Abstract
T cells restricted to neurotropic viruses are potentially harmful as their activity may result in the destruction of neurons. In the Borna disease virus (BDV) model, antiviral CD8 T cells entering the brain of infected mice cause neurological disease but no substantial loss of neurons unless the animals lack interferon-γ (IFN-γ). We show here that glutamate receptor antagonists failed to prevent BDV-induced neuronal loss in IFN-γ-deficient mice, suggesting that excitotoxicity resulting from glutamate receptor overstimulation is an unlikely explanation for the neuronal damage. Experiments with IFN-γ-deficient mice lacking eosinophils indicated that these cells, which specifically accumulate in the infected brains of IFN-γ-deficient mice, are not responsible for CA1 neuronal death. Interestingly, BDV-induced damage of CA1 neurons was reduced significantly in IFN-γ-deficient mice lacking perforin, suggesting a key role for CD8 T cells in this pathological process. Specific death of hippocampal CA1 neurons could be triggered by adoptive transfer of BDV-specific CD8 T cells from IFN-γ-deficient mice into uninfected mice that express transgene-encoded BDV antigen at high level in astrocytes. These results indicate that attack by CD8 T cells that cause the death of CA1 neurons might be directed toward regional astrocytes and that IFN-γ protects vulnerable CA1 neurons from collateral damage resulting from exposure to potentially toxic substances generated as a result of CD8 T cell-mediated impairment of astrocyte function.
Controlling viral infections in the central nervous system is a very demanding task for the immune system as there is a substantial risk of collateral damage.1 It is often difficult to assess the extent of damage caused by the antiviral immune response to nonrenewable cells in the brain. The reason for this difficulty is that the cytopathic activities of viruses and immune cells do not necessarily result in distinct pathologies. In experimental settings, the complexity of the system can be reduced if model viruses are used that have adopted noncytolytic replication strategies. A suitable pathogen for studying these issues is Borna disease virus (BDV).
BDV can replicate in neurons and other cell types of the central and peripheral nervous system of mice and other mammals without damaging the host cells.2,3 The noncytotoxic nature of BDV was most convincingly demonstrated in MRL/MpJ (MRL) mice lacking functional alleles of the β2-microglobulin gene, which, as a consequence, lack mature CD8 T cells. Unlike wild-type MRL mice that frequently develop fatal neurological disease after infection with BDV, mutant MRL mice lacking mature CD8 T cells are completely resistant to BDV-induced disease although the virus replicates to very high levels in the brain of such mice.4 Neurological disease in this model system thus seems to result exclusively from the activity of antiviral CD8 T cells, which recognize virus-infected cells in the brain. This view was recently confirmed by a study in which some features of the BDV-induced neurological disease were successfully recapitulated by adoptive transfer of virus-specific CD8 T cells into recipient mice that express viral antigen derived from a transgene that is active in either astrocytes or neurons.5
Immune cell infiltrates are abundantly present in the brain of diseased wild-type MRL mice, but the infected organ usually shows no striking pathological alterations. However, in infected MRL mice lacking functional alleles of the IFN-γ gene, a selective and characteristic destruction of neurons was noted in the CA1 region of the hippocampus in about 50% of the mice.4 This destruction of neurons was paralleled by an influx of eosinophils, a cell type that is usually not found in the brain of BDV-infected wild-type mice.4 Interestingly, the CA1 neurons in these mice usually remain uninfected in mice.4
The cause of the selective death of hippocampal neurons during BDV-induced brain inflammation in the absence of IFN-γ is not known. Several possible causes have been discussed, including excitotoxicity resulting from glutamate receptor overstimulation, as described for CA1 damage after measles virus,6 Sindbis virus7,8 and HIV infection,9 and the possibility that the influx of eosinophils caused the neuronal damage possibly by secretion of toxic granule proteins, cytokines, or lipid mediators.10 Here we report that excitotoxicity and eosinophil influx are most likely not the cause of the observed CA1 neuron damage. Rather, our data suggest that the disease-inducing CD8 T cells play a decisive role. Interestingly, CA1 neuron damage was observed if CD8 T cells from IFN-γ-deficient but not from wild-type mice were adoptively transferred into uninfected mice that express transgene-encoded viral antigen selectively in astrocytes. These results suggest that the observed CA1 neuron death is a bystander phenomenon of T cell-mediated recognition of antigen-expressing astrocytes that only becomes evident if IFN-γ is absent.
Materials and Methods
Mice
MRL and B10.BR mice were originally purchased from The Jackson Laboratory (Bar Harbor, Maine). Transgenic mice expressing the nucleoprotein N of BDV in either astrocytes (Astro-N) or neurons (Neuro-N) were described earlier.11 MRL-GKOxPKO mice were generated from MRL-GKO4 and MRL-PKO12 single knockout mice. BALB-GKOxΔDblGATA mice were generated by breeding BALB-GKO and BALB-ΔDblGATA mice.13 The knockout allele of the IFN-γ gene14 available in C57BL/6 mice was introduced into B10.BR mice by a single round of backcrossing. Breeding colonies were maintained in our local animal facility. All animal experiments were approved by local institutional animal care and use committees.
Viruses and Infections
If not indicated differently, mice were infected intracerebrally into the thalamic region of the left brain hemisphere by injecting 10-μl samples of a 10% brain homogenate containing 300 focus forming units of mouse-adapted BDV strain #9715 or with 1000 focus forming units of BDV strain CRNP516 using a Hamilton syringe. For immune priming, B10.BR mice were injected intramuscularly with 2 × 106 plaque forming units of recombinant parapoxvirus ovis strain D1701-VrV-p40 expressing BDV-N (PPV-N).17 N-specific immunity was boosted 1 week later by infecting the animals intraperitoneally with 2 × 107 pfu of recombinant vaccinia virus expressing BDV-N.18 Two weeks after the booster immunization, mice were sacrificed and splenocytes were isolated for re-stimulation of N-specific CD8 T cells.
