Abstract
Phosphoinositide 3-kinase (PI3K), PTEN and localized phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3] play key roles in chemotaxis, regulating cell motility by controlling the actin cytoskeleton in Dictyostelium and mammalian cells. PtdIns(3,4,5)P3, produced by PI3K, acts via diverse downstream signaling components, including the GTPase Rac, Arf-GTPases and the kinase Akt (PKB). It has become increasingly apparent, however, that chemotaxis results from an interplay between the PI3K-PTEN pathway and other parallel pathways in Dictyostelium and mammalian cells. In Dictyostelium, the phospholipase PLA2 acts in concert with PI3K to regulate chemotaxis, whereas phospholipase C (PLC) plays a supporting role in modulating PI3K activity. In adenocarcinoma cells, PLC and the actin regulator cofilin seem to provide the direction-sensing machinery, whereas PI3K might regulate motility.
Keywords: Dictyostelium; Phospholipase; PTEN; Phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3]; Ras; Cytoskeleton
Introduction
Chemotaxis plays a central role in various biological processes, such as cellular morphogenesis, innate immunity, inflammation and metastasis of cancer cells (Böttcher and Niehrs, 2005; Eccles, 2004; Martin and Parkhurst, 2004; Sasaki and Firtel, 2006). It involves local production and degradation of phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3] at the plasma membrane, resulting in a net accumulation of PtdIns(3,4,5)P3 at the leading edge. This leads ultimately to actin polymerization, formation of pseudopodia and directional cell movement. In Dictyostelium, the levels of PtdIns(3,4,5)P3 are regulated by the phosphoinositide 3-kinase (PI3K) and the phosphatase PTEN [phosphatase and tensin homolog (mutated in multiple advanced cancers)]. Initial experiments in Dictyostelium suggested that the activity of PI3K and PTEN, and the subsequent formation of localized intracellular PtdIns(3,4,5)P3 gradients, are required for chemotaxis, especially for external-gradient sensing during chemotaxis (Funamoto et al., 2001; Huang et al., 2003; Iijima and Devreotes, 2002). However, studies over the past year indicate that PI3K and PTEN activity and localized PtdIns(3,4,5)P3 gradients, although important, are dispensable for chemotaxis under many conditions (Andrew and Insall, 2007; Hoeller and Kay, 2007; Loovers et al., 2006; Takeda et al., 2007; Van Haastert and Veltman, 2007; Wessels et al., 2007). Here, we first discuss the importance of PI3K, PTEN and PtdIns(3,4,5)P3 for cell motility and cytoskeletal regulation. In addition, we highlight recently identified pathways that have also been found to control chemotaxis. We concentrate on Dictyostelium, a simple eukaryotic model for the study of chemotaxis, and on mammalian neutrophils and adenocarcinoma cells, in which the understanding of chemotaxis has progressed substantially in recent years.
Chemotaxis in the absence of PI3K and/or PTEN
In Dictyostelium, PI3K1, PI3K2 and PI3K3 are the major PI3Ks implicated in chemotaxis (Takeda et al., 2007). Studies on pi3k1− pi3k2− double-knockout or pi3k1− pi3k2− pi3k3− triple-knockout cells confirm that PI3K plays a pivotal role in regulating cell speed and locomotion and is involved in directional sensing, especially in shallow, linear gradients (as generated in a Dunn chamber), whereas, in steep, exponential gradients (as emitted by a micropipette), other pathways might be sufficient to allow chemotactic movement (Hoeller and Kay, 2007; Loovers et al., 2006; Takeda et al., 2007). The importance of PI3K also depends on the developmental stage of the cells. Early in development, pi3k1− pi3k2− cells show defects in cell speed and directionality, whereas, later in development, they behave almost like wild-type cells. These differences might be due in part to a difference in the relative activity of Akt (PKB; protein kinase B) and the related kinase PKBR1 (PKB related protein 1) (Meili et al., 2000; Takeda et al., 2007). The kinases are genetically redundant but are preferentially, although not exclusively, expressed during growth and multicellular development, respectively. Cells lacking both kinases exhibit severe defects in growth and motility (Meili et al., 2000). Like its mammalian counterpart, Dictyostelium Akt is regulated by PI3K, whereas PKBR1 is found constitutively on the plasma membrane and is PI3K-independent.
A multiple-knockout strain lacking all five Dictyostelium class I pi3k genes as well as the phosphatase PTEN is still able to undergo chemotaxis in strong chemoattractant gradients, but shows reduced speed (Hoeller and Kay, 2007). Therefore, the polarization of membrane PtdIns(3,4,5)P3 is not essential for directed chemotaxis, but ensures rapid movement in response to chemoattractants. Dictyostelium cells achieve chemotaxis by biasing decisions between randomly generated pseudopodia (Andrew and Insall, 2007; Varnum-Finney et al., 1987). PI3K probably controls the rate at which pseudopodia are randomly generated, but not the direction of pseudopod formation, whereas PTEN suppresses lateral pseudopod formation, keeping cells on track (Wessels et al., 2007). In Dictyostelium, PI3K, PTEN and polarized PtdIns(3,4,5)P3 are thus mainly involved in the control of cell speed and pseudopod formation. This is highly important in basic cell motility and shallow gradients but less so in the presence of a strong chemotactic signal (Sasaki et al., 2007; Takeda et al., 2007).
In mammalian cells, localized PtdIns(3,4,5)P3 production and PI3K activity control polarization and migration during chemotactic movement (Hirsch et al., 2000; Sadhu et al., 2003; Sasaki et al., 2000; Servant et al., 2000; Wang et al., 2002). Experiments on B cells reveal that the PI3K regulatory subunit p85δ plays a major role in regulating chemokine responses and cell motility (Matheu et al., 2007). In neutrophils, PtdIns(3,4,5)P3 accumulates at the leading edge in a PI3Kγ-dependent manner (Ferguson et al., 2007; Nishio et al., 2007). Neutrophils lacking PI3Kγ move more slowly than wild-type cells, but do not have defects in directional sensing, similar to the Dictyostelium multiple pi3k knockout. PI3K is also important for stabilizing the leading edge of chemotaxing neutrophils, as in Dictyostelium (Sasaki et al., 2004; Van Keymeulen et al., 2006). In addition, Ferguson et al. have demonstrated that pi3kγ− neutrophils exhibit strong defects in adhesion. The decrease in speed observed in these cells might reflect the involvement of PI3K in regulating integrin-based adhesion (Ferguson et al., 2007). In Dictyostelium, adhesion has been implicated in chemotactic movement controlled by the Arp2-Arp3 (Arp2/3) complex and Rap1 (Jeon et al., 2007; Langridge and Kay, 2007). Whether PI3K is involved has yet to be determined.
In Dictyostelium, the reciprocal localizations of PI3K and PTEN help to establish a steep gradient of PtdIns(3,4,5)P3 in chemotaxing cells (Funamoto et al., 2002; Iijima and Devreotes, 2002). Recent studies shed light on the identities of the PtdIns(3,4,5)P3-degrading enzymes that regulate chemotaxis in neutrophils (Nishio et al., 2007; Subramanian et al., 2007). Subramanian et al. found that mouse pten− neutrophils have enhanced PtdIns(3,4,5)P3 levels, Akt phosphorylation and actin polymerization, resulting in an increased speed, but they have only small directionality defects during chemotaxis (Subramanian et al., 2007). The authors conclude that PTEN plays only a minor role in directional sensing, but has considerable effects on the sensitivity of the neutrophil response to chemoattractants (Subramanian et al., 2007). By contrast, Nishio et al. found that pten− neutrophils do not exhibit enhanced PtdIns(3,4,5)P3 levels and that most PtdIns(3,4,5)P3 degradation in neutrophils is accomplished by the phosphatase SHIP1 (Src homology 2 domain-containing inositol-5-phosphatase 1, also known as INPP5D) (Nishio et al., 2007). Neutrophils lacking SHIP1 migrate more slowly than wild-type cells, have reduced polarity, and exhibit defects in spatially restricted F-actin assembly but not in gradient sensing. Therefore, although PTEN is the dominant phosphatase for PtdIns(3,4,5)P3 degradation in Dictyostelium, SHIP1 seems to be the main PtdIns(3,4,5)P3 phosphatase in neutrophil chemotaxis. A recent study on PI3K and PTEN in macrophages uncovered a pathway mediated by the small GTPase RhoA, by which the PI3K catalytic subunit p110δ keeps PTEN lipid phosphatase activity in check via a mechanism involving RhoA and its effector kinase ROCK (Rho kinase) (Papakonstanti et al., 2007). Upon stimulation with CSF-1 (colony-stimulating factor-1), PI3K activity inhibits RhoA activation, thereby limiting ROCK activation of PTEN, a process that is crucial for cell polarization and chemotaxis. These observations demonstrate a bidirectional relationship between PI3K and PTEN in which PTEN degrades the lipids produced by PI3K and PI3K reciprocally controls PTEN. A related study demonstrated that RhoA/ROCK and CDC42 together control the localization and activity of PTEN in mouse neutrophils in response to the chemoattractant N-formyl-methionyl-leucyl-phenylalanine (fMLP) (Li et al., 2005).
