Abstract
This protocol describes a simple and efficient way to label specific cell surface proteins with biophysical probes on mammalian cells. Cell surface proteins tagged with a 15-amino acid peptide are biotinylated by Escherichia coli biotin ligase (BirA), whereas endogenous proteins are not modified. The biotin group then allows sensitive and stable binding by streptavidin conjugates. This protocol describes the optimal use of BirA and streptavidin for site-specific labeling and also how to produce BirA and monovalent streptavidin. Streptavidin is tetravalent and the cross-linking of biotinylated targets disrupts many of streptavidin’s applications. Monovalent streptavidin has only a single functional biotin-binding site, but retains the femtomolar affinity, low off-rate and high thermostability of wild-type streptavidin. Site-specific biotinylation and streptavidin staining take only a few minutes, while expression of BirA takes 4 d and expression of monovalent streptavidin takes 8 d.
INTRODUCTION
It is a great chemical challenge to modify a single protein amid the many others present on the mammalian cell surface. A number of approaches have been taken to tackle this1. We have focused on using enzymes to achieve high specificity and rapid labeling. In particular, we have adopted the enzyme biotin ligase (BirA) from Escherichia coli 2–4. Site-specific biotinylation is an efficient, rapid and sensitive way for imaging proteins in mammalian cells. It is also effective for protein detection by western blot3 and for protein isolation with exceptional purity5. Proteins that are genetically fused with only a 15-amino acid recognition sequence (the acceptor peptide or AP, also known as Avitag) are recognized by BirA6. Biotinylation then allows sensitive recognition by streptavidin conjugated to a range of biophysical probes, including small molecule fluorophores or quantum dots (Fig. 1), which can allow imaging of the target protein at the single molecule level3,7,8.
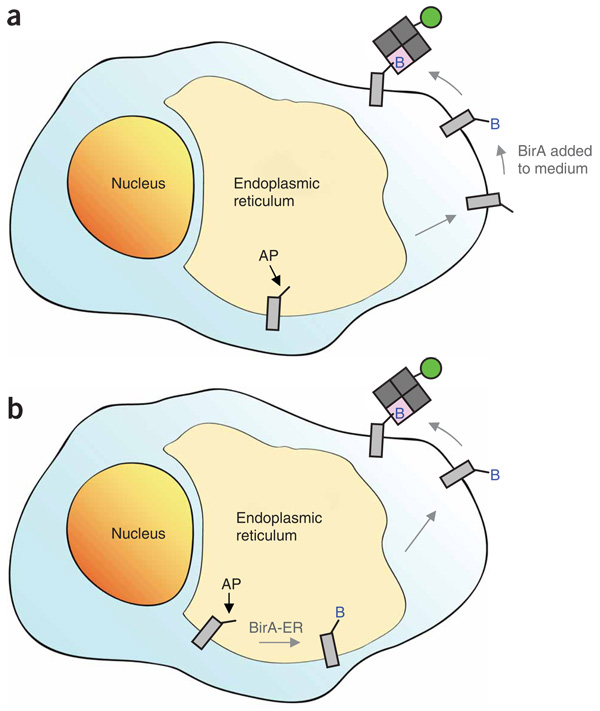
Figure 1.
Schematic of protein imaging with BirA and streptavidin. (a) Biotinylation at the cell surface. The 15-amino acid acceptor peptide (AP) is genetically encoded on the protein of interest. The protein folds in the endoplasmic reticulum (ER) and traffics to the cell surface. Recombinantly expressed biotin ligase (BirA) is added to the cell medium with biotin–AMP or ATP and biotin, biotinylating the AP (blue B = biotin). Excess biotin is removed by washing. Monovalent streptavidin (gray and pink squares, not to scale) conjugated to a probe of interest (green circle) is added to bind the biotinylated surface proteins. (b) Biotinylation with BirA in the ER. The protein folds in the ER and is biotinylated by ER-retained biotin ligase (BirA-ER). The biotinylated receptor traffics to the cell surface and is bound by the monovalent streptavidin-probe conjugate.
Diversity of applications
BirA labeling
BirA labeling has been successfully applied to a wide range of cell surface proteins, with AP insertion at the N terminus, C terminus or within loops7 of the protein and is specific in all the mammalian cell types we have tested, including human, mouse, rat and hamster cell lines, and primary neuron cultures3. BirA labeling has also been successful in nonmammalian cells8–11. BirA labels the AP-tag specifically in different cellular compartments: at the cell surface3, in the endoplasmic reticulum12–14(Fig. 1b), in the cytosol and in the nucleus15. BirA labeling may also be used in living animals, for example in a transgenic mouse expressing cytosolic BirA16.
The advantage of streptavidin labeling is its high affinity and stability17; hence, the benefit of streptavidin labeling over Ab labeling will be most evident for proteins expressed at low levels or where long-term imaging is desired. BirA may also be used to study protein dynamics, either by labeling proteins both before and after a stimulus using streptavidins conjugated to differently colored fluorophores3, or by biotinylating surface proteins, allowing time for internalization, and then using streptavidin to quantify how much biotinylated receptor remains on the cell surface. In nonimaging contexts, the fast on-rate and high stability of the streptavidin–biotin interaction mean that BirA biotinylation allows isolation of protein complexes with greatly improved signal to noise ratios5,16.
An alternative protocol using biotin ligase involves endogenous mammalian biotin ligase (holocarboxylase synthetase) recognition of a small protein domain, for targeting probes in living animals, such as for MRI18. This protocol has the advantage that there is no need to introduce a new biotin ligase but has the disadvantages of the increased size of the acceptor domain (129 amino acids), relative to the AP for BirA, the restricted location of attachment of this acceptor domain and the inability to control when biotinylation occurs18.
Specific biotinylation can also be achieved in cells using reaction with C-terminal Cys generated by intein cleavage19, but this approach limits the site of biotin attachment to the C terminus of the protein, requires incubation of cells with high concentration of thiols, which disrupts the folding of many proteins, and may be restricted to cytosolic labeling.
Monovalent streptavidin
Receptor cross-linking can affect receptor signaling function20. Cross-linking can also change the dynamics of movement within the membrane21,22 and internalization from the membrane. Cross-linking can be avoided to some extent using ligand in excess, but if ligand dissociates over time or new pools of receptor are exocytosed, cross-linking becomes problematic again. This has long been a problem with the use of antibodies in labeling. Some studies have used Fab Ab fragments that will not cross-link, but very often dissociate rapidly23, making study problematic for more than a few minutes. In the case of BirA labeling, using excess wild-type streptavidin will sometimes be sufficient to avoid significant cross-linking of biotinylated proteins (Fig. 2). For more reliable studies we suggest using monovalent streptavidin.
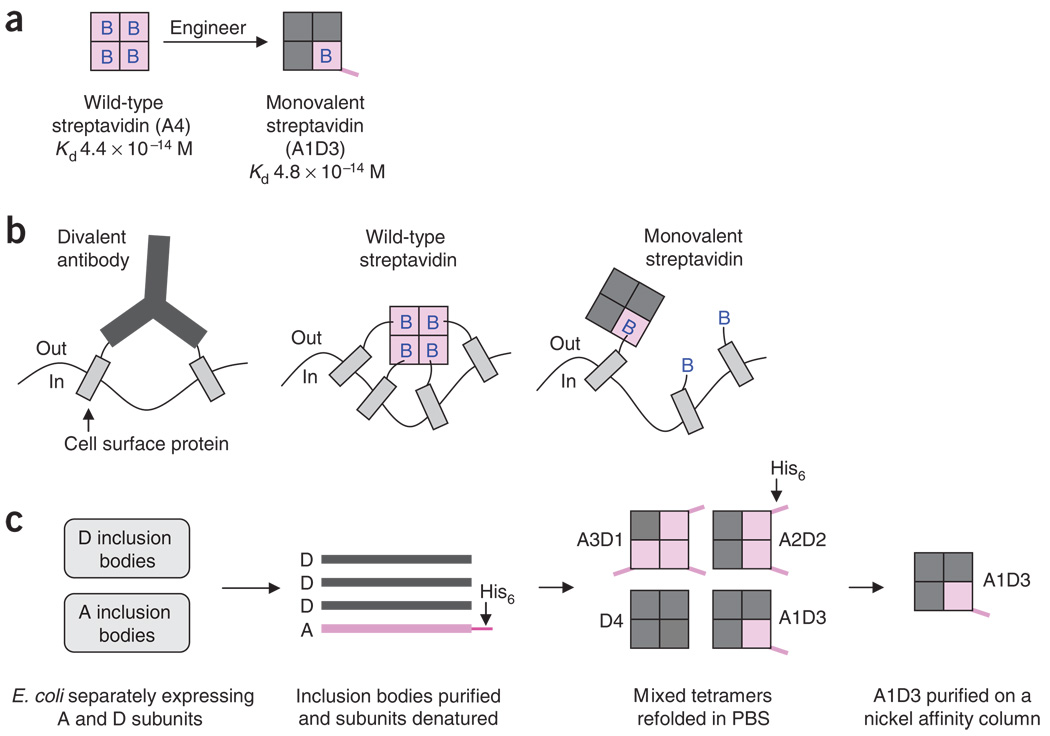
Figure 2.