Glutamate Receptor Inhibitors
Every 12 hours animals received intraperitoneal injections of either 0.5 μg of MK801 per gram body weight, 10 μg of GYKI52466 per gram body weight, or 0.25 μg of MK801 combined with 5 μg of GYKI52466 per gram body weight. MK801 was dissolved in PBS. GYKI52466 was dissolved in dimethylsulfoxide (DMSO) and diluted 1:20 in PBS. These concentrations of inhibitors were well tolerated, whereas twofold higher doses elicited neurological symptoms. Control mice were injected with 10 μl of 5% DMSO in PBS per gram body weight.
Isolation of Mononuclear Cells from the Brains of Diseased Animals
Mononuclear cells were prepared as previously described.18
Flow Cytometry
A peptide with the amino acid sequence TELEISSI was purchased from Neosystem (Strasbourg, France) at a purity of >65%. TELEISSI/H-2Kk-tetrameric complexes labeled with phycoerythrin were kindly provided by the National Institute of Allergy and Infectious Diseases tetramer facility (Bethesda, MD). Tetramers (5 μg/ml for 105 to 106 lymphocytes in a volume of 50 μl) were used together with allophycocyanin-conjugated anti-CD8α antibodies (1 μg/ml, clone 53–6.7, BD Biosciences). Incubation was for 30 minutes at room temperature. All other antibodies were obtained from BD Biosciences. Analysis of stained cells was performed with a FACSort flow cytometer (BD Biosciences).
Short-Term CD8 T Cell Cultures
Splenic lymphocytes from immunized mice (responder cells) were obtained by gently pressing the organ through a metal grid (60 mesh, Sigma). Responder splenocytes were seeded in IMDM medium supplemented with 10% fetal calf serum, penicillin/streptomycin and 1 mmol/L β-mercaptoethanol (complete IMDM) into 24-well plates at 3 × 106 cells/well and mixed with 3 × 105 naive splenocytes pulsed with 10−6 mol/l TELEISSI peptide (stimulator cells). After 5 days in culture, 25 units of murine interleukin-2 (Peprotech, London, England) were added per ml medium. Two days later, all cells were harvested, pooled, and seeded again at 3 × 106 responders/well mixed with 3 × 105 TELEISSI-pulsed stimulator cells. Cells were then incubated with one third of conditioned medium from the first week of culture and two thirds of fresh complete IMDM medium. Cultures were again supplemented with 25 units/ml of murine interleukin-2, incubated for another 7 days and analyzed by flow cytometry using TELEISSI/H-2Kk tetramer and anti-CD8α antibody. Only cultures containing at least 50% of TELEISSI-specific CD8 T cells were used for adoptive transfer experiments.
Adoptive Transfer
Short-term cultures containing at least 50% TELEISSI-specific CD8 T cells were washed, pelleted, resuspended in serum-free IMDM, and filtered through a plastic mesh with a 100-μm pore size. Filtered cells were counted, pelleted, and resuspended in serum-free IMDM at the desired concentration. For intracerebral transfer, a maximal volume of 20 μl was injected into the left brain hemisphere of anesthetized adult mice using a Hamilton syringe.
Immunohistochemical Analyses
Brain sectioning and immunohistochemistry were done as described earlier.19 The degree of virus infection was assessed by immunohistological staining of 5-μm paraffin-embedded brain sections with a monoclonal antibody Bo1820 directed against the viral nucleoprotein. Immunostained sections were counterstained with Mayer’s hematoxylin.
For the specific detection of eosinophils, we took advantage of the fact that peroxidase activity of eosinophils is highly resistant to cyanide treatment, whereas peroxidase of other granulocytes, mast cells, and endothelial cells can be poisoned by cyanide.21 Frozen sections of mouse brains were fixed with a 1:1 (v/v) mixture of acetone/methanol, air dried, and incubated for 20 minutes with cyanide containing diaminobenzidine substrate solution (5 μl of 30% H2O2 and 5 drops of 100 mmol/L KCN for 5 ml of diaminobenzidine substrate solution) at room temperature. After extensive washing, the sections were counterstained with Mayer’s hematoxylin.
For the detection of microglial cells and macrophages, frozen mouse brain sections were fixed with a 1:1 (v/v) mixture of acetone/methanol. Air-dried sections were incubated with anti-CD11b (Mac-1) antibodies and counterstained with Mayer’s hematoxylin. For the detection of apoptotic cells, the in situ cell death detection kit (Roche) was used basically as described in the manufacturer’s protocol.
Scoring of Neurological Disease, Hippocampal Damage, CNS Viral Load and Inflammation
Animals were scored as exhibiting clinical symptoms if two or more of the following abnormalities were observed: uncontrolled movements of extremities when the animal was lifted by the tail, hunched posture, pronounced weight loss, severe ataxia, torticollis, paraparesis, or apathy. Hippocampus damage was assessed on H&E-stained sagittal brain sections. It was considered substantial if the nuclei in the CA1 region appeared shrunken or were largely absent.
Viral load in the CNS was assessed by immunohistochemical staining of BDV-N with monoclonal antibody Bo18. The staining was scored on an arbitrary scale from 0 to 3 as follows: 0 indicates no virus-positive cells detected in at least three brain sections, 1 indicates less than 100 BDV-N positive cells per brain section, 2 indicates a large number of antigen-positive cells in certain brain areas only and, 3 indicates a large number of antigen-positive cells in most brain areas.
