Abstract
The severity of malaria is multi-factorial. It is associated with parasite-induced alteration in pro-inflammatory and anti-inflammatory cytokine and chemokine levels in host serum and cerebrospinal fluid. It is also associated with sequestration and cytoadherence of parasitized erythrocytes (pRBCs) in post-capillary venules and blood brain barrier (BBB) dysfunction. The role of these factors in development of vascular injury and tissue damage in malaria patients is unclear. While some studies indicate a requirement for pRBC adhesion to vascular endothelial cells (EC) in brain capillaries to induce apoptosis and BBB damage, others show no role of apoptosis resulting from adhesion of pRBC to EC. In the present study, the hypothesis that soluble factors from Plasmodium falciparum-infected erythrocytes induce apoptosis in human brain vascular endothelial (HBVEC) and neuroglia cells (cellular components of the BBB) was tested. Apoptotic effects of parasitized (pRBC) and non-parasitized erythrocyte (RBC) conditioned medium on HBVEC and neuroglia cells were determined in vitro by evaluating nuclear DNA fragmentation (TUNEL assay) in cultured cells. Soluble factors from Plasmodium falciparum-infected erythrocytes in conditioned medium induced extensive DNA fragmentation in both cell lines, albeit to a greater extent in HBVEC than neuroglia, indicating that extended exposure to high levels of these soluble factors in serum may associated with vascular, neuronal and tissue injury in malaria patients.
Malaria is a debilitating and life-threatening infection of Plasmodium falciparum resulting in over 2.5 million deaths annually, with young children and pregnant women at greatest risk [1,2]. Cerebral malaria (CM) is a serious complication of malaria resulting from increased sequestration of parasitized erythrocytes in micro vessels as well as petechial and ring hemorrhages in the brain. Although severe forms of malaria (severe malaria anemia and CM) have been studied extensively, many of the pathophysiological mechanisms leading to host tissue damage and death remain unclear. Several studies suggest that parasite induced apoptosis plays an important role in immunopathogenesis of severe forms of malaria [3–6]. However, examination of post mortem tissues from Ghanaian (West Africa) children who died of severe forms of malaria showed sequestered parasitized erythrocytes in the CM cases than in the severe malaria anemia cases [7]. In one hypothesis sequestration of parasitized red blood cells (pRBC) leads to activation of vascular endothelial cells causing BBB dysfunction [3,8,9]. However, other evidence suggests that parasite sequestration alone does not fully account for immunopathogenesis of malaria [10,11]. At present, there is no uncontested hypothesis(es) explaining how sequestered parasites induce the neurological symptoms associated with severe forms of malaria. Recent studies using ex vivo co-cultures of human lung endothelial cells and P. falciparum demonstrate that pRBC adhesion to human endothelial cells is responsible for observed induction of apoptosis [12]. Immunohistochemistry on post-mortem tissue from Vietnamese adults [8] and Malawian children [13] showed a loss of endothelial cell junctional proteins ZO-1, occludin and vinculin, most notably in vessels containing sequestered pRBC. Further, P. falciparum schizont-rich extract-induced apoptosis was detected in lymphocytes harvested from uninfected subjects [14]. Tripathi et al. [15] demonstrated that pRBCs perturbed BBB integrity by multi-factorial and multi-step processes. They also determined that, in addition to adherence of pRBCs to brain endothelium, parasite specific factors played an important role in the pathogenesis of CM by decreasing BBB integrity although the specific factors remain to be identified. Other studies determined that a direct cytotoxic effect of pRBCs on brain endothelial cells [2] leads to the decrease in the integrity of the BBB monolayer [15]. Recently, Taoufiq et al [6] demonstrated that the Rho kinase pathway was involved in pathogenic interactions between endothelium and P. falciparum during human CM and multiorgan failure syndrome [6,12]. Other studies using P. falciparum field isolates found that these isolates can induce EC apoptosis which is associated with neurological manifestation [16]. They and others found that pRBC adhesion is required to induce endothelial cell apoptosis [6,12,16]. Other studies have demonstrated induction of EC apoptosis indirectly in the absence of physical interaction with pRBCs [17]. In contrast to these evidences, other studies have shown that caspase 3 activity in ECs by pRBCs could also result in suppression of apoptosis during cytoadherence [18]. The aim of the present study was to determine whether adherence of pRBC to human brain endothelial cells (HBVEC) and neuroglia cells is a pre-requisite for the induction of apoptosis by Plasmodium falciparum-infected erythrocytes soluble factors. The results of this study indicate that soluble factors from Plasmodium falciparum-infected erythrocytes induce apoptosis of HBVEC and neuroglia in the absence of pRBC adhesion.