Monovalent streptavidin structure and purification. (a) Wild-type streptavidin is a tetramer with four biotin binding sites (B = biotin). Monovalent streptavidin has three inactive subunits (dark gray squares) and one subunit that binds biotin with femtomolar affinity (pink square). (b) Schematic of the cross-linking of cell surface proteins by antibodies and wild-type streptavidin, but not by monovalent streptavidin. Wild-type or monovalent streptavidin are shown bound to biotinylated acceptor peptide (AP)-fusion proteins. (c) Process for making monovalent streptavidin. Inactive streptavidin subunits (D = Dead streptavidin) and wild-type streptavidin subunits (A = Alive streptavidin) are separately expressed in Escherichia coli and form inclusion bodies. The inclusion bodies are purified and then dissolved in guanidinium hydrochloride. Dead and Alive subunits at a ratio of 3:1 are refolded by diluting into PBS, to give a mixture of streptavidin heterotetramers. Streptavidin tetramers bearing a single His6-tagged wild-type subunit are purified using a nickel-affinity column.
Streptavidin is a tetramer containing four biotin-binding sites, each with femtomolar affinity for biotin17. Mutations that make streptavidin monomeric reduce biotin-binding affinity by ~ 104-fold24,25. Similarly, monomeric avidin has an enormously reduced biotin-binding affinity26. In our approach, we kept the streptavidin tetramer intact but inactivated three of the biotin-binding sites by mutation4, to give a monovalent streptavidin containing a single biotin-binding site with an affinity equivalent to the original tetramer (Fig. 2a). Monovalent streptavidin is purified by refolding of inclusion bodies expressed in E. coli and a single nickel affinity column (Fig. 2c). We give methods for the application of monovalent streptavidin in cellular imaging, but monovalent streptavidin is also likely to find application in constructing protein arrays, protein delivery in living animals where cross-linking can activate complement destruction of the cell27 and in nanotechnology for controlled assembly of biological28 and nonbiological components29 on the nanoscale.
Limitations
The size of streptavidin
Streptavidin is 56 kDa, and even though this is significantly smaller than an IgG Ab (160 kDa), the size still has the potential to affect protein function. Where possible, controls should be performed to determine this. For example, one can test that binding of streptavidin to AP-tagged epidermal growth factor (EGF) receptor does not change Tyr phosphorylation in response to EGF. We have developed an alternative to streptavidin labeling, where BirA transfers a ketone analog of biotin. The ketone group can then be specifically labeled with a hydrazide linked to a small molecule of interest, such as a fluorophore or a photocrosslinker2. Therefore, the overall change in protein size is very small, although the kinetics of hydrazide labeling mean that this approach lacks sensitivity. We do not provide a protocol for ketone labeling here. An alternative approach, based on the use of lipoic acid ligase, allows labeling with a similar small increase in protein size but with more sensitivity than ketone labeling30.
Exogenous expression
Unlike for Ab labeling of endogenous proteins, the AP-fusion must be transfected into the cell, raising the specter of artifacts from overexpression. One can either test that overexpression does not significantly change the cell behavior of interest, or one can control transgene expression to endogenous levels by the use of suitable promoters or selection of appropriately expressing stable cell lines.
Experimental design
To ensure the specificity of your signal, valuable negative controls are either to perform the above procedure without added or cotransfected BirA, or to pretreat the streptavidin with excess free biotin so that there should be no binding to biotinylated cellular proteins. If you are using a fluorescent protein cotransfection marker, streptavidin staining should be restricted to the cells expressing the fluorescent protein. However, note that the correlation between the cells that are transfected with two different plasmids is not always perfect. The best streptavidin conjugate depends on the usage of filters for your microscope. We have always had the best results with streptavidin-Alexa Fluor 568 (similar spectrum to Texas Red) which is bright and emits well away from cell autofluorescence. Streptavidin-fluorescein and streptavidin-Texas Red gave much poorer signals. Streptavidin-quantum dots will only be better for general imaging than Alexa Fluor 568 under these circumstances: (i) if you have a broad excitation filter particularly for quantum dots, as recommended by Invitrogen; (ii) if the cells are not expressing high levels of cyan fluorescent protein or yellow fluorescent protein, which can bleed into the quantum dot channel more than one would expect; or (iii) if you want to image at the single molecule level3. Avidin conjugates will bind to biotinylated proteins in place of streptavidin, but will show higher nonspecific binding31 and lower stability of biotin binding32.
MATERIALS
REAGENTS
pET21a streptavidin-Alive4, available on request from Ting lab
pET21a streptavidin-Dead4, available on request from Ting lab
pDisplay BirA-ER (M. Howartz et al., unpublished data), available on request from Ting lab
Coomassie brilliant blue
Isopropyl-β-d-thiogalactopyranoside, 100 mg ml−1 in water
 Store at −20 °C (1,000× stock).
Store at −20 °C (1,000× stock).B-PER (Pierce). Store at room temperature.
Lysozyme (Sigma)
 Store powder at −20 °C and dissolve at 10 mg ml−1 in PBS just before use.
Store powder at −20 °C and dissolve at 10 mg ml−1 in PBS just before use.6 M Guanidium hydrochloride, pH 1.5
 Harmful.
Harmful.Nickel-nitrilotriacetic acid agarose (Qiagen)
Phenylmethylsulfonylfluoride. Make up at 100 mM in isopropanol (100 × stock)
 Harmful, store at −20 °C.
Harmful, store at −20 °C.Protease inhibitor cocktail
 The protease inhibitor must be EDTA-free, as EDTA can remove nickel from the nickel-affinity column.
The protease inhibitor must be EDTA-free, as EDTA can remove nickel from the nickel-affinity column.Luria–Broth (LB) medium and LB-Amp plates (ampicillin 100 µg ml−1)
Alexa Fluor 568 succinimidyl ester (Invitrogen)
E. coli BL21 (DE3) pLysS (Novagen)
 Streptavidin does not express well in many other strains of BL21.
Streptavidin does not express well in many other strains of BL21.Standard media for mammalian cell culture
Standard reagents for DNA cloning
Lipofectamine 2000 (Invitrogen) or other preferred transfection reagent
Ammonium sulfate
BirA (Avidity)
Streptavidin-Alexa Fluor 568 (Invitrogen)
Biotin (Sigma)
ATP (Sigma)
Biotin–adenosine monophosphate (biotin–AMP; Ting lab)
Nickel-binding buffer
BSA (Omnipur, fraction V; EMD Biosciences)
Inclusion wash buffer
6 × SDS-loading buffer
Tyrode’s buffer
1 M Imidazole EQUIPMENT
EQUIPMENT
18-Gauge (pink) needle for picking up coverslips
Standard equipment for mammalian cell culture
Bacterial incubator
Floor centrifuge, capable of spinning 30-ml tubes at 17,700g and spinning down 1 l of bacterial culture at 8,000g
Centrifuge tubes appropriate to your centrifuge for spinning liter cultures and for spinning 30 ml at 17,700g
Bacterial shaker
Motorized pipettor
 A powerful pipettor is crucial to efficient washing of inclusion bodies. We have found Pipet-Aid XP (Drummond) most effective.
A powerful pipettor is crucial to efficient washing of inclusion bodies. We have found Pipet-Aid XP (Drummond) most effective.Microscope with filters appropriate to streptavidin-dye (Texas Red filter for Alexa Fluor 568)
Spectrophotometer to measure A280 for protein concentration and A600 to monitor bacterial growth
Sonicator
Poly-prep chromatography column (Bio-Rad)
Protein electrophoresis apparatus, such as Invitrogen X-Lock
Dialysis membrane, 10-kDa cutoff
Microdialysis tubes, 10-kDa cutoff
Stir plate and large stir bar, for dialysis and for refolding of inclusion bodies
DNA electrophoresis equipment
NAP-5 size exclusion column (GE Healthcare)
REAGENT SETUP
BirA
BirA was purchased from Avidity.com, or expressed from pET21a-BirA (plasmid available on request from Ting lab) in E. coli as described in Box 1. Store BirA protein aliquots at −80 °C.
BOX 1 BirA EXPRESSION
Overview: BirA is an Escherichia coli protein; hence, it expresses to a high level and remains soluble, when overexpressed as a His6-fusion. The His6-fusion protein is isolated in good purity and yield using a nickel-affinity column. Note that BirA can alternatively be purchased from Avidity.
- Transform plasmid, amplify bacteria and harvest bacteria
 2 d for Steps (i)–(ii); 7 h for Steps (iii)–(vi)
2 d for Steps (i)–(ii); 7 h for Steps (iii)–(vi)
- Transform competent BL21 (DE3) pLysS E. coli with pET21a-BirA onto Luria–Broth (LB)-Amp plates and incubate at 37 °C overnight.
-
Start overnight culture: inoculate one colony from the plate into 10 ml LB-Amp medium (LB with 100 µg ml−1 ampicillin) and shake at 220 r.p.m. at 37 °C overnight.
 Do not use plates that have been stored at 4 °C and do not use glycerol stocks. Use only freshly transformed plates to ensure optimal expression.
Do not use plates that have been stored at 4 °C and do not use glycerol stocks. Use only freshly transformed plates to ensure optimal expression. -
Start expression culture: dilute 4 ml of this overnight culture into 400 ml LB-Amp.
 Dilute overnight culture at least 100-fold. Shake at 37 °C until A600 is 0.5–0.7 (~ 3 h).Take a 50 µl sample of the bacteria as the preinduction control for testing BirA expression by SDS-PAGE.