Brain mononuclear cell infiltrates were scored on H&E-stained sagittal brain sections on an arbitrary scale from 0 to 3 as follows: 0 indicates no infiltrates, 1 indicates up to two perivascular infiltrates per brain section with only one or two layers of cells affected and some mononuclear cells in the meninges, 2 indicates three to five perivascular infiltrates per brain section with multilayer appearance, spread into parenchyma and intermediate meningitis, and 3 indicates six or more perivascular infiltrates per brain section with multiple layers of cells affected, strong infiltration of parenchyma at multiple sites and strong meningitis.
Results
Apoptotic Death of Hippocampal Neurons in BDV-Infected GKO Mice
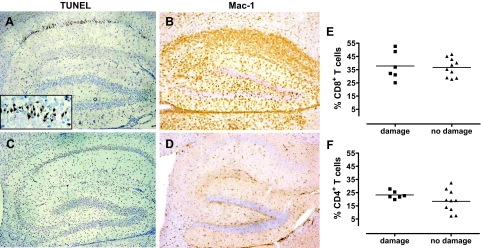
We previously showed that about 50% of BDV-infected MRL mice lacking a functional IFN-γ gene (designated MRL-GKO) develop hippocampal damage.4 Damaged hippocampi either contained only few immune cells and a large number of shrunken nuclei in the CA1 region or else massive immune cell infiltration accompanied with an almost complete loss of hippocampal CA1 neurons.4 These two different presentations of brain pathology may reflect early and late stages of the destruction process. Here, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining was performed to determine whether the shrunken appearance of the CA1 neurons in affected brains is a sign of apoptosis. We found that a high percentage of damaged neurons were strongly positive in the TUNEL reaction (Figure 1A). Interestingly, staining was not restricted to the nuclei of dying CA1 neurons but rather seemed to spread to the cytoplasm as described earlier for apoptotic rat nerves.22 Virtually no TUNEL-positive cells were observed in brains of control animals, including uninfected healthy MRL-GKO mice and BDV-infected wild-type MRL mice showing strong neurological disease (Figure 1C). Staining for Mac-1, a marker of activated microglia and macrophages was prominent throughout the hippocampus of BDV-infected MRL-GKO mice with neuronal damage (Figure 1B) but virtually absent in BDV-infected MRL-GKO mice without visible neuronal damage (Figure 1D), indicating strong local activation of microglia and/or infiltration of macrophages in damaged brain regions. To determine the composition of the brain infiltrates in diseased mice with and without hippocampus damage, brain mononuclear cells were prepared and analyzed by flow cytometry. No significant differences were observed in the frequencies of CD8 T cells (Figure 1E) and CD4 T cells (Figure 1F).
Figure 1.
Hippocampal neurons of BDV-infected GKO mice die by apoptosis, which is accompanied by activation of microglia. MRL-GKO mice were infected with BDV strain #97 and sacrificed when disease developed 3 to 5 weeks later. Sagittal brain sections were used for (A) TUNEL staining or (B) staining against Mac-1 as a marker for activated microglia and macrophages. Inset shows a higher magnification of the apoptotic CA1 region. C: BDV-infected MRL wild-type mice served as negative controls for the TUNEL staining. D: BDV-infected MRL-GKO mice without damaged hippocampi were used as negative controls for the Mac-1 staining. Results of one representative animal are shown each. Brain mononuclear cells from BDV-infected MRL-GKO mice with or without damaged hippocampi were analyzed for the frequency of (E) CD8 T cells and (F) CD4 T cells. Each symbol represents an individual animal. The horizontal lines indicate the arithmetic means of each group.
Hippocampal Damage Cannot Be Blocked by Glutamate Receptor Antagonists
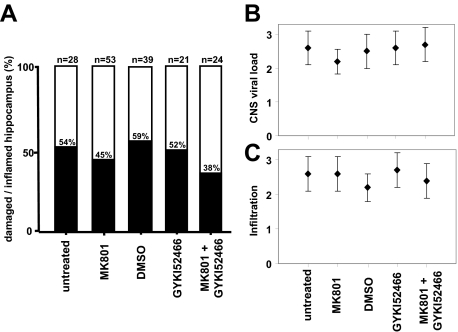
To evaluate the hypothesis that neuronal damage is mediated by excitotoxicity resulting from glutamate receptor overstimulation, MRL-GKO mice were infected with BDV, followed by intraperitoneal treatment with the NMDA receptor antagonist MK801 or the AMPA receptor antagonist GYKI52466 every 12 hours from day 7 postinfection until the animals were diseased. Controls included infected animals kept untreated or treated with 5% DMSO, the solvent used for GYKI52466. When H&E-stained sections of brains that contained a substantial number of immune cells were analyzed, we found that 45% (25/53) of the MK801-treated mice and 54% (15/28) of the untreated MRL-GKO control mice showed damaged hippocampal CA1 neurons (Figure 2A). Fifty-two percent (11/21) of the GYKI52466-treated mice contained substantially damaged hippocampal CA1 neurons, as compared with 59% (23/39) of the DMSO-treated control group (Figure 2A). When BDV-infected MRL-GKO mice were treated with a combination of both glutamate receptor antagonists, 38% (9/24) of the animals with immune infiltrates had damaged hippocampal CA1 neurons (Figure 2A). Although a trend toward protection was noted, the observed differences in frequency of hippocampal damage in the various experimental groups did not reach statistical significance.
Figure 2.
