Abstract
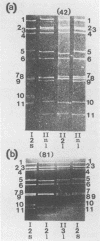
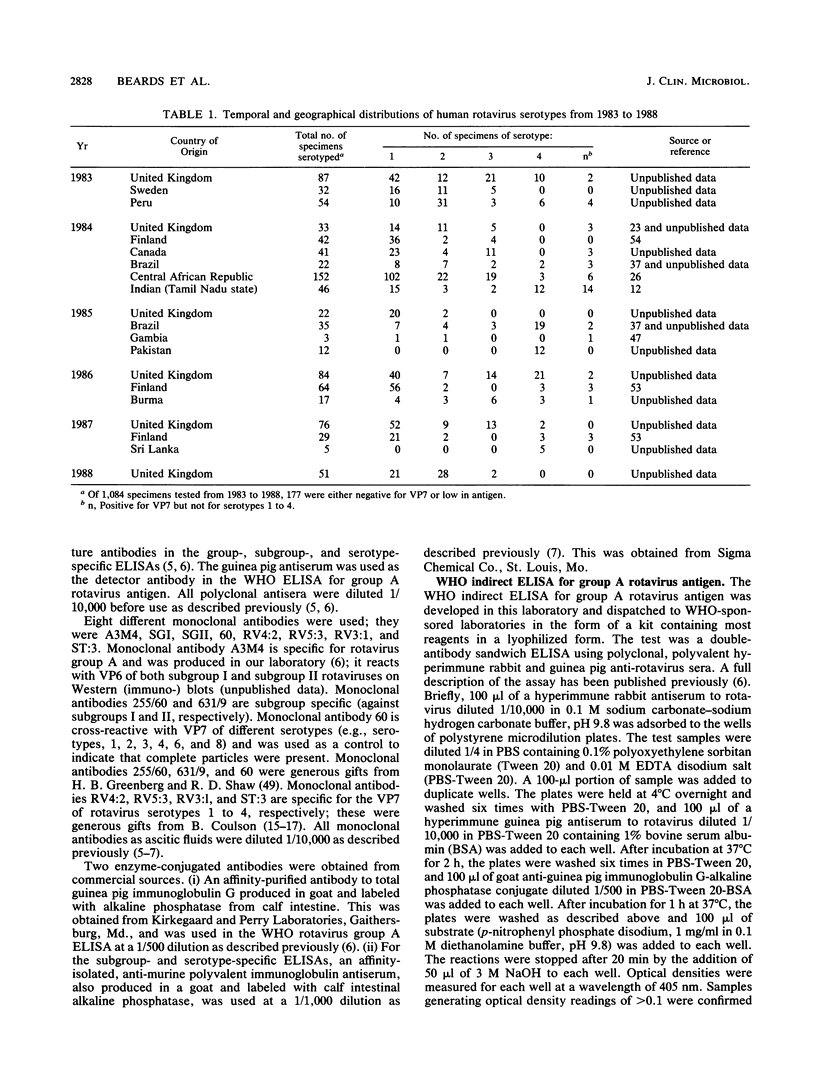
Between 1983 and 1988, subgroups and serotypes were determined for 907 of 1,084 clinical specimens of rotaviruses collected in various countries of Europe, North and South America, Africa, and Asia. Enhanced enzyme immunoassays based on monoclonal antibodies specific for rotavirus proteins VP6 and VP7 were used. Significant differences in the prevalent serotypes were detected from year to year in the United Kingdom and Brazil and also in different countries during the same year. Throughout the study, rotavirus serotype 1 was detected most often (53.8%), followed in frequency by serotype 2 (17.8%), serotype 3 (12.1%), serotype 4 (11.1%), and serotypes other than 1 to 4 (5.1%). No individual serotype was found to predominate consistently in any one location. In the United Kingdom, rotavirus serotypes varied in prevalence in a regular but not predictable way. We suggest that a similar epidemiology might be found in other settings. Seventeen unusual strains were detected. Of these, five strains did not react with reference monoclonal antibodies specific for subgroup I and subgroup II, but they reacted with rotavirus group A-specific polyclonal and monoclonal antibodies; four strains were of subgroup II, serotype 2, and at least one had a "long" electropherotype; two strains were of subgroup I, serotype 2 with a long electropherotype; and one strain was of subgroup I, serotype 3. Five group C rotaviruses were detected.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboudy Y., Shif I., Zilberstein I., Gotlieb-Stematsky T. Use of polyclonal and monoclonal antibodies and analysis of viral RNA in the detection of unusual group A human rotaviruses. J Med Virol. 1988 Jul;25(3):351–359. doi: 10.1002/jmv.1890250312. [DOI] [PubMed] [Google Scholar]
- Albert M. J., Unicomb L. E., Tzipori S. R., Bishop R. F. Isolation and serotyping of animal rotaviruses and antigenic comparison with human rotaviruses. Brief report. Arch Virol. 1987;93(1-2):123–130. doi: 10.1007/BF01313898. [DOI] [PubMed] [Google Scholar]
- Anderson E. L., Belshe R. B., Bartram J., Crookshanks-Newman F., Chanock R. M., Kapikian A. Z. Evaluation of rhesus rotavirus vaccine (MMU 18006) in infants and young children. J Infect Dis. 1986 May;153(5):823–831. doi: 10.1093/infdis/153.5.823. [DOI] [PubMed] [Google Scholar]
- Beards G. M., Campbell A. D., Cottrell N. R., Peiris J. S., Rees N., Sanders R. C., Shirley J. A., Wood H. C., Flewett T. H. Enzyme-linked immunosorbent assays based on polyclonal and monoclonal antibodies for rotavirus detection. J Clin Microbiol. 1984 Feb;19(2):248–254. doi: 10.1128/jcm.19.2.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards G. M., Flewett T. H. Serological characterisation of human rotaviruses propagated in cell cultures. Brief report. Arch Virol. 1984;80(2-3):231–237. doi: 10.1007/BF01310663. [DOI] [PubMed] [Google Scholar]
- Beards G. M., Pilfold J. N., Thouless M. E., Flewett T. H. Rotavirus serotypes by serum neutralisation. J Med Virol. 1980;5(3):231–237. doi: 10.1002/jmv.1890050307. [DOI] [PubMed] [Google Scholar]
- Beards G. M. Polymorphism of genomic RNAs within rotavirus serotypes and subgroups. Arch Virol. 1982;74(1):65–70. doi: 10.1007/BF01320783. [DOI] [PubMed] [Google Scholar]