PtdIns(3,4,5)P3 signaling controls cell motility
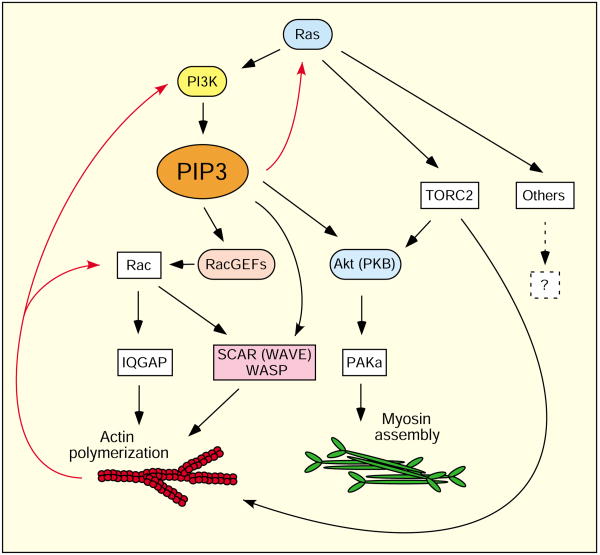
Even though PtdIns(3,4,5)P3 signaling is now believed to be dispensable for chemotaxis in steep gradients, there is no doubt that PtdIns(3,4,5)P3 strongly influences cell motility via the regulation of the cytoskeleton. Recent findings suggest that PtdIns(3,4,5)P3 signaling is a general regulator of cytoskeleton dynamics in a variety of cellular processes. Sasaki et al. recently demonstrated that PtdIns(3,4,5)P3 participates in a positive-feedback loop, similar to the one controlling migration during Dictyostelium chemotaxis (Sasaki et al., 2007). The loop, which comprises the small GTPase Ras, PI3K, PtdIns(3,4,5)P3 and F-actin, underlies basic cell motility as well as changes in cell shape during cytokinesis (Fig. 1) (Sasaki et al., 2007; Soll et al., 2002). Randomly moving vegetative Dictyostelium cells that lack expression of their only G-protein β subunit (Gβ)-encoding gene, and therefore any G-protein-coupled receptor (GPCR) signaling, exhibit co-localized activation of PI3K and Ras, as well as reciprocal localization of PI3K and PTEN. In consequence, PtdIns(3,4,5)P3 accumulates at sites of F-actin polymerization, causing transient pseudopod extension (Sasaki et al., 2007). It is therefore suggested that this positive-feedback loop might constitute a core regulatory pathway used by cells to modulate the cytoskeleton, whether activated by an extracellular stimulus or not. PI3K also modulates basal-level lymphocyte motility in the lymph node (Matheu et al., 2007). Whether Ras is involved in this process is unknown. However, the recent findings that Ras is required for maximal PI3K signaling in Drosophila (Orme et al., 2006), that it directly binds to and activates PI3Kγ in neutrophils (Suire et al., 2006), and that it is required for PtdIns(3,4,5)P3 and lamellipodium production in adenocarcinoma cells (Yip et al., 2007) support a universal role for Ras in the regulation of PI3K function.
Fig. 1.
PtdIns(3,4,5)P3 (PIP3) controls cell motility. Basic cell motility is regulated by a Ras–PI3K–PtdIns(3,4,5)P3–F-actin circuit. During chemotaxis, this circuit becomes restricted to the leading edge, allowing directed movement. Several downstream effectors of PtdIns(3,4,5)P3, such as RacGEFs and Akt, activate F-actin polymerization and myosin assembly (see text for details). Positive-feedback loops (red arrows) allow signal amplification, enhanced actin polymerization at the leading edge and the production of pseudopodia. In addition, Ras effectors, such as TORC2 (target of rapamycin complex 2), regulate the actin cytoskeleton and myosin assembly independently of PI3K and PtdIns(3,4,5)P3 (Lee et al., 2005). TORC2 functions, in part, by phosphorylating Akt in the C-terminal hydrophobic domain (Bhaskar and Hay, 2007). In Dictyostelium, Akt is thus regulated by two Ras-mediated pathways, PI3K and TORC2 (Lee et al., 2005).
Although the activation of the Ras-PI3K circuit is apparently stochastic in vegetative Dictyostelium cells and promotes random motility in the absence of an extracellular stimulus, chemotactic signaling probably induces a biased localized activation of the Ras-PI3K circuit, thereby restricting it to the leading edge of migrating cells and allowing them to move directionally. This hypothesis is supported by analyses of the behavior of Dictyostelium cells, fibroblasts and neutrophils migrating up shallow chemoattractant gradients (Andrew and Insall, 2007; Varnum-Finney et al., 1987). Under these conditions, pseudopodia are produced fairly randomly and independently of the chemotactic signaling, and directional sensing leads to the maintenance of the best-aligned existing pseudopod rather than the production of a new one (Andrew and Insall, 2007; Varnum-Finney et al., 1987). Although pharmacological inhibition of PI3K leads to aberrant migration, it does not appear to affect the accuracy of chemotaxis, causing only a reduction in the frequency of pseudopod formation.
The suggestion that PtdIns(3,4,5)P3 signaling affects the rate, but not the accuracy, of chemotaxis fits with the findings of Wessels et al., who suggest that PTEN is not involved in gradient sensing but rather in the suppression of lateral pseudopodia (Wessels et al., 2007). Their high-resolution computer-assisted study of the behavior of Dictyostelium cells revealed that pten− cells detect a chemoattractant gradient quite well, but that the efficiency of chemotaxis is poor because they produce multiple lateral pseudopodia that take them off track. Even in the absence of chemoattractants, the developed, aggregation-competent pten− cells exhibit reduced velocity and persistence of movement, because of their failure to repress lateral pseudopodia, compared with wild-type cells, which produce only one large pseudopod at a time (Wessels et al., 2007). Elevated levels of PtdIns(3,4,5)P3 that is randomly distributed along the plasma membrane in pten− cells potentially drives the formation of new pseudopodia, many of which are randomly aligned relative to the gradient of chemoattractant – as occurs in Dictyostelium cells and leukocytes expressing a lipid-tagged PI3K that is uniformly localized along the plasma membrane (Funamoto et al., 2002; Lee et al., 2005; Lee et al., 1999; Sasaki et al., 2004). However, Wessels et al. found that pten− cells are defective in myosin II assembly at the cell cortex in response to chemoattractant, providing insight into the possible mechanism underlying the lack of repression of lateral pseudopodia in these cells (Wessels et al., 2007). Indeed, myosin II normally localizes to the sides and rear of chemotactic neutrophils and Dictyostelium cells, where it prevents the formation of lateral pseudopodia and promotes cell body contraction and posterior retraction (Heid et al., 2005; Heid et al., 2004; Stites et al., 1998; Uchida et al., 2003; Wessels et al., 1988; Xu et al., 2003). Given its sequence similarity to the actin-binding protein tensin, Wessels et al. further suggest that PTEN could directly interact with and modulate the F-actin–myosin cytoskeleton, implying that PTEN could play a role in cytoskeleton regulation that is independent of its PtdIns(3,4,5)P3 phosphatase activity.