Dilute overnight culture at least 100-fold. Shake at 37 °C until A600 is 0.5–0.7 (~ 3 h).Take a 50 µl sample of the bacteria as the preinduction control for testing BirA expression by SDS-PAGE.
- Induce protein expression: add isopropyl-β-d-thiogalactopyranoside to a final concentration of 100 µg ml−1.
- Shake at 30 °C for 3 h. This lower temperature improves the folding efficiency of BirA.
-
Harvest bacteria: take a 50 µl sample of the bacteria as the postinduction sample for testing BirA expression by 12% SDS-PAGE. Centrifuge the culture at 8,000g for 5 min at 4 °C. Decant supernatant. Keep pellet on ice.
 The pellet can also be frozen at −80 °C and processed whenever is convenient. Storage at −20 °C is not recommended. You may want to perform 12% SDS-PAGE at this stage, to confirm that the BirA has induced, before proceeding with the nickel-affinity column.
The pellet can also be frozen at −80 °C and processed whenever is convenient. Storage at −20 °C is not recommended. You may want to perform 12% SDS-PAGE at this stage, to confirm that the BirA has induced, before proceeding with the nickel-affinity column.
- Purify BirA on a nickel-affinity column
 2 h for Steps (i)–(vi); 16 h for Steps (vii)–(ix)
2 h for Steps (i)–(vi); 16 h for Steps (vii)–(ix)
- Lyse bacteria: resuspend pellet in 4 ml ice-cold nickel-binding buffer with phenylmethylsulfonylfluoride and protease inhibitor cocktail using a motorized pipettor (avoid foaming) and transfer to a 15-ml centrifuge tube. Try to keep bacteria/bacterial lysate at 4 °C for the rest of the purification. Sonicate on ice using a microtip for 6 × 15 s on 50% pulse. Let the cells cool for 3 min between pulses. This long rest time helps minimize foaming and improves your yield. Do not let the microtip touch the tube walls while it is sonicating. Ensure your solution bubbles during sonication. Addition of 0.5-mm glass beads increases the lysis efficiency. Spin down the bacterial lysate in 1.5-ml microcentrifuge tubes at 14,000g for 10 min at 4 °C.
- Load a Poly-prep column with 0.4 ml (packed volume) nickel-nitrilotriacetic acid (Ni-NTA) agarose resin. Allow five column volumes of ice-cold nickel-binding buffer to flow through the Ni-NTA (all flow is driven only by gravity). Avoid letting the column run dry for >30 s, else purification will be impaired.
-
Load bacterial supernatant onto the column and let it flow through the column.

- Run 10 column volumes of ice-cold BirA wash buffer (nickel-binding buffer with 30 mM imidazole) through the column to remove nonspecifically bound proteins.
- Elute BirA from the column with 2 ml ice-cold BirA elution buffer (nickel wash buffer plus 100 mM imidazole).
- Store eluted fractions at 4 °C. Check fractions for protein by A280 using elution buffer as the blank or by 12% SDS-PAGE. BirA molecular weight is 35 kDa. With a 1-cm cuvette, 0.1 mg ml−1 of BirA gives A280 = 0.13 (ref. 6). Pool the positive fractions. Determine the protein concentration of your pooled sample and then dilute to 100 µM (3.5 mg ml−1) in PBS (if the concentration is too high, there will be a lot of aggregation when you dialyze).
- Dialyze BirA three times, each for > 3 h, against 1,000-fold excess PBS at 4 °C.
- Measure BirA concentration from A280. Use PBS as a blank. Dilute BirA to 50 µM.
- Aliquot BirA and store at −80 °C, where it is stable indefinitely. Once thawed, the enzyme can be stored at 4 °C for a few days with little loss of activity.
Streptavidin-Alexa Fluor 568
Make up in PBS at 2 mg ml−1. Store 50-µl aliquots at −20 °C. Aliquots are stable at 4 °C for 1–2 months.
Avoid repeated freeze–thaws, which increase nonspecific binding (monova-lent streptavidin and monovalent streptavidin-Alexa Fluor 568 preparation is described in Box 2).
BOX 2 MONOVALENT STREPTAVIDIN EXPRESSION
Overview: Monovalent streptavidin is a heterotetramer consisting of three Dead subunits which do not bind biotin and one subunit which binds biotin with high affinity. Dead (D) and Alive (A) subunits are expressed separately in Escherichia coli, where they form inclusion bodies (Fig. 2c). D and A inclusion bodies are dissolved in guanidinium hydrochloride, mixed in an appropriate ratio, and refolded by rapid dilution into PBS. The refold creates a statistical mixture of tetramers, and monovalent streptavidin is purified from the other tetramers by a nickel-affinity column, as its single His6 tag gives it different elution properties to the tetramers with 0, 2, 3 or 4 His6 tags.
-
Express Alive and Dead streptavidin subunits
 2 d for Steps (i)–(ii); 8 h for Steps (iii)–(v); 3 h for Steps (vi)–(vii)Instructions are for a 1.5-l culture. If you are growing more, scale up all volumes appropriately. To make monovalent streptavidin, grow a 1 l culture of A subunit and 2 or 3 l of D subunit.
2 d for Steps (i)–(ii); 8 h for Steps (iii)–(v); 3 h for Steps (vi)–(vii)Instructions are for a 1.5-l culture. If you are growing more, scale up all volumes appropriately. To make monovalent streptavidin, grow a 1 l culture of A subunit and 2 or 3 l of D subunit.- Transform BL21 (DE3) pLysS with pET21a streptavidin (Alive or Dead) onto LB-Amp plates and incubate at 37 °C overnight.
-
Start overnight culture. Inoculate a colony from the plate in 15 ml LB-Amp medium and shake overnight at 220 r.p.m. and 37 °C.
 Do not use a colony that has ever been at 4 °C. Do not use glycerol stocks to start your overnight culture.
Do not use a colony that has ever been at 4 °C. Do not use glycerol stocks to start your overnight culture. -
Grow up culture. Put the 15 ml culture in 1.5-l LB-Amp and shake at 37 °C until A600 0.9 (~ 3 h).
 Dilute the overnight culture at least 1:100.
Dilute the overnight culture at least 1:100.
- Induce culture. Take 50 µl for the preinduction gel sample and freeze at −20 °C. Add 1.5 ml 1,000 isopropyl-β-D-thiogalactopyranoside (final concentration of 100 µg ml−1) and shake at 37 °C for 4 h.
-
Harvest bacteria. Take 50 µl for postinduction gel sample and freeze at −20 °C. Spin down the rest of the bacteria at 8,000g for 5 min, discard the supernatant and freeze the pellet at −80 °C.
 The pellet can be stored indefinitely at −80 °C.
The pellet can be stored indefinitely at −80 °C. -
Test induction. Run a 16% SDS-PAGE to test induction. The molecular weight of D is 13.2 kDa; A is 14.1 kDa (due to the extra His6 tag) (Fig. 4a).
 On 12% gels the streptavidin monomer band will be hard to resolve from the dye-front.
On 12% gels the streptavidin monomer band will be hard to resolve from the dye-front. -
The preinduction 50 µl sample is concentrated by spinning down at 14,000g for 1 min, the supernatant is removed, and the pellet is resuspended in 20 µl PBS. This 20 µl is mixed with 4 µl of 6 × SDS-PAGE loading buffer and heated at 95 °C for 5 min before loading 12 µl on the gel. Postinduction sample of 50 µl is mixed with 10 µl 6 × SDS-PAGE loading buffer and boiled similarly before loading 12 µl on the gel.

- Purify A and D from inclusion bodies
 3 h
3 h
-
Resuspend the pellet in 10 ml B-PER (Pierce). Then add an extra 10 ml B-PER. Put on a rocker for 10 min. Centrifuge at 27,000g for 10 min at 4 °C. There is no need to perform any of these steps in the cold or to add protease inhibitors—inclusion bodies are aggregates that are not susceptible to proteases.
 Inefficient washing of inclusion bodies will lead to both poor refolding and poor final purity. Many motorized pipettors are too slow to wash inclusion bodies thoroughly. Keep the pipettor plugged in or it will run down. Resuspend pellets fully in small volumes, and only when it is fully suspended, make up to the final desired volume. If the pellet is initially mixed with 100 ml buffer, it will be impossible to resuspend it properly. Resuspend using 10 ml, not 25 ml, pipettes to maximize shear force. Ideally no lumps should be visible before you proceed to the next step, but do not spend more than a few minutes pipetting at each stage. If you are centrifuging in multiple tubes, try to dislodge the pellets to get them in the same tube so that you only make one effort to suspend them all. Avoid as much as possible making B-PER or wash buffer foam.
Inefficient washing of inclusion bodies will lead to both poor refolding and poor final purity. Many motorized pipettors are too slow to wash inclusion bodies thoroughly. Keep the pipettor plugged in or it will run down. Resuspend pellets fully in small volumes, and only when it is fully suspended, make up to the final desired volume. If the pellet is initially mixed with 100 ml buffer, it will be impossible to resuspend it properly. Resuspend using 10 ml, not 25 ml, pipettes to maximize shear force. Ideally no lumps should be visible before you proceed to the next step, but do not spend more than a few minutes pipetting at each stage. If you are centrifuging in multiple tubes, try to dislodge the pellets to get them in the same tube so that you only make one effort to suspend them all. Avoid as much as possible making B-PER or wash buffer foam. -
Again resuspend the pellet from all the centrifuge tubes in a total volume of 10 ml B-PER.