The study samples were obtained from the Korle-Bu Teaching Hospital’s Child Health Department, Accra, Ghana, after ethical approval by Morehouse School of Medicine and University of Ghana Medical School. Children (22–72 months) reporting with malaria to the Child Health Department’s diagnostic laboratory were selected for participation in the study after informed consent was obtained from their parents or guardian. Malaria cases were confirmed by thick film slides microscopy and Plasmodium falciparum HRP II (PfHRP II) ELISA assay. Blood specimens (5ml) were collected prior to enrollment of subjects. Blood cells (infected and uninfected) and plasma were separated using Vacutainer Cell Preparation Tubes (CPT) with Sodium Citrate (Becton Dickinson, US). Hemolysed blood samples were excluded from this study. The red blood cells (infected and uninfected) were washed three times with isotonic PBS to remove serum albumin and other serum proteins and incubated in Roswell Park Memorial Institute (RPMI) 1640 media (Atlanta Biologicals, Atlanta, GA) at 37°C for 4hr. The RBC-RPMI mixture was spun down at 1500 × g and the supernatant (RBC and pRBC conditioned media) collected and stored at −80°C until ready for use. In all ten (10) malaria positive and 10 malaria negative children were enrolled in the study.
Human Brain Vascular Endothelial Cells (HBVEC; Biowhittaker, Walkersville, MD) were cultured at 37°C with 5% CO2 in Complete Serum-free Medium Kit (Cell Systems, Kirkland, WA) supplemented with 2% fetal bovine serum (FBS; ATCC, Manassas, VA) and 100 U/ml of streptomycin, 100 U/ml of penicillin (Gibco, Grand Island, NY) and were harvested and passaged at about 70–90% confluence. At confluence, HBVECs (2×105 cells/ml) were transferred into 35mm tissue culture dish containing collagen-coated cover slip and incubated at 37°C in 5% CO2 for 24–48hr.
Human neuroglia cells were obtained from the American Type Culture Collection (M059K, ATCC, Manassas, VA) and cultured at 37°C under 5% CO2 in a humidified atmosphere in D-MEM/F-12 medium (ATCC, Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum and 100 U/ml of streptomycin, 100 U/ml of penicillin. At about 90% confluence, the cells were transferred into a 35mm tissue culture dish containing a collagen coated cover slip at a density of 2×105 cells/ml and incubated at 37°C in 5% CO2 for 24–48hr.
The apoptotic effect of pooled RBC conditioned medium (RBC-SUP) and pRBC conditioned medium (pRBC-SUP) from 10 malaria negative and 10 positive children respectively on HBVEC were compared at different concentrations to determine the LD50 concentrations of the conditioned medium. In a second set of experiments, HBVEC and neuroglia cells were co-incubated with 100µl/ml (LD50) of RBC-SUP or pRBC-SUP in 35mm tissue culture dish containing collagen coated cover slip at 37°C in 5% CO2 for 24hr. Subsequently, the cultures were assayed for apoptosis using the Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. All experiments were done in triplicate.
