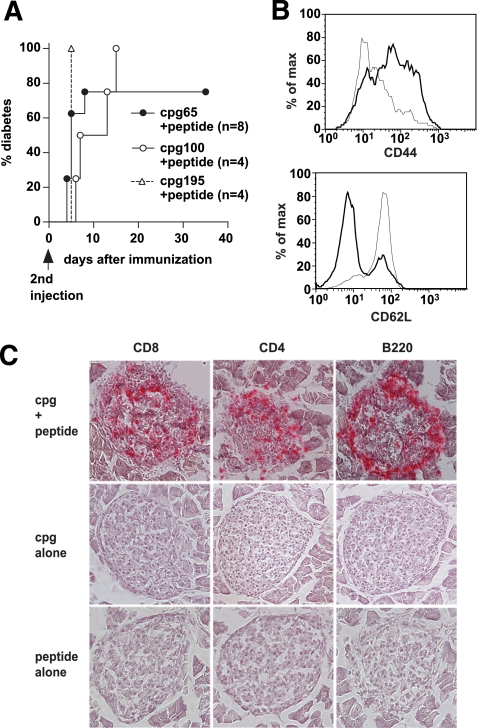
The G9 cells in TCR transgenic mice express a naïve phenotype and function, and the G9Cα−/−.NOD mice do not develop spontaneous diabetes. Therefore, we sought to test whether activation of the insulin-reactive transgenic T-cells, other than by adding activated CD4 T-cells, could cause diabetes. We injected the mice with insulin B15-23 peptide together with type B CpG oligonucleotides and showed that this approach promoted very rapid diabetes development (Fig. 5A). This was a specific response requiring peptide, as CpG alone did not induce disease (data not shown). Similarly, the CpG was required to activate the APCs, as injection with peptide alone also did not induce disease (data not shown). The combined CpG and peptide injection activated G9 transgenic T-cells, and their activation profile shows downregulation of CD62L and upregulation of CD44 (illustrated in Fig. 5B). On development of diabetes, most islets were seen to have extensive infiltration and destruction, whereas little or no insulitis was seen in the islets of mice that were immunized with either CpG or peptide alone (shown in Fig. 5C).
FIG. 5.
Activated CD8 T-cells from G9Cα−/−.NOD mice cause diabetes. A: G9Cα−/−.NOD mice were immunized with B15-23 peptide (50 μg) together with CpG oligonucleotides at different doses (two injections, 9–21 days apart). Diabetes was seen after the second injection in the majority of mice and the graph illustrates the mice, following the second injection, immunized with peptide plus 65 μg CpG (●), peptide plus 100 μg CpG (○), and peptide plus 195 μg CpG (▵). Two of four mice immunized with peptide, and high-dose CpG (195 μg) actually developed diabetes before the second injection and the remaining two mice are shown in the graph. None of the control mice injected with peptide alone (n = 11) or the various doses of CpG alone (n = 13) developed diabetes (data not shown). P < 0.0001 when all groups were compared. B: Flow cytometry of spleen cells from a nonimmunized G9Cα−/−.NOD mouse, gated on CD8 T-cells, shown in the thin line, compared with cells from a diabetic mouse after CpG and peptide immunization, shown in the thick line. The upper panel shows upregulation of CD44 and the lower panel illustrates downregulation of CD62L. C: Histology showing sections from diabetic G9Cα−/−.NOD mouse immunized with CpG and peptide (top row), CpG alone (middle row), and peptide alone (bottom row), stained with anti-CD8, anti-CD4, and anti-B220 (B-cells). (A high-quality representation of this figure is available in the online issue.)
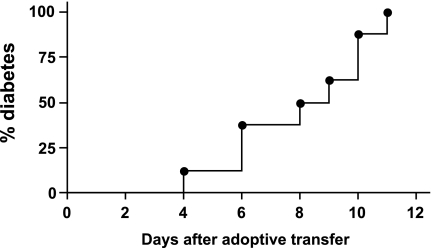
Furthermore, the G9Cα−/−.NOD CD8 T-cells could become potent diabetogenic T-cells after activation with bone marrow–derived dendritic cells that are matured by CpG. Following a 3-day culture with CpG-matured bone marrow dendritic cells in the presence of insulin B15-23 peptide, the G9Cα−/−.NOD CD8 transgenic cells were able to transfer diabetes rapidly without the aid of CD4 T-cells into 6-week-old NOD.scid mice (Fig. 6), recapitulating the phenotype of the original CD8 T-cell clones (23). G9Cα−/−.NOD CD8 T-cells that were not preactivated did not transfer diabetes when a similar number of nonactivated G9Cα−/−.NOD CD8 T-cells were transferred and observed for 12 weeks after transfer (data not shown). This suggests that activation increases the functional avidity of G9 cells (shown by the data in Fig. 3A), and this enhances their cytotoxic function and diabetogenicity.