 You can stop inclusion body purification at any stage: put the pellet at −80 °C and resume next day.Add an extra 10 ml B-PER. Add 800 µl lysozyme (Sigma; 10 mg ml−1 in PBS) and incubate at room temperature for 5 min. Add 100 ml inclusion wash buffer. Mix well by repeatedly inverting the bottle, as the solution is very viscous. Centrifuge at 27,000g for 10 min at 4 °C.
You can stop inclusion body purification at any stage: put the pellet at −80 °C and resume next day.Add an extra 10 ml B-PER. Add 800 µl lysozyme (Sigma; 10 mg ml−1 in PBS) and incubate at room temperature for 5 min. Add 100 ml inclusion wash buffer. Mix well by repeatedly inverting the bottle, as the solution is very viscous. Centrifuge at 27,000g for 10 min at 4 °C. - Resuspend the pellet from all the centrifuge tubes in a total volume of 10 ml inclusion wash buffer. Then add 90 ml extra inclusion wash buffer. Mix well. Centrifuge at 27,000g for 10 min at 4 °C. At this stage the pellet may be diffuse and membranous. Remove as much supernatant, while retaining these membranes, because the membranes are also bound to much valuable protein. If there is still lots of supernatant, resuspend the pellet in this left over supernatant, before adding any extra wash buffer. In subsequent washes the size of this membrane should decrease, until it is not visible in final washes. If the membranous material persists, do an extra incubation with lysozyme as in Step B(ii).
- Repeat Step B(iii).
- Repeat Step B(iii) again, to complete the washing of inclusion bodies.
-
- Mix A and D in guanidinium hydrochloride and refold
 1 h for Steps (i–ii); 16 h for Step (iii)
1 h for Steps (i–ii); 16 h for Step (iii)
-
Dissolve the inclusion bodies. Resuspend the pellet in 6 ml 6 M guanidinium hydrochloride pH 1.5.
 Harmful. Spin this solubilized streptavidin at 17,700g for 20 min at 4 °C. Keep the supernatant.
Harmful. Spin this solubilized streptavidin at 17,700g for 20 min at 4 °C. Keep the supernatant. Streptavidin redissolved in guanidinium hydrochloride can be stored at 4 °C for months.
Streptavidin redissolved in guanidinium hydrochloride can be stored at 4 °C for months. - Mix A and D in the appropriate ratio. For each sample in guanidinium, determine the A280. To do this, blank the spectrophotometer with guanidinium. Dilute an aliquot of the streptavidin sample ~ 60-fold in guanidinium before measuring, so that A280 < 1. This is only an estimate of protein concentration because it will depend on how well your protein induced and how well you have washed your inclusion bodies. A sample calculation follows: 2 µl A gave A280 = 0.3, and 2 µl D gave A280 = 0.5. 5 ml D gives 5 × 0.5 = 2.5 units. Three equivalents D per equivalent A, so you want 2.5/3 = 0.833 units A. 0.833/0.3= 2.78 ml A. Therefore, mix 5 ml of D with 2.78 ml of A.
-
Refold streptavidin. Cool 250 ml PBS to 4 °C in a 500-ml Duran bottle in a cold room. Start the PBS spinning rapidly, so that the vortex reaches down to the top of the stir bar. Add guanidinium supernatant, drop by drop using a 200-µl pipette, to the fastest moving part of the PBS.
 Adding streptavidin too rapidly to the PBS will promote aggregation. The point is to dilute each drop of guanidinium as quickly as possible. The fraction of a second that the protein is at a high concentration and in an intermediate concentration of guanidinium is when it is most prone to aggregate. When all the guanidinium has been added, turn down the stirring speed so that there is no vortex. If your refolding has been very successful, the PBS should be largely clear. If the PBS is very cloudy or even worse contains white threads, your refolding yield will be poor.Note: Continue stirring overnight at 4 °C.
Adding streptavidin too rapidly to the PBS will promote aggregation. The point is to dilute each drop of guanidinium as quickly as possible. The fraction of a second that the protein is at a high concentration and in an intermediate concentration of guanidinium is when it is most prone to aggregate. When all the guanidinium has been added, turn down the stirring speed so that there is no vortex. If your refolding has been very successful, the PBS should be largely clear. If the PBS is very cloudy or even worse contains white threads, your refolding yield will be poor.Note: Continue stirring overnight at 4 °C.
-
-
Precipitate streptavidin from refold
 1dThis is to remove what has not folded, to remove other contaminating proteins that were in the inclusion bodies, and to concentrate your streptavidin from 250 ml to 1 ml.
1dThis is to remove what has not folded, to remove other contaminating proteins that were in the inclusion bodies, and to concentrate your streptavidin from 250 ml to 1 ml.- Centrifuge the 250 ml refold at 17,700g for 15 min at 4 °C to remove streptavidin that did not fold and other contaminating proteins. Keep the supernatant.
- Precipitating streptavidin. Add 62.7 g solid ammonium sulfate, ~ 10 g at a time, to the supernatant, while stirring at 4 °C. Stir for > 3 h. Remove insoluble debris by filtering through tissue paper, using a funnel, into a 500-ml Duran bottle and keep the flow-through. It is not necessary to use high quality filter paper for this filtration—regular paper towels perform fine.
- Add a further 59 g ammonium sulfate all at once to the flow-through and leave stirring gently > 3 h at 4 °C. Next, centrifuge at 17,700g for 15 min at 4 °C. Streptavidin is the precipitate! You can discard the supernatant. Let the container drain upside-down on tissue paper, to remove the remaining supernatant.
-
Resuspend the precipitate in a minimum volume of PBS at room temperature (~1 ml). Remove the last of the insoluble material by spinning at 14,000g for 5 min at 4 °C. Keep the supernatant. Measure A280 with a blank of PBS (with a 1-cm cuvette, 0.1 mg ml−1 of streptavidin gives A280 = 0.355 (ref. 41)). The yield of total soluble streptavidin should be 5–20 mg l−1 of starting culture. If the concentration is > 3 mg ml−1, dilute to 3 mg ml−1 to reduce aggregation. Dialyze the supernatant three times in 2 l PBS, each dialysis for > 3 h. This dialysis is only because excess ammonium is reported to interfere with the nickel-nitrilotriacetic acid (Ni-NTA) column. Shorter dialysis or running through a NAP-5 column (GE Healthcare) may be sufficient.
 Mixed streptavidin tetramers in PBS may be stored indefinitely at −80 °C.
Mixed streptavidin tetramers in PBS may be stored indefinitely at −80 °C. -
Testing the streptavidin refold. Test purity and yield on an 8% SDS-PAGE gel, loading samples without boiling. The streptavidin tetramer is 56 kDa. It will stay as a tetramer if you add SDS-PAGE loading buffer but do not boil, although it may not run at the same rate as an unfolded 56 kDa protein42.
 Do not allow sufficient ice to melt that the electrophoresis equipment begins to float in the ice box or there is a risk of electrical shock.You should see a ladder of different heterotetramers (Fig. 4c, ‘mix’ lane). Ideally D4, A1D3 and A2D2 will be present in approximately equal amounts, so that the yield of A1D3 is optimized. If D4 is in excess, use more A relative to D in the refold next time. If there is little D4 present, use more D relative to A in the refold next time.
Do not allow sufficient ice to melt that the electrophoresis equipment begins to float in the ice box or there is a risk of electrical shock.You should see a ladder of different heterotetramers (Fig. 4c, ‘mix’ lane). Ideally D4, A1D3 and A2D2 will be present in approximately equal amounts, so that the yield of A1D3 is optimized. If D4 is in excess, use more A relative to D in the refold next time. If there is little D4 present, use more D relative to A in the refold next time. When analyzing streptavidin tetramers by SDS-PAGE, it helps to make sure the gel box is well cooled, because the gel can heat up to 50 °C. Because of the presence of SDS, this temperature is enough to break up the tetramer. Thus surround the gel box with ice, run at 170 V, and make sure every 15 min that the ice is still in contact with the gel box.
When analyzing streptavidin tetramers by SDS-PAGE, it helps to make sure the gel box is well cooled, because the gel can heat up to 50 °C. Because of the presence of SDS, this temperature is enough to break up the tetramer. Thus surround the gel box with ice, run at 170 V, and make sure every 15 min that the ice is still in contact with the gel box.
- Purify monovalent streptavidin on the nickel-affinity column
 2 h for Step E(i–v); 1 d for Step E(vi); 3 h for Step E(vii); 1 d for Step E(viii)
2 h for Step E(i–v); 1 d for Step E(vi); 3 h for Step E(vii); 1 d for Step E(viii)
- Load a Poly-prep column with 1.6 ml (packed volume) Ni-NTA agarose.
- Wash with 8 ml nickel-binding buffer, using gravity flow at room temperature. Meanwhile lay out microcentrifuge tubes to collect the fractions.
-
Keep 50 µl of your streptavidin for a later gel. Load the remainder of the streptavidin on the column and allow to flow into the column.
 Loading the streptavidin onto the resin in batch mode, by rocking, will make the separation of monovalent streptavidin from divalent streptavidin less efficient.