Frequency of hippocampus damage in BDV-infected GKO mice is not influenced significantly by administration of glutamate receptor antagonists MK801 and GYKI52466. MRL-GKO mice were infected with BDV strain #97 and treated by intraperitoneal administration of glutamate receptor antagonists every 12 hours from day 7 postinfection until disease developed 3 to 5 weeks later. Untreated mice served as controls for the MK801 group. Mice treated with 5% DMSO served as controls for the groups receiving GYKI52466 either alone or in combination with MK801. A: H&E-stained sagittal brain sections were analyzed for mononuclear cell infiltration and assessed for hippocampal damage if they contained at least three to five perivascular infiltrates per brain section with multilayer appearance. Columns illustrate the frequency of hippocampus damage in inflamed brains under various treatment conditions. The numbers of animals in each treatment group are indicated above the individual columns. Data shown in the figure represent a summary of four independent experiments. B: CNS viral load was determined by staining brain sections against BDV-N and scoring on an arbitrary scale from 0 to 3. C: The degree of mononuclear cell infiltration was scored on H&E-stained brain sections on an arbitrary scale from 0 to 3. Geometric means ± standard deviations are indicated.
We analyzed properly stained brain sections to determine whether the glutamate receptor antagonists affected the frequency of virus-infected neurons and brain inflammation. We found that these parameters were not significantly different between the various experimental groups (Figure 2B). To analyze whether the composition of the immune cell subsets was altered in the presence of glutamate receptor antagonists, mononuclear cells were isolated from the brain of all diseased animals and analyzed by flow cytometry for expression of CD8, CD4, or CD25. Furthermore, the fraction of BDV-specific CD8 T cells recognizing the BDV-N derived major epitope peptide TELEISSI18 was determined. We found that the frequency of cells expressing these markers was also not significantly different in the various experimental groups (data not shown).
Hippocampal Damage in the Absence of Infiltrating Eosinophils
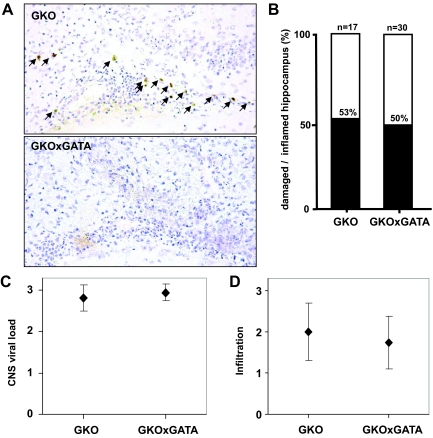
ΔDblGATA mice lack eosinophils due to a targeted deletion of a GATA1-responsive element in the promoter of the GATA1 gene.13 We bred ΔDblGATA mutant mice with animals that lack a functional IFN-γ gene to generate double-knockout strain BALB-GKOxΔDblGATA. To determine whether eosinophils, which infiltrate the brain of BDV-infected GKO but not wild-type mice,4 might be responsible for the observed hippocampal damage, we infected BALB-GKO and BALB-GKOxΔDblGATA mice with BDV strain CRNP5 and sacrificed the animals when they showed signs of neurological disease. Strain CRNP5 was used for these experiments because it induces hippocampal damage in BALB/c mice with higher efficiency than our standard BDV strain (unpublished results), presumably due to a better adaptation to the mouse brain. To determine the extent of eosinophil infiltration, frozen brain sections were assayed for cyanide-resistant peroxidase activity as described.21 As expected from the genotypes of the animals used for this study, the brains of BDV-infected BALB-GKO with neurological disease contained eosinophils (Figure 3A, top panel), whereas brains of diseased BALB-GKOxΔDblGATA did not (Figure 3A, bottom panel). Brains from diseased animals of either strain contained comparable numbers of BDV-infected brain cells (Figure 3C) and infiltrating immune cells (Figure 3D). When H&E-stained brain sections were analyzed for hippocampal damage, we found that 53% (9/17) of BALB-GKO and 50% (15/30) of BALB-GKOxΔDblGATA mice contained damaged CA1 neurons (Figure 3B). The finding that a similar frequency of hippocampus damage was observed in the presence and absence of eosinophils excludes an essential role of this cell type in the apoptotic death of CA1 neurons in BDV-infected GKO mice.
Figure 3.
Unaltered frequency of hippocampal damage in diseased GKO mice lacking eosinophils due to a genetic defect. BALB-GKO and BALB-GKOxΔDblGATA mice, which contain or lack eosinophils, respectively, were infected with BDV strain CRNP5. Animals were sacrificed when showing clear signs of neurological disease at 3 to 8 weeks postinfection. A: Staining of sagittal brain sections for eosinophils (arrows) confirmed the expected absence of these cells in BALB-GKOxΔDblGATA mice. B: Brain sections were analyzed for mononuclear cell infiltration and assessed for hippocampus damage if they contained at least two perivascular infiltrates per brain section and some mononuclear cells in the meninges. Columns illustrate the frequency of hippocampus damage in inflamed brains. The numbers of animals in each group are indicated above the individual columns. C: CNS viral load was determined by staining the brain sections against BDV-N and scoring on an arbitrary scale from 0 to 3. D: The degree of mononuclear cell infiltration was scored on H&E-stained brain sections on an arbitrary scale from 0 to 3. Geometric means ± SDs are indicated.