FIG. 6.
Adoptive transfer of purified activated G9Cα−/−.NOD CD8 T-cells into NOD.scid mice. G9Cα−/−.NOD CD8 T-cells were purified and cultured with matured bone marrow–derived dendritic cells together with 0.5 μg/ml insulin B15-23 peptide for 3 days. After washing, the cells were intravenously injected into 6-week-old NOD.scid mice (n = 8). Mice were tested daily for glycosuria and diabetes confirmed by blood glucose >13.9mmol/l.
Activation of insulin-reactive CD8 T-cells by increasing antigen presentation within islets.
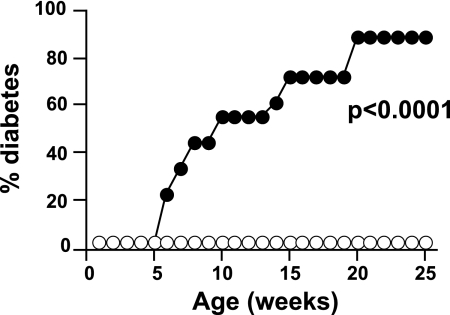
We have previously suggested that expression of the costimulator B7-1 on the islets is able to activate low-avidity T-cells and cause accelerated diabetes (30). To test whether the coexpression of RIP-B7.1 could induce diabetes in G9Cα−/−.NOD mice, we crossed the G9Cα−/−.NOD mice to RIP-B7.1.Cα−/− mice that had been generated for this purpose. In the presence of B7.1 in the pancreas, the G9Cα−/−.NOD mice developed spontaneous diabetes (shown in Fig. 7). To determine that this was a peripheral effect and not related to changes in selection of the G9TCR T-cells, we had previously demonstrated that the naïve G9TCR CD8 T-cells from G9Cα−/−.NOD mice could readily transfer diabetes to NOD.scid mice that expressed RIP-B7.1 (30), indicating that the naïve G9TCR CD8 T-cells can be activated in situ in the islets and become highly pathogenic. These results support the data presented earlier that increasing the functional avidity of G9TCR T-cells through activation can promote diabetogenicity of transgenic G9 T-cells.
FIG. 7.
Diabetes incidence in G9Cα−/−.RIP-B7.1 NOD mice. G9Cα−/−.NOD mice that also expressed RIP-B7.1 transgene (●, n = 18) were compared with nontransgenic G9Cα−/−.NOD mice (○, n = 20) and observed for diabetes. Mice were tested weekly for glycosuria and diabetes confirmed by blood glucose >13.9 mmol/l (P < 0.0001).
Activation of insulin-reactive CD8 T-cells in vivo in NOD mice.
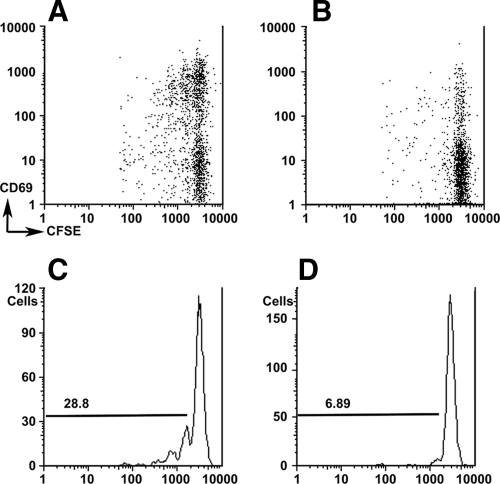
To investigate the homing and activation of insulin-reactive G9 CD8 T-cells in vivo, we used CFSE-labeled, purified CD8 T-cells from G9Cα−/−.NOD mice transferred into 4- to 6-week-old NOD mice and analyzed CFSE dilution in different lymphoid tissues. We showed that the G9 CD8 T-cells proliferated, as shown by the decrease in CFSE staining, mainly in the pancreatic lymph nodes (PLNs) but not in the spleen or other lymph nodes. More importantly, the dividing G9 CD8 T-cells express early activation marker CD69 (Fig. 8). This indicates that NOD mice as young as 4 weeks of age have the capability of activating G9-like cells in the pancreatic lymph nodes. However, this single transfer of nonactivated cells into 4- to 6-week-old NOD mice did not result in acceleration of diabetes, when the mice were followed to 15 weeks (the age by which acceleration of disease would be observed).