Loading the streptavidin onto the resin in batch mode, by rocking, will make the separation of monovalent streptavidin from divalent streptavidin less efficient. - Let 8 ml SA wash buffer (nickel-binding buffer plus 10 mM imidazole) flow through the column, eluting the rest of D4 and impurities from the inclusion bodies.
- Add 12 ml SA elution buffer (nickel-binding buffer plus 75 mM imidazole), eluting monovalent streptavidin. Collect 0.5 ml fractions in 1.5-ml microcentrifuge tubes. To purify divalent streptavidin, follow the elution conditions we described previously4. To purify trivalent streptavidin, it is best to put the His6 tag on the Dead subunit (Dead-His6 plasmid available on request from the Ting lab), which will lead to more efficient nickel-affinity separation.
- Test fractions on an 8% SDS-PAGE gel. Mix 10 µl samples of each fraction with 2 µl 6 × SDS-loading buffer and load without boiling onto 8% SDS-PAGE gels. In one lane on each gel load the mix of streptavidin tetramers reserved in Step E(iii), as a reference as to which band is which (Fig. 4b). Pool the fractions containing monovalent streptavidin and dialyze in PBS three times. For a final preparation of 1–2 mg ml−1, only pool the most concentrated fractions at this stage. If total yield is more important, pool all fractions that show good purity.
- Finally, run the pooled and dialyzed monovalent streptavidin again on an 8% gel, with comparison to a mixed refold, to confirm the purity (Fig. 4c). The yield of the desired heterotetramer should be ~2 mg l−1 of initial culture. Monovalent streptavidin can be stored for a month at 4 °C or indefinitely at −80 °C. Avoid multiple freeze–thaw cycles.
-
To label monovalent streptavidin with Alexa Fluor 568, use Alexa Fluor 568 succinimidyl ester exactly according to the protocol for labeling antibodies in the ‘Amine Reactive Probes’ protocol on the Invitrogen website. This uses a dye:streptavidin molar ratio of 10:1. After reaction, purify labeled streptavidin away from free dye on an NAP-5 column, following manufacturer’s instructions, and then dialyze three times, each time for > 3h in > 500-fold excess PBS. This typically gives us three dye molecules attached per streptavidin tetramer.
 Ensure that the monovalent streptavidin has been thoroughly dialyzed before labeling and that there are no nucleophiles present, such as Tris or imidazole, which may disrupt succinimidyl ester labeling.
Ensure that the monovalent streptavidin has been thoroughly dialyzed before labeling and that there are no nucleophiles present, such as Tris or imidazole, which may disrupt succinimidyl ester labeling.
Biotin
Store powder at 4 °C. Store 100 mM stocks in DMSO long term at −20 °C but aliquots are stable for at least a few months at 4 °C. It is hard to dissolve biotin to > 1 mM in aqueous solution.
ATP
Make single-use 100-mM aliquots at −80 °C (100 × stock).  Avoid freeze–thawing. ATP will rapidly be hydrolyzed at pH > 8.5.
Avoid freeze–thawing. ATP will rapidly be hydrolyzed at pH > 8.5.
Biotin–AMP
No commercial supplier. Synthesize according to Coleman and Huang33 or available on request from Ting lab. Powder should be stored desiccated at −20 °C. Dissolve at 1 mM in PBS pH 7.4 (100 × stock).
Solutions are best stored in aliquots at −80 °C.  Avoid pH > 8.5 or biotin–AMP will hydrolyze to biotin and AMP.
Avoid pH > 8.5 or biotin–AMP will hydrolyze to biotin and AMP.
Ampicillin, sodium salt
Dissolve at 100 mg ml−1 in water and filter sterilize.
Nickel-binding buffer
50 mM Tris base, 300 mM NaCl, adjusted to pH 7.8 with 1 M HCl. Store at 4 °C.
1 M Imidazole
in nickel-binding buffer, adjusted with 5 M HCl to pH 7.8. Dilute the imidazole stock solution in nickel-binding buffer to make:
| Buffer | Imidazole concentration (mM) |
| BirA wash buffer | 30 |
| BirA elution buffer | 100 |
| SA wash buffer | 10 |
| SA elution buffer | 75 |
BSA
Make 20 ml of 10% BSA stock in PBS and then dialyze four times in 2 l of PBS, for 24 h each time, to remove traces of biotin. Store 1 ml aliquots at −20 °C, but aliquots are stable at 4 °C for 2 weeks. For neurons, Sigma cell culture grade BSA is preferable.
Inclusion wash buffer
100 mM NaCl, 50 mM Tris pH 8.0, 0.5% Triton X-100, stable at room temperature.
Tyrode’s buffer
145 mM NaCl, 1.25 mM CaCl2, 3 mM KCl, 1.25 mM MgCl2, 0.5 mM NaH2PO4 · H2O, 10 mM glucose, 10 mM HEPES, adjusted to pH 7.4 with 1 M HCl and filter-sterilized, store at 4 °C.
6 × SDS-loading buffer
0.23 M Tris–HCl pH 6.8, 24% vol/vol glycerol, 120 µM bromophenol blue, 0.4 M DTT, 0.23 M SDS, store at −20 °C.
EQUIPMENT SETUP
Glass coverslips
For cell culture, 22 × 22 mm2 no. 0 or no. 1 thickness coverslips from VWR, cut to ~ 7 × 7 mm2 by hand using a diamond knife. We commonly perform biotinylation reactions on cells grown on 7 × 7 mm2 coverslips in 48-well plates. This means the reaction volume is only 100 µl and uses only a small number of cells. For imaging, we transfer the coverslips to homemade imaging dishes (made by punching a 15-mm diameter hole in a 35-mm cell culture plate and covering the hole with a glass coverslip attached with candle wax), so that there are only two thin pieces of glass between the cells and the objective lens. (Imaging quality is always very poor through plastic.) The coverslips are sterilized in the 48-well plate by a few minutes of UV irradiation from the lamp in the cell culture hood. Alternatively, glass-bottom multiwell culture plates (MatTek Corporation) or chamber slides (Lab-Tek) may be used. Note that for single molecule imaging of dyes or fluorescent proteins, coverslip preparation is crucial34,35.
PROCEDURE
Biotinylation of cell surface proteins with BirA  3d
3d
-
1
Insert the acceptor peptide sequence into your protein of interest. BirA can biotinylate the acceptor peptide, AP (GLNDIFEAQKIEWHE) at the N terminus, C terminus2,3 or in a loop of a protein7. It does not matter if there are a few residues preceding the AP at the N terminus or following the AP at the C terminus. However, it is probable that the presence of a big folded domain right next to the AP may hinder BirA access. So if possible, add a few Gly or Ser on either side of the AP sequence (to give GSGLNDIFEAQKIEWHEGSG) to insulate the AP tag from the folding influence of the rest of the protein. Insertion can be done either by standard methods, involving ligation of hybridized primers into a restriction site2, or by inverse PCR. Inverse PCR can introduce a short peptide sequence into a gene in one step independent of restriction sites present, and so gives great flexibility in the site of AP insertion36. The AP sequence must be on the extracellular/luminal face of the protein. Instead of cloning the AP fusion by yourself, you can use commercial sources of ~20,000 human genes with AP inserted at the N terminus or the C terminus. Note that N-terminal fusions will not allow AP display at the cell surface because they will precede the signal sequence.
Biotinylation of AP
-
2There are two possibilities for AP biotinylation in mammalian cells: (i) Adding recombinant BirA at the cell surface, or (ii) expression of BirA in the secretory pathway (Fig. 1). Cotransfection with BirA has the advantage of simplicity—there is no need to prepare BirA protein or biotin–AMP—and the whole experiment is quicker. The advantage of biotinylation by adding BirA protein to the cell surface is that only the cell surface pool of proteins is biotinylated; hence, this pool can be tracked specifically for several hours with only a 0.24-kDa modification. On the basis of experiments with a number of proteins (EphA3, GluR2, low-density lipoprotein receptor), we have not observed a difference in the sensitivity of the two methods of biotinylation. The procedure follows.
- AP-fusion biotinylation with BirA added to the cell surface
 2 d for Step 2A(i–ii); 2 min to 2 h for Step 2A(iii–iv)
2 d for Step 2A(i–ii); 2 min to 2 h for Step 2A(iii–iv)
- Plate cells on 7 × 7 mm2 coverslips in 48-well plates for transfection.
- Transfect cells with the AP-fusion or establish a stable clone. Transient expression is typically optimal 24–48 h after transfection. Cotransfection with a plasmid encoding a fluorescent protein is a useful way to test transfection efficiency and to identify transfected cells under the microscope.
- BirA biotinylation. Wash the cells in PBS pH 7.4 with 5 mM MgCl2 (PBS–Mg). (For neurons, replace PBS–Mg at every step with Tyrode’s buffer.)
- Add PBS–Mg with 0.3 µM BirA (from Avidity, or expressed as in Box 1) and 10 µM biotin–AMP for 1–60 min at room temperature or 37 °C. If biotin–AMP is not available, 1 mM ATP and 10 µM biotin can be used instead, although the ATP may cause purinoreceptor activation3,37,38. The efficiency of biotinylation will depend on the concentration of your target protein at the cell surface and the steric hindrance around the AP. We can typically detect biotinylation after 1-min incubation with BirA and 15-min incubation is saturating. If you need faster biotinylation, you can increase [BirA] to 2 µM.