- Beards G. M. Serotyping of rotavirus by NADP-enhanced enzyme-immunoassay. J Virol Methods. 1987 Nov;18(2-3):77–85. doi: 10.1016/0166-0934(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Birch C. J., Heath R. L., Gust I. D. Use of serotype-specific monoclonal antibodies to study the epidemiology of rotavirus infection. J Med Virol. 1988 Jan;24(1):45–53. doi: 10.1002/jmv.1890240107. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Stallings E. P., Lewis C., Miles A. J., Chanock R. M., Kapikian A. Z. Pediatric viral gastroenteritis during eight years of study. J Clin Microbiol. 1983 Jul;18(1):71–78. doi: 10.1128/jcm.18.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C., Pedley S., McCrae M. A. Group C rotaviruses in humans. J Clin Microbiol. 1986 Apr;23(4):760–763. doi: 10.1128/jcm.23.4.760-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Mathan M. M., Mathew M., Martin R., Beards G. M., Mathan V. I. Rotavirus epidemiology in Vellore, south India: group, subgroup, serotype, and electrophoretype. J Clin Microbiol. 1988 Nov;26(11):2410–2414. doi: 10.1128/jcm.26.11.2410-2414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanock R. M. Joseph E. Smadel memorial lecture. Strategy for development of respiratory and gastrointestinal tract viral vaccines in the 1980s. J Infect Dis. 1981 Mar;143(3):364–374. doi: 10.1093/infdis/143.3.364. [DOI] [PubMed] [Google Scholar]
- Chiba S., Yokoyama T., Nakata S., Morita Y., Urasawa T., Taniguchi K., Urasawa S., Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986 Aug 23;2(8504):417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Fowler K. J., Bishop R. F., Cotton R. G. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J Virol. 1985 Apr;54(1):14–20. doi: 10.1128/jvi.54.1.14-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Tursi J. M., McAdam W. J., Bishop R. F. Derivation of neutralizing monoclonal antibodies to human rotaviruses and evidence that an immunodominant neutralization site is shared between serotypes 1 and 3. Virology. 1986 Oct 30;154(2):302–312. doi: 10.1016/0042-6822(86)90456-3. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Unicomb L. E., Pitson G. A., Bishop R. F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol. 1987 Mar;25(3):509–515. doi: 10.1128/jcm.25.3.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desselberger U., Hung T., Follett E. A. Genome analysis of human rotaviruses by oligonucleotide mapping of isolated RNA segments. Virus Res. 1986 Jun;4(4):357–368. doi: 10.1016/0168-1702(86)90082-1. [DOI] [PubMed] [Google Scholar]
- Feachem R. G., Hogan R. C., Merson M. H. Diarrhoeal disease control: reviews of potential interventions. Bull World Health Organ. 1983;61(4):637–640. [PMC free article] [PubMed] [Google Scholar]
- Follett E. A., Sanders R. C., Beards G. M., Hundley F., Desselberger U. Molecular epidemiology of human rotaviruses. Analysis of outbreaks of acute gastroenteritis in Glasgow and the west of Scotland 1981/82 and 1982/83. J Hyg (Lond) 1984 Apr;92(2):209–222. doi: 10.1017/s0022172400064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarg-Chenon A., Bricout F., Nicolas J. C. Serological characterization of human reassortant rotaviruses. J Virol. 1986 Aug;59(2):510–513. doi: 10.1128/jvi.59.2.510-513.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul S. K., Simpson T. F., Woode G. N., Fulton R. W. Antigenic relationships among some animal rotaviruses: virus neutralization in vitro and cross-protection in piglets. J Clin Microbiol. 