Reduced Hippocampal Damage in BDV-Infected GKO Mice Lacking Perforin
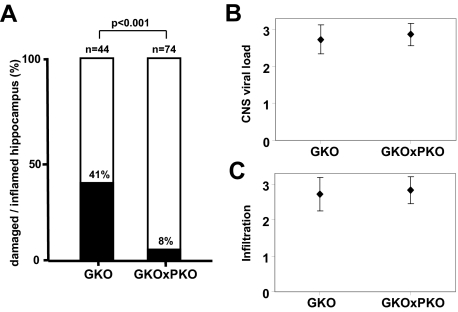
To determine whether the disease-inducing cytotoxic T cells might represent the critical cell type that mediates CA1 neuronal damage in BDV-infected GKO mice, we generated MRL mice with homozygous deletions of both IFN-γ and perforin genes (designated MRL-GKOxPKO). We reasoned that if T cells are involved in the killing of CA1 neurons, the frequency of hippocampal damage should decrease if the granule exocytosis pathway of T cells is compromised. MRL-GKO and MRL-GKOxPKO mice were infected with BDV and sacrificed when they showed neurological disease. H&E-stained brain sections that contained a substantial number of immune cells were then analyzed for hippocampal damage. Brains from diseased GKO and GKOxPKO mice contained comparable numbers of BDV-infected brain cells (Figure 4B) and infiltrating immune cells (Figure 4C). Forty-one percent (18/44) of the MRL-GKO mice but only 8% (6/74) of the MRL-GKOxPKO mice presented with a damaged hippocampus (Figure 4A). This difference was highly significant (P < 0.001), suggesting that perforin-mediated killing plays an important role in the pathological process.
Figure 4.
Decreased frequency of hippocampus damage in BDV-infected GKO mice lacking perforin due to a genetic defect. MRL-GKO and MRL-GKOxPKO mice, which contain or lack functional perforin genes, respectively, were infected with BDV strain #97 and sacrificed when diseased between 3 to 7 weeks postinfection. A: H&E-stained sagittal brain sections were analyzed for mononuclear cell infiltration and assessed for hippocampus damage if they contained at least three to five perivascular infiltrates per brain section with multilayer appearance. Columns illustrate the frequency of hippocampus damage in inflamed brains. The numbers of animals in each group are indicated above the individual columns. The difference was statistically significant (P < 0.001). B: CNS viral load was determined by staining the brain sections against BDV-N and scoring on an arbitrary scale from 0 to 3. C: The degree of mononuclear cell infiltration was scored on H&E-stained brain sections on an arbitrary scale from 0 to 3. Geometric means ± SDs are indicated.
Cultured IFN-γ-Deficient Antiviral CD8 T Cells Can Induce Hippocampal Damage
If cytotoxic T cells are responsible for the CA1 neuron damage in BDV-infected GKO mice, it should be possible to induce such damage by adoptive transfer of BDV-specific CD8 T cells from GKO mice into appropriate recipients. Unlike MRL mice, B10.BR mice fail to spontaneously generate CD8 T cells against BDV due to immunological ignorance.23 However, the asymptomatic state of persistently infected B10.BR mice can be terminated by several means, including adoptive transfer of CD8 T cells that recognize the major epitope of the viral nucleoprotein, designated TELEISSI.5 We therefore used the B10.BR model system for a more detailed analysis of the pathological processes, which ultimately result in CA1 neuronal damage.
To generate BDV-specific CD8 T cells lacking IFN-γ, we back-crossed the defective IFN-γ gene from C57BL/6 into B10.BR mice, yielding strain B10.BR-GKO. Mice were immunized sequentially with two different poxvirus vectors expressing the nucleoprotein of BDV as described.24 The spleen cells of immunized mice were then restimulated in vitro with peptide TELEISSI. After 2 weeks in culture, 50% to 70% of the surviving CD8 T cells usually had the desired specificity as previously described for cells from wild-type mice.5 As expected,5 all persistently infected B10.BR mice developed strong neurological symptoms within 2 to 3 days after transfer of TELEISSI-specific CD8 T cells from B10.BR-GKO mice, whereas all uninfected mice stayed healthy (Table 1). Histological analysis revealed substantial damage of CA1 neurons in about one of five persistently infected mice that received CD8 T cell grafts originating from B10.BR-GKO mice, irrespective of whether persistently infected B10.BR-GKO or wild-type B10.BR mice were used as recipients (Table 1). For unknown reasons, the frequency of hippocampus damage in this experimental system thus remained substantially lower than during BDV infection of MRL-GKO mice (compare Figures 2, 3, and 4). Nevertheless, our T cell transfer study with B10.BR mice clearly confirmed the conclusion from the above-described experiments with MRL mice that CD8 T cells are the decisive cell type, which induces CA1 neuronal damage in the absence of IFN-γ.
Table 1.
Adoptively Transferred CD8 T Cells from GKO Mice Induce Hippocampus Damage in Brains of Mice Presenting Cognate Antigen
| Donor | Recipient | Neurological disease (frequency) | Hippocampus damage (frequency) |
|---|---|---|---|
| B10.BR-GKO | Uninfected B10.BR | 0/18 (0%) | 0/18 (0%) |
| Infected B10.BR-wt | 12/12 (100%) | 2/12 (17%) | |
| Infected B10.BR-GKO | 13/13 (100%) | 3/13 (23%) | |
| Astro-N | 21/21 (100%) | 8/21 (38%) | |
| Neuro-N | 14/14 (100%) | 0/14 (0%) | |
| B10.BR-wt | Astro-N | 17/17 (100%) | 0/17 (0%) |
Approximately 5 × 106 CD8 T cells with specificity for BDV-N-derived peptide TELEISSI from either GKO or wild-type mice were transferred by the intracerebral route into the indicated recipients. Mice were sacrificed when severe neurological symptoms were observed. Uninfected control recipients were sacrificed at 14 days post-transfer. H&E-stained sagittal brain sections were prepared and analyzed for hippocampal damage.