An increasingly well-characterized aspect of the regulation of the actin cytoskeleton by PtdIns(3,4,5)P3 in Dictyostelium, neutrophils and fibroblasts is Rac signaling. Different Rac guanine nucleotide exchange factors (GEFs), as well as Rac effectors such as SCAR (WAVE) and WASP proteins, bind to and are regulated by PtdIns(3,4,5)P3, leading to localized polymerization of F-actin (Fig. 1) (Charest and Firtel, 2007). Moreover, a recent study shows that ArhGAP15, a PH-domain-containing Rac-GTPase-activating protein (GAP), binds to and is activated by PtdIns(3,4,5)P3 in migrating macrophages, suggesting that PtdIns(3,4,5)P3 also regulates the GAP-promoted inactivation of Rac during chemotaxis (Costa et al., 2007). Studies in both Dictyostelium and neutrophils have uncovered a positive-feedback loop between PI3K and Rac via F-actin (Fig. 1) that amplifies the signal and, in part via modulation of IQGAP as well as SCAR and WASP proteins, leads to massive F-actin polymerization at the leading edge of chemotaxing cells and the production of pseudopodia (Charest and Firtel, 2006). Evidence now points to the presence of a similar positive-feedback loop between Rac and PI3K in integrin-mediated fibroblast migration, which is implicated in the cytoskeletal rearrangements that lead to efficient formation of focal complexes, cell spreading and polarization (Smerling et al., 2007). A Rac-PI3K feedback loop might thus be part of a general signal-amplifying mechanism used by cells to produce extensive and localized polymerization of F-actin. Such a mechanism could be parallel to or intertwined with the Ras-PI3K feedback loop. Interestingly, the adhesion-related receptor kinase Axl induces neuronal migration via a pathway involving PI3K-mediated Ras-dependent activation of Rac (Nielsen-Preiss et al., 2007). In this case, however, PI3K is proposed to act upstream of Ras in the stimulation of Rac activity, which the authors suggest occurs via the direct modulation of RacGEFs by Ras. Such a mechanism was previously suggested to occur in the Ras-dependent activation of the RacGEF Tiam1 in fibroblasts, in which Tiam1 interacts directly with GTP-Ras via its Ras-binding domain (Lambert et al., 2002).
PtdIns(3,4,5)P3 also provides membrane-binding sites for regulators and effectors of the small GTPase Arf6, which regulates actin remodeling in several types of motile cell (D'Souza-Schorey and Chavrier, 2006; Donaldson, 2003; Jackson et al., 2000). These effects of Arf6 on the actin cytoskeleton are partially mediated by its activation of phospholipase D (PLD) and phosphatidylinositol-4-phosphate 5-kinase (PIP5K) (Brown et al., 2001; Honda et al., 1999; Santy and Casanova, 2001), as well as by modulation of Rac signaling (Boshans et al., 2000; D'Souza-Schorey et al., 1997; Koo et al., 2007; Otsuki et al., 2001; Radhakrishna et al., 1999; Santy and Casanova, 2001; Santy et al., 2005). Cross-talk between Arf6 and Rac might involve the indirect regulation of the RacGEF DOCK180-Elmo (engulfment and cell motility protein) complex in MDCK cells by the ArfGEF ARNO and Arf6 (Santy et al., 2005). Arf6 also interacts directly with the RacGEF Kalirin, facilitating the activation of Rac in COS cells (Koo et al., 2007).
The other well-known downstream effector of PI3K signaling is Akt, which directly binds to and is positively regulated by PtdIns(3,4,5)P3, in addition to being regulated by the target of rapamycin complex 2 (TORC2) (Cantley, 2002; Manning and Cantley, 2007; Bhaskar and Hay, 2007). Although Akt is mostly known for its role in cell growth and survival, increasing evidence suggests that it is involved in cell migration (Sasaki and Firtel, 2006). Earlier studies performed in Dictyostelium suggested that Akt regulates myosin II assembly by activating PAKa (p21-activated kinase-a) (Fig. 1) (Chung and Firtel, 1999; Chung et al., 2001). More recently, mammalian Akt2 (PKBβ) was found to phosphorylate myosin 5a in response to insulin stimulation; this phosphorylation enhances the interaction of myosin 5a with the actin cytoskeleton and leads to increased glucose transport (Yoshizaki et al., 2007). Akt is also proposed to regulate actin dynamics via its direct binding to and phosphorylation of actin (Cenni et al., 2003; Vandermoere et al., 2007) or via phosphorylation of the actin-binding protein Girdin (Akt phosphorylation enhancer; APE). APE localizes to the leading edge and is essential for the integrity of the actin cytoskeleton in migrating fibroblasts (Anai et al., 2005; Enomoto et al., 2005). Interestingly, Akt has also been suggested to promote microtubule stabilization in these cells (Onishi et al., 2007).
Chemotaxis pathways that work in parallel with PtdIns(3,4,5)P3 signaling
The recent experimental observations described in the previous two sections support a model in which the PI3K-PTEN pathway is important for regulating the actin cytoskeleton in Dictyostelium chemotaxis and in mammalian cells, but it is not the only pathway regulating chemotaxis. In support of this, other parallel pathways are now thought to contribute to chemotactic movement and gradient sensing.
Phospholipase A2 and phospholipase C in Dictyostelium
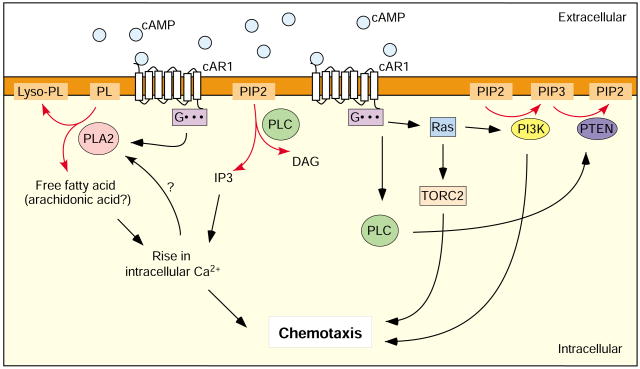
The chemoattractant cAMP stimulates several second-messenger systems in Dictyostelium, including adenylyl cyclase, guanylyl cyclase, the uptake of Ca2+ and its release from internal stores, phospholipase C (PLC), PI3K and phospholipase A2 (PLA2; encoded by plaA). Two groups have independently demonstrated that PLA2 regulates chemotaxis in parallel with PI3K (Fig. 2) (Chen et al., 2007; van Haastert et al., 2007). Further analysis of the two pathways revealed that inhibition of either pathway in shallow gradients inhibits chemotaxis, whereas, in steep gradients, both pathways must be inhibited to prevent proper chemotaxis.
Fig. 2.
PLA2 and PI3K/PTEN regulate chemotaxis in Dictyostelium. In Dictyostelium, chemotaxis is regulated by at least two intertwined and partly redundant pathways involving PI3K and PLA2. Both pathways are regulated by extracellular cAMP. The PI3K pathway is regulated, via PtdIns(4,5)P2 (PIP2)/PTEN, by PLC. The PLA2 pathway depends on cytosolic Ca2+, which is regulated by IP3 (thus partly by PLC), fatty acids and Ca2+ uptake. In steep gradients, either pathway is dispensable; in shallow gradients, both pathways are necessary to allow efficient chemotaxis (see text for details). Red arrows indicate enzymatic reactions. PL, phospholipids; Lyso-PL, lyso-phospholipids; Gαβγ, heterotrimeric G protein; cAR1, cAMP receptor; PIP3, PtdIns(3,4,5)P3.
Previous studies in Dictyostelium have suggested that products of PLA2 action, such as arachidonic acid, affect chemoattractant-induced Ca2+ influx and can trigger Ca2+ influx directly (Schaloske and Malchow, 1997). In mammalian cells, arachidonic acid is involved in the release of Ca2+ from internal stores by regulating calcium channels (Osterhout and Shuttleworth, 2000; Shuttleworth and Thompson, 1999). Cells lacking PLA2 display a decrease in the levels of 3H-arachidonic acid, or a closely related derivative, after stimulation (Chen et al., 2007). Although disrupting PLA2 has no effect on Ca2+ uptake (Chen et al., 2007), simultaneous inhibition of Ca2+ uptake and Ca2+ release does make cells sensitive to PI3K inhibitors (van Haastert et al., 2007). These results suggest that the PLA2-dependent pathway involves a rise in intracellular Ca2+ that might be regulated by arachidonic acid or derivatives via the release of Ca2+ from internal stores. Cytosolic Ca2+ might also have a regulatory effect on PLA2 activation (Chen et al., 2007; van Haastert et al., 2007).
