Abstract
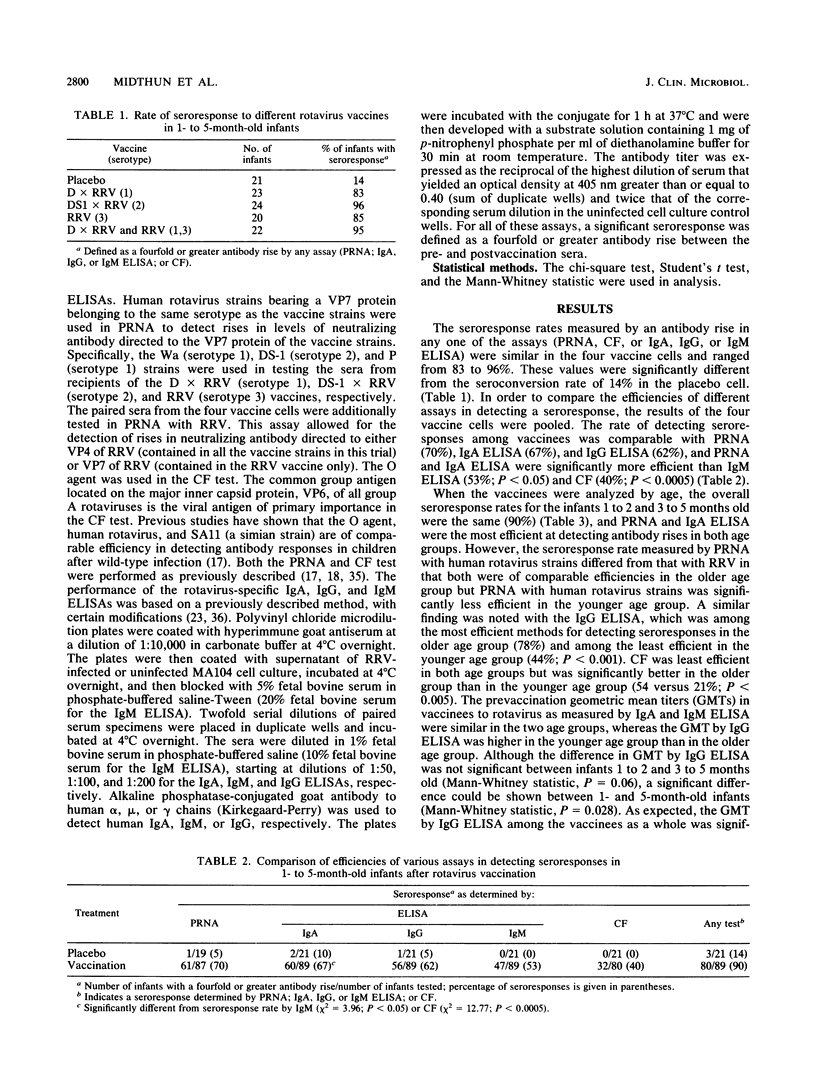
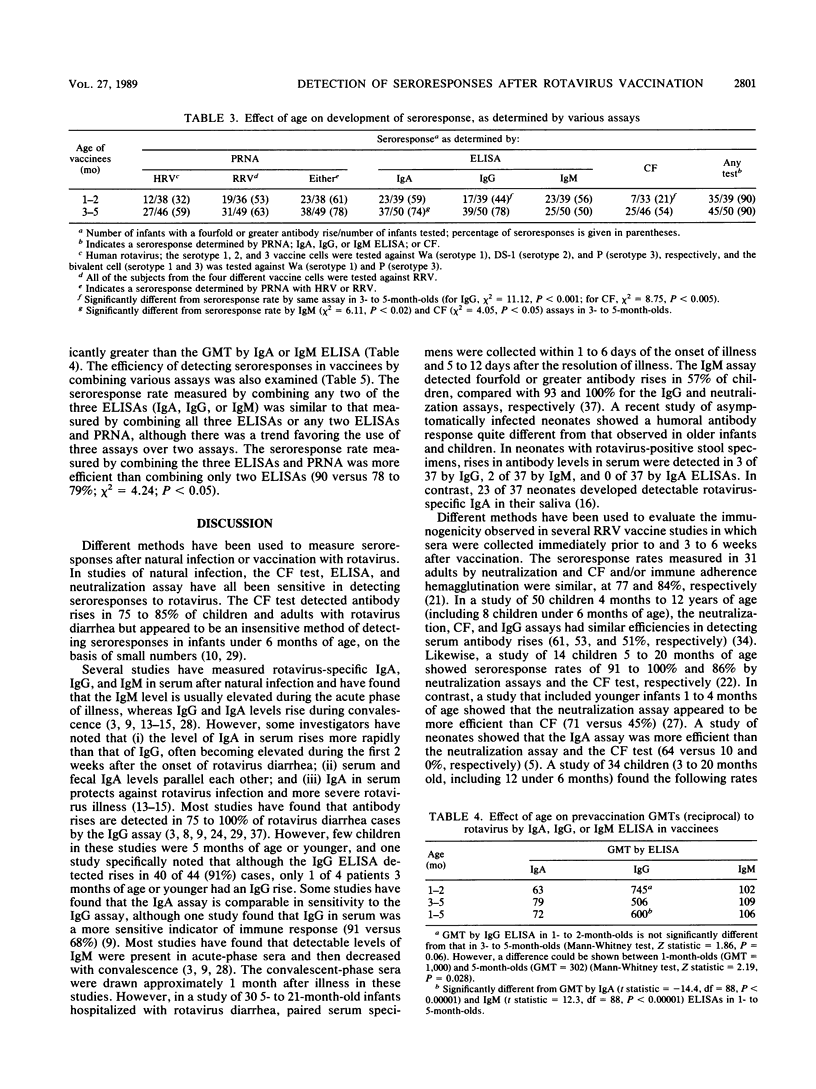
In a phase 1 study to evaluate human-rhesus rotavirus reassortant vaccines, 116 infants 1 to 5 months of age received one of the following five preparations: the serotype 1 reassortant, the serotype 2 reassortant, rhesus rotavirus (serotype 3), a bivalent preparation (serotypes 1 and 3), or a placebo. Seroresponses to the different vaccines were measured by plaque reduction neutralization assay (PRNA); rotavirus-specific immunoglobulin A (IgA), IgG, and IgM enzyme-linked immunosorbent assays (ELISAs); and complement fixation (CF). The seroresponse rate, calculated by using a fourfold or greater antibody rise by any assay, was similar in the four vaccine groups (83 to 96%). When the data from all the vaccinees were pooled, IgA ELISA, IgG ELISA, and PRNA were comparable in detecting seroresponses (67, 62, and 70%, respectively) and more efficient than IgM ELISA (53%) and CF (44%). When the vaccinees were analyzed by age, the overall seroresponse rates were the same for infants 1 to 2 and 3 to 5 months old (90%). The IgA ELISA and PRNA were the most efficient for detecting antibody rises in both age groups. IgG ELISA was among the least efficient methods for detecting antibody rises in the younger age group but among the most efficient in the older age group (44 versus 78%). CF was among the least efficient methods in both age groups but was significantly better in the older age group than in the younger age group (54 versus 21%). Our findings show that ELISA, in particular rotavirus-specific IgA ELISA, is a sensitive indicator of vaccine takes in 1- to 5 month-old infants, the target population for vaccination. ELISA should also be very useful in demonstrating natural rotavirus infections in field studies in which a stool specimen from a diarrheal episode is not always available. The ELISA has the advantages of being easier and quicker and requiring less serum than PRNA, but it does not give serotype-specific information about the immune response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark H. F., Borian F. E., Bell L. M., Modesto K., Gouvea V., Plotkin S. A. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988 Sep;158(3):570–587. doi: 10.1093/infdis/158.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Furukawa T., Bell L. M., Offit P. A., Perrella P. A., Plotkin S. A. Immune response of infants and children to low-passage bovine rotavirus (strain WC3). Am J Dis Child. 1986 Apr;140(4):350–356. doi: 10.1001/archpedi.1986.02140180084030. [DOI] [PubMed] [Google Scholar]