Apoptosis in treated and untreated cells were determined by utilizing an In Situ Cell Death Detection Kit (Fluorescein; Roche Diagnostics, Indianapolis, IN) which employs the TUNEL technology to detect and quantify apoptosis at single cell level. The procedure was performed according to the manufacturer’s instructions. The cells were visualized by epiflourescence on a computer-controlled Zeiss Axioskop microscope (Carl Zeiss, Thornwood, NY) linked to MagnaFire 2.1C (Olympus American, Melville, NY) and Image-Pro Plus 4.1 (Media Cybernetics, Silver Springs, MD) imaging software via a charged coupled device (CCD) camera (MC 100 SPOT 60910; Photonic Science, East Sussex, UK).
A hallmark of apoptosis is internucleosomal cleavage, leading to the generation of discrete DNA fragments that are multiples of approximately 100 bp. To assay for DNA ladder formation, nuclear extracts were obtained from treated cells. The nuclear extracts were centrifuged at 15,340 × g for 20 min at 4°C, and then RNA removed by RNase A (Sigma, St. Louis, MO) digestion at 37°C for 2hr. All of the protein present was eliminated from the mixture by proteinase K (Promega, Madison, WI.) treatment at 56°C for 2hr. DNA precipitation was initiated by an overnight treatment with 4 M ammonium acetate and a 0.7 volume of isopropanol at −20°C. DNA purification was achieved by performing several 70% ethanol washes, followed by centrifugation at 15,340 × g for 30 min. Purified DNA was air dried, resuspended in sterile water, and quantitatively and qualitatively analyzed by spectrophotometry (HP 8453 spectrophotometer; HP ChemStation, Palo Alto, CA). Twenty micrograms (20µg) of DNA from each treatment was prepared in gel loading buffer that gave a final concentration of 0.02% bromophenol blue, 5% glycerol, 0.1% SDS, and 50µg of ethidium bromide. The buffered DNA samples were resolved in a neutral 1.5% agarose gel at 6 V/cm for 4hr. DNA ladder markers (Promega, Madison, WI) were used as size standards. Images were taken using FD67110 Fotodyne FOTO/Analyst Luminary Workstations Systems (Fotodyne, Inc., Hartland, WI) and the negative image was generated via Adobe Photoshop Software (version 5.0.2).