Inhibition of both PLA2 and PLC almost completely inhibits the cAMP-mediated PtdIns(3,4,5)P3 response and causes drastic chemotactic defects (van Haastert et al., 2007), although inhibition of PLC alone does not affect chemotaxis (Drayer et al., 1994). This result implicates PLC in the regulation of the PI3K-mediated chemotaxis pathway – it probably acts by regulating phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] levels and PTEN (Fig. 2). In addition, PLC signaling might cross-talk with the PLA2 pathway at the level of intracellular Ca2+ regulation. PLC controls intracellular Ca2+ levels by generating inositol phosphates [inositol (1,4,5)-trisphosphate; Ins(1,4,5)P3] and diacylglycerol (DAG), which activates Ca2+-sensitive enzymes such as protein kinase C (PKC) (Drin and Scarlata, 2007).
The exact role of PLA2 and its derivatives in chemotaxis and the degree to which the pathways have overlapping functions or influence different aspects of chemotaxis remain to be determined. The fact that cells are still able to sense gradients and move with a high directionality in the absence of PI3K or PLA2 confirms that the machinery responsible for directional sensing must act downstream of G-protein activation by chemoattractants but upstream of the PI3K-PTEN and PLA2 pathways. Components of this direction-sensing machinery could include Ras proteins, which help to control chemotaxis in Dictyostelium, in part via the regulation of PI3K and TORC2 (Funamoto et al., 2002; Lee et al., 2005; Lee et al., 1999; Sasaki et al., 2004). Interestingly, the expression of a dominant-negative RasG in Dictyostelium cells lacking the RasGEF Aimless (AleA) severely impairs directional sensing (Sasaki et al., 2004).
The regulation of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 levels by PLC is also involved in the response to chemorepellents in Dictyostelium (Keizer-Gunnink et al., 2007). cAMP analogs, such as 8-para-chlorphenylthio-cAMP (8CPT-cAMP), can induce a repellent response in Dictyostelium by binding to the cAMP receptor cAR1 (Johnson et al., 1992). This induces a localized inhibition of PLC, which is normally activated by cAMP. Inhibition of PLC is proposed to cause the local accumulation of PtdIns(4,5)P2, PTEN binding and PtdIns(3,4,5)P3 degradation at the front of the cell. This leads to dominant PtdIns(3,4,5)P3 signaling at the rear of the cell, resulting in a movement away from the repellent source. PLC therefore can act as a polarity switch, controlling the response to signals in the environment.
PLC and PLD in mammalian chemotaxis
PtdIns(4,5)P2 has a pivotal role in both the PLC and PLD cellular signaling pathways. It serves as the major substrate for PLC proteins and simultaneously influences the subcellular localization and activity of PLD proteins. Studies on mouse neutrophils lacking PLC-β2 and PLC-β3 isoforms have indicated that the PLC pathways play an important role in chemoattractant-mediated production of superoxide, and in the regulation of protein kinases and chemokine-induced Ca2+ signaling, but not in chemotaxis (Li et al., 2000; McNeill et al., 2007). Nevertheless, treatment of human neutrophils with PLC inhibitors blocks chemotactic responses to interleukin 8 (IL8) and leukotriene B4 (LTB4) (Hou et al., 2004). Thus, the function of PLC in neutrophil chemotaxis is not fully understood and might vary according to the chemoattractant. By contrast, PLC-β is clearly necessary for T-cell chemotaxis. It acts by transiently raising cytoplasmic Ca2+ concentrations via inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3, IP3] but does not influence PKC activity (Bach et al., 2007; Smit et al., 2003). The identities of the Ca2+-dependent downstream components (such as calmodulin kinases, myosin light-chain kinase or Rho kinase) that mediate the chemotactic response in T-cells remain elusive.
The PH domain of PLC is thought to target it to particular lipids and membrane surfaces (Rebecchi and Scarlata, 1998). There is evidence that, in differentiating promyelocytes, PLC-β2 interacts with actin via the PH domain of PLC-β2 (Brugnoli et al., 2007). This promotes the association with cytoskeleton-associated PtdIns(4,5)P2 in the plasma membrane, resulting in hydrolysis of PtdIns(4,5)P2 and consequent cytoskeletal rearrangements and motility.
PLD hydrolyzes the phosphodiester bond in phosphatidylcholine (PC), resulting in the production of choline and the second messenger phosphatidic acid (PA) (Oude Weernink et al., 2007). The function of PLD during neutrophil chemotaxis has not been fully determined. Azuma et al. (Azuma et al., 2007) propose that PLD is required for activation of p38MAPK upon stimulation with fMLP under conditions that stimulate superoxide production, but not chemotaxis. By contrast, Powner et al. (Powner et al., 2007) have recently demonstrated that PLD regulates integrins that support stable adhesion during neutrophil migration. In that study, PA produced by PLD activity stimulated the generation of PtdIns(4,5)P2 by stimulating phosphatidylinositol 4-phosphate 5-kinase activity in response to fMLP PtdIns(4,5)P2 promoted the binding of talin to the surface-expressed β2-integrin CD18 and hence caused activation of the integrins CD11b/CD18, required for stable adhesion and migration. The study also pointed to the involvement of PLD in the distribution and function of actin stress fibers. In Dictyostelium, inhibition of PLD causes a dramatic decrease in PtdIns(4,5)P2 synthesis, resulting in severe defects in actin-based motility (Zouwail et al., 2005). In mast cells, human PLD binds to actin, which is important for the regulation of PLD1b activity (Farquhar et al., 2007). Evidence thus supports a link between phosphatidylcholine hydrolysis and remodeling of the actin cytoskeleton in a variety of processes and cell types.
In neutrophils, PI3Kγ becomes localized to the plasma membrane in response to stimulation by fMLP, increasing the formation of PtdIns(3,4,5)P3 and thereby the recruitment of other factors that activate PLD (e.g. Rho and Arf-GTPases, as well as PKC isoforms) (Chen and Exton, 2004; Henage et al., 2006). The activity of PLD can be inhibited by prostaglandin E2 (PGE2), which stimulates protein kinase A (PKA). This in turn inhibits the translocation of the PLD-activating factors, possibly by inhibiting PI3K (Burelout et al., 2007; Burelout et al., 2004). However, it is unclear how PI3K might be inhibited, because the PI3K regulatory and catalytic subunits are not targets for PKA phosphorylation in vitro and are unlikely targets in vivo. PI3K activity is regulated by the binding of the subunits p101/p110γ to the Gβγ subunits released upon receptor activation (Brock et al., 2003; Stephens et al., 1997), but Gβγ-subunit protein sequences do not contain consensus sites for PKA phosphorylation, and phosphorylation by PKA has not been demonstrated. The fMLP receptor is also not phosphorylated by PKA (Burelout et al., 2007). Therefore, the mechanism that underlies the inhibitory effect of PKA on PI3K requires further analysis.
PLC and cofilin in adenocarcinoma cells
In adenocarcinoma cells, epidermal growth factor (EGF) stimulation induces two peaks of actin polymerization, which is similar to the biphasic F-actin-polymerization response to cAMP that is seen in Dictyostelium (Chan et al., 2000; Chan et al., 1998; Chen et al., 2003). The second peak is dependent on PI3K activity, both in carcinoma cells and in Dictyostelium (Chen et al., 2003; Hill et al., 2000). In carcinoma cells, the first peak was recently demonstrated to depend on PLC-γ and cofilin (Mouneimne et al., 2004). These results, and those from other studies, strongly argue that PLC, together with cofilin, mediates gradient sensing in these cells (Ghosh et al., 2004; Mouneimne et al., 2006). By contrast, in Dictyostelium PLC regulates PIP2 levels and therefore cell motility, but not directional sensing. In Dictyostelium, cofilin is involved in actin remodeling and localizes to the leading edge during chemotaxis (Aizawa et al., 1995; Aizawa et al., 1997) but there is no evidence that it regulates gradient sensing and, in contrast to mammalian cofilin, Dictyostelium cofilin lacks the regulatory Ser at position 3. Interestingly, in cells lacking both PLA2 and PI3K activities (plaA−/pi3k1−/2− cells), the first peak of actin polymerization is significantly decreased, although both plaA− cells and pi3k1−/2− cells have nearly normal first peaks of actin polymerization, indicating that both pathways might be involved in the initial actin polymerization (Chen et al., 2007).