- Davidson G. P., Hogg R. J., Kirubakaran C. P. Serum and intestinal immune response to rotavirus enteritis in children. Infect Immun. 1983 May;40(2):447–452. doi: 10.1128/iai.40.2.447-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mol P., Zissis G., Butzler J. P., Mutwewingabo A., André F. E. Failure of live, attenuated oral rotavirus vaccine. Lancet. 1986 Jul 12;2(8498):108–108. doi: 10.1016/s0140-6736(86)91643-0. [DOI] [PubMed] [Google Scholar]
- Flores J., Daoud G., Daoud N., Puig M., Martinez M., Perez-Schael I., Shaw R., Greenberg H. B., Midthun K., Kapikian A. Z. Reactogenicity and antigenicity of rhesus rotavirus vaccine (MMU-18006) in newborn infants in Venezuela. Pediatr Infect Dis J. 1988 Nov;7(11):776–780. doi: 10.1097/00006454-198811000-00006. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Blanco M., Vilar M., Garcia D., Perez M., Daoud N., Midthun K., Kapikian A. Z. Reactions to and antigenicity of two human-rhesus rotavirus reassortant vaccine candidates of serotypes 1 and 2 in Venezuelan infants. J Clin Microbiol. 1989 Mar;27(3):512–518. doi: 10.1128/jcm.27.3.512-518.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Gonzalez M., Garcia D., Perez M., Daoud N., Cunto W., Chanock R. M., Kapikian A. Z. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet. 1987 Apr 18;1(8538):882–884. doi: 10.1016/s0140-6736(87)92858-3. [DOI] [PubMed] [Google Scholar]
- Grauballe P. C., Hornsleth A., Hjelt K., Krasilnikoff P. A. Detection by ELISA of immunoglobulin G subclass-specific antibody responses in rotavirus infections in children. J Med Virol. 1986 Mar;18(3):277–281. doi: 10.1002/jmv.1890180309. [DOI] [PubMed] [Google Scholar]
- Grimwood K., Lund J. C., Coulson B. S., Hudson I. L., Bishop R. F., Barnes G. L. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol. 1988 Apr;26(4):732–738. doi: 10.1128/jcm.26.4.732-738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust I. D., Pringle R. C., Barnes G. L., Davidson G. P., Bishop R. F. Complement-fixing antibody response to rotavirus infection. J Clin Microbiol. 1977 Feb;5(2):125–130. doi: 10.1128/jcm.5.2.125-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey N. A., Anderson E. L., Sears S. D., Steinhoff M., Wilson M., Belshe R. B., Midthun K., Kapikian A. Z., Chanock R. M., Samorodin R. Human-rhesus reassortant rotavirus vaccines: safety and immunogenicity in adults, infants, and children. J Infect Dis. 1988 Dec;158(6):1261–1267. doi: 10.1093/infdis/158.6.1261. [DOI] [PubMed] [Google Scholar]
- Hanlon P., Hanlon L., Marsh V., Byass P., Shenton F., Hassan-King M., Jobe O., Sillah H., Hayes R., M'Boge B. H. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987 Jun 13;1(8546):1342–1345. doi: 10.1016/s0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- Hjelt K., Grauballe P. C., Andersen L., Schiøtz P. O., Howitz P., Krasilnikoff P. A. Antibody response in serum and intestine in children up to six months after a naturally acquired rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1986 Jan;5(1):74–80. doi: 10.1097/00005176-198601000-00014. [DOI] [PubMed] [Google Scholar]