FIG. 8.
Insulin-reactive CD8 T-cells proliferate only in PLNs but not in the spleen, MLN, or ALN when adoptively transferred into NOD mice. CFSE-labeled purified G9Cα−/−.NOD CD8 T-cells were injected iv into 4-week-old NOD mice and the lymphoid tissue extracted and cells analyzed in PLNs (A) and spleen (B). The cells were gated on CD8 T-cells, and CFSE-labeled cells, costained with anti-CD69, are shown from PLNs (A) and spleen (B). Percentage proliferation of divided cells is shown after gating on CD8 T-cells from PLNs (C) and spleen (D). Proliferation in MLN and ALN was similar to spleen (data not shown). All groups were compared by ANOVA P = 0.014.
DISCUSSION
Our study has shown that G9 TCR transgenic CD8 T-cells that react to an insulin B-chain peptide B15-23, one of the earliest antigenic epitopes to which CD8 T-cells respond (at 3–4 weeks of age), are selected in the thymus. Once released in the periphery, they can be stimulated to become highly pathogenic. The G9 cells are activated by the B15-23 peptide, which has low-affinity binding to the MHC-Kd, and, interestingly, as expected from a naturally processed and presented peptide, transgenic G9 cells can be stimulated by whole insulin protein cross-presented by dendritic cells. Although G9Cα−/− mice do not spontaneously develop diabetes, they do so when naturally activated polyclonal CD4 T-cells from diabetic NOD mice are transferred, indicating a requirement for CD4 T-cells. Moreover, when transgenic G9 cells are fully activated in vitro, they gain potent diabetogenicity and cause diabetes in immunodeficient NOD.scid mice, without additional CD4 T-cell help, recapitulating the phenotype of the parental clone cells. Importantly, these cells can also be activated in vivo and cause rapid diabetes development by providing exogenous insulin peptide in the presence of immunostimulatory CpG. A similar effect can be achieved if the costimulatory molecule B7.1 is expressed within the islets.
Why are these low-avidity T-cells present in the NOD mouse and where are the cells stimulated in vivo? Recently, a multitude of peripheral antigens were shown to be expressed in medullary epithelial cells of the thymus due to the autoimmune regulator gene Aire (32). High-avidity T-cells are removed at the cortico-medullary junction, protecting against autoimmunity, and deficiency of Aire leads to widespread multiorgan autoimmune disease (32). However, some autoreactive T-cells still escape from the thymus, and, using model antigens, it has been shown that low-avidity T-cells are not deleted in the thymus (33,34). PI2 is expressed in the thymus (17,35), and even with the thymic expression, G9-like low-avidity insulin-reactive T-cells can clearly escape from negative selection. Furthermore, in our studies, low-avidity insulin-specific CD8 T-cells are found in the periphery and are not subject to peripheral deletion. As with other islet autoantigen-reactive reactive CD8 (36) and CD4 (37) T-cells in the NOD mouse, G9 cells are activated and proliferate preferentially in the pancreatic lymph nodes compared with the spleen and other lymph nodes, including the mesenteric lymph nodes. However, our earlier study and those of others (1,3,24) demonstrated that the numbers of these cells remain small in lymphoid tissue, but they are measurable in the islets from an early age. It is possible that only in the islets is sufficient antigen presented to these cells for their expansion, as a relatively high concentration of the low-affinity peptide is required to stimulate them. These cells can be sufficiently activated in the pancreatic draining lymph nodes, possibly by dendritic cells that stimulate them to traffic to the islets where a higher concentration of the insulin peptide within the islets induces G9 cell expansion and damage to the islets in the early phases. However, other specificities that may be of higher avidity, such as IGRP-reactive CD8 T-cells, expand more and overtake these cells in number and percentage representation in the islet infiltrate in later phases of the disease development.