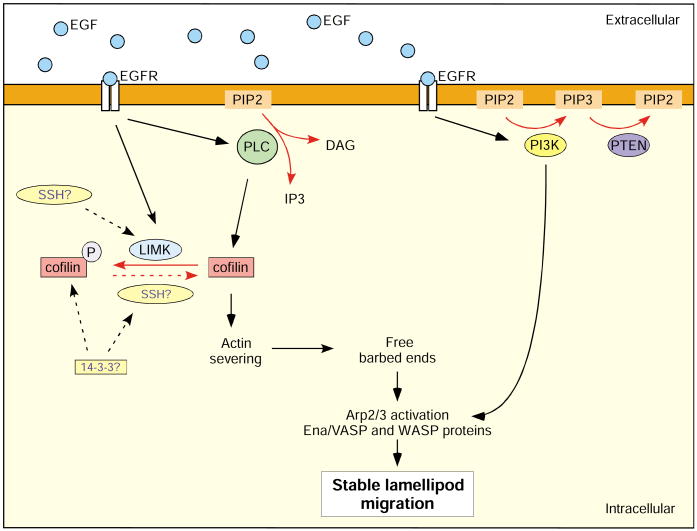
In carcinoma cells, cofilin is activated via PLC-γ, which acts by locally decreasing PtdIns(4,5)P2. Cofilin is essential for the localized formation of barbed ends, which act as sites for new local actin polymerization; cofilin thus determines the direction of cell protrusion and movement (Condeelis, 2001; DesMarais et al., 2005; Ghosh et al., 2004) (Fig. 3). Cofilin activity seems to be mainly dependent on PLC-γ-mediated PtdIns(4,5)P2 hydrolysis and does not involve an IP3-mediated Ca2+ release (Ma et al., 2000; Mouneimne et al., 2006; Yonezawa et al., 1991). In addition to activation by PLC-γ, mammalian cofilin is also phosphorylated at Ser3 by LIM kinase (LIMK), which inhibits the ability of cofilin to bind actin (Zebda et al., 2000), and is dephosphorylated by the phosphatase Slingshot (SSH) (Nishita et al., 2005; Niwa et al., 2002; Ohta et al., 2003). Phosphorylation of cofilin increases upon EGF stimulation (Mouneimne et al., 2004; Song et al., 2006) and is required for chemotactic sensing, although the exact function is not fully understood (Mouneimne et al., 2006). In Jurkat T-cells, cofilin is thought to be inactivated by phosphorylation by LIMK after stimulation with stromal cell-derived factor-1α (SDF-1α). This results in the formation of F-actin-rich lamellipodial protrusions (Nishita et al., 2005). Via the association with F-actin, the phosphatase Slingshot-1L becomes locally activated in the protrusions and dephosphorylates, and thereby re-activates, cofilin in the lamellipodium. This allows actin-filament turnover and ensures the dynamic nature of the lamellipodium (Nishita et al., 2005).
Fig. 3.
PLC and cofilin are the gradient-sensing machinery in adenocarcinoma cells. In response to EGF stimulation, PLC becomes activated. By hydrolyzing PtdIns(4,5)P2 (PIP2), it activates cofilin. By severing actin filaments, cofilin increases the number of free barbed ends, producing the platform for the Arp2/3 complex and Ena/VASP proteins. This allows initial protrusion and determines the direction of movement. Activation of PI3K via EGF and its signaling to Arp2/3 promotes the formation of a stable lamellipod and efficient migration (see text for details). The phosphatase SSH and 14-3-3 proteins might be involved in regulating the phosphorylation state of cofilin. Red arrows indicate enzymatic reactions. EGFR, epidermal growth factor receptor; PIP3, PtdIns(3,4,5)P3; SSH, Slingshot.
Recent studies of carcinoma cells indicate that the initial activation of cofilin does not involve dephosphorylation in response to chemoattractant stimulation (Mouneimne et al., 2004; Song et al., 2006). Cofilin is instead thought to be locally released and activated by hydrolysis of PtdIns(4,5)P2 by PLC-γ and, simultaneously, be globally inactivated via phosphorylation by LIMK (Hitchcock-DeGregori, 2006; Mouneimne et al., 2006). This leads to an asymmetric distribution of cofilin activity, setting the direction of lamellipodium formation and subsequent migration. This model is consistent with earlier findings that cofilin is recruited to the leading edge immediately before lamellipod extension and is followed by the Arp2/3 complex and the extension of the lamellipod (DesMarais et al., 2004). However, it is not fully understood whether or not cofilin is completely deactivated by LIMK and whether the phosphatase SSH or 14-3-3 proteins might be involved in this process (Soosairajah et al., 2005).
Whereas the inhibition of PLC-γ/cofilin leads to defects in gradient sensing, inhibition of PI3K or PTEN decreases motility and speed in carcinoma cells, as it does in Dictyostelium (Mouneimne et al., 2006; Mouneimne et al., 2004). Full lamellipod extension requires PI3K activity, because the second peak of actin polymerization is dependent on PI3K (Hill et al., 2000). PI3K has been postulated to signal to WAVE and the Arp2/3 complex, which is necessary for lamellipod protrusion (Bailly et al., 2001; Higgs and Pollard, 2001; Takenawa and Miki, 2001). The PLC-γ/cofilin and PI3K/Arp2/3 signaling pathways thus cooperate in chemotactic gradient sensing and efficient lamellipod generation in response to EGF stimulation. By hydrolyzing PtdIns(4,5)P2, PLC-γ activates cofilin, which promotes F-actin severing. This creates free barbed ends, defining the site for Arp2/3 activation. The Arp2/3 complex nucleates new filaments, which become elongated by Ena/VASP proteins, creating a branched actin network that allows stable lamellipod protrusion and migration.
Conclusions
The recent advances outlined here emphasize the crucial role of phospholipid signaling and lipid metabolism in the control of chemotaxis and cell motility. This involves more than the PI3K/PtdIns(3,4,5)P3-dependent pathway, although PI3K signaling clearly plays a central role in the regulation of cytoskeleton dynamics. The findings that distinct pathways act in parallel to control chemotaxis in different cells, and that the nature of some of these pathways varies with the type of cell and signal, highlight previously unappreciated levels of complexity in this important cellular behavior. The challenge of deciphering the distinct events that take part in directional sensing versus cell motility consequently appears greater than previously appreciated, because the multiple parallel pathways are probably interlinked and could seem redundant while serving slightly different and complementary purposes. Hence, future research should aim at understanding the relationship between the different signaling pathways that underlie chemotaxis and cell motility, focusing particularly on the phospholipid-dependent pathways but without excluding the possibility that phospholipid-independent pathways play crucial roles as well.
Acknowledgments
V.K. was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft. P.G.C. was supported by a postdoctoral fellowship from the Fonds de la Recherche en Santé du Québec. This work was supported, in part, by USPHS grants to R.A.F.