Statistical analysis was performed with SigmaPlot 2006 (version 10.0) with SigmaStat (version 3.5) integration (Chicago, IL) software for windows. Data were analyzed by One-Way ANOVA using the Holm-Sidak method to compare group pairs. Results were expressed as means ± standard deviations of individual experimental groups. A value of p < 0.05 was considered significant.
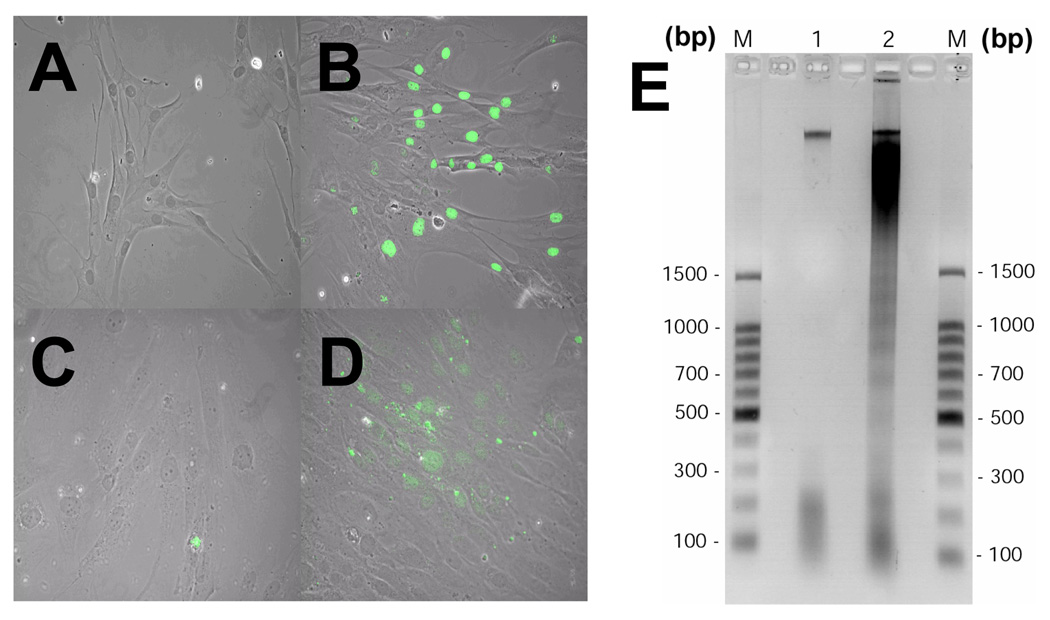
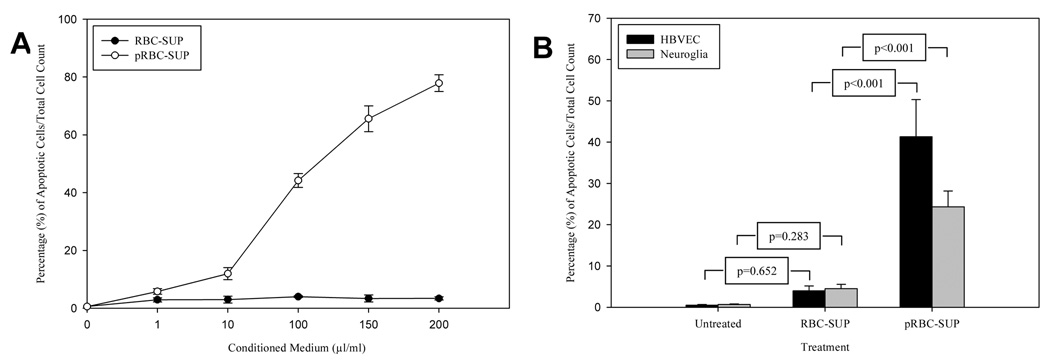
RBC-SUP and pRBC-SUP treated cells and untreated cells were evaluated for apoptosis by TUNEL. The pRBC-SUP treated cultures showed significant TUNEL labeling (Figure 1B/D) and DNA fragmentation (Figure 1E-lane 3) compared to RBC-SUP treated controls (Figure 1A/C; E-lane 2). Similar DNA fragmentation data was observed for neuroglia cells (data not shown). Dose-response analysis of HBVEC exposed to pRBC-SUP was evaluated using TUNEL (Figure 2A). 10% of HBVEC displaying apoptosis were observed at a minimum threshold of 10µl/ml (v/v) pRBC-SUP with an LD50 of approximately 100µl/ml (v/v) observed (Figure 2A open circles). HBVEC that were exposed to equal concentrations of RBC-SUP, exhibited no change in the percentage labeling cells (Figure 2A closed circles). Thus, TUNEL labeling of HBVEC exposed to pRBC-SUP was dose-dependent. pRBC conditioned media induced significant apoptosis (p<0.001 for both cell lines) at LD50 concentration (Figure 2B, pRBC-SUP) compared with RBC conditioned media and untreated cells (Figure 2B, RBC-SUP). pRBC conditioned media induced greater apoptosis in HBVECs than in neuroglia (Figure 2B). RBC conditioned media (Figure 2B,RBC-SUP) induced apoptosis similar to background apoptosis observed in untreated cells (HBVECs, p=0.652; neuroglia cells, p=0.283; Figure 2B, untreated). Thus, the TUNEL and DNA electrophoresis data showed that soluble factors from P. falciparum infected RBC in conditioned media significantly induced apoptosis in HBVECs and neuroglia cells in the absence of intact RBC’s.
Figure 1.
