Abstract
Recent studies identified an association between genetic variants in the lymphotoxin-α (LTα) gene and leprosy. To study the influence of LTα on the control of experimental leprosy, both low- and high-dose Mycobacterium leprae foot pad (FP) infections were evaluated in LTα-deficient chimeric (cLTα−/−) and control chimeric (cB6) mice. Cellular responses to low-dose infection in cLTα−/− mice were dramatically different, with reduced accumulation of CD4+ and CD8+ lymphocytes and macrophages and failure to form granulomas. Growth of M. leprae was contained for 6 months, but augmented late in infection. In contrast, tumor necrosis factor knockout and tumor necrosis factor receptor 1 knockout FPs exhibited extensive inflammatory infiltration with an increase in M. leprae growth throughout infection. Following high-dose infection, cB6 FP induration peaked at 4 weeks and was maintained for 12 weeks. Induration was not sustained in cLTα−/− FPs that contained few lymphocytes and no granulomas. There was a reduction in the expression levels of inflammatory cytokines, chemokines, and chemokine receptors, including nitric oxide synthase 2, vascular cell adhesion molecule, and intercellular cell adhesion molecule. Furthermore, cLTα−/− popliteal lymph nodes contained a higher proportion of naïve CD44loCD62Lhi T cells than cB6 mice, suggestive of reduced T cell activation. Therefore, both LTα and tumor necrosis factor are essential for the regulation of the granuloma, but they have distinctive roles in the recruitment of lymphocytes and maintenance of the granulomatous response during chronic M. leprae infection.
On infection with many intracellular organisms, especially mycobacterial pathogens, a granulomatous response ensues. This complex process involves the participation of cell-mediated immunity (CMI), activation of endothelial cells, enhancement of adhesion molecule expression, management of macrophage and lymphocyte infiltration, and induction of the microbiostatic and microbicidal effects of macrophages.1 In addition, extensive intercellular communication, chiefly through cytokine and chemokine signals, is required to orchestrate this cellular accumulation and results in a three-dimensional structure that limits or prevents dissemination of the pathogen and is largely protective.
The contributions of both soluble and membrane-bound tumor necrosis factor (TNF) to granuloma development have been established.2,3,4,5 Lymphotoxin (LT)-α is also a member of the TNF superfamily, but as compared with TNF, much less is known about its function. LTα is required for the development of secondary lymphoid tissue6 through its association with LTβ, to form heterotrimeric LTα1β2 and LTα2β1, which enable interaction between lymphocytes and surrounding fibroblasts, and epithelial and myeloid cells that express the LTβ receptor. Current evidence also suggests that LTα plays a regulatory role in CMI7 and serves as an initiating stimulus in chronic inflammation8 through the activation of signaling cascades involving adhesion molecules, cytokines, and chemokines, for the recruitment and retention of lymphocytes. LTα3, the secreted form of lymphotoxin, is produced by activated lymphocytes, primarily CD4+ T cells, natural killer cells, and B cells, and binds with equal affinity as TNF to TNF receptor (TNFR) 1 and TNFR2. LTα3 also binds to the herpes virus entry mediator.9 This receptor has a wide tissue distribution, being expressed predominantly on T cells, B cells, and monocytes. Signaling through herpes virus entry mediator induces activation of nuclear factor-κB and activator protein 1 and may play a role in T cell activation.
LTα signaling has proven important in controlling infections with diverse intracellular pathogens, including Mycobacterium tuberculosis, Listeria monocytogenes, Candida albicans, Toxoplasma gondii, Leishmania donovani, Plasmodium chabaudi, and influenza virus.10,11,12,13,14,15,16 The role of LTα in host defense against leprosy is unknown; however, LTα has been detected in leprosy lesions, primarily at the tuberculoid end of the immunopathological spectrum and in Type 1 reactions,17,18 which supports its purported role as a proinflammatory cytokine. Importantly, a recent study found that a polymorphism, LTA + 80 within the promoter region of the gene encoding LTα, is an important risk factor for developing leprosy per se.19
In an effort to understand the mechanisms underlying this association between LTα and leprosy, we used low and high dose models to examine M. leprae infection in mice deficient in this cytokine. Peripheral lymph nodes (LNs) do not develop in mice with a targeted disruption in the LTα gene.6,20,21 Therefore, to ensure that such defects did not contribute to our results, chimeric knockout (KO) mice were generated by reconstitution of irradiated RAG mice with LTα−/− bone marrow cells (cLTα−/−). cLTα−/− mice have intact peripheral lymphoid organs and are fully reconstituted with immune cells, but are deficient in hematopoietically derived soluble and membrane-bound forms of LTα.10,11,13,14,22 Irradiated RAG mice reconstituted with C57Bl/6 (B6) bone marrow cells served as chimeric controls (cB6). Our studies examined the long term effects of LTα deficiency into the chronic phase of M. leprae infection and its effect on the granulomatous response. In contrast to TNF KO (TNF−/−) and TNFR1 KO (TNFR1−/−) mice, which showed extensive lymphocytic infiltration and augmented growth of M. leprae throughout the infection period, LTα-deficient mice were unable to recruit lymphocytes into the infected foot pad (FP), develop and maintain granulomas, or optimally control the growth of M. leprae in the chronic stage; thus, we show an aberrant phenotype in an animal model that has a defect in a gene that was found by whole genome screening to be significantly associated with the development of leprosy in humans.
Materials and Methods
Mice
Chimeric LTα−/− (cLTα−/−) mice were generated by reconstitution of irradiated (5.5 Gy) C57Bl/6.RAG-1−/− mice with 1 × 107 LTα−/− bone marrow cells. Irradiated RAG mice reconstituted with C57Bl/6 (B6) bone marrow (cB6) served as controls. Cellular reconstitution was verified by analysis of peripheral blood by flow cytometry (data not shown) as described previously13 and by comparison of immune responses of cB6 mice with C57Bl/6J (B6) mice (Table 1). TNF knockout (TNF−/−) mice were generated as previously described.2 TNFR1−/− and B6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Experiments were approved by the University of Sydney Animal Care and Ethics Committee and performed in compliance with all applicable state and federal laws and regulations in accordance with USPHS Policy on Humane Care and Use of Laboratory Animals. The National Hansen’s Disease Program’s Assurance of Compliance (#A3032-01) is registered with the Office of Laboratory Animal Welfare.
Table 1.
Cellular Composition of the M. leprae-Infected FP
| Marker | cB6 | B6 |
|---|---|---|
| CD3+ | 46.18 ± 3.43 | 51.85 ± 1.97 |
| CD3+CD4+ | 70.45 ± 0.60 | 58.55 ± 1.41 |
| CD4+CD44loCD62Lhi | 3.36 ± 1.09 | 5.77 ± 2.18 |
| CD4+CD44hiCD62Llo | 87.77 ± 1.62 | 77.17 ± 2.47 |
| CD3+CD8+ | 18.24 ± 1.91 | 20.33 ± 1.95 |
| CD8+CD44loCD62Lhi | 6.27 ± 2.73 | 9.18 ± 1.20 |
| CD8+CD44hiCD62Llo | 82.76 ± 1.93 | 71.84 ± 3.18 |
| B220+ | 0.87 ± 0.26 | 1.80 ± 0.19 |
| NK1.1.1+ | 2.21 ± 0.36 | 4.15 ± 0.71 |
| CD11b+Ly6G- | 76.63 ± 2.74 | 75.42 ± 2.45 |
| CD11b+IAb+ | 76.73 ± 4.37 | 80.70 ± 1.71 |
| Ly6G+ | 5.42 ± 1.69 | 4.99 ± 0.60 |
| CD11c+ | 1.45 ± 0.27 | 1.73 ± 0.13 |
FP from individual B6 and cB6 mice were harvested at 12 weeks post infection and digested to yield single cell suspensions. Values are percentages of cells in the CD4, CD8, lymphocyte, or myeloid gate. Gates were determined based on forward- and side-scatter parameters.
Maintenance of a Viable M. leprae Inoculum
M. leprae strain Thai-53 is maintained in continuous programmed passage in athymic nu/nu mice (Harlan Sprague-Dawley, Inc., Indianapolis, IN). M. leprae viability was determined by radiorespirometry23 and vital staining.24 The bacilli were stored at 4°C and used within 24 hours.
Infection of Mice with M. leprae
Low dose (Shepard) growth model: Mice were infected with 6 × 103 M. leprae in 0.03 ml PBS in each hind FP. At 3, 6, and 9 months, and 12 months (TNFR1−/− only), post-infection, FPs were harvested for enumeration of acid fast bacilli (AFB), and histopathological and immunohistochemical analyses; popliteal LN cells were analyzed by flow cytometry.
High dose infection model: Each hind FP was inoculated with 3 × 107 M. leprae in 0.03 ml PBS,25 and induration was measured weekly using a Vernier caliper. At 3 months post-infection, FP tissues were harvested for histopathological and immunohistochemical analyses, flow cytometry, and RNA expression; popliteal LN cells were evaluated by flow cytometry.
Enumeration of AFB
AFB were enumerated in individual FPs. Briefly, each hind FP was homogenized, three smears were prepared, and 20 microscopic fields per smear were counted to determine the yield for that individual mouse. The threshold of counting ability in this system is 4.8 × 103 AFB per FP, equivalent to 1 AFB in 60 microscopic fields.
Histopathology
The feet from M. leprae-infected mice were fixed in 10% buffered formalin, decalcified in a solution of 10% (w/v) sodium citrate and 22.5% (v/v) formic acid, and embedded in paraffin. Four-μm sections were prepared of cross-sections at the distal, mid, and proximal area of the metatarsals of the infected foot and stained with H&E or Fite-Faraco acid fast stain. The histological feature of a granuloma was defined as an aggregation of macrophages with M. leprae and lymphocytes. In the sections the lymphocytic infiltrates, bacterial index of granuloma, and granuloma fraction were assessed as previously described.26,27 Sections were viewed and captured on a Zeiss Axioplan microscope (Zeiss, Germany) equipped with a MicroFire camera and PictureFrame software.25
Immunohistochemistry
FP tissue was embedded in optimal cutting temperature medium (Tissue Tek, Inc., Torrence, CA), snap frozen in liquid nitrogen, and stored at −70C. Frozen tissue was cut (Frigocut 2800E, Leica Microsystem, Inc., Bannockburn, IL) into 4-μm sections and fixed in cold acetone. After blocking with avidin and biotin (Vector Laboratories, Burlingame, CA), rat anti-mouse CD4 (PharMingen, San Diego, CA) or rat anti-mouse CD8 (PharMingen) was applied to serial sections. Biotin-labeled mouse anti-rat F(ab)2 fragments (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used as the secondary antibody. Immunohistochemical staining was performed with the Vectastain Elite ABC kit (Vector), the AEC Substrate kit (Vector), and a hematoxylin counterstain.
Flow Cytometry
LN cells were prepared by passing LNs from individual mice through a tissue screen. FP tissues were digested with collagenase/DNase to produce single cell suspensions.25 Cells were treated with Fc Block (CD16/CD32 [Fcγ III/II receptor]) (BD Biosciences, San Jose, CA) and stained with appropriate isotype control antibodies or anti-CD3 (clone 17A2), anti-CD4 (clone RM4-5), anti-CD8a (clone 53-6.7), anti-CD44 (clone (IM7), anti-CD62L (clone MEL-14), anti-CD244.2 (clone 2B4), anti-CD11b (clone M1/70), anti-I-Ab (clone 25-9-17), and anti-Ly-6G (clone 1A8), all from BD Biosciences. Analyses were performed using a FACS Aria interfaced with a Hewlett Packard 4100 running FACS Diva software (BD Biosciences).
Isolation of total RNA and Real-Time Reverse Transcription PCR
RNA was extracted from FP tissue of individual mice using an RNAgents kit (Promega Corp., Madison, WI). RNA was reconstituted in RNase-free distilled water, treated with DNase (DNA-free kit, Ambion, Austin, TX), and RNA concentrations were determined by reading the optical density at 260 nm in a SynergyHT Multidetection Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT). cDNA was generated from 0.5 μg RNA using random hexamers and an reverse transcription (RT) for PCR kit (Clontech, Palo Alto, CA) at 42°C for 1 hour in a thermocycler (9600, Perkin-Elmer Corp., Norwalk, CT). Controls for DNA contamination were prepared from RNA samples using the reverse transcription reagents minus the reverse transcriptase. All sample and control preparations were aliquoted and stored at −70°C. Real-time RT-PCR for cytokine and chemokine transcripts were performed on these cDNA via Taqman technology using specific primer sets and probes (Applied BioSystems, Foster City, CA) and Universal Master Mix (Applied BioSystems) in an ABI PRISM 7700 Sequence Detection System (Applied BioSystems). Semiquantitative analyses of the data were accomplished using the standard curve comparative method. Standard curves were generated using tenfold dilutions of cDNA from Concanavalin A-stimulated mouse spleen cells or interferon (IFN)γ and lipopolysaccharide-stimulated mouse macrophage. Data were normalized for template variation by dividing each gene transcript value by that of the glyceraldehyde-3-phosphate dehydrogenase RNA RT-PCR value for the same template.25
Statistical Analyses
Growth of M. leprae was analyzed using the non-parametric Mann-Whitney test. Differences in induration were determined using pairwise t-tests of least square means. All other data were analyzed using unpaired t-tests with or without Welch’s correction. Data were considered significant at P < 0.05.25
Results
LTα-Deficient Chimeras Show Impaired Restriction of M. leprae Growth during Chronic Infection, Whereas TNF−/− and TNFR1−/− FPs Exhibit Enhanced Bacillary Growth throughout Infection
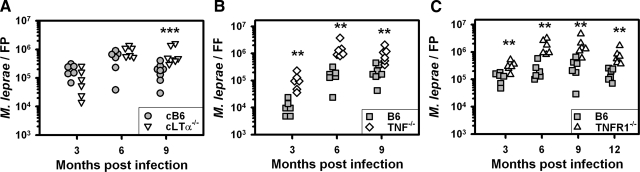
Mice were infected with 6 × 103 viable M. leprae per FP and harvested at 3, 6, and 9 months, and 12 months (TNFR1−/− only), post-infection. As shown in Figure 1A, multiplication of M. leprae in cB6 mice reached approximately 105 bacilli by 3 months and remained at this level through 9 months. There was no difference between cB6 and cLTα−/− mice in the number of AFB recovered at 3 and 6 months; however, M. leprae counts were significantly higher in the cLTα−/− FPs at 9 months (P = 0.0006). Hence, LTα is not necessary for the restriction of acute M. leprae multiplication; however, the influence of LTα deficiency on bacterial growth is apparent as infection progresses into the chronic stage.
Figure 1.
LTα-deficient chimeras show impaired restriction of M. leprae growth during chronic infection, whereas TNF−/− and TNFR1−/− FPs exhibit enhanced bacillary growth throughout infection. cB6 (circles) and cLTα−/− (inverted triangles) mice (A), B6 (squares) and TNF−/− (diamonds) mice (B), and B6 (squares) and TNFR1−/− (triangles) mice (C), were infected in each hind FP with 6 × 103 viable M. leprae (low dose growth model). At 3, 6, and 9 months, and 12 months (C only), the number of AFB per FP was determined. Each symbol represents one mouse. The threshold of counting ability is 4.8 × 103 AFB per FP. Experiments shown are representative of two independent experiments each. **P < 0.01; ***P < 0.001.
In contrast, growth of M. leprae was enhanced throughout infection in TNF−/− and TNFR1−/− FPs. As shown in Figure 1, B and C, multiplication of M. leprae in the FPs of B6 mice reached on the order of 104 to 105 bacilli per FP at 3 months, peaked at 105 AFB by 6 months and remained at this level at 9 to 12 months. Numbers of M. leprae recovered from the FP of TNF−/− mice (Figure 1B) were ∼tenfold higher at 3 months (P = 0.0022), 6 months (P = 0.0022), and 9 months (P = 0.0013). Likewise, AFB were markedly elevated in the TNFR1−/− mice (Figure 1C) at all time points as compared with B6 mice (3 months, P = 0.0087; 6 months, P = 0.0022; 9 months, P = 0.0043; 12 months, P = 0.0022). Thus, TNF signaling is required for optimal restriction of M. leprae growth during both the acute and chronic stages of infection.
M. leprae-Infected cLTα−/− FPs Exhibit a Reduced Granulomatous Response throughout Infection
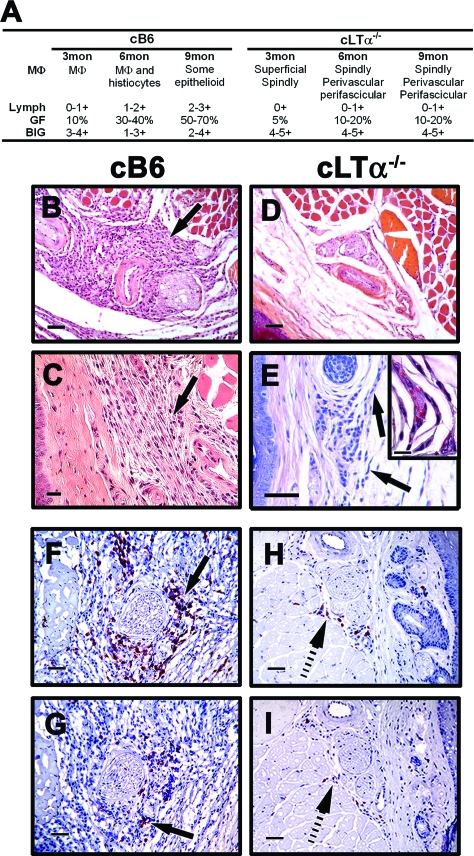
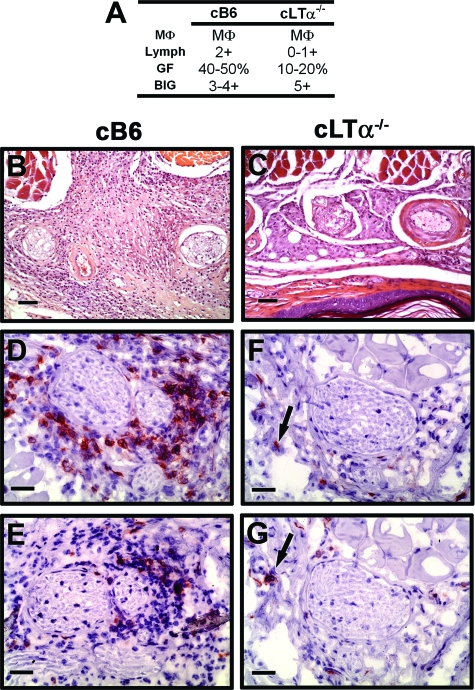
The cellular infiltrates that developed in the cB6 mice on infection with M. leprae displayed characteristics similar to those seen in B6 FPs previously reported.25,28,29 cB6 FPs exhibited scattered macrophage infiltration with a nil to mild lymphocyte response at 3 months (Figure 2A), which by 9 months (Figure 2, A and B) developed into a moderate to dense lymphocyte infiltration among plump histiocytes and macrophages (Figure 2C) containing bacilli. In contrast, cLTα−/− mice exhibited very poor cellular infiltration throughout infection (Figure 2A). At 3 months, the FPs showed scattered histiocytes and macrophages in the superficial dermis; many of the macrophages were spindle shaped. Lymphocytic infiltration was absent. By 6 months, FPs showed small perivascular and perifascicular clusters of macrophages with histiocytes in the superficial dermis, and in some areas the cells were disposed in streaks. Strikingly, in cLTα−/− mice the lymphocyte response remained poor. This overall picture continued at 9 months (Figure 2, A and D), where the FPs exhibited spindled macrophages (Figure 2E) containing clumps of AFB (Figure 2E inset) and few lymphocytes. The poor lymphocytic response in cLTα−/− FPs was supported by immunohistochemical staining. As shown in Figure 2F, cB6 FP infiltrates contained primarily CD4+ T cells with a lower influx of CD8+ T cells (Figure 2G). In stark contrast, there were very few T cells, either CD4+ (Figure 2H) or CD8+ (Figure 2I), present in the cLTα−/− FPs. These data suggest that hematopoietically derived LTα−/− is crucial for cellular recruitment into the M. leprae-induced granuloma.
Figure 2.
M. leprae-infected cLTα−/− FP exhibit a reduced granulomatous response throughout infection. cB6 and cLTα−/− mice were infected in each hind FP with 6 × 103 viable M. leprae. A: FP granulomas were scored for macrophage morphology (MΦ), lymphocyte infiltration (Lymph), granuloma fraction (GF), and bacilli in the granulomas (BIG). B–E: Histopathological (H&E) and (F–I) immunohistochemical staining in FP at 9 months post-infection. In cB6 mice, a mild chronic inflammatory infiltrate (arrows, B and C) was observed in the subcutis; in cLTα−/− mice, the cellular infiltrate was greatly reduced (D), and spindle shaped MΦ (arrows, E) contained clumps of AFB (E inset, Fite-Faraco acid fast stain). In cB6 mice, the T cell infiltrate consisted primarily of CD4+ cells (arrow, F) with a lower influx of CD8+ cells (arrow, G). cLTα−/− FP contained few CD4+ (hatched arrow, H) or CD8+ (hatched arrow, I) cells. For B, D, F–I, scale bars = 50 μm; C and E, scale bars = 20 μm; and E inset, scale bar = 10 μm. Experiment shown is representative of two independent experiments.
M. leprae-Infected TNF−/− and TNFR1−/− FPs Present an Intense and Diffuse Granulomatous Response
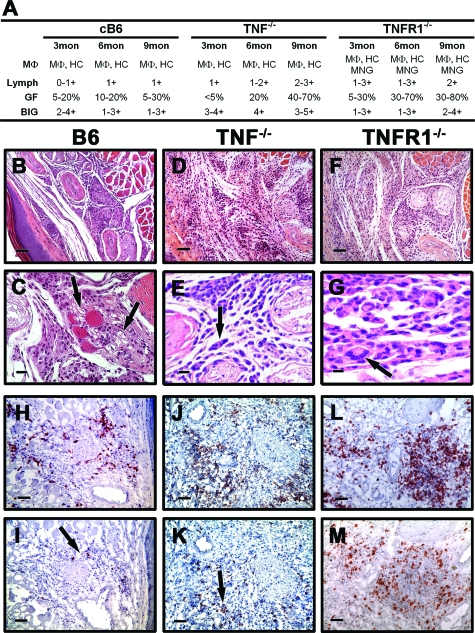
In B6 mice, scattered histiocytes and macrophages were present (Figure 3A), the lymphocytic response was nil to mild, and the inflammatory cells were predominantly in the superficial dermis at 3 months. By 6 months, focal accumulation of foamy macrophages, histiocytes, and few lymphocytes were observed, and this continued at 9 months (Figure 3, A–C). The cellular infiltrates were perifascicular and the nerves were not inflamed. Immunohistochemical staining showed the lymphocytes to be primarily CD4+ T cells (Figure 3H), with a lower level of infiltration of CD8+ T cells (Figure 3I).
Figure 3.
M. leprae-infected TNF−/− and TNFR1−/− FP present a diffuse granulomatous response. B6, TNF−/− and TNFR1−/− mice were infected in each hind FP with 6 × 103 viable M. leprae. A: Differential granulomatous response was determined by scoring as in Figure 2. B–G: Histopathological (H&E) and (H–M) immunohistochemical staining in FP. B6 FP were comprised of histiocytes, MΦ, and a mild lymphocytic response (B and C, arrows in C); in TNF−/− and TNFR1−/− mice, there was a diffuse and dense infiltration (D and F) with giant cell formation (arrows, E and G). In all three mouse strains, the T cell infiltrate consisted primarily of CD4+ (H, J, and L) cells with a lower influx of CD8+ (arrows, I and K) cells. For (B, D, F, H–M) scale bars = 50 μm; (C) scale bar = 20 μm; and (E and G) scale bars = 10 μm. Experiment shown is representative of two independent experiments.
TNF−/− and TNFR1−/− mice, in contrast, exhibited a more rapid granulomatous response (Figure 3A). Early in infection, FPs contained large, unorganized cellular accumulations composed of histiocytes and macrophages scattered with lymphocytes around blood vessels and nerves and infiltrating the muscle bundles. The cellular infiltrates occupied up to 70% of the dermis. As the infection progressed, the granulomatous response consisted of diffuse and dense infiltration by lymphocytes (Figure 3A, D, and F) extending to the dorsal side of the foot. In areas there was well-defined granuloma formation with focal aggregation of histiocytic cells, small multinucleate giant cells and lymphocytes (Figure 3, E and G). The cellular infiltrate cuffed the nerves but there was no intraneural lymphocytic inflammation (Figure 3F). The lymphocytic response was composed of both CD4+ (Figure 3, J and L) and CD8+ (Figure 3, K and M) T cells, and occupied up to 80% of the dermis. These data indicate that TNF is essential for organizing and restraining the granulomatous response to M. leprae infection, and that TNF, rather than LTα, is the primary signal for the TNFR1 receptor in this model.
LTα-Deficient Chimeras Exhibit Impaired FP Induration and a Reduced Granulomatous Response Following High Dose Infection
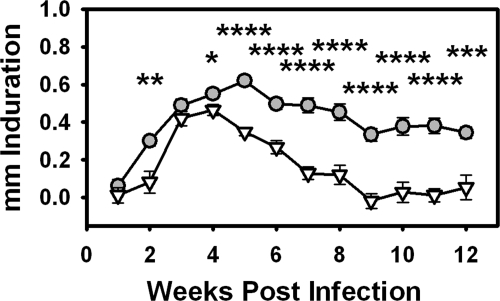
To further analyze the mechanisms involved in the aberrant granulomatous response in cLTα−/− FP, the effects of LTα deficiency were evaluated using a high dose infection model.25 On inoculation of M. leprae into cB6 mice, the FP induration increased gradually over the first 5 weeks, peaked and remained relatively constant up to 12 weeks post-infection (Figure 4). This pattern of induration is similar to that seen in B6 mice25 and LTα transcripts could be detected in these FPs (see supplemental Figure S1 at http://ajp.amjpathol.org). In contrast, an initial delay in FP induration was observed in cLTα−/− mice, which increased to match that of cB6 at 3 to 4 weeks. Thereafter FP induration steadily waned in cLTα−/− mice and was significantly less than that in cB6 throughout the remainder of the infection period.
Figure 4.
LTα-deficient chimeras exhibit impaired FP induration. cB6 (circles) and cLTα−/− (inverted triangles) FP were inoculated with 3 × 107 M. leprae and FP induration was measured using a Vernier caliper. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
Histopathologically, high dose cB6 FPs, as in the low dose model, contained macrophages and a moderate lymphocyte infiltration around blood vessels, nerves, and muscle bundles (Figure 5, A and B). The lymphocytes were primarily CD4+ T cells (Figure 5D) with a lower influx of CD8+ cells (Figure 5E). In contrast, LTα−/− FPs exhibited small collections of macrophages (Figure 5, A and C) with only a few scattered lymphocytes (Figure 5, F and G). There was no intraneural inflammation in either strain. Thus, the lack of cellular infiltration was not dependent on dose of M. leprae as the granulomatous response failed to develop in cLTα−/− mice even with a high level of infection.
Figure 5.
cLTα−/− mice exhibit a reduced granulomatous response following high dose infection. A: Differential granulomatous response was determined by scoring as in Figure 2. B–C: Histopathological (H&E) and (D–G) immunohistochemical staining in FP at 12 weeks post-inoculation. In cB6 mice, a moderate lymphocyte infiltration was observed (B); in cLTα−/− mice, there were few scattered lymphocytes and small collections of MΦ (C). In cB6 mice, the T cell infiltrate consisted primarily of CD4+ cells (D) with a lower influx of CD8+ cells (E). cLTα−/− FP contained few CD4+ (arrow, F) or CD8+ (arrow, G) cells. For (B and C) scale bars = 50 μm; and (D–G) scale bars = 30 μm. Experiment shown is representative of two independent experiments.
Immune Cell Composition of the M. leprae-Infected FPs
We next examined the cellular composition of the M. leprae-infected FPs. Flow cytometric analyses of cB6 FP cells harvested at 12 weeks revealed a cellular makeup similar to that of B6 mice, further demonstrating that these chimeras were fully reconstituted and generated a comparable granulomatous response to M. leprae infection (Table 1,25). cB6 FP cellular infiltrates were composed primarily of a myeloid population, with a high forward/side scatter (9894.7 ± 384.0 gated events per 15,000 total events), and a lymphocyte population (1363.3 ± 169.1 gated event per 15,000 total events). In the myeloid gate, CD11b+ macrophages accounted for 76.63 ± 2.74% of the cells, and 76.63 ± 2.74 of these macrophages expressed I-Ab. Few neutrophils were present (5.42 ± 1.69%). The lymphocyte gate was comprised primarily of CD3+ T cells (46.18 ± 3.43%) with few natural killer cells (2.21 ± 0.36%) and fewer B cells (0.87 ± 0.26%). Of the CD3+ cells present, the majority were CD4+ (70.45 ± 1.05%) compared with CD8+ (18.24 ± 3.31%), confirming the results obtained by the immunohistochemical staining (Figures 2, 3, and 5). In addition, CD4+ and CD8+ cells were examined for T cell activation markers. Although the repertoire of T cell activation markers is rapidly expanding,30 the relative levels of CD44 and CD62L are frequently used to distinguish naïve (CD44loCD62Lhi) from effector (CD44hiCD62Llo) T cells.31,32,33,34,35,36,37 In the M. leprae-infected FPs, the majority of CD4+ and CD8+ cells were of the effector phenotype, expressing CD44hiCD62Llo (87.77 ± 1.62% and 82.76 ± 1.93, respectively). We also attempted to evaluate the cellular composition of the cLTα−/− FPs by flow cytometry; however, we were unsuccessful as these preparations lacked sufficient numbers of leukocytes for a reliable assessment.
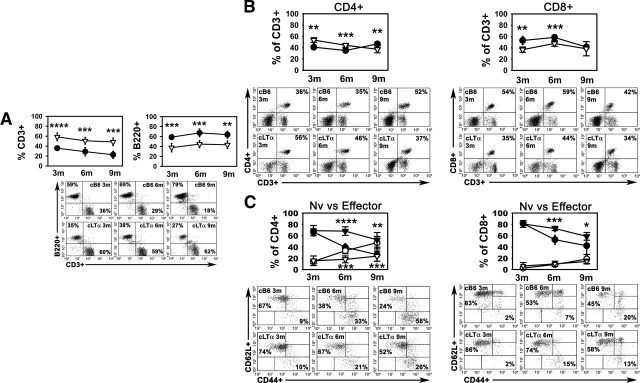
cLTα−/− Mice Exhibit Altered Lymphocyte Profiles in the Draining Popliteal LNs Following M. leprae Infection in the FPs
Since we were unable to evaluate the cLTα−/− FP populations, we examined the T cell profiles in the popliteal LN, which drains the FP, using our two infection models. The number and composition of lymphocytes in the LNs of cB6 mice was comparable to that seen in B6 LNs (unpublished data), again indicating that the chimeric mice were fully restored. In the low dose model (Figure 6), similar numbers of cells were also obtained from the LNs in both cB6 (7.18 × 106 ± 4.82 × 106, 1.38 × 107 ± 1.61 × 107, and 4.66 × 106 ± 2.34 × 106, at 3, 6, and 9 months, respectively) and cLTα−/− (2.36 × 106 ± 1.66 × 106, 7.17 × 105 ± 3.76 × 105, and 3.77 × 106 ± 4.15 × 106, at 3, 6, and 9 months, respectively) mice, except at 6 months post-infection when fewer cells were recovered from the deficient mice (P = 0.0152). LNs from infected cB6 mice exhibited a roughly 40% T cell to 60% B cell ratio at 3 months, which decreased as infection progressed (Figure 6A). On infection of cLTα−/− FPs, however, the T cell to B cell ratio in the LNs was essentially reversed with cLTα−/− LNs containing significantly higher percentages (∼60%) of CD3+ cells (P = 0.0001, P = 0.0006, P = 0.0006 at 3, 6, and 9 months, respectively) and lower percentages (∼40%) of B220+ cells (P = 0.0003, P = 0.0004, P = 0.0024 at 3, 6, and 9 months, respectively). In addition, the CD4:CD8 ratio was altered in cLTα−/− LNs. At 3 and 6 months in cLTα−/− LNs, there was a higher CD4:CD8 T cell ratio as compared with cB6 LNs (Figure 6B). CD4+ and CD8+ cells were also examined for T cell activation markers. The ratio of naïve to effector CD4+ cells was high in both strains of mice early in infection at 3 months. In the cB6 LNs, this ratio inverted over the course of infection, and by 9 months, the majority of CD4+ cells were of the effector phenotype. In contrast, in the absence of LTα, few effector CD4+ cells developed and the majority retained the naïve phenotype. Within the CD8+ population, although the reduction in the naïve phenotype was slower, the expression of the activation markers progressed in cLTα−/− LNs as in control mice (Figure 6C). These data indicate an aberrant evolution of CD4+ lymphocytes in the cLTα−/− mice in response to M. leprae infection.
Figure 6.
cLTα−/− mice exhibit altered lymphocyte profiles in the draining popliteal LN following M. leprae infection in the FP. cB6 (circles) and cLTα−/− (inverted triangles) FP were inoculated with 6 × 103 viable M. leprae. At 3, 6, and 9 months post-infection, popliteal LN from individual mice were harvested and single cell suspensions were prepared. For analysis by flow cytometry, the lymphocyte gate was determined based on forward- and side-scatter parameters. Cell numbers obtained from cB6 (7.18 × 106 ± 4.82 × 106, 1.38 × 107 ± 1.61 × 107, and 4.66 × 106 ± 2.34 × 106, at 3, 6, and 9 months, respectively) and cLTα−/− (2.36 × 106 ± 1.66 × 106, 7.17 × 105 ± 3.76 × 105, and 3.77 × 106 ± 4.15 × 106, at 3, 6, and 9 months, respectively) mice were similar, except at 6 months post-infection when fewer cells were recovered from the deficient mice (P = 0.0152). The percentage of (A) CD3+ and B220+ cells within the lymphocyte gate, (B) CD3+ cells that were CD4+ or CD8+, and (C) CD4+ and CD8+ cells expressing CD44loCD62Lhi (Naïve [Nv]; closed symbols) and CD44hiCD62Llo (Effector; open symbols) were determined. Each graph depicts group means ± SE of 4 to 5 mice per group and is representative of two independent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. Below each graph, scatter plots demonstrate flow cytometry data and percentages of an individual representative mouse from each group.
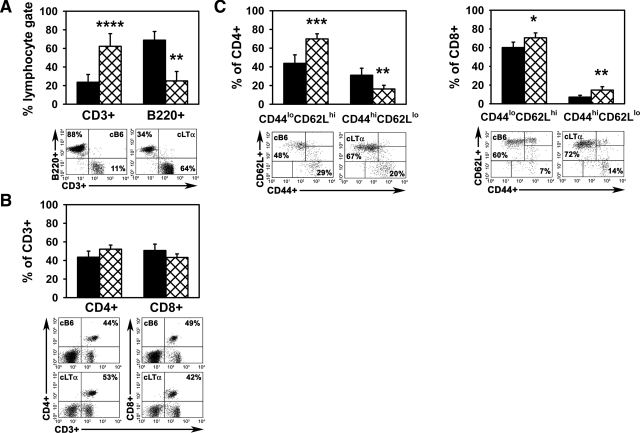
We likewise compared the popliteal LN populations in the two strains of mice in the high dose infection model. The LNs from the M. leprae-infected cLTα−/− mice were smaller than those from the cB6 mice and contained fewer total cells (cB6: 4.25 × 107 ± 2.32 × 107 cells; cLTα−/−: 1.50 × 106 ± 1.27 × 106 cells, P = 0.004). As shown in Figure 7A, the cB6 LNs were comprised primarily of B220+ cells (68.79 ± 9.39%) with a smaller population of CD3+ cells (23.62 ± 8.36%) present. In contrast, the popliteal LNs of cLTα−/− mice contained a markedly higher percentage of CD3+ cells (62.25 ± 13.60, P = 0.0022) with a corresponding reduction in the percentage of B220+ cells (25.05 ± 10.16, P < 0.0001). While there was little difference in the ratio of CD4+ cells (43.49 ± 6.57 in cB6 and 52.10 ± 4.47 in cLTα−/−) to CD8+ cells (50.69 ± 6.86 in cB6 and 43.18 ± 3.84 in cLTα−/−) between the two strains (Figure 7B), the percentages of both naïve CD4+CD44loCD62Lhi cells and naïve CD8+CD44loCD62Lhi cells were higher in the cLTα−/− LNs (Figure 7C). Collectively, these data support a crucial role for LTα in T cell evolution and lymphocyte trafficking, not only within the local microenvironment of M. leprae infection in the FP but also in the regional lymphoid tissue, and demonstrate that its influence is sustained throughout long term infection.
Figure 7.
Lymphocyte composition of the popliteal LN of cB6 and cLTα−/− mice in the high dose infection model. cB6 (closed bars) and cLTα−/− (hatched bars) FP were inoculated with 3 × 107 M. leprae. Popliteal LNs from individual mice were harvested and single cell suspensions were analyzed by flow cytometry. The lymphocyte gate was determined based on forward- and side-scatter parameters. The LNs from the M. leprae-infected cLTα−/− mice were smaller than those from cB6 mice and contained fewer total cells (cB6: 4.25 × 107 ± 2.32 × 107 cells; cLTα−/−: 1.50 × 106 ± 1.27 × 106 cells, P = 0.004). The percentages of (A) CD3+ and B220+ cells within the lymphocyte gate, (B) CD3+ cells that were CD4+ or CD8+, and (C) CD4+ and CD8+ cells expressing CD44 and CD62L were determined at 12 weeks post-infection. Experiment shown is representative of two independent experiments. Bars depict group means ± SE of four mice per group. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. Below each graph, corresponding scatter plots demonstrate flow cytometry data and percentages of an individual representative mouse from each group.
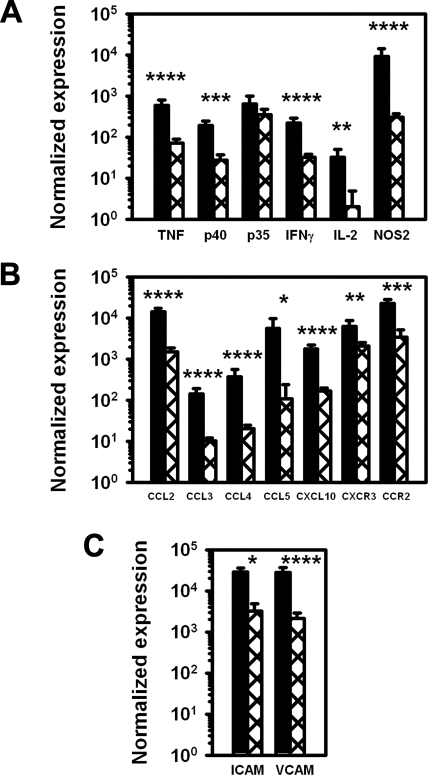
Inability to Maintain the Granulomatous Response to M. leprae in cLTα−/− Mouse FP Is Associated with Reduced Cytokine, Chemokine, and CAM Expression
Expression of various cytokine and chemokine transcripts in the FP was examined by real time RT-PCR. Like B6 mice,25,29 cB6 mice expressed Th1 cytokines, including interleukin (IL)-12/23p40, IL-12p35, IFNγ, IL-2, and TNF, as well as the activated macrophage product, NOS2 (Figure 8A). In contrast, there was a 6- to 33-fold lower level of expression of all tested cytokine transcripts in the cLTα−/− FPs, with the exception of IL-12p35, which was expressed at a level similar to cB6 mice. As shown in Figure 8B, cB6 FPs expressed CCL2, CCL3, CCL4, and CXCL10 and the chemokine receptors, CXCR3 and CCR2. For each of these chemokines, there was a 9 to 50-fold lower level of expression in the cLTα−/− FPs, together with reduced expression for CXCR3 and CCR2. In addition, there was a reduced expression of the cell adhesion molecules, VCAM and ICAM, in the LTα−/− FP (Figure 8C) as compared with cB6 FPs.
Figure 8.
Inability to maintain the granulomatous response to M. leprae in cLTα−/− mouse FPs is associated with reduced cytokine, chemokine, and CAM expression. cB6 (closed bars) and cLTα−/− (hatched bars) FPs were inoculated with 3 × 107 M. leprae and FP tissue was harvested at 12 weeks post-infection. RNA was purified and subjected to real-time RT-PCR for (A) Th1 cytokines and NOS2, (B) chemokines and chemokine receptors, and (C) CAMs. Gene expression is reported as a ratio to glyceraldehyde-3-phosphate dehydrogenase mRNA expression. Data shown are means ± SE of four mice per group and are representative of two independent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P < 0.0001.
Discussion
Clinical and histopathological criteria are used to classify a patient’s disease status in the broad spectrum of leprosy.38 In addition, associations have been made between tuberculoid leprosy and Th1 cytokine generation, and lepromatous leprosy and Th2 cytokine generation.17 However, whether the cytokine responses expressed across the leprosy spectrum are the cause or an effect of the disease is poorly understood.
In our ongoing studies, we use two models to analyze the effects of M. leprae infection in mice. In the low dose growth model, published by Shepard in 1960,39 an inoculum of a few thousand M. leprae grows locally and plateaus with the onset of adaptive immunity by ≤6 months post-infection in immunocompetent mice. For many years, this infection model was the only method available for determining M. leprae viability and growth, and it was used to study basic immunological mechanisms and evaluate new anti-leprosy drugs.40 We use this low dose model to compare growth of M. leprae among the KO strains, as well as to evaluate histopathological and immunohistochemical responses in the FP.
Recently, we described a second model loosely based on the Lepromin Test.25 Unlike the 48-hours purified protein derivative test, which is diagnostic for M. tuberculosis exposure, the Lepromin Test in humans measures the ability to mount a granulomatous response to a large inoculum of dead M. leprae, and induration is generally measured at 28 days. The Lepromin Test has been used both clinically and in research settings to assess the host’s capacity to mount a CMI response to the bacilli.38 In our model, we inoculate a high dose of viable M. leprae into the FP. In immunocompetent mice (25 this study, unpublished data), FP induration peaks and plateaus at approximately 28 days; however, we have found that the response in many strains of immunocompromised mice evolves dramatically over the course of several weeks, so we routinely monitor the infections for a longer duration. Furthermore, the FP and the draining popliteal LNs develop distinct and characteristic cellular profiles.25 The FP, which contains immune cells recruited to this site only after M. leprae infection, consists of macrophages and effector T cells, primarily CD4+; whereas, the LN is comprised of B cells and naive CD4+ and CD8+ T lymphocytes.
Since cytokines, as well as chemokines and CAMs, are instrumental for the induction, function, regulation, and maintenance of granulomas,1 we have used our two models to examine M. leprae growth and especially granuloma formation in mouse strains with knockouts in various cytokines important in CMI, including IFNγ−/−, IL-12p40−/−, and TNF−/−, as well as NOS2−/− mice. While the contribution of each of the cytokines to CMI is well established, none of these knockouts allowed an unrestricted growth of the bacilli as occurs when CMI is totally dysfunctional, such as in the athymic nu/nu mouse in which bacterial counts reach ≥1010 AFB.38 Yet, the granulomatous responses that developed in the infected FP, and the cytokine and chemokine profiles generated, were quite distinct among the various knockouts. M. leprae-infected IFNγ−/− mice developed a large, unorganized cellular infiltration in the FP and a TH2-type cytokine profile.29 In contrast, NOS2−/− FP exhibited a granulomatous inflammation that infiltrated the musculature of the foot and a Th1-type cytokine and chemokine response.28 Of note, as shown here, M. leprae-infected TNF-deficient mice generated an intense and diffuse T cell inflammation and growth of the bacilli was augmented throughout infection, phenomena quite different from those observed in the LTα-deficient mice. These data indicate that TNF and LTα exert dramatically different effects in the execution of CMI in experimental leprosy. Our results also suggest that signaling via TNFR1 is primarily a function of TNF rather than LTα, as LTα did not compensate for the lack of TNF in the TNF−/− mice. These findings are currently being explored further.
Cytokines also influence chemokine gradients, which facilitate the temporal and spatial coordination of antigen-presenting cells and Ag-specific T cells in granulomas. In humans, CCL-3 and CCL-4 are chemoattractants for CD4+ and CD8+ lymphocytes, respectively.41 CCL2 promotes macrophage migration, and CCL5 and CXCL10 induce activated T cell migration to the granulomas.1 The contribution of TNF especially to the regulation of M. tuberculosis-induced granuloma structural organization and maintenance via the induction of chemokines has been extensively studied42,43,44; LTα3 can also mediate chemokine expression.45 In addition, LTα3induces the expression of CAMs, such as VCAM and ICAM, on endothelial cells,45 which are up-regulated by IFNγ.46 Moreover, because in vivo administration of a transgenic LT preparation induced ICAM and VCAM expression, it was postulated that LT is an initiating factor in chronic inflammation.8 Therefore, LTα is not only critically involved in the early steps of effector T cell generation via modulation of cytokine induction, but also in the effective recruitment of macrophage and T cells by regulating chemotactic signals and CAM expression, all of which could significantly alter appropriate granuloma formation. In our model, M. leprae-infected LTα-deficient mice generated a markedly depressed cytokine, chemokine, and CAM response and were unable to optimally develop effector CD4+ T cells and maintain an inflammatory accumulation around this persistent, virtually indestructible organism.
The poor granulomatous response in the FP of M. leprae infected cLTα−/− mice is reminiscent of that in the lungs of M. tuberculosis infected cLTα−/− mice.13 Although CD4+ cells migrated to the lung, they collected in the perivascular and peribronchial regions and not in the well-defined granulomas characteristic of wild-type mice. Lung lesions in the M. tuberculosis-infected cLTα−/− mice, however, consisted of neutrophils and necrotic material; in contrast, we saw little neutrophil accumulation in the M. leprae-infected FP. This difference may reflect the rather innocuous nature of the leprosy bacillus compared with the more proinflammatory tubercle bacillus. In addition, growth of M. tuberculosis in the lungs was enhanced >3 log10 in cLTα−/− while M. leprae growth in the FP was only enhanced late in infection. It is noteworthy that despite the poor infiltration of mononuclear cells into the site of infection, the bacilli survived and multiplied in the cLTα−/− FP suggesting that a large macrophages response is not necessary to support the growth or persistence of M. leprae. This is not unreasonable as a single macrophage can accommodate more than 100 viable M. leprae and remain physiologically normal and functional.47,48 Furthermore, in immunocompetent mouse FP as well as in some KO mouse models, which on M. leprae infection develop an enlarged FP with a strong macrophage influx (eg, IFNγ−/−, NOS2−/−), a large percentage of these cells are, in fact, uninfected (unpublished data).
These findings are especially interesting in light of a recent study in human leprosy, which, using whole genome based genetic approaches and positional cloning, identified a polymorphism, LTA + 80 located in the promoter region of the gene encoding LTα, that was significantly associated with an increase in leprosy risk.19 This association was seen in patients of three different ethnic populations (Brazilian, Vietnamese, and Indian) and was strongest in persons <16 years of age. Importantly, this study provided strong evidence that susceptibility to this complex disease is dependent on a genetic predisposition in the host.49 This genetic susceptibility could influence the early responses to M. leprae, a pathogen extremely dependent on host cellular processes for its survival, and control key immunoregulatory events that determine the long term outcome of this slowly progressing infection. Our results correlate this clinically identified susceptibility gene for leprosy with a significantly reduced granulomatous response and growth control in an experimental model of chronic infection. Studying the immunoregulatory mechanisms of leprosy, both in the subclinical and unstable borderline areas of the spectrum, should contribute to our understanding of the complex interactions between pathogen and host.
Supplementary Material
Footnotes
Address reprint requests to Linda B. Adams, DHHS, HRSA, BPHC, National Hansen’s Disease Programs Laboratory Research Branch at LSU-SVM, Skip Bertman Drive, Baton Rouge, LA 70803. E-mail: ladams1@lsu.edu.
Supported by National Institutes of Health (NIH) grant AI-50027 (L. Adams) and the NHMRC of Australia (B. Saunders and W. Britton).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address for D.A.H. is Mycobacterial Research Laboratories, Anandaban Hospital, Kathmandu, Nepal.
References
- Boros DL. Boros DL, editor. Washington DC: ASM Press,; The cellular immunological aspects of the granulomatous response. 2003:pp. 1–28. [Google Scholar]
- Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Saunders BM, Tran S, Ruuls S, Sedgwick JD, Briscoe H, Britton WJ. Transmembrane TNF is sufficient to initiate cell migration and granuloma formation and provide acute, but not long-term, control of Mycobacterium tuberculosis infection. J Immunol. 2005;174:4852–4859. doi: 10.4049/jimmunol.174.8.4852. [DOI] [PubMed] [Google Scholar]
- Zganiacz A, Santosuosso M, Wang J, Yang T, Chen L, Anzulovic M, Alexander S, Gicquel B, Wan Y, Bramson J, Inman M, Xing Z. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest. 2004;113:401–413. doi: 10.1172/JCI18991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins. LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M. The evolving crosstalk between co-stimulatory and co-inhibitory receptors: hVEM-BTLA. Trends Immunol. 2005;26:292–294. doi: 10.1016/j.it.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Clark K, Kulk N, Amante F, Haque A, Engwerda C. Lymphotoxin alpha and tumour necrosis factor are not required for control of parasite growth, but differentially regulate cytokine production during Plasmodium chabaudi chabaudi AS infection. Parasite Immunol. 2007;29:153–158. doi: 10.1111/j.1365-3024.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- Engwerda CR, Ato M, Stager S, Alexander CE, Stanley AC, Kaye PM. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in the control of Leishmania donovani infection. Am J Pathol. 2004;165:2123–2133. doi: 10.1016/s0002-9440(10)63262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund FE, Partida-Sanchez S, Lee BO, Kusser KL, Hartson L, Hogan RJ, Woodland DL, Randall TD. Lymphotoxin-alpha-deficient mice make delayed, but effective. T and B cell responses to influenza. J Immunol. 2002;169:5236–5243. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
- Roach DR, Briscoe H, Saunders B, France MP, Riminton S, Britton WJ. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J Exp Med. 2001;193:239–246. doi: 10.1084/jem.193.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach DR, Briscoe H, Saunders BM, Britton WJ. Independent protective effects for tumor necrosis factor and lymphotoxin alpha in the host response to Listeria monocytogenes infection. Infect Immun. 2005;73:4787–4792. doi: 10.1128/IAI.73.8.4787-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D, Kwok LY, Lutjen S, Soltek S, Hoffmann S, Korner H, Deckert M. Both lymphotoxin-alpha and TNF are crucial for control of Toxoplasma gondii in the central nervous system. J Immunol. 2003;170:6172–6182. doi: 10.4049/jimmunol.170.12.6172. [DOI] [PubMed] [Google Scholar]
- Vonk AG, Netea MG, van Krieken JH, van der Meer JW, Kullberg BJ. Delayed clearance of intraabdominal abscesses caused by Candida albicans in tumor necrosis factor-alpha- and lymphotoxin-alpha-deficient mice. J Infect Dis. 2002;186:