References
- Aizawa H, Sutoh K, Tsubuki S, Kawashima S, Ishii A, Yahara I. Identification, characterization, and intracellular distribution of cofilin in Dictyostelium discoideum. J Biol Chem. 1995;270:10923–10932. doi: 10.1074/jbc.270.18.10923. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Fukui Y, Yahara I. Live dynamics of Dictyostelium cofilin suggests a role in remodeling actin latticework into bundles. J Cell Sci. 1997;110:2333–2344. doi: 10.1242/jcs.110.19.2333. [DOI] [PubMed] [Google Scholar]
- Anai M, Shojima N, Katagiri H, Ogihara T, Sakoda H, Onishi Y, Ono H, Fujishiro M, Fukushima Y, Horike N, et al. A novel protein kinase B (PKB)/Akt-binding protein enhances PKB kinase activity and regulates DNA synthesis. J Biol Chem. 2005;280:18525–18535. doi: 10.1074/jbc.M500586200. [DOI] [PubMed] [Google Scholar]
- Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 2007;9:193–200. doi: 10.1038/ncb1536. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Kosaka K, Kashimata M. Phospholipase D-dependent and -independent p38MAPK activation pathways are required for superoxide production and chemotactic induction, respectively, in rat neutrophils stimulated by fMLP. Eur J Pharmacol. 2007;568:260–268. doi: 10.1016/j.ejphar.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Bach TL, Chen QM, Kerr WT, Wang Y, Lian L, Choi JK, Wu D, Kazanietz MG, Koretzky GA, Zigmond S, et al. Phospholipase Cbeta is critical for T cell chemotaxis. J Immunol. 2007;179:2223–2227. doi: 10.4049/jimmunol.179.4.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly M, Ichetovkin I, Grant W, Zebda N, Machesky LM, Segall JE, Condeelis J. The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr Biol. 2001;11:620–625. doi: 10.1016/s0960-9822(01)00152-x. [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Boshans RL, Szanto S, van Aelst L, D'Souza-Schorey C. ADP-ribosylation factor 6 regulates actin cytoskeleton remodeling in coordination with Rac1 and RhoA. Mol Cell Biol. 2000;20:3685–3694. doi: 10.1128/mcb.20.10.3685-3694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, Schultz G, Nurnberg B. Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma. J Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnoli F, Bavelloni A, Benedusi M, Capitani S, Bertagnolo V. PLC-[beta]2 activity on actin-associated polyphosphoinositides promotes migration of differentiating tumoral myeloid precursors. Cell Signal. 2007;19:1701–1712. doi: 10.1016/j.cellsig.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Burelout C, Thibault N, Levasseur S, Simard S, Naccache PH, Bourgoin SG. Prostaglandin E2 inhibits the phospholipase D pathway stimulated by formyl-methionyl-leucyl-phenylalanine in human neutrophils. Involvement of EP2 receptors and phosphatidylinositol 3-kinase gamma. Mol Pharmacol. 2004;66:293–301. doi: 10.1124/mol.66.2.293. [DOI] [PubMed] [Google Scholar]
- Burelout C, Thibault N, Harbour D, Naccache PH, Bourgoin SG. The PGE2-induced inhibition of the PLD activation pathway stimulated by fMLP in human neutrophils is mediated by PKA at the PI3-K[gamma] level. Biochem Pharmacol. 2007;74:730–741. doi: 10.1016/j.bcp.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Cenni V, Sirri A, Riccio M, Lattanzi G, Santi S, de Pol A, Maraldi NM, Marmiroli S. Targeting of the Akt/PKB kinase to the actin skeleton. Cell Mol Life Sci. 2003;60:2710–2720. doi: 10.1007/s00018-003-3349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AY, Raft S, Bailly M, Wyckoff JB, Segall JE, Condeelis JS. EGF stimulates an increase in actin nucleation and filament number at the leading edge of the lamellipod in mammary adenocarcinoma cells. J Cell Sci. 1998;111:199–211. doi: 10.1242/jcs.111.2.199. [DOI] [PubMed] [Google Scholar]
- Chan AY, Bailly M, Zebda N, Segall JE, Condeelis JS. Role of cofilin in epidermal growth factor-stimulated actin polymerization and lamellipod protrusion. J Cell Biol. 2000;148:531–542. doi: 10.1083/jcb.148.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16:339–347. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Charest PG, Firtel RA. Big roles for small GTPases in the control of directed cell movement. Biochem J. 2007;401:377–390. doi: 10.1042/BJ20061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JS, Exton JH. Regulation of phospholipase D2 activity by protein kinase C alpha. J Biol Chem. 2004;279:22076–22083. doi: 10.1074/jbc.M311033200. [DOI] [PubMed] [Google Scholar]
- Chen L, Janetopoulos C, Huang YE, Iijima M, Borleis J, Devreotes PN. Two phases of actin polymerization display different dependencies on PI(3,4,5)P3 accumulation and have unique roles during chemotaxis. Mol Biol Cell. 2003;14:5028–5037. doi: 10.1091/mbc.E03-05-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Iijima M, Tang M, Landree MA, Huang YE, Xiong Y, Iglesias PA, Devreotes PN. PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev Cell. 2007;12:603–614. doi: 10.1016/j.devcel.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Firtel RA. PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis. J Cell Biol. 1999;147:559–576. doi: 10.1083/jcb.147.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Potikyan G, Firtel RA. Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol Cell. 2001;7:937–947. doi: 10.1016/s1097-2765(01)00247-7. [DOI] [PubMed] [Google Scholar]
- Condeelis J. How is actin polymerization nucleated in vivo? Trends Cell Biol. 2001;11:288–293. doi: 10.1016/s0962-8924(01)02008-6. [DOI] [PubMed] [Google Scholar]
- Costa C, Barberis L, Ambrogio C, Manazza AD, Patrucco E, Azzolino O, Neilsen PO, Ciraolo E, Altruda F, Prestwich GD, et al. Negative feedback regulation of Rac in leukocytes from mice expressing a constitutively active phosphatidylinositol 3-kinase {gamma} Proc Natl Acad Sci USA. 2007;104:14354–14359. doi: 10.1073/pnas.0703175104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Boshans RL, McDonough M, Stahl PD, Van Aelst L. A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J. 1997;16:5445–5454. doi: 10.1093/emboj/16.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesMarais V, Macaluso F, Condeelis J, Bailly M. Synergistic interaction between the Arp2/3 complex and cofilin drives stimulated lamellipod extension. J Cell Sci. 2004;117:3499–3510. doi: 10.1242/jcs.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesMarais V, Ghosh M, Eddy R, Condeelis J. Cofilin takes the lead. J Cell Sci. 2005;118:19–26. doi: 10.1242/jcs.01631. [DOI] [PubMed] [Google Scholar]
- Donaldson JG. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J Biol Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- Drayer AL, Van der Kaay J, Mayr GW, Van Haastert PJ. Role of phospholipase C in Dictyostelium: formation of inositol 1,4,5-trisphosphate and normal development in cells lacking phospholipase C activity. EMBO J. 1994;13:1601–1609. doi: 10.1002/j.1460-2075.1994.tb06423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Scarlata S. Stimulation of phospholipase C[beta] by membrane interactions, interdomain movement, and G protein binding - How many ways can you activate an enzyme? Cell Signal. 2007;19:1383–1392. doi: 10.1016/j.cellsig.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles SA. Parallels in invasion and angiogenesis provide pivotal points for therapeutic intervention. Int J Dev Biol. 2004;48:583–598. doi: 10.1387/ijdb.041820se. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K, Takahashi M. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Farquhar MJ, Powner DJ, Levine BA, Wright MH, Ladds G, Hodgkin MN. Interaction of PLD1b with actin in antigen-stimulated mast cells. Cell Signal. 2007;19:349–358. doi: 10.1016/j.cellsig.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, Crabbe T, Finan P, Jones G, Jackson S, et al. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007;9:86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- Funamoto S, Milan K, Meili R, Firtel RA. Role of phosphatidylinositol 3′ kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in Dictyostelium. J Cell Biol. 2001;153:795–810. doi: 10.1083/jcb.153.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- Heid PJ, Wessels D, Daniels KJ, Gibson DP, Zhang H, Voss E, Soll DR. The role of myosin heavy chain phosphorylation in Dictyostelium motility, chemotaxis and F-actin localization. J Cell Sci. 2004;117:4819–4835. doi: 10.1242/jcs.01358. [DOI] [PubMed] [Google Scholar]