Why do these TCR transgenic mice not develop diabetes spontaneously? In the NOD mouse, different islet antigen–specific T-cells are likely to be involved in early phases of the autoimmune process, and it is likely that each may amplify the autoimmune response of the others by producing interleukin-2 locally, which may also increase cells by homeostatic expansion (38). Insulin, as a β-cell–specific autoantigen, is very different from other β-cell–associated antigens. In addition to MHC class I–restricted antigen presentation by the β-cells, insulin is secreted by islet β-cells in response to high glucose from the portal system and circulates in the body at low concentrations, dependent on ambient glucose. The peptide recognized by the insulin-specific T-cells in this study has a low affinity of binding to H2-Kd molecules (39). The widely studied 8.3 TCR transgenic T-cells recognize an epitope of IGRP 206-214, an enzyme within β-cells with high avidity (40). Much less is known of the other autoantigen, dystrophia myotonica kinase, which stimulates the CD8 T-cells from the TCR transgenic mouse AI-4 (3). Both of these mice develop diabetes spontaneously, although 8.3TCR transgenic mice develop more diabetes in the presence of CD4 T-cells (27), whereas this is not a requirement for the AI-4 transgenic mouse (41). In contrast, for CD4 TCR transgenic mice, which also have varying incidence of diabetes, the highly pathogenic BDC2.5 cells develop spontaneous diabetes when expressed on a C57BL/6 genetic background, but the incidence of diabetes is much lower on the NOD genetic background (42), although it is sharply increased when regulatory T-cells are removed by expression on a NOD.scid genetic background. Similarly, the 4.1 TCR transgenic mice recognizing an unknown islet antigen develop diabetes a little earlier on a RAG2−/− genetic background (27), and the 12-4.1 TCR transgenic mice, recognizing an insulin peptide only, develop diabetes on a RAG2−/− genetic background (43). Not surprisingly, these mice have varying phenotypes, differing between CD4 and CD8 transgenic T-cells that are likely to reflect important aspects of the biology of the native cells within the normal mice and also interactions with other cells of the immune system, emphasizing the need to study more than one specificity of pathogenic T-cell.
The fact that the G9TCR transgenic mice do not develop spontaneous diabetes is highly reminiscent of the LCMVgp/p14 double transgenic mouse, in which a transgenic TCR that recognizes a peptide of lymphocytic choriomeningitis virus (LCMV) glycoprotein was coexpressed with the target LCMVgp as an autoantigen in the pancreas using the rat insulin promoter (RIP) (44). In that model, the TCR transgenic cells were “ignorant” and diabetes did not occur spontaneously but could be induced to occur on infection with LCMV or administration of the LCMVgp peptide with lipopolysaccharide (44). Here, we show that this mechanism can operate for a naturally occurring autoantigen (insulin) and low-avidity (G9) T-cells. In the NOD mouse, which develops diabetes spontaneously, infection is clearly not the strong stimulus necessary for activation of low-avidity cells, and, indeed, many infections prevent diabetes in these mice. However, there may be endogenous stimuli of the innate immune system that could activate low-avidity cells (45,46), leading to further immune activation in both diabetes and other autoimmune diseases. Indeed, we have shown that activation, using a ligand for TLR9, can stimulate the insulin-reactive cells to become highly potent cytotoxic T-cells. The challenge will be to identify how this process occurs in vivo.
What relevance does this low-avidity T-cell have in diabetes? A recent study (15) showed that diabetes was inhibited if NOD mice were deficient in both PI1 and PI2 but expressed a transgenic insulin that was mutated at position 16 of the B-chain. The alteration of B16, in fact, will undoubtedly affect both pathogenic CD4 cells recognizing insulin B9-23 (47) as well as pathogenic CD8 T-cells recognizing insulin B15-23 (23). This B16 mutation overlaps the CD8 epitope B15-23 and is the major MHC-Kd binding residue for the B15-23 epitope (39). Its effects in protecting against diabetes were likely to be linked to removing not only the CD4 epitope affecting diabetogenic CD4 cells but also the CD8 epitope, abolishing the ability of G9-like CD8 T-cells to recognize and bind natural B15-23, a weak binder to the MHC-Kd (39). Thus, although the study by Nakayama et al. (15) did not distinguish effects on CD4 insulin– versus CD8 insulin–reactive T-cells, CD8 T-cells are highly likely to be affected (48,49). G9-like cells are present in the islets at a very early stage of islet infiltration (1), and others have shown that reactivity to B15-23 is the earliest CD8 antigenic specificity so far identified when compared with the more dominant IGRP-reactive T-cells (24). Cytotoxic T-cells specific for IGRP were not detected in PItg NOD mice that were tolerant to PI, suggesting that immune responses against PI are required for IGRP-specific T-cells to develop (50). The current study reports insulin-reactive CD8 transgenic T-cells can cause rapid diabetes after activation, and this study does not address whether insulin is a “prime autoantigen” for CD8 T-cells in NOD mice. Further work is ongoing to establish whether these cells require “help” from CD4 insulin–reactive cells or whether other specificities can provide this.