 Adding more biotin or biotin–AMP can impair the signal. With 1 mM biotin, we regularly observe lower signals than with 10 µM biotin. We suspect this is because 1 mM biotin is hard to wash off and may use up some of the streptavidin. Adding more biotin–AMP is unwise for a different reason. Biotin–AMP is an activated intermediate and at 100 µM there is nonspecific biotinylation of Lys on cell surface proteins.
Adding more biotin or biotin–AMP can impair the signal. With 1 mM biotin, we regularly observe lower signals than with 10 µM biotin. We suspect this is because 1 mM biotin is hard to wash off and may use up some of the streptavidin. Adding more biotin–AMP is unwise for a different reason. Biotin–AMP is an activated intermediate and at 100 µM there is nonspecific biotinylation of Lys on cell surface proteins.
-
AP-fusion biotinylation with BirA in the secretory pathway
 2dCotransfect the AP fusion with the plasmid delivering BirA to the secretory pathway (pDisplay BirA-ER). We typically transfect with an equal amount of plasmid encoding the AP fusion and BirA-ER. After transfection in serum-free medium, using Lipofectamine 2000, we remove the transfection mix and add back normal growth medium, supplementing this with 10 µM biotin. For neuron culture in Neurobasal medium (Invitrogen), there is no need to add any supplementary biotin. Cells are then analyzed the next day.
2dCotransfect the AP fusion with the plasmid delivering BirA to the secretory pathway (pDisplay BirA-ER). We typically transfect with an equal amount of plasmid encoding the AP fusion and BirA-ER. After transfection in serum-free medium, using Lipofectamine 2000, we remove the transfection mix and add back normal growth medium, supplementing this with 10 µM biotin. For neuron culture in Neurobasal medium (Invitrogen), there is no need to add any supplementary biotin. Cells are then analyzed the next day.
Detect biotinylation with streptavidin  15 min to 2 h
15 min to 2 h
-
3
Wash the cells four times with PBS–Mg to remove any residual biotin/biotin–AMP.
-
4
Incubate with PBS–Mg, 1% dialyzed BSA and 10 µg ml−1 (monovalent) streptavidin-Alexa Fluor 568 or an alternative streptavidin conjugate for 5 min at 24 °C.
-
5
Wash three times with PBS–Mg to remove free streptavidin. You can then incubate the cells for varying times, to allow internalization and trafficking of the AP-fusion protein, or image them immediately, using the same buffer (Fig. 3, panel a).
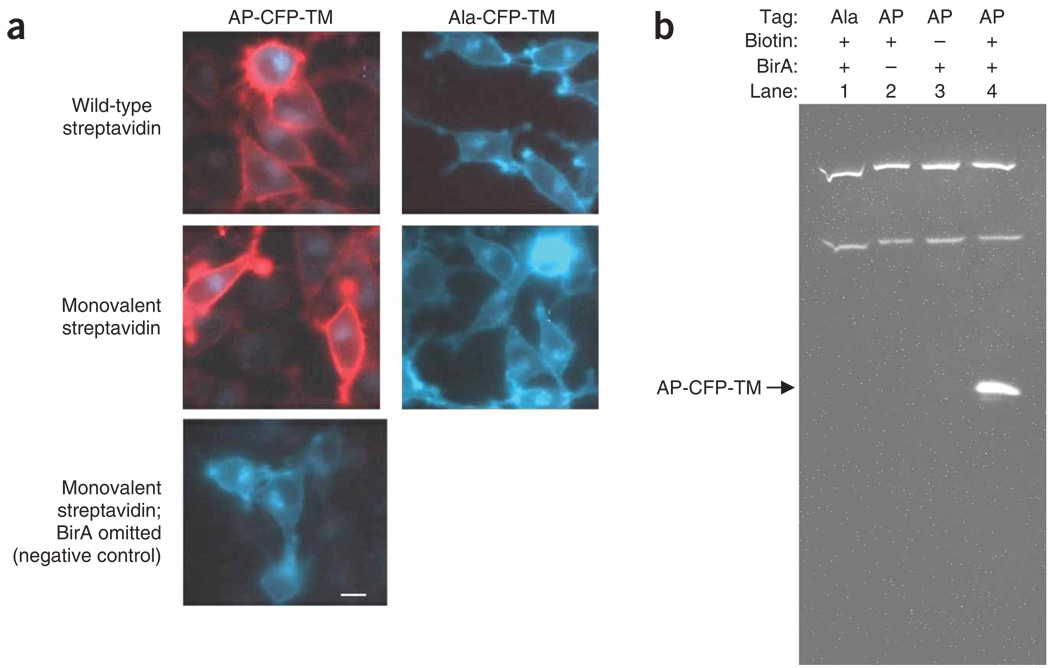
Figure 3.
Specificity of cell surface labeling with BirA and streptavidin. (a) BirA specificity by fluorescence imaging. AP-CFP-TM is a construct with cyan fluorescent protein (CFP) with an acceptor peptide (AP), and the transmembrane helix of platelet-derived growth factor receptor. HeLa cells transfected with AP-CFP-TM or Ala-CFP-TM (a control with an Ala point mutation in the AP) were biotinylated with BirA for 10 min and stained with wild-type (top row) or monovalent streptavidin (middle row) conjugated to Alexa Fluor 568. Alexa Fluor 568 (red) and CFP (cyan) images are overlaid. No streptavidin staining of AP-CFP-TM was observed when BirA was omitted (bottom row). Scale bar 10 µm. (b) BirA specificity tested by immunoblot. HeLa transfected with AP-CFP-TM were incubated with BirA for 5 min and total cell lysates were blotted with wild-type streptavidin–horseradish peroxidase. Data are displayed with (1) Ala-CFP-TM, (2) no BirA, (3) no biotin or (4) with all components present. Upper bands represent endogenous biotinylated proteins.
 Centrifuge all streptavidin conjugates at 14,000g for 5 min at 4 °C immediately before use, to remove aggregates.
Centrifuge all streptavidin conjugates at 14,000g for 5 min at 4 °C immediately before use, to remove aggregates.
 Extensive incubation of cells at >4 °C will allow nonspecific internalization of streptavidin-dye conjugate, leading to high nonspecific background. The streptavidin–biotin on-rate is very fast, so 5 min at room temperature should be sufficient. If further receptor trafficking or signaling is not required, streptavidin can be applied for 10 min at 4 °C and washed at 4 °C, for minimum nonspecific background from internalization. Cell lines will generally maintain their morphology for ≥1 h at 4 °C, while you stain and image.
Extensive incubation of cells at >4 °C will allow nonspecific internalization of streptavidin-dye conjugate, leading to high nonspecific background. The streptavidin–biotin on-rate is very fast, so 5 min at room temperature should be sufficient. If further receptor trafficking or signaling is not required, streptavidin can be applied for 10 min at 4 °C and washed at 4 °C, for minimum nonspecific background from internalization. Cell lines will generally maintain their morphology for ≥1 h at 4 °C, while you stain and image.
 Dilute BirA or streptavidin on the day of the experiment. Proteins are unstable at low concentration and get stuck to the sides of the tubes.
Dilute BirA or streptavidin on the day of the experiment. Proteins are unstable at low concentration and get stuck to the sides of the tubes.
![]()
Cell fixation (optional)
-
6
After staining with streptavidin, there is no problem to fix with formaldehyde or methanol, using standard methods.
If you intend to biotinylate, then fix with formaldehyde, and then add streptavidin, it is possible to get specific AP-biotin staining, but you need to be careful: even without adding a detergent, such as Triton X-100, formaldehyde can permeabilize the cells. Using electron microscopy-grade methanol-free formaldehyde can help to stop permeabilization. Cells have endogenous biotinylated proteins in the mitochondria and the cytosol39, but not in the secretory pathway. If the streptavidin is able to penetrate the plasma membrane, this will cause high background staining. Be sure to do a negative control, omitting BirA to test the specificity of your signal.
If you fix with formaldehyde, then add BirA, and stain with streptavidin, you will probably not get a specific signal because the formaldehyde will react with the Lys on the AP tag and block the biotinylation.