- Heid PJ, Geiger J, Wessels D, Voss E, Soll DR. Computer-assisted analysis of filopod formation and the role of myosin II heavy chain phosphorylation in Dictyostelium. J Cell Sci. 2005;118:2225–2237. doi: 10.1242/jcs.02342. [DOI] [PubMed] [Google Scholar]
- Henage LG, Exton JH, Brown HA. Kinetic analysis of a mammalian phospholipase D: allosteric modulation by monomeric GTPases, protein kinase C, and polyphosphoinositides. J Biol Chem. 2006;281:3408–3417. doi: 10.1074/jbc.M508800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs HN, Pollard TD. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu Rev Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- Hill K, Welti S, Yu J, Murray JT, Yip SC, Condeelis JS, Segall JE, Backer JM. Specific requirement for the p85-p110alpha phosphatidylinositol 3-kinase during epidermal growth factor-stimulated actin nucleation in breast cancer cells. J Biol Chem. 2000;275:3741–3744. doi: 10.1074/jbc.275.6.3741. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Hitchcock-DeGregori SE. Chemotaxis: cofilin in the driver's seat. Curr Biol. 2006;16:R1030–R1032. doi: 10.1016/j.cub.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Hoeller O, Kay RR. Chemotaxis in the absence of PIP3 gradients. Curr Biol. 2007;17:813–817. doi: 10.1016/j.cub.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, et al. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Hou C, Kirchner T, Singer M, Matheis M, Argentieri D, Cavender D. In vivo activity of a phospholipase C inhibitor, 1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione (U73122), in acute and chronic inflammatory reactions. J Pharmacol Exp Ther. 2004;309:697–704. doi: 10.1124/jpet.103.060574. [DOI] [PubMed] [Google Scholar]
- Huang YE, Iijima M, Parent CA, Funamoto S, Firtel RA, Devreotes P. Receptor-mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol Biol Cell. 2003;14:1913–1922. doi: 10.1091/mbc.E02-10-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Kearns BG, Theibert AB. Cytohesins and centaurins: mediators of PI 3-kinase-regulated Arf signaling. Trends Biochem Sci. 2000;25:489–495. doi: 10.1016/s0968-0004(00)01644-3. [DOI] [PubMed] [Google Scholar]
- Jeon TJ, Lee DJ, Merlot S, Weeks G, Firtel RA. Rap1 controls cell adhesion and cell motility through the regulation of myosin II. J Cell Biol. 2007;176:1021–1033. doi: 10.1083/jcb.200607072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Van Haastert PJ, Kimmel AR, Saxe CL, 3rd, Jastorff B, Devreotes PN. The cyclic nucleotide specificity of three cAMP receptors in Dictyostelium. J Biol Chem. 1992;267:4600–4607. [PubMed] [Google Scholar]
- Keizer-Gunnink I, Kortholt A, Van Haastert PJM. Chemoattractants and chemorepellents act by inducing opposite polarity in phospholipase C and PI3-kinase signaling. J Cell Biol. 2007;177:579–585. doi: 10.1083/jcb.200611046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo TH, Eipper BA, Donaldson JG. Arf6 recruits the Rac GEF Kalirin to the plasma membrane facilitating Rac activation. BMC Cell Biol. 2007;8:29. doi: 10.1186/1471-2121-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, Collard JG, Der CJ. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- Langridge PD, Kay RR. Mutants in the Dictyostelium Arp2/3 complex and chemoattractant-induced actin polymerization. Exp Cell Res. 2007;313:2563–2574. doi: 10.1016/j.yexcr.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Lee S, Parent CA, Insall R, Firtel RA. A novel Ras-interacting protein required for chemotaxis and cyclic adenosine monophosphate signal relay in Dictyostelium. Mol Biol Cell. 1999;10:2829–2845. doi: 10.1091/mbc.10.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Comer FI, Sasaki A, McLeod IX, Duong Y, Okumura K, Yates JR, 3rd, Parent CA, Firtel RA. TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Mol Biol Cell. 2005;16:4572–4583. doi: 10.1091/mbc.E05-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-2 and -3 and PI3K in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- Loovers HM, Postma M, Keizer-Gunnink I, Huang YE, Devreotes PN, van Haastert PJ. Distinct roles of PI(3,4,5)P3 during chemoattractant signaling in Dictyostelium: a quantitative in vivo analysis by inhibition of PI3-kinase. Mol Biol Cell. 2006;17:1503–1513. doi: 10.1091/mbc.E05-09-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HT, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Akt/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Matheu MP, Deane JA, Parker I, Fruman DA, Cahalan MD. Class IA phosphoinositide 3-kinase modulates basal lymphocyte motility in the lymph node. J Immunol. 2007;179:2261–2269. doi: 10.4049/jimmunol.179.4.2261. [DOI] [PubMed] [Google Scholar]
- McNeill E, Conway SJ, Roderick HL, Bootman MD, Hogg N. Defective chemoattractant-induced calcium signalling in S100A9 null neutrophils. Cell Calcium. 2007;41:107–121. doi: 10.1016/j.ceca.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Meili R, Ellsworth C, Firtel RA. A novel Akt/PKB-related kinase is essential for morphogenesis in Dictyostelium. Curr Biol. 2000;10:708–717. doi: 10.1016/s0960-9822(00)00536-4. [DOI] [PubMed] [Google Scholar]
- Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, Yip SC, Ghosh M, Eddy R, Backer JM, Condeelis J. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J Cell Biol. 2004;166:697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouneimne G, DesMarais V, Sidani M, Scemes E, Wang W, Song X, Eddy R, Condeelis J. Spatial and temporal control of cofilin activity is required for directional sensing during chemotaxis. Curr Biol. 2006;16:2193–2205. doi: 10.1016/j.cub.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Nielsen-Preiss SM, Allen MP, Xu M, Linseman DA, Pawlowski JE, Bouchard RJ, Varnum BC, Heidenreich KA, Wierman ME. Adhesion-related kinase induction of migration requires phosphatidylinositol-3-kinase and ras stimulation of rac activity in immortalized gonadotropin-releasing hormone neuronal cells. Endocrinology. 2007;148:2806–2814. doi: 10.1210/en.2007-0039. [DOI] [PubMed] [Google Scholar]
- Nishio M, Watanabe K, Sasaki J, Taya C, Takasuga S, Iizuka R, Balla T, Yamazaki M, Watanabe H, Itoh R, et al. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat Cell Biol. 2007;9:36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- Nishita M, Tomizawa C, Yamamoto M, Horita Y, Ohashi K, Mizuno K. Spatial and temporal regulation of cofilin activity by LIM kinase and Slingshot is critical for directional cell migration. J Cell Biol. 2005;171:349–359. doi: 10.1083/jcb.200504029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Kousaka K, Nagata-Ohashi K, Ohashi K, Muramoto A, Shima Y, Niwa R, Uemura T, Mizuno K. Differential activities, subcellular distribution and tissue expression patterns of three members of Slingshot family phosphatases that dephosphorylate cofilin. Genes Cells. 2003;8:811–824. doi: 10.1046/j.1365-2443.2003.00678.x. [DOI] [PubMed] [Google Scholar]
- Onishi K, Higuchi M, Asakura T, Masuyama N, Gotoh Y. The PI3K-Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells. 2007;12:535–546. doi: 10.1111/j.1365-2443.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- Orme MH, Alrubaie S, Bradley GL, Walker CD, Leevers SJ. Input from Ras is required for maximal PI(3)K signalling in Drosophila. Nat Cell Biol. 2006;8:1298–1302. doi: 10.1038/ncb1493. [DOI] [PubMed] [Google Scholar]
- Osterhout JL, Shuttleworth TJ. A Ca(2+)-independent activation of a type IV cytosolic phospholipase A(2) underlies the receptor stimulation of arachidonic acid-dependent noncapacitative calcium entry. J Biol Chem. 2000;275:8248–8254. doi: 10.1074/jbc.275.11.8248. [DOI] [PubMed] [Google Scholar]