In conclusion, we have shown that a CD8 T-cell responsive to a natural peptide of insulin, a major autoantigen in autoimmune diabetes, is selected in the thymus and can become highly pathogenic once activated in the periphery, causing diabetes. These T-cells are “ignorant” in a naïve state, but this can be readily overcome by stimuli that include direct activation or activation of antigen-presenting cells, either in pancreatic lymph nodes or in the islets. CD4 T-cells are likely to play a significant role, and this is currently under investigation. Further experiments aim to identify the endogenous stimulators that facilitate the “switch” of naïve and “ignorant” G9 cells into potent β-cell–destructive effector cells in the NOD mouse. Our study provides an important model not only for the study of immunopathogenic role of insulin-specific CD8 T-cells in autoimmune diabetes but also for better design of insulin-based immunotherapy.
Supplementary Material
ACKNOWLEDGMENTS
These studies were funded by the Wellcome Trust, the Juvenile Diabetes Research Foundation, and the National Institute of Diabetes and Digestive and Kidney Diseases (DERC, transgenic core, and NOD mouse genetic core). Tetramers were obtained from the National Institutes of Health Core Facility.
No potential conflicts of interest relevant to this article were reported.
In memory of Charles A. Janeway, Jr., without whose support in the early stages, this work would not have been possible. We thank Dr. Andrew Herman and the University of Bristol Cellular and Molecular Medicine Flow Cytometry Facility for assistance in these studies and Dr. Alexander Chervonsky for helpful comments on the manuscript. We also thank Ewan Basterfield, Jenny Radcliffe, Louise Phelon, and Craig Husher for expert care of the animals.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, Shastri N, Pamer EG, Janeway CA, Jr: Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library [see comments]. Nat Med 1999; 5: 1026– 1031 [DOI] [PubMed] [Google Scholar]
- 2.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, Santamaria P, DiLorenzo TP: Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A 2003; 100: 8384– 8388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieberman SM, Takaki T, Han B, Santamaria P, Serreze DV, DiLorenzo TP: Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J Immunol 2004; 173: 6727– 6734 [DOI] [PubMed] [Google Scholar]
- 4.Quinn A, McInerney MF, Sercarz EE: MHC class I-restricted determinants on the glutamic acid decarboxylase 65 molecule induce spontaneous CTL activity. J Immunol 2001; 167: 1748– 1757 [DOI] [PubMed] [Google Scholar]
- 5.Jarchum I, Baker JC, Yamada T, Takaki T, Marron MP, Serreze DV, DiLorenzo TP: In vivo cytotoxicity of insulin-specific CD8+ T-cells in HLA-A*0201 transgenic NOD mice. Diabetes 2007; 56: 2551– 2560 [DOI] [PubMed] [Google Scholar]
- 6.Takaki T, Marron MP, Mathews CE, Guttmann ST, Bottino R, Trucco M, DiLorenzo TP, Serreze DV: HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J Immunol 2006; 176: 3257– 3265 [DOI] [PubMed] [Google Scholar]
- 7.Mallone R, Martinuzzi E, Blancou P, Novelli G, Afonso G, Dolz M, Bruno G, Chaillous L, Chatenoud L, Bach JM, van Endert P: CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes 2007; 56: 613– 621 [DOI] [PubMed] [Google Scholar]
- 8.Baker C, Petrich de Marquesini LG, Bishop AJ, Hedges AJ, Dayan CM, Wong FS: Human CD8 responses to a complete epitope set from preproinsulin: implications for approaches to epitope discovery. J Clin Immunol 2008; 28: 350– 360 [DOI] [PubMed] [Google Scholar]
- 9.Skowera A, Ellis RJ, Varela-Calvino R, Arif S, Huang GC, Van-Krinks C, Zaremba A, Rackham C, Allen JS, Tree TI, Zhao M, Dayan CM, Sewell AK, Unger W, Drijfhout JW, Ossendorp F, Roep BO, Peakman M: CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest 2008; 118: 3390– 3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh N, Hanafusa T, Miyazaki A, Miyagawa J, Yamagata K, Yamamoto K, Waguri M, Imagawa A, Tamura S, Inada M, et al. : Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest 1993; 92: 2313– 2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sibley RK, Sutherland DE, Goetz F, Michael AF: Recurrent diabetes mellitus in the pancreas iso- and allograft: a light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest 1985; 53: 132– 144 [PubMed] [Google Scholar]