Adoptive CD8 T Cell Transfer Can Induce CA1 Neuronal Damage in Transgenic Mice Expressing the Cognate Antigen on Astrocytes
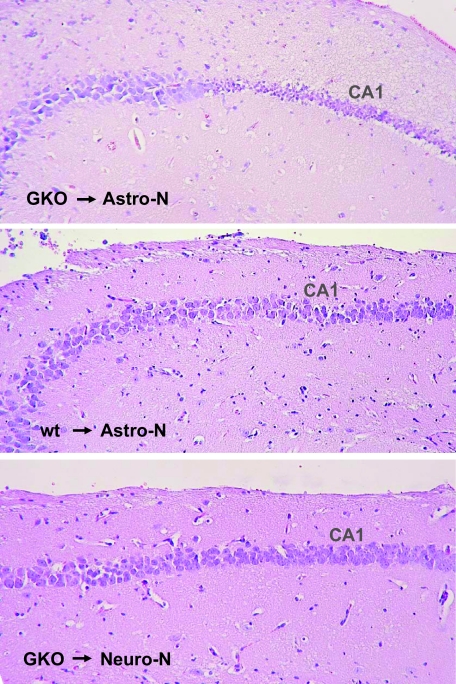
CA1 neurons of mice are usually not infected by BDV.4 It thus appeared unlikely that these cells represent suitable target cells for antiviral CD8 T cells. To further evaluate the possibility that the CD8 T cell-dependent CA1 neuronal death in GKO mice occurred via indirect mechanisms, we adoptively transferred CD8 T cells from wild-type and GKO mice into uninfected Astro-N mice that transgenically express the nucleoprotein of BDV at a high level in astrocytes.11 We previously reported that these transgenic animals develop severe neurological disease after intracerebral transfer of TELEISSI-specific wild-type CD8 T cells.5 We observed that the transfer of CD8 T cells derived from B10.BR-GKO mice similarly resulted in strong neurological disease within 1 to 3 days in all 21 Astro-N recipients that were used for this experiment (Table 1). We observed CA1 neuron damage in 38% (8/21) of the Astro-N mice receiving IFN-γ-deficient CD8 T cells (Table 1). On histological analysis, the hippocampus damage in Astro-N mice following adoptive transfer of CD8 T cells from GKO mice looked virtually identical to the damage seen in BDV-infected MRL-GKO mice (Figure 5, top panel). Importantly, none of the 17 Astro-N control mice that received similar numbers of CD8 T cells originating from wild-type mice developed hippocampal damage (Table 1 and Figure 5, middle panel), excluding the possibility that non-specific effects contributed to the CA1 neuron damage.
Figure 5.
CA1 neuron damage after adoptive transfer of IFN-γ-deficient CD8 T cells into transgenic mice expressing viral antigen in astrocytes. Approximately 5 × 106 CD8 T cells with specificity for BDV-N-derived peptide TELEISSI from B10.BR-GKO mice were transferred by the intracerebral route into Astro-N or Neuro-N mice as indicated. As an additional control, CD8 T cells of identical specificity from wild-type B10.BR mice were applied to Astro-N mice. Mice were sacrificed when severe neurological symptoms were observed. H&E-stained sagittal brain sections were prepared and analyzed for hippocampus damage. The figure shows representative examples of the data set displayed in Table 1.
To determine whether antiviral T cells might cause the same pathology if antigen is present on neurons only, we adoptively transferred CD8 T cells into Neuro-N mice, which specifically express a transgene encoding the nucleoprotein of BDV in neurons.11 As predicted from previous studies with T cells from wild-type mice,5 the transfer of CD8 T cells derived from B10.BR-GKO cells resulted in strong neurological disease in all 14 Neuro-N recipients that were used for this experiment (Table 1). However, no CA1 neuron damage was observed in any of these mice (Table 1 and Figure 5, bottom panel), further supporting the view that the antiviral T cells induce neuronal cell death by a mechanism that involves antigen recognition on astrocytes rather than neurons. Taken together these results indicated that antigen recognition by T cells on astrocytes triggered the death of bystander CA1 neurons that are neither infected with BDV nor expressing virus-derived antigen. They further suggested that IFN-γ possesses an activity, which renders CA1 neurons resistant to collateral damage during brain inflammation.
Discussion
In this report we provided evidence that IFN-γ exhibits neuroprotective activity when antiviral CD8 T cells attack antigen-expressing astrocytes in the brain. Neuroprotective activity of IFN-γ has previously been observed in cell culture systems where IFN-γ was shown to protract neuronal cell death due to nerve growth factor deprivation.25 To our knowledge, neuroprotective activity of IFN-γ had never before been recorded in vivo.
The loss of neurons from the hippocampal CA1 region in IFN-γ-deficient mice was accompanied by a suspicious brain influx of eosinophils that was not observed in infected wild-type animals.4 The influx of eosinophil granulocytes was likely caused by elevated levels of interleukin-13 and eotaxin in the brains of BDV-infected MRL GKO mice.4 Before this study was completed, it remained unclear whether eosinophils play an active role in the BDV-induced pathological process. We showed here that the frequency of CA1 neuron damage in BDV-infected GKO mice lacking eosinophils due to a genetic defect was not significantly different from the damage observed in wild-type mice, making clear that these brain-infiltrating immune cells are not responsible for the damage.