![]()
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 5 | No streptavidin staining visible | Poor transfection | Are you sure that there is good transfection? Can you cotransfect a plasmid encoding a cytosolic fluorescent protein to see what proportion of cells is being transfected? |
| Acceptor peptide (AP)-tagged protein not getting to the cell surface | Many proteins do not traffic well to the cell surface when expressed in unnatural cell types, for example, AMPA receptors traffic poorly in HEK 293 cells. Some proteins express better in the presence of a partner, for example, GluR1 with GluR2. Can you use an Ab to an extracellular epitope of your protein to stain live cells and show that your protein is getting to the cell surface? We found that incubating HEK 293 or HeLa cells at 32 °Cor even 25 °C overnight can improve the surface export of many proteins. If the temperature of your incubator cannot be changed, it may be possible to get CO2-independent medium (e.g., from Invitrogen), so that cells just need to be placed at the desired temperature. Some proteins do not tolerate peptide insertion at certain sites, leading to protein misfolding, mistargeting or degradation. See if there is any precedent for successful or unsuccessful peptide or fluorescent protein insertion at your site of interest | ||
| BirA not working | Use a positive control of cells transfected with AP-CFP-TM (plasmid available from the Ting lab2), where you can see transfected cells from their cyan fluorescent protein (CFP) expression (Fig. 3a). If this does not give you a very clear signal, there is a problem with your BirA reaction or streptavidin detection scheme. We do this control every time we have a new reagent, DNA construct or cell line. Note that AP-CFP-TM does not work well in neurons: use AP-neuroligin 2 as a positive control instead | ||
| Streptavidin not working | If there is any doubt that your streptavidin-dye is functional and visible on your microscope system, the strongest positive control is to incubate cells in PBS with and without 1 mg ml−1 EZ-Link Sulfo-NHS-LC-Biotin (Pierce) for 10 min at 24 °C, wash four times in PBS and incubate with 10 µg ml−1 streptavidin-Alexa Fluor 568 in PBS–Mg with 1% BSA for 5 min at 24 °C. Wash three times in PBS and image. There should be a dramatic difference in streptavidin signal between cells with and without Sulfo-NHS-LC-Biotin, which reacts nonspecifically with hundreds of proteins on the cell surface | ||
| AP is not accessible to BirA | BirA can detect the AP at the N terminus, C terminus or in a loop of a protein. It does not matter if there are a few residues preceding the AP at the N terminus or following the AP at the C terminus. So if a given construct does not work, perhaps you could add another few spacer residues (e.g., GlySerSerGly) between the AP tag and the rest of the protein. Alternatively, place the AP at a different extracellular location on the protein | ||
| Nonspecific streptavidin staining | Bad batch of streptavidin or too much biotin–AMP | Do you get the same background if you omit BirA? If so, you should reduce your streptavidin concentration, obtain a fresh batch of streptavidin, add streptavidin for a shorter time or add streptavidin at 4 °C. Make sure you are adding 1% BSA along with the streptavidin. Do you get the same background if you add biotin–AMP but no BirA? If so, biotin–AMP is probably reacting nonspecifically, so you need to reduce the biotin–AMP concentration (1 µM also yields good results) and check that your buffer is pH 7.4 and not pH 8.0: at lower pH, nonspecific biotinylation is reduced | |
| Poor blocking of cells | Poor blocking can cause clumps of streptavidin-dye staining. Whenever adding streptavidin to cells, ensure that BSA is present. 0.5% dialyzed bovine N,N-dimethyl casein (Calbiochem) is also good at blocking nonspecific binding. Alternatively, nonspecific signals may be from cell debris, because of significant cell death. Alternatively, it may be from a poor quality streptavidin preparation: do you see significant precipitate when you centrifuge the streptavidin-conjugate at 14,000g for 5 min? If so, your preparation is going bad and you need to get another. Avoid refreezing streptavidin and do not store at > 3 mg ml−1, which promotes aggregation. If you conjugated dye by yourself to streptavidin, conjugating too much dye favors aggregation. Test whether you see similar clumps if you omit BirA biotinylation | ||
| Cell toxicity | We have not observed toxicity from biotinylation and streptavidin staining, even with sensitive cell types, such as neurons. However, if you do see impaired cell health at the end of the biotinylation, one of the following adjustments may help: (i) replace PBS–Mg with Tyrode’s medium, well-buffered DMEM or RPMI or even cell growth medium containing 10% serum. For neurons, Tyrode’s medium is preferable to PBS–Mg. (ii) Be gentle when washing the cells: let the medium flow gently down the side of the well rather than pipetting directly on to the cells. Do not leave cells out of medium for more than a few seconds. (iii) Replace biotin + ATP with biotin–AMP. ATP can impair viability of some cell types. Alternatively, adding high concentrations of BirA, without added ATP or biotin–AMP, can achieve reasonable biotinylation. Recombinant BirA copurifies with biotin–AMP in the active site (Kd 10−10 M)40, and we have shown that this BirA–biotin–AMP complex can efficiently label AP-tagged proteins without addition of ATP to cells3. (iv) If you are incubating with BirA for 30–60 min, try reducing this to 5–10 min and see if the streptavidin signal is still sufficient. (v) Perform the incubation with BirA at 37 °C. Surprisingly, we have found that BirA works faster at 30 °C than at 37 °C. However, the reaction is still fast at 37 °C, so use that temperature if you want your cells to be happiest. BirA works perfectly well at room temperature. BirA has some activity even at 4 °C, so do not assume that putting the reaction on ice will completely stop biotinylation—it is better to wash the BirA away | ||
| Box 1 A(iii) and (vi) | Bacteria do not reach A600 = 0.5, BirA does not induce | Ensure that you are starting your overnight culture from freshly transformed plates. Try an alternative strain of Escherichia coli BL21, such as BL21 (DE3) RIPL (Stratagene). Ensure that your ampicillin and isopropyl-β-D-thiogalactopyranoside (IPTG) stocks are active | |
| Box 1 B(iii) | Bacterial lysate hardly flows through nickel-affinity column | Inefficient bacterial lysis and/or large amounts of DNA making the lysate viscous | You can incubate your lysate with DNase I, or bind the lysate to the nickel resin in batch mode. Increase the centrifugation time, after lysis, to 20 min, and do not use the bottom fraction of the supernatant, which is the most viscous |
| Box 2 A(iii) and (vii) | Bacteria do not reach A600 = 0.9, streptavidin does not induce | Ensure that you are starting your overnight culture from freshly transformed plates. Try an alternative strain of E. coli BL21, such as BL21 (DE3) RIPL (Stratagene). Ensure that your ampicillin and IPTG stocks are active. Induction should be very obvious (Fig. 4a). If the 10-kDa molecular weight marker is not clearly resolved from the dye-front on SDS-PAGE, cast your gel with a higher percentage of acrylamide, so that it is easy to see the streptavidin induction band away from the dye-front. Plates and media containing 34 µg ml−1 chloramphenicol will help to ensure that the pLysS plasmid is not lost | |
| Box 2 C(iii) | All of the streptavidin aggregates on refolding into PBS | Inadequate washing of inclusion bodies | The pellet must be fully resuspended at every wash to remove contaminating debris. See the notes in Box 2 B(i) on pipetting, to help you to achieve this. Aggregation can also be caused by adding too much guanidinium hydrochloride to the PBS or adding guanidinium hydrochloride too fast |
ANTICIPATED RESULTS
Typical results for biotinylation of cell surface proteins with BirA, after imaging with monovalent streptavidin, are shown in Figure 3a.
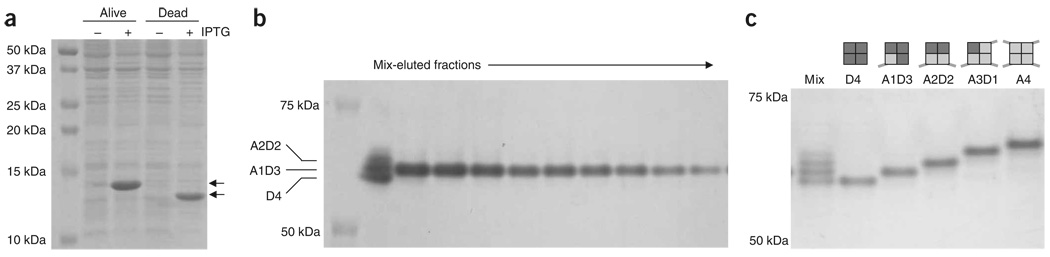
The typical yield from culturing 1 l of bacteria is 40 mg of purified BirA. The typical yield from culturing 1 l of Dead streptavidin and 1/3 l of Alive streptavidin is 2 mg of purified monovalent streptavidin. Induction of Alive and Dead streptavidin is shown in Figure 4a. Elution of monovalent streptavidin from a nickel-affinity column is shown in Figure 4b and SDS-PAGE of purified monovalent streptavidin is displayed in Figure 4c.
Figure 4.
Monovalent streptavidin expression and purification. (a) Induction of Alive and Dead streptavidin. Alive and Dead streptavidin were expressed in Escherichia coli from a pET plasmid, and expression was tested by SDS-PAGE and Coomassie staining. Each lysate is shown pre- or postinduction. The streptavidin band is marked with arrows. Alive runs higher because of an extra His6 tag. (b) Elution of monovalent streptavidin from a nickel-affinity column. A mixture of heterotetramers was loaded on to a nickel-affinity column. The identity of each fraction eluted by imidazole was determined by SDS-PAGE and Coomassie staining, relative to the unpurified mixture in the left lane (mix). (c) Final purified monovalent streptavidin (A1D3), shown relative to Dead streptavidin (D4), the original mixture of tetramers after refolding (mix), divalent streptavidin (A2D2), trivalent streptavidin (A3D1) or tetravalent streptavidin (A4), after SDS-PAGE and Coomassie staining. IPTG, isopropyl-β-d-thiogalactopyranoside.
ACKNOWLEDGMENTS
We thank all past and current members of the Ting lab who have contributed to methods described here, particularly Marta Fernandez Suarez for cloning pET21a-BirA and Yi Zheng for supplying Figure 4b. Funding was provided by the National Institutes of Health (R01 GM072670-01 and P20 GM072029-01), the McKnight Foundation, the Dreyfus Foundation and the Massachusetts Institute of Technology. M.H. was supported by a Computational and Systems Biology Initiative MIT-Merck postdoctoral fellowship. We thank Tanabe USA for biotin.