- Hjelt K., Grauballe P. C., Paerregaard A., Nielsen O. H., Krasilnikoff P. A. Protective effect of preexisting rotavirus-specific immunoglobulin A against naturally acquired rotavirus infection in children. J Med Virol. 1987 Jan;21(1):39–47. doi: 10.1002/jmv.1890210106. [DOI] [PubMed] [Google Scholar]
- Hjelt K., Grauballe P. C., Schiøtz P. O., Andersen L., Krasilnikoff P. A. Intestinal and serum immune response to a naturally acquired rotavirus gastroenteritis in children. J Pediatr Gastroenterol Nutr. 1985 Feb;4(1):60–66. doi: 10.1097/00005176-198502000-00012. [DOI] [PubMed] [Google Scholar]
- Jayashree S., Bhan M. K., Kumar R., Raj P., Glass R., Bhandari N. Serum and salivary antibodies as indicators of rotavirus infection in neonates. J Infect Dis. 1988 Nov;158(5):1117–1120. doi: 10.1093/infdis/158.5.1117. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Kim H. W., Kalica A. R., Wyatt R. G., Vankirk D. H., Chanock R. M., James H. D., Jr, Vaughn A. L. Antigenic relationships among five reovirus-like (RVL) agents by complement fixation (CF) and development of new substitute CF antigens for the human RVL agent of infantile gastroenteritis. Proc Soc Exp Biol Med. 1976 Sep;152(4):535–539. doi: 10.3181/00379727-152-39434. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Mebus C. A., Wyatt R. G., Kalica A. R., James H. D., Jr, VanKirk D., Chanock R. M. New complement-fixation test for the human reovirus-like agent of infantile gastroenteritis. Nebraska calf diarrhea virus used as antigen. Lancet. 1975 May 10;1(7915):1056–1061. doi: 10.1016/s0140-6736(75)91827-9. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Hoshino Y., Glass R. I., Midthun K., Gorziglia M., Chanock R. M. Rotavirus: the major etiologic agent of severe infantile diarrhea may be controllable by a "Jennerian" approach to vaccination. J Infect Dis. 1986 May;153(5):815–822. doi: 10.1093/infdis/153.5.815. [DOI] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Kapikian A. Z., Midthun K., Ferra P. J., Fortier D. N., Hoffman K. M., Baig A., Levine M. M. Safety, infectivity, transmissibility and immunogenicity of rhesus rotavirus vaccine (MMU 18006) in infants. Pediatr Infect Dis. 1986 Jan-Feb;5(1):25–29. doi: 10.1097/00006454-198601000-00005. [DOI] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Lim Y., Krall G., Kapikian A. Z., Levine M. M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988 Jun;7(6):388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- McLean B., Sonza S., Holmes I. H. Measurement of immunoglobulin A, G, and M class rotavirus antibodies in serum and mucosal secretions. J Clin Microbiol. 