The CA1 hippocampal neurons are known to exhibit the highest degree of susceptibility among central nervous system neurons toward various brain insults like epilepsy,26 ischemia,27 Huntington disease,28 Parkinson’s disease,29 and Alzheimer’s disease.30 In the mouse model system, persistent infection with BDV usually has no detrimental effect on CA1 neurons or any other cells of the central nervous system. Only if the mice lack functional IFN-γ genes, CA1 neurons are selectively lost in the infected animals.4 Since BDV replicates more vigorously in brains of mice that lack IFN-γ than in wild-type mice,4 it was difficult to distinguish between the possibility that neurons are damaged in GKO mice because the virus replicates to higher titers and the possibility that neuronal damage is not a direct consequence of viral replication. All available evidence now argues in favor of the second possibility. In the present study we showed that the hippocampal CA1 neurons of BDV-infected GKO mice die by apoptosis, which is accompanied by strong activation of microglia and/or infiltration of macrophages. We further demonstrated that CA1 neuron damage can be mimicked in an experimental system in which no replicating BDV is present and in which viral antigen in the brain is derived from a transgene that is selectively expressed in either neurons or astrocytes. Using this experimental system it became clear that CA1 neuron damage occurred only if CD8 T cells were present in the brain, if these cells recognized the antigen on astrocytes and if the T cells lacked IFN-γ. Interestingly, the damaging activity of CD8 T cells was not observed if the cognate antigen was expressed on neurons or if the T cells possessed a functional IFN-γ gene. Collectively, these observations suggest that the T cell-mediated impairment of astrocyte function generates a microenvironment that negatively affects the survival of CA1 neurons. Since the adoptive transfer of antiviral CD8 T cells from GKO mice induced CA1 neuron damage with similar frequency in persistently infected wild-type and GKO mice, endogenous IFN-γ production of the recipient cannot compensate for the lack of T cell-produced IFN-γ. This result shows that most if not all neuroprotective IFN-γ in the brain originates from the infiltrating T cells.
It is unclear why only about 50% of the severely diseased, BDV-infected MRL-GKO mice and only about 38% of Astro-N mice receiving antiviral CD8 T cells from GKO mice developed recognizable damage of hippocampal CA1 neurons. We speculate that CA1 neuron damage occurs only if sufficiently high numbers of antiviral T cells are present in the hippocampus. The slightly lower rate of hippocampus damage in Astro-N mice might be explained by the fact that disease progression in Astro-N mice after CD8 T cell transfer was much faster than in BDV-infected MRL-GKO mice without T cell transfer (2 to 3 days vs. 20 to 30 days). Since the intracerebral injections were not done stereotactically, the adoptively transferred antiviral T cells were most probably not delivered uniformly to all brain regions. Thus, CD8 T cells probably had less time for migration to the hippocampus in Astro-N mice than in infected MRL-GKO mice.
A truly remarkable finding of our present study with uninfected transgenic mice that express the viral antigen in astrocytes is that cell death induced by antiviral T cells was primarily detectable in bystander neurons rather than in the antigen-positive cells, raising the question regarding the mechanisms involved in the pathological process. Using glutamate receptor antagonists, we found that excitotoxicity resulting from glutamate receptor overstimulation is most likely not responsible. This is in contrast to the mouse models of measles virus and Sindbis virus infection where administration of MK801 was shown to reduce CA1 damage.7 Since neuronal damage was far less pronounced if the perforin gene was defective and since it is highly unlikely that cytotoxic factors of CD8 T cells can act on distant cells not presenting the cognate antigen, we assume that astrocytes are the primary target of such cytotoxic CD8 T cells and that the protective effect on CA1 neurons might be an indirect one. Functional impairment or killing of astrocytes especially in the CA1 region might impair regional detoxification and result in local accumulation of potentially toxic substances and death of CA1 neurons. Evidence for such a scenario has been reported in a model of transient forebrain ischemia.31 A third possibility is that the loss or functional impairment of astrocytes deprives neurons of factors, which are essential for the survival of CA1 neurons, as discussed for HIV-induced dementia where infection of astrocytes probably induces neuronal dysfunction due to suppression of growth factors.32,33 Importantly, our results suggest that the presence of IFN-γ during immune attack of CD8 T cells on astrocytes is closely associated with survival of CA1 neurons.
Acknowledgments
We thank the NIAID Tetramer Facility (Atlanta, GA) for producing H-2Kk-TELEISSI tetramers, Alison Humbles for supplying ΔDblGATA mice, Rosita Frank for excellent technical assistance, and Georg Kochs and Otto Haller for helpful comments on the manuscript.
Footnotes
Address reprint requests to Dr. Peter Staeheli, Department of Virology, University of Freiburg, Hermann-Herder-Straße 11, D-79104 Freiburg, Germany. E-mail: peter.staeheli@uniklinik-freiburg.de.
Supported by the Deutsche Forschungsgemeinschaft (SFB 620).
Present address for K.R.: Institute for Microbiology, ETH Zürich, Wolfgang-Pauli-Str. 10, CH-8093 Zürich, Switzerland; Present address for J.H.: Bavarian Nordic GmbH, Fraunhoferstrasse 13, D-82152 Martinsried, Germany.