- 12.Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, Podolin PL, Zijlstra M, Jaenisch R, Peterson LB: β2-Microglobulin–deficient NOD mice do not develop insulitis or diabetes. Diabetes 1994; 43: 500– 504 [DOI] [PubMed] [Google Scholar]
- 13.Serreze DV, Gallichan WS, Snider DP, Croitoru K, Rosenthal KL, Leiter EH, Christianson GJ, Dudley ME, Roopenian DC: MHC class I-mediated antigen presentation and induction of CD8+ cytotoxic T-cell responses in autoimmune diabetes–prone NOD mice. Diabetes 1996; 45: 902– 908 [DOI] [PubMed] [Google Scholar]
- 14.Kay TW, Parker JL, Stephens LA, Thomas HE, Allison J: RIP-beta 2-microglobulin transgene expression restores insulitis, but not diabetes, in beta 2-microglobulin null nonobese diabetic mice. J Immunol 1996; 157: 3688– 3693 [PubMed] [Google Scholar]
- 15.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS: Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005; 435: 220– 223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugliese A: Central and peripheral autoantigen presentation in immune tolerance. Immunology 2004; 111: 138– 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B: Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med 2005; 202: 33– 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, Wickramasinghe S, Colle E, Polychronakos C: Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 1997; 15: 289– 292 [DOI] [PubMed] [Google Scholar]
- 19.Moriyama H, Abiru N, Paronen J, Sikora K, Liu E, Miao D, Devendra D, Beilke J, Gianani R, Gill RG, Eisenbarth GS: Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse. Proc Natl Acad Sci U S A 2003; 100: 10376– 10381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thebault-Baumont K, Dubois-Laforgue D, Krief P, Briand JP, Halbout P, Vallon-Geoffroy K, Morin J, Laloux V, Lehuen A, Carel JC, Jami J, Muller S, Boitard C: Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest 2003; 111: 851– 857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French MB, Allison J, Cram DS, Thomas HE, Dempsey-Collier M, Silva A, Georgiou HM, Kay TW, Harrison LC, Lew AM: Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes 1997; 46: 34– 39 [DOI] [PubMed] [Google Scholar]
- 22.Jaeckel E, Lipes MA, von Boehmer H: Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol 2004; 5: 1028– 1035 [DOI] [PubMed] [Google Scholar]
- 23.Wong FS, Visintin I, Wen L, Flavell RA, Janeway CA, Jr: CD8 T cell clones from young nonobese diabetic (NOD) islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. J Exp Med 1996; 183: 67– 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trudeau JD, Kelly-Smith C, Verchere CB, Elliott JF, Dutz JP, Finegood DT, Santamaria P, Tan R: Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J Clin Invest 2003; 111: 217– 223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P: Progression of autoimmune diabetes driven by avidity maturation of a T- cell population. Nature 2000; 406: 739– 742 [DOI] [PubMed] [Google Scholar]
- 26.Anderson B, Park BJ, Verdaguer J, Amrani A, Santamaria P: Prevalent CD8(+) T cell response against one peptide/MHC complex in autoimmune diabetes. Proc Natl Acad Sci U S A 1999; 96: 9311– 9316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P: Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med 1997; 186: 1663– 1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kouskoff V, Signorelli K, Benoist C, Mathis D: Cassette vectors directing expression of T cell receptor genes in transgenic mice. J Immunol Methods 1995; 180: 273– 280 [DOI] [PubMed] [Google Scholar]
- 29.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, Hayday AC, Owen MJ: Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science 1992; 256: 1448– 1452 [DOI] [PubMed] [Google Scholar]
- 30.Thomas IJ, Petrich de Marquesini LG, Ravanan R, Smith RM, Guerder S, Flavell RA, Wraith DC, Wen L, Wong FS: CD86 has sustained costimulatory effects on CD8 T cells. J Immunol 2007; 179: 5936– 5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derbinski J, Schulte A, Kyewski B, Klein L: Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2001; 2: 1032– 1039 [DOI] [PubMed] [Google Scholar]