Fluorescence Images of treated cells were observed on a Zeiss Axioskop microscope and pictures were taken using a charged coupled device (CCD) camera, MagnaFire, Model S99806 (Olympus American) at a magnification of X200. (A) Image of HBVECs treated with 100µl/ml (v/v) RBC-SUP. (B) HBVECs (nucleus stained green) treated with 100µl/ml (v/v) pRBC-SUP undergoing apoptosis. (C) Neuroglia cells treated with 100µl/ml (v/v) RBC-SUP. (D) Neuroglia treated with 100µl/ml (v/v) pRBC-SUP undergoing apoptosis. (E) DNAs from HBVECs extracts were resolved by agarose gel electrophoresis, stained with ethidium bromide and visualized by exposure to UV light. Images were taken using FD67110 Fotodyne FOTO/Analyst Luminary Workstations Systems (Fotodyne, Inc., Hartland, WI.) and the negative image was generated via Adobe Photoshop Software 5.0 and formated with Adobe Illustrator 10.0 software. The results are typical representations of at least two independent experiments. The lanes are as follows: DNA ladder marker (lane M), RBC-SUP (lane 1) and pRBC-SUP (lane 2).
Figure 2.
(A) Dose-response curve showing effect of pRBC conditioned medium (pRBC-SUP) (µl/ml) on apoptosis in HBVEC. HBVEC exposed to equal concentrations of RBC conditioned medium (RBC-SUP) exhibited no change in percent labeling of cells. (B) Apoptotic effect of uninfected, RBC-SUP and pRBC-SUP on HBVECs and neuroglia cells. Shown are results of TUNEL assay of apoptotic HBVECs and neuroglia cells incubated in 100µl/ml pRBC-SUP or RBCSUP. Results are expressed as percentage of apoptotic cells. Bars represent standard deviations of three experiment.
Approximately 1–2.5 million children die of malaria annually and bulk of these deaths can be attributed to severe forms of malaria including severe malaria anemia (SMA) and CM in endemic countries. The mechanism(s) mediating severity of malaria are unclear. For example, adhesion of pRBC to the vascular endothelium in the brain results in the sequestration of pRBCs [19,20]; a major factor associated with CM severity. Brown et al. [8], determined that adhesion of pRBCs to endothelial cells via cellular adhesion molecules induces a deleterious effects on vascular endothelial cells in the brain that could directly result in disruption of the BBB. Adhesion of pRBCs to EC activates expression of pro-inflammatory cytokines and chemokines [21–24], apoptosis and tissue damage [6,8,12,14,15,25–29]. It is not clear how pRBCs adhesion to EC induces apoptosis [16]. Others suggest that, cross-linking of the adhesion molecules at the EC surface can trigger intracellular signaling pathways leading to a variety of cellular processes, including apoptosis [16,30]. Recently, fifty-nine (59) putative Plasmodium apoptogenic genes were identified with potential involvement in parasite induced cell death through cytoadherence [28]. Sequestration of pRBCs within the brain capillary network is critical in severe malaria pathogenesis [27]. However, cytoadherence appears to be essential but not sufficient to explain outcome of CM since only a subset of malaria cases lead to malaria complication [6,31]. In a study using P. falciparum field isolates on EC, they found that the degree of cytoadherence was higher among individuals with apoptosis than the individuals without apoptosis [16]. However, they also found out that the correlation between the degree of cytoadherence and the capacity to induce apoptosis was not significant [16]. Interestingly, a recent study revealed that increasing the adhesion of pRBC to endothelial cells in vitro failed to increase endothelial cell apoptosis [27]. Thus, the degree of pRBC adherence did not correlate with endothelial cells apoptosis [27]. In this study, soluble factors from P. falciparum infected RBC induced HBVECs as well as neuroglia cell apoptosis without a need for adherence of pRBCs to the cells. The HBVEC-based apoptosis assay revealed that pRBC-SUP induced cell death