References
- Walker PR, Calzascia T, de Tribolet N, Dietrich PY. T-cell immune responses in the brain and their relevance for cerebral malignancies. Brain Res Brain Res Rev. 2003;42:97–122. doi: 10.1016/s0165-0173(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Hallensleben W, Schwemmle M, Hausmann J, Stitz L, Volk B, Pagenstecher A, Staeheli P. Borna disease virus-induced neurological disorder in mice: infection of neonates results in immunopathology. J Virol. 1998;72:4379–4386. doi: 10.1128/jvi.72.5.4379-4386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog S, Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- Hausmann J, Pagenstecher A, Baur K, Richter K, Rziha HJ, Staeheli P. CD8 T cells require gamma interferon to clear borna disease virus from the brain and prevent immune system-mediated neuronal damage. J Virol. 2005;79:13509–13518. doi: 10.1128/JVI.79.21.13509-13518.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur K, Rauer M, Richter K, Pagenstecher A, Gotz J, Hausmann J, Staeheli P. Antiviral CD8 T cells recognize borna disease virus antigen transgenically expressed in either neurons or astrocytes. J Virol. 2008;82:3099–3108. doi: 10.1128/JVI.02479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson T, Schwarcz R, Love A, Kristensson K. Measles virus-induced hippocampal neurodegeneration in the mouse: a novel, subacute model for testing neuroprotective agents. Neurosci Lett. 1993;154:109–112. doi: 10.1016/0304-3940(93)90183-l. [DOI] [PubMed] [Google Scholar]
- Nargi-Aizenman JL, Havert MB, Zhang M, Irani DN, Rothstein JD, Griffin DE. Glutamate receptor antagonists protect from virus-induced neural degeneration. Ann Neurol. 2004;55:541–549. doi: 10.1002/ana.20033. [DOI] [PubMed] [Google Scholar]
- Nargi-Aizenman JL, Griffin DE. Sindbis virus-induced neuronal death is both necrotic and apoptotic and is ameliorated by N-methyl-d-aspartate receptor antagonists. J Virol. 2001;75:7114–7121. doi: 10.1128/JVI.75.15.7114-7121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res. 1996;706:303–307. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- Kita H. The eosinophil: a cytokine-producing cell? J Allergy Clin Immunol. 1996;97:889–892. doi: 10.1016/s0091-6749(96)80061-3. [DOI] [PubMed] [Google Scholar]
- Rauer M, Gotz J, Schuppli D, Staeheli P, Hausmann J. Transgenic mice expressing the nucleoprotein of Borna disease virus in either neurons or astrocytes: decreased susceptibility to homotypic infection and disease. J Virol. 2004;78:3621–3632. doi: 10.1128/JVI.78.7.3621-3632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann J, Schamel K, Staeheli P. CD8(+) T lymphocytes mediate Borna disease virus-induced immunopathology independently of perforin. J Virol. 2001;75:10460–10466. doi: 10.1128/JVI.75.21.10460-10466.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Hofer M, Hausmann J, Staeheli P, Pagenstecher A. Cerebral expression of interleukin-12 induces neurological disease via differential pathways and recruits antigen-specific T cells in virus-infected mice. Am J Pathol. 2004;165:949–958. doi: 10.1016/S0002-9440(10)63356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino Y, Kobasa D, Rubin SA, Pletnikov MV, Carbone KM. Enhanced neurovirulence of borna disease virus variants associated with nucleotide changes in the glycoprotein and L polymerase genes. J Virol. 2002;76:8650–8658. doi: 10.1128/JVI.76.17.8650-8658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel M, Planz O, Fischer T, Stitz L, Rziha HJ. Prevention of virus persistence and protection against immunopathology after Borna disease virus infection of the brain by a novel Orf virus recombinant. J Virol. 2005;79:314–325. doi: 10.1128/JVI.79.1.314-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamel K, Staeheli P, Hausmann J. Identification of the immunodominant H-2K(k)-restricted cytotoxic T-cell epitope in the Borna disease virus nucleoprotein. J Virol. 2001;75:8579–8588. doi: 10.1128/JVI.75.18.8579-8588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassnacht U, Ackermann A, Staeheli P, Hausmann J. Immunization with dendritic cells can break immunological ignorance toward a persisting virus in the central nervous system and induce partial protection against intracerebral viral challenge. J Gen Virol. 2004;85:2379–2387. doi: 10.1099/vir.0.80115-0. [DOI] [PubMed] [Google Scholar]
- Haas B, Becht H, Rott R. Purification and properties of an intranuclear virus-specific antigen from tissue infected with Borna disease virus, J Gen Virol. 1986;67:235–241. doi: 10.1099/0022-1317-67-2-235. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D, Grusky G. The identification of eosinophil colonies in soft-agar cultures by differential staining for peroxidase. J Histochem Cytochem. 1976;24:1270–1272. doi: 10.1177/24.12.63511. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Takeyama A, Xiao C, Takano-Yamamoto T, Ichikawa H. Electron microscopic demonstration of nick end-labeled DNA fragments during capsaicin-induced apoptosis of trigeminal primary neurons in neonatal rats. Brain Res. 1999;818:147–152. doi: 10.1016/s0006-8993(98)01227-x. [DOI] [PubMed] [Google Scholar]
- Hausmann J, Hallensleben W, de la Torre JC, Pagenstecher A, Zimmermann C, Pircher H, Staeheli P. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc Natl Acad Sci USA. 1999;96:9769–9774. doi: 10.1073/pnas.96.17.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann J, Baur K, Engelhardt KR, Fischer T, Rziha HJ, Staeheli P. Vaccine-induced protection against Borna disease in wild-type and perforin-deficient mice. J Gen Virol. 2005;86:399–403. doi: 10.1099/vir.0.80566-0. [DOI] [PubMed] [Google Scholar]
- Chang JY, Martin DP, Johnson EM., Jr Interferon suppresses sympathetic neuronal cell death caused by nerve growth factor deprivation. J Neurochem. 1990;55:436–445. doi: 10.1111/j.1471-4159.1990.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Carpenter S, Zatorre RJ, Cashman NR. Temporal lobe epilepsy caused by domoic acid intoxication: evidence for glutamate receptor-mediated excitotoxicity in humans. Ann Neurol. 1995;37:123–126. doi: 10.1002/ana.410370125. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Beal MF. Huntington’s disease, energy, and excitotoxicity. Neurobiol Aging. 1994;15:275–276. doi: 10.1016/0197-4580(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Beal MF. Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis. Ann Neurol. 1998;44:S110–S114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg BM, Gelbard HA, Epstein LG. HIV-1 infection of the developing nervous system: central role of astrocytes in pathogenesis. Virus Res. 1994;32:253–267. doi: 10.1016/0168-1702(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Deshpande M, Zheng J, Borgmann K, Persidsky R, Wu L, Schellpeper C, Ghorpade A. Role of activated astrocytes in neuronal damage: potential links to HIV-1-associated dementia. Neurotox Res. 2005;7:183–192. doi: 10.1007/BF03036448. [DOI] [PubMed] [Google Scholar]