- Otsuki Y, Tanaka M, Yoshii S, Kawazoe N, Nakaya K, Sugimura H. Tumor metastasis suppressor nm23H1 regulates Rac1 GTPase by interaction with Tiam1. Proc Natl Acad Sci USA. 2001;98:4385–4390. doi: 10.1073/pnas.071411598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Weernink PA, Han L, Jakobs KH, Schmidt M. Dynamic phospholipid signaling by G protein-coupled receptors. Biochim Biophys Acta Biomembr. 2007;1768:888–900. doi: 10.1016/j.bbamem.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Papakonstanti EA, Ridley AJ, Vanhaesebroeck B. The p110delta isoform of PI 3-kinase negatively controls RhoA and PTEN. EMBO J. 2007;26:3050–3061. doi: 10.1038/sj.emboj.7601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powner DJ, Pettitt TR, Anderson R, Nash GB, Wakelam MJ. Stable adhesion and migration of human neutrophils requires phospholipase D-mediated activation of the integrin CD11b/CD18. Mol Immunol. 2007;44:3211–3221. doi: 10.1016/j.molimm.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Scarlata S. Pleckstrin homology domains: a common fold with diverse functions. Annu Rev Biophys Biomol Struct. 1998;27:503–528. doi: 10.1146/annurev.biophys.27.1.503. [DOI] [PubMed] [Google Scholar]
- Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol. 2003;170:2647–2654. doi: 10.4049/jimmunol.170.5.2647. [DOI] [PubMed] [Google Scholar]
- Santy LC, Casanova JE. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J Cell Biol. 2001;154:599–610. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santy LC, Ravichandran KS, Casanova JE. The DOCK180/Elmo complex couples ARNO-mediated Arf6 activation to the downstream activation of Rac1. Curr Biol. 2005;15:1749–1754. doi: 10.1016/j.cub.2005.08.052. [DOI] [PubMed] [Google Scholar]
- Sasaki AT, Firtel RA. Regulation of chemotaxis by the orchestrated activation of Ras, PI3K, and TOR. Eur J Cell Biol. 2006;85:873–895. doi: 10.1016/j.ejcb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol. 2004;167:505–518. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki AT, Janetopoulos C, Lee S, Charest PG, Takeda K, Sundheimer LW, Meili R, Devreotes PN, Firtel RA. G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility. J Cell Biol. 2007;178:185–191. doi: 10.1083/jcb.200611138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Schaloske R, Malchow D. Mechanism of cAMP-induced Ca2+ influx in Dictyostelium: role of phospholipase A2. Biochem J. 1997;327:233–238. doi: 10.1042/bj3270233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL. Discriminating between capacitative and arachidonate-activated Ca(2+) entry pathways in HEK293 cells. J Biol Chem. 1999;274:31174–31178. doi: 10.1074/jbc.274.44.31174. [DOI] [PubMed] [Google Scholar]
- Smerling C, Tang K, Hofmann W, Danker K. Role of the alpha(1) integrin cytoplasmic tail in the formation of focal complexes, actin organization, and in the control of cell migration. Exp Cell Res. 2007;313:3153–3165. doi: 10.1016/j.yexcr.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Verdijk P, van der Raaij-Helmer EM, Navis M, Hensbergen PJ, Leurs R, Tensen CP. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood. 2003;102:1959–1965. doi: 10.1182/blood-2002-12-3945. [DOI] [PubMed] [Google Scholar]
- Soll DR, Wessels D, Heid PJ, Zhang H. A contextual framework for characterizing motility and chemotaxis mutants in Dictyostelium discoideum. J Muscle Res Cell Motil. 2002;23:659–672. doi: 10.1023/a:1024459124427. [DOI] [PubMed] [Google Scholar]
- Song X, Chen X, Yamaguchi H, Mouneimne G, Condeelis JS, Eddy RJ. Initiation of cofilin activity in response to EGF is uncoupled from cofilin phosphorylation and dephosphorylation in carcinoma cells. J Cell Sci. 2006;119:2871–2881. doi: 10.1242/jcs.03017. [DOI] [PubMed] [Google Scholar]
- Soosairajah J, Maiti S, Wiggan O, Sarmiere P, Moussi N, Sarcevic B, Sampath R, Bamburg JR, Bernard O. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J. 2005;24:473–486. doi: 10.1038/sj.emboj.7600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AS, Thelen M, Cadwallader K, Tempst P, et al. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Stites J, Wessels D, Uhl A, Egelhoff T, Shutt D, Soll DR. Phosphorylation of the Dictyostelium myosin II heavy chain is necessary for maintaining cellular polarity and suppressing turning during chemotaxis. Cell Motil Cytoskeleton. 1998;39:31–51. doi: 10.1002/(SICI)1097-0169(1998)39:1<31::AID-CM4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, You J, Mizgerd JP, Luo HR. Tumor suppressor PTEN is a physiological suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suire S, Condliffe AM, Ferguson GJ, Ellson CD, Guillou H, Davidson K, Welch H, Coadwell J, Turner M, Chilvers ER, et al. Gbetagammas and the Ras binding domain of p110gamma are both important regulators of PI(3)Kgamma signalling in neutrophils. Nat Cell Biol. 2006;8:1303–1309. doi: 10.1038/ncb1494. [DOI] [PubMed] [Google Scholar]
- Takeda K, Sasaki AT, Ha H, Seung HA, Firtel RA. Role of PI3 kinases in chemotaxis in Dictyostelium. J Biol Chem. 2007;282:11874–11884. doi: 10.1074/jbc.M610984200. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- Uchida KS, Kitanishi-Yumura T, Yumura S. Myosin II contributes to the posterior contraction and the anterior extension during the retraction phase in migrating Dictyostelium cells. J Cell Sci. 2003;116:51–60. doi: 10.1242/jcs.00195. [DOI] [PubMed] [Google Scholar]
- Vandermoere F, El Yazidi-Belkoura I, Demont Y, Slomianny C, Antol J, Lemoine J, Hondermarck H. Proteomics exploration reveals that actin is a signaling target of the kinase Akt. Mol Cell Proteomics. 2007;6:114–124. doi: 10.1074/mcp.M600335-MCP200. [DOI] [PubMed] [Google Scholar]
- Van Haastert PJM, Veltman DM. Chemotaxis: navigating by multiple signaling pathways. Sci STKE. 2007;2007:pe40. doi: 10.1126/stke.3962007pe40. [DOI] [PubMed] [Google Scholar]
- van Haastert PJM, Keizer-Gunnink I, Kortholt A. Essential role of PI3-kinase and phospholipase A2 in Dictyostelium discoideum chemotaxis. J Cell Biol. 2007;177:809–816. doi: 10.1083/jcb.200701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol. 2006;174:437–445. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum-Finney BJ, Voss E, Soll DR. Frequency and orientation of pseudopod formation of Dictyostelium discoideum amebae chemotaxing in a spatial gradient: further evidence for a temporal mechanism. Cell Motil Cytoskeleton. 1987;8:18–26. doi: 10.1002/cm.970080104. [DOI] [PubMed] [Google Scholar]
- Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4:513–518. doi: 10.1038/ncb810. [DOI] [PubMed] [Google Scholar]
- Wessels D, Soll DR, Knecht D, Loomis WF, De Lozanne A, Spudich J. Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Dev Biol. 1988;128:164–177. doi: 10.1016/0012-1606(88)90279-5. [DOI] [PubMed] [Google Scholar]
- Wessels D, Lusche DF, Kuhl S, Heid P, Soll DR. PTEN plays a role in the suppression of lateral pseudopod formation during Dictyostelium motility and chemotaxis. J Cell Sci. 2007;120:2517–2531. doi: 10.1242/jcs.010876. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- Yip SC, El-Sibai M, Coniglio SJ, Mouneimne G, Eddy RJ, Drees BE, Neilsen PO, Goswami S, Symons M, Condeelis JS, et al. The distinct roles of Ras and Rac in PI 3-kinase-dependent protrusion during EGF-stimulated cell migration. J Cell Sci. 2007;120:3138–3146. doi: 10.1242/jcs.005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa N, Homma Y, Yahara I, Sakai H, Nishida E. A short sequence responsible for both phosphoinositide binding and actin binding activities of cofilin. J Biol Chem. 1991;266:17218–17221. [PubMed] [Google Scholar]
- Yoshizaki T, Imamura T, Babendure JL, Lu JC, Sonoda N, Olefsky JM. Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bbeta) substrate modulating GLUT4 vesicle translocation. Mol Cell Biol. 2007;27:5172–5183. doi: 10.1128/MCB.02298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebda N, Bernard O, Bailly M, Welti S, Lawrence DS, Condeelis JS. Phosphorylation of ADF/cofilin abolishes EGF-induced actin nucleation at the leading edge and subsequent lamellipod extension. J Cell Biol. 2000;151:1119–1128. doi: 10.1083/jcb.151.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouwail S, Pettitt TR, Dove SK, Chibalina MV, Powner DJ, Haynes L, Wakelam MJ, Insall RH. Phospholipase D activity is essential for actin localization and actin-based motility in Dictyostelium. Biochem J. 2005;389:207–214. doi: 10.1042/BJ20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]