1982 Sep;16(3):495–503. doi: 10.1128/jcm.16.3.495-503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Courbot M. C., Beraud A. M., Beards G. M., Campbell A. D., Gonzalez J. P., Georges A. J., Flewett T. H. Subgroups, serotypes, and electrophoretypes of rotavirus isolated from children in Bangui, Central African Republic. J Clin Microbiol. 1988 Apr;26(4):668–671. doi: 10.1128/jcm.26.4.668-671.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. K., Naik T. N. Detection of a large number of subgroup 1 human rotaviruses with a "long" RNA electropherotype. Arch Virol. 1989;105(1-2):119–127. doi: 10.1007/BF01311122. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Wyatt R. G., Kapikian A. Z., Kalica A. R., Flores J., Jones R. Rescue and serotypic characterization of noncultivable human rotavirus by gene reassortment. Infect Immun. 1982 Jul;37(1):104–109. doi: 10.1128/iai.37.1.104-109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R., Birch C., Gust I. Antigenic analysis of rotavirus isolates using monoclonal antibodies specific for human serotypes 1, 2, 3 and 4, and SA11. J Gen Virol. 1986 Nov;67(Pt 11):2455–2466. doi: 10.1099/0022-1317-67-11-2455. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Chanock R. M., Kapikian A. Z. Analysis by plaque reduction neutralization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J Clin Microbiol. 1987 Feb;25(2):290–294. doi: 10.1128/jcm.25.2.290-294.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Flores J., Midthun K., Kapikian A. Z. Serotypic characterization of rotaviruses derived from asymptomatic human neonatal infections. J Clin Microbiol. 1985 Mar;21(3):425–430. doi: 10.1128/jcm.21.3.425-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Hoshino Y., Glass R. I., Midthun K., Gorziglia M., Chanock R. M. Rotavirus: the major etiologic agent of severe infantile diarrhea may be controllable by a "Jennerian" approach to vaccination. J Infect Dis. 1986 May;153(5):815–822. doi: 10.1093/infdis/153.5.815. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Greenberg H. B., Kalica A. R., Kim H. W., Brandt C. D., Rodriguez W. J., Parrott R. H., Chanock R. M. Approaches to immunization of infants and young children against gastroenteritis due to rotaviruses. Rev Infect Dis. 1980 May-Jun;2(3):459–469. doi: 10.1093/clinids/2.3.459. [DOI] [PubMed] [Google Scholar]
- Linhares A. C., Gabbay Y. B., Mascarenhas J. D., Freitas R. B., Flewett T. H., Beards G. M. Epidemiology of rotavirus subgroups and serotypes in Belem, Brazil: a three-year study. Ann Inst Pasteur Virol. 1988 Jan-Mar;139(1):89–99. doi: 10.1016/s0769-2617(88)80009-1. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., McCorquodale J. G. The molecular biology of rotaviruses. II. Identification of the protein-coding assignments of calf rotavirus genome RNA species. Virology. 1982 Mar;117(2):435–443. doi: 10.1016/0042-6822(82)90482-2. [DOI] [PubMed] [Google Scholar]
- Midthun K., Valdesuso J., Hoshino Y., Flores J., Kapikian A. Z., Chanock R. M. Analysis by RNA-RNA hybridization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J Clin Microbiol. 1987 Feb;25(2):295–300. doi: 10.1128/jcm.25.2.295-300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Hoshino Y., Flores J., Kapikian A. Z. Genetic analysis of a human rotavirus that belongs to subgroup I but has an RNA pattern typical of subgroup II human rotaviruses. J Clin Microbiol. 1987 Jul;25(7):1159–1164. doi: 10.1128/jcm.25.7.1159-1164.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Oyamada H., Suto T. Relative frequency of human rotavirus subgroups 1 and 2 in Japanese children with acute gastroenteritis. J Med Virol. 1985 Sep;17(1):29–34. doi: 10.1002/jmv.1890170105. [DOI] [PubMed] [Google Scholar]