in HBVEC at a minimum threshold concentration of 10µl/ml (v/v). DNA fragmentation laddering assays revealed that, extensive apoptosis occurred after treatment with pRBC-SUP but not with RBC-SUP. The TUNEL assay in HBVEC and neuroglia revealed that soluble factor(s) from infected RBC induce apoptosis in both cell lines in the absence of host immune modulators or pRBC adherence. The evidence suggests that a soluble, apoptotic factor(s) is secreted or shed by the infected RBC due to the presence of parasites in host blood. Increased production or release of this factor(s) could directly mediate the neuropathogenesis and BBB damage associated with severe forms of the disease. Several recent studies support this hypothesis. A recent in vitro study showed that parasite extracts induced apoptosis in mononuclear cell cultures [14,29]. Additionally, Tripathi et al. [15] observed soluble factors from pRBC decreased electrical resistance in HBVEC’s while Ghigo et al. [32] showed that a soluble factor (>100 kDa) released from pRBCs activates human umbilical vein endothelial cells. However, in this study, soluble apoptotic factors from infected RBC induced neuroglia cell apoptosis without a need for adherence of pRBCs was also observed. Axonal injury due to CM has also been observed in human cerebral cortices and the cerebellum and to a lesser extent in the midbrain structures or the brain stem [26]. These studies show that severe forms of malaria such as CM can be initiated or amplified by factor(s) secreted directly into host blood and subsequently released into the cerebrospinal fluid (CSF) by pRBC’s or a more complex interplay between parasite factors and host pro-inflammatory immune modulators [14]. Changes in the BBB and CSF resulting from such interactions would allow cytokines and malaria antigens to enter the brain compartment, from which they are normally excluded [33]. This would lead to the activation of microglia or damage to astrocytes, a phenomenon that has been observed in murine and human CM [34–37]. Astrocytes are critical in regulating the interstitial fluid milieu, contributing to the maintenance of the BBB and they also synthesize neuroprotective molecules. Thus, impairment of astrocytes function may cause disruption of neuronal activity [33]. Thus, we propose a hypothesis in which increased expression and secretion of soluble factors from infected RBC contributes to HBVEC activation and apoptosis which perturbs the BBB and subsequently directly or indirectly induce neuroglial apoptosis. Studies are underway to characterize the apoptotic factor(s) and the mechanisms mediating this apoptotic pathway. Interestingly the HBVECs used with this study were more susceptible to apoptosis than neuroglia cells (Figure 2B). Thus, these cell types may have different susceptibilities to the apoptotic factors. It also seems that during severe malaria, sequestration of pRBCs may cause amplified local apoptotic effects in the brain that could result in BBB dysfunction.
In conclusion, the present study demonstrates that soluble apoptotic factor(s) from infected RBC have apoptogenic effects on human brain endothelial and neuroglia cells. This observation suggests that pRBCs adherence to host endothelium is not a pre-requisite for inducing apoptosis.
Acknowledgments
The authors acknowledge Clement Adu-Gyamfi, Stella Baidoo and Opuni Asiedu for technical assistance and Chelsea Glass, Nathan McGinnis, and September Hesse of Morehouse School of Medicine for participation in sample collection and the patients and their guardians for providing permission to use samples. The authors are grateful to the laboratory staff of the Korle-Bu Teaching Hospital’s Child Health Department and the support of the Minority International Health Disparities Research Training (MIHRT) Program at Howard University. The support of the Center for Disease Diagnosis and Research (CDDR) in Ghana is acknowledged.