1980 Sep;12(3):314–319. doi: 10.1128/jcm.12.3.314-319.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Hoshino Y., Kapikian A. Z., Chanock R. M. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J Clin Microbiol. 1986 Nov;24(5):822–826. doi: 10.1128/jcm.24.5.822-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Schael I., Gonzalez M., Daoud N., Perez M., Soto I., Garcia D., Daoud G., Kapikian A. Z., Flores J. Reactogenicity and antigenicity of the rhesus rotavirus vaccine in Venezuelan children. J Infect Dis. 1987 Feb;155(2):334–338. doi: 10.1093/infdis/155.2.334. [DOI] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Bogger-Goren S., Li P., Carmody P. J., Barrett H. J., Ogra P. L. Development of serum and intestinal antibody response to rotavirus after naturally acquired rotavirus infection in man. J Med Virol. 1981;8(3):215–222. doi: 10.1002/jmv.1890080309. [DOI] [PubMed] [Google Scholar]
- Sarkkinen H. K., Meurman O. H., Halonen P. E. Solid-phase radioimmunoassay of IgA, IgG, and IgM antibodies to human rotavirus. J Med Virol. 1979;3(4):281–289. doi: 10.1002/jmv.1890030406. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Fong K. J., Losonsky G. A., Levine M. M., Maldonado Y., Yolken R., Flores J., Kapikian A. Z., Vo P. T., Greenberg H. B. Epitope-specific immune responses to rotavirus vaccination. Gastroenterology. 1987 Nov;93(5):941–950. doi: 10.1016/0016-5085(87)90555-5. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., d'Hondt E., André F. E., Beards G. M., Flewett T. H. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985 Aug;107(2):189–194. doi: 10.1016/s0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Tajima T., Thompson J., Kokubun K., Kapikian A., Karzon D. T. Candidate rotavirus vaccine (rhesus rotavirus strain) in children: an evaluation. Pediatrics. 1987 Oct;80(4):473–480. [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Kim H. W., Kapikian A. Z., Chanock R. M. Immunological response to infection with human reovirus-like agent: measurement of anti-human reovirus-like agent immunoglobulin G and M levels by the method of enzyme-linked immunosorbent assay. Infect Immun. 1978 Feb;19(2):540–546. doi: 10.1128/iai.19.2.540-546.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B. J., Han S. X., Yan Y. K., Liang X. R., Ma G. Z., Yang Y., Ng M. H. Development of neutralizing antibodies and group A common antibodies against natural infections with human rotavirus. J Clin Microbiol. 1988 Aug;26(8):1506–1512. doi: 10.1128/jcm.26.8.1506-1512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