Footnotes
COMPETING INTERESTS STATEMENTS The authors declare competing financial interests (see the HTML version of this article for details).
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Marks KM, Nolan GP. Chemical labeling strategies for cell biology. Nat. Methods. 2006;3:591–596. doi: 10.1038/nmeth906. [DOI] [PubMed] [Google Scholar]
- 2.Chen I, Howarth M, Lin W, Ting AY. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat. Methods. 2005;2:99–104. doi: 10.1038/nmeth735. [DOI] [PubMed] [Google Scholar]
- 3.Howarth M, Takao K, Hayashi Y, Ting AY. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl. Acad. Sci. USA. 2005;102:7583–7588. doi: 10.1073/pnas.0503125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howarth M, et al. A monovalent streptavidin with a single femtomolar biotin binding site. Nat. Methods. 2006;3:267–273. doi: 10.1038/NMETHXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Werven FJ, Timmers HT. The use of biotin tagging in Saccharomyces cerevisiae improves the sensitivity of chromatin immunoprecipitation. Nucleic Acids Res. 2006;34:e33. doi: 10.1093/nar/gkl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckett D, Kovaleva E, Schatz PJ. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 1999;8:921–929. doi: 10.1110/ps.8.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates IR, et al. Membrane lateral diffusion and capture of CFTR within transient confinement zones. Biophys. J. 2006;91:1046–1058. doi: 10.1529/biophysj.106.084830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgar R, et al. High-sensitivity bacterial detection using biotin-tagged phage and quantum-dot nanocomplexes. Proc. Natl. Acad. Sci. USA. 2006;103:4841–4845. doi: 10.1073/pnas.0601211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athavankar S, Peterson BR. Control of gene expression with small molecules: biotin-mediated acylation of targeted lysine residues in recombinant yeast. Chem. Biol. 2003;10:1245–1253. doi: 10.1016/j.chembiol.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Jaramillo A, Shi R, Kwok WW, Mohanakumar T. In vivo biotinylation of the major histocompatibility complex (MHC) class II/peptide complex by coexpression of BirA enzyme for the generation of MHC class II/tetramers. Hum. Immunol. 2004;65:692–699. doi: 10.1016/j.humimm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Mito Y, Henikoff JG, Henikoff S. Genome-scale profiling of histone H3.3 replacement patterns. Nat. Genet. 2005;37:1090–1097. doi: 10.1038/ng1637. [DOI] [PubMed] [Google Scholar]
- 12.Parrott MB, Barry MA. Metabolic biotinylation of secreted and cell surface proteins from mammalian cells. Biochem. Biophys. Res. Commun. 2001;281:993–1000. doi: 10.1006/bbrc.2001.4437. [DOI] [PubMed] [Google Scholar]
- 13.Barat B, Wu AM. Metabolic biotinylation of recombinant antibody by biotin ligase retained in the endoplasmic reticulum. Biomol. Eng. 2007;24:283–291. doi: 10.1016/j.bioeng.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nesbeth D, et al. Metabolic biotinylation of lentiviral pseudotypes for scalable paramagnetic microparticle-dependent manipulation. Mol. Ther. 2006;13:814–822. doi: 10.1016/j.ymthe.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Grosveld F, et al. Isolation and characterization of hematopoietic transcription factor complexes by in vivo biotinylation tagging and mass spectrometry. Ann. NY Acad. Sci. 2005;1054:55–67. doi: 10.1196/annals.1345.008. [DOI] [PubMed] [Google Scholar]
- 16.de Boer E, et al. Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. USA. 2003;100:7480–7485. doi: 10.1073/pnas.1332608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green NM. Avidin and streptavidin. Meth. Enzymol. 1990;184:51–67. doi: 10.1016/0076-6879(90)84259-j. [DOI] [PubMed] [Google Scholar]
- 18.Tannous BA, et al. Metabolic biotinylation of cell surface receptors for in vivo imaging. Nat. Methods. 2006;3:391–396. doi: 10.1038/nmeth875. [DOI] [PubMed] [Google Scholar]
- 19.Chattopadhaya S, Tan LP, Yao SQ. Strategies for site-specific protein biotinylation using in vitro, in vivo and cell-free systems: toward functional protein arrays. Nat. Protoc. 2006;1:2386–2398. doi: 10.1038/nprot.2006.338. [DOI] [PubMed] [Google Scholar]
- 20.Klemm JD, Schreiber SL, Crabtree GR. Dimerization as a regulatory mechanism in signal transduction. Annu. Rev. Immunol. 1998;16:569–592. doi: 10.1146/annurev.immunol.16.1.569. [DOI] [PubMed] [Google Scholar]
- 21.Iino R, Koyama I, Kusumi A. Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Biophys. J. 2001;80:2667–2677. doi: 10.1016/S0006-3495(01)76236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxton MJ, Jacobson K. Single-particle tracking: applications to membrane dynamics. Annu. Rev. Biophys. Biomol. Struct. 1997;26:373–399. doi: 10.1146/annurev.biophys.26.1.373. [DOI] [PubMed] [Google Scholar]
- 23.Schwesinger F, et al. Unbinding forces of single antibody-antigen complexes correlate with their thermal dissociation rates. Proc. Natl. Acad. Sci. USA. 2000;97:9972–9977. doi: 10.1073/pnas.97.18.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu SC, Wong SL. Engineering soluble monomeric streptavidin with reversible biotin binding capability. J. Biol. Chem. 2005;280:23225–23231. doi: 10.1074/jbc.M501733200. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi MH, Wong SL. Design, production, and characterization of a monomeric streptavidin and its application for affinity purification of biotinylated proteins. Protein Expr. Purif. 2002;25:409–415. doi: 10.1016/s1046-5928(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 26.Laitinen OH, et al. Rational design of an active avidin monomer. J. Biol. Chem. 2003;278:4010–4014. doi: 10.1074/jbc.M205844200. [DOI] [PubMed] [Google Scholar]
- 27.Muzykantov VR, Smirnov MD, Samokhin GP. Avidin-induced lysis of biotinylated erythrocytes by homologous complement via the alternative pathway depends on avidin’s ability of multipoint binding with biotinylated membrane. Biochim. Biophys. Acta. 1992;1107:119–125. doi: 10.1016/0005-2736(92)90336-k. [DOI] [PubMed] [Google Scholar]
- 28.Niemeyer CM. Bioorganic applications of semisynthetic DNA-protein conjugates. Chemistry. 2001;7:3188–3195. doi: 10.1002/1521-3765(20010803)7:15<3188::aid-chem3188>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Keren K, Berman RS, Buchstab E, Sivan U, Braun E. DNA-templated carbon nanotube field-effect transistor. Science. 2003;302:1380–1382. doi: 10.1126/science.1091022. [DOI] [PubMed] [Google Scholar]
- 30.Fernández-Suárez M, et al. Redirecting lipoic acid ligase for cell surface protein labeling with small-molecule probes. Nat. Biotechnol. 2007;25:1483–1487. doi: 10.1038/nbt1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marttila AT, et al. Recombinant NeutraLite avidin: a non-glycosylated, acidic mutant of chicken avidin that exhibits high affinity for biotin and low non-specific binding properties. FEBS Lett. 2000;467:31–36. doi: 10.1016/s0014-5793(00)01119-4. [DOI] [PubMed] [Google Scholar]
- 32.Nordlund HR, Hytonen VP, Laitinen OH, Kulomaa MS. Novel avidin-like protein from a root nodule symbiotic bacterium, Bradyrhizobium japonicum. J. Biol. Chem. 2005;280:13250–13255. doi: 10.1074/jbc.M414336200. [DOI] [PubMed] [Google Scholar]
- 33.Coleman TM, Huang F. RNA-catalyzed thioester synthesis. Chem. Biol. 2002;9:1227–1236. doi: 10.1016/s1074-5521(02)00264-8. [DOI] [PubMed] [Google Scholar]
- 34.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 36.Gama L, Breitwieser GE. Generation of epitope-tagged proteins by inverse polymerase chain reaction mutagenesis. Methods Mol. Biol. 2002;182:77–83. doi: 10.1385/1-59259-194-9:077. [DOI] [PubMed] [Google Scholar]
- 37.Rathbone MP, et al. Trophic effects of purines in neurons and glial cells. Prog. Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 38.Burnstock G. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman-Smith A, Cronan JE., Jr In vivo enzymatic protein biotinylation. Biomol. Eng. 1999;16:119–125. doi: 10.1016/s1050-3862(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Beckett D. Kinetics of biotinyl-5′-adenylate synthesis catalyzed by the Escherichia coli repressor of biotin biosynthesis and the stability of the enzyme-product complex. Biochemistry. 1994;33:7354–7360. doi: 10.1021/bi00189a041. [DOI] [PubMed] [Google Scholar]
- 41.Green NM, Melamed MD. Optical rotatory dispersion, circular dichroism and far-ultraviolet spectra of avidin and streptavidin. Biochem. J. 1966;100:614–621. doi: 10.1042/bj1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayer EA, Ehrlich-Rogozinski S, Wilchek M. Sodium dodecyl sulfate-polyacrylamide gel electrophoretic method for assessing the quaternary state and comparative thermostability of avidin and streptavidin. Electrophoresis. 1996;17:1319–1324. doi: 10.1002/elps.1150170808. [DOI] [PubMed] [Google Scholar]