Financial support: This investigation received financial support from WHO/UNDP/TDR Collaborative Research Grant (A00524) and National Institutes of Health grant numbers NIH-RCMI (RR03034), NIHNIGM-MBRS (SO6GM08248), and NIH-FIC (R21TW006804-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Murray CK, Gasser RA, Jr, Magill AJ, et al. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev. 2008;21:97–110. doi: 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wassmer SC, Combes V, Candal FJ, et al. Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect Immun. 2006;74:645–653. doi: 10.1128/IAI.74.1.645-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berendt AR, Tumer GD, Newbold CI. Cerebral malaria: the sequestration hypothesis. Parasitol Today. 1994;10:412–414. doi: 10.1016/0169-4758(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 4.Clark IA, Rockett KA. The cytokine theory of human cerebral malaria. Parasitol Today. 1994;10:410–412. doi: 10.1016/0169-4758(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 5.Grau GE, de KS. Cerebral malaria: mediators, mechanical obstruction or more? Parasitol Today. 1994;10:408–409. doi: 10.1016/0169-4758(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 6.Taoufiq Z, Gay F, Balvanyos J, et al. Rho kinase inhibition in severe malaria: thwarting parasite-induced collateral damage to endothelia. J Infect Dis. 2008;197:1062–1073. doi: 10.1086/528988. [DOI] [PubMed] [Google Scholar]
- 7.Armah H, Dodoo AK, Wiredu EK, et al. High-level cerebellar expression of cytokines and adhesion molecules in fatal, paediatric, cerebral malaria. Ann Trop Med Parasitol. 2005;99:629–647. doi: 10.1179/136485905X51508. [DOI] [PubMed] [Google Scholar]
- 8.Brown H, Hien TT, Day N, et al. Evidence of blood-brain barrier dysfunction in human cerebral malaria. Neuropathol Appl Neurobiol. 1999;25:331–340. doi: 10.1046/j.1365-2990.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- 9.Turner GD, Morrison H, Jones M, et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 10.Milner DA, Jr, Dzamalala CP, Liomba NG, et al. Sampling of supraorbital brain tissue after death: improving on the clinical diagnosis of cerebral malaria. J Infect Dis. 2005;191:805–808. doi: 10.1086/427814. [DOI] [PubMed] [Google Scholar]
- 11.Seydel KB, Milner DA, Jr, Kamiza SB, et al. The distribution and intensity of parasite sequestration in comatose Malawian children. J Infect Dis. 2006;194:208, 215. doi: 10.1086/505078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pino P, Vouldoukis I, Kolb JP, et al. Plasmodium falciparum--infected erythrocyte adhesion induces caspase activation and apoptosis in human endothelial cells. J Infect Dis. 2003;187:1283–1290. doi: 10.1086/373992. [DOI] [PubMed] [Google Scholar]
- 13.Brown H, Rogerson S, Taylor T, et al. Blood-brain barrier function in cerebral malaria in Malawian children. Am J Trop Med Hyg. 2001;64:207–213. doi: 10.4269/ajtmh.2001.64.207. [DOI] [PubMed] [Google Scholar]
- 14.Balde AT, Aribot G, Tall A, et al. Apoptosis modulation in mononuclear cells recovered from individuals exposed to Plasmodium falciparum infection. Parasite Immunol. 2000;22:307–318. doi: 10.1046/j.1365-3024.2000.00307.x. [DOI] [PubMed] [Google Scholar]
- 15.Tripathi AK, Sullivan DJ, Stins MF. Plasmodium falciparum-infected erythrocytes decrease the integrity of human blood-brain barrier endothelial cell monolayers. J Infect Dis. 2007;195:942–950. doi: 10.1086/512083. [DOI] [PubMed] [Google Scholar]
- 16.Toure FS, Ouwe-Missi-Oukem-Boyer O, Bisvigou U, et al. Apoptosis: a potential triggering mechanism of neurological manifestation in Plasmodium falciparum malaria. Parasite Immunol. 2008;30:47–51. doi: 10.1111/j.1365-3024.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 17.Hemmer CJ, Lehr HA, Westphal K, et al. Plasmodium falciparum Malaria: reduction of endothelial cell apoptosis in vitro. Infect Immun. 2005;73:1764–1770. doi: 10.1128/IAI.73.3.1764-1770.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakravorty SJ, Carret C, Nash GB, et al. Altered phenotype and gene transcription in endothelial cells, induced by Plasmodium falciparum-infected red blood cells: pathogenic or protective? Int J Parasitol. 2007;37:975–987. doi: 10.1016/j.ijpara.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baruch DI, Gormely JA, Ma C, et al. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ockenhouse CF, Tegoshi T, Maeno Y, et al. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armah HB, Wilson NO, Sarfo BY, et al. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J. 2007;6:147. doi: 10.1186/1475-2875-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown H, Turner G, Rogerson S, et al. Cytokine expression in the brain in human cerebral malaria. J Infect Dis. 1999;180:1742–1746. doi: 10.1086/315078. [DOI] [PubMed] [Google Scholar]
- 23.Hanum PS, Hayano M, Kojima S. Cytokine and chemokine responses in a cerebral malaria-susceptible or -resistant strain of mice to Plasmodium berghei ANKA infection: early chemokine expression in the brain. Int Immunol. 2003;15:633–640. doi: 10.1093/intimm/dxg065. [DOI] [PubMed] [Google Scholar]
- 24.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 25.Balde AT, Sarthou JL, Roussilhon C. Acute Plasmodium falciparum infection is associated with increased percentages of apoptotic cells. Immunol Lett. 1995;46:59–62. doi: 10.1016/0165-2478(95)00017-y. [DOI] [PubMed] [Google Scholar]
- 26.Medana IM, Day NP, Hien TT, et al. Axonal injury in cerebral malaria. Am J Pathol. 2002;160:655–666. doi: 10.1016/S0002-9440(10)64885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pino P, Taoufiq Z, Brun M, et al. Effects of hydroxyurea on malaria, parasite growth and adhesion in experimental models. Parasite Immunol. 2006;28:675–680. doi: 10.1111/j.1365-3024.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- 28.Siau A, Toure FS, Ouwe-Missi-Oukem-Boyer O, et al. Whole-transcriptome analysis of Plasmodium falciparum field isolates: identification of new pathogenicity factors. J Infect Dis. 2007;196:1603–1612. doi: 10.1086/522012. [DOI] [PubMed] [Google Scholar]
- 29.Toure-Balde A, Sarthou JL, Aribot G, et al. Plasmodium falciparum induces apoptosis in human mononuclear cells. Infect Immun. 1996;64:744–750. doi: 10.1128/iai.64.3.744-750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meredith J, Jr, Mu Z, Saido T, et al. Cleavage of the cytoplasmic domain of the integrin beta3 subunit during endothelial cell apoptosis. J Biol Chem. 1998;273:19525–19531. doi: 10.1074/jbc.273.31.19525. [DOI] [PubMed] [Google Scholar]
- 31.Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–840. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- 32.Ghigo D, Todde R, Ginsburg H, et al. Erythrocyte stages of Plasmodium falciparum exhibit a high nitric oxide synthase (NOS) activity and release an NOS-inducing soluble factor. J Exp Med. 1995;182:677–688. doi: 10.1084/jem.182.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt NH, Golenser J, Chan-Ling T, et al. Immunopathogenesis of cerebral malaria. Int J Parasitol. 2006;36:569–582. doi: 10.1016/j.ijpara.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Medana IM, Chan-Ling T, Hunt NH. Redistribution and degeneration of retinal astrocytes in experimental murine cerebral malaria: relationship to disruption of the blood-retinal barrier. Glia. 1996;16:51–64. doi: 10.1002/(SICI)1098-1136(199601)16:1<51::AID-GLIA6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 35.Medana IM, Hunt NH, Chan-Ling T. Early activation of microglia in the pathogenesis of fatal murine cerebral malaria. Glia. 1997;19:91–103. doi: 10.1002/(sici)1098-1136(199702)19:2<91::aid-glia1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 36.Schluesener HJ, Kremsner PG, Meyermann R. Widespread expression of MRP8 and MRP14 in human cerebral malaria by microglial cells. Acta Neuropathol. 1998;96:575–580. doi: 10.1007/s004010050938. [DOI] [PubMed] [Google Scholar]
- 37.Deininger MH, Kremsner PG, Meyermann R, et al. Macrophages/microglial cells in patients with cerebral malaria. Eur Cytokine Netw. 2002;13:173–185. [PubMed] [Google Scholar]