- Nakagomi T., Nakagomi O. RNA-RNA hybridization identifies a human rotavirus that is genetically related to feline rotavirus. J Virol. 1989 Mar;63(3):1431–1434. doi: 10.1128/jvi.63.3.1431-1434.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Birch C., McLean B., Holmes I. H. Molecular epidemiology of human rotaviruses in Melbourne, Australia, from 1973 to 1979, as determined by electrophoresis of genome ribonucleic acid. J Clin Microbiol. 1981 Feb;13(2):272–278. doi: 10.1128/jcm.13.2.272-278.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M. G., Goh S. G., Williams K., Campbell A. D., Beards G. M., Sanders R. C., Flewett T. H. Epidemiological aspects of rotavirus infection in young Gambian children. Ann Trop Paediatr. 1985 Mar;5(1):23–28. doi: 10.1080/02724936.1985.11748354. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Stoner-Ma D. L., Estes M. K., Greenberg H. B. Specific enzyme-linked immunoassay for rotavirus serotypes 1 and 3. J Clin Microbiol. 1985 Aug;22(2):286–291. doi: 10.1128/jcm.22.2.286-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele A. D., Alexander J. J. The relative frequency of subgroup I and II rotaviruses in black infants in South Africa. J Med Virol. 1988 Mar;24(3):321–327. doi: 10.1002/jmv.1890240309. [DOI] [PubMed] [Google Scholar]
- Unicomb L. E., Bishop R. F. Epidemiology of rotavirus strains infecting children throughout Australia during 1986-1987. A study of serotype and RNA electropherotype. Arch Virol. 1989;106(1-2):23–34. doi: 10.1007/BF01311035. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K., Wakasugi F., Kobayashi N., Chiba S., Sakurada N., Morita M., Morita O., Tokieda M. Survey of human rotavirus serotypes in different locales in Japan by enzyme-linked immunosorbent assay with monoclonal antibodies. J Infect Dis. 1989 Jul;160(1):44–51. doi: 10.1093/infdis/160.1.44. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., d'Hondt E., André F. E., Beards G. M., Flewett T. H. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985 Aug;107(2):189–194. doi: 10.1016/s0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Kapikian A. Z., Delem A., Zissis G. A comparative trial of rhesus monkey (RRV-1) and bovine (RIT 4237) oral rotavirus vaccines in young children. J Infect Dis. 1986 May;153(5):832–839. doi: 10.1093/infdis/153.5.832. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Zissis G., Brandt C. D., Rodriguez W. J., Kim H. W., Parrott R. H., Urrutia J. J., Mata L., Greenberg H. B. Epidemiology of human rotavirus Types 1 and 2 as studied by enzyme-linked immunosorbent assay. N Engl J Med. 1978 Nov 23;299(21):1156–1161. doi: 10.1056/NEJM197811232992103. [DOI] [PubMed] [Google Scholar]
- Zheng B. J., Lam W. P., Yan Y. K., Lo S. K., Lung M. L., Ng M. H. Direct identification of serotypes of natural human rotavirus isolates by hybridization using cDNA probes derived from segment 9 of the rotavirus genome. J Clin Microbiol. 1989 Mar;27(3):552–557. doi: 10.1128/jcm.27.3.552-557.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zoysa I., Feachem R. G. Interventions for the control of diarrhoeal diseases among young children: rotavirus and cholera immunization. Bull World Health Organ. 1985;63(3):569–583. [PMC free article] [PubMed] [Google Scholar]