- 32.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D: Projection of an immunological self shadow within the thymus by the aire protein. Science 2002; 298: 1395– 1401 [DOI] [PubMed] [Google Scholar]
- 33.von Herrath MG, Dockter J, Oldstone MB: How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity 1994; 1: 231– 242 [DOI] [PubMed] [Google Scholar]
- 34.Zehn D, Bevan MJ: T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity 2006; 25: 261– 270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Throsby M, Homo-Delarche F, Chevenne D, Goya R, Dardenne M, Pleau JM: Pancreatic hormone expression in the murine thymus: localization in dendritic cells and macrophages. Endocrinology 1998; 139: 2399– 2406 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, O'Brien B, Trudeau J, Tan R, Santamaria P, Dutz JP: In situ beta cell death promotes priming of diabetogenic CD8 T lymphocytes. J Immunol 2002; 168: 1466– 1472 [DOI] [PubMed] [Google Scholar]
- 37.Turley S, Poirot L, Hattori M, Benoist C, Mathis D: Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med 2003; 198: 1527– 1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamimura D, Bevan MJ: Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med 2007; 204: 1803– 1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong FS, Moustakas AK, Wen L, Papadopoulos GK, Janeway CA, Jr: Analysis of structure and function relationships of an autoantigenic peptide of insulin bound to H-2K(d) that stimulates CD8 T cells in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A 2002; 99: 5551– 55562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han B, Serra P, Yamanouchi J, Amrani A, Elliott JF, Dickie P, Dilorenzo TP, Santamaria P: Developmental control of CD8 T cell-avidity maturation in autoimmune diabetes. J Clin Invest 2005; 115: 1879– 1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graser RT, DiLorenzo TP, Wang F, Christianson GJ, Chapman HD, Roopenian DC, Nathenson SG, Serreze DV: Identification of a CD8 T cell that can independently mediate autoimmune diabetes development in the complete absence of CD4 T cell helper functions. J Immunol 2000; 164: 3913– 3918 [DOI] [PubMed] [Google Scholar]
- 42.Zucchelli S, Holler P, Yamagata T, Roy M, Benoist C, Mathis D: Defective central tolerance induction in NOD mice: genomics and genetics. Immunity 2005; 22: 385– 396 [DOI] [PubMed] [Google Scholar]
- 43.Jasinski JM, Yu L, Nakayama M, Li MM, Lipes MA, Eisenbarth GS, Liu E: Transgenic insulin (B:9-23) T-cell receptor mice develop autoimmune diabetes dependent upon RAG genotype, H-2g7 Homozygosity, and insulin 2 gene knockout. Diabetes 2006; 55: 1978– 1984 [DOI] [PubMed] [Google Scholar]
- 44.Garza KM, Chan VS, Ohashi PS: T cell tolerance and autoimmunity. Rev Immunogenet 2000; 2: 2– 17 [PubMed] [Google Scholar]
- 45.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS: Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity 2007; 27: 321– 333 [DOI] [PubMed] [Google Scholar]
- 46.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ: Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 2007; 8: 487– 496 [DOI] [PubMed] [Google Scholar]
- 47.Wegmann DR, Norbury-Glaser M, Daniel D: Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol 1994; 24: 1853– 1857 [DOI] [PubMed] [Google Scholar]
- 48.Nakayama M, Beilke JN, Jasinski JM, Kobayashi M, Miao D, Li M, Coulombe MG, Liu E, Elliott JF, Gill RG, Eisenbarth GS: Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest 2007; 117: 1835– 1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi M, Abiru N, Arakawa T, Fukushima K, Zhou H, Kawasaki E, Yamasaki H, Liu E, Miao D, Wong FS, Eisenbarth GS, Eguchi K: Altered B:9-23 insulin, when administered intranasally with cholera toxin adjuvant, suppresses the expression of insulin autoantibodies and prevents diabetes. J Immunol 2007; 179: 2082– 2088 [DOI] [PubMed] [Google Scholar]
- 50.Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, Colman PG, Harrison LC, Lew AM, Thomas HE, Kay TW: Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 2006; 116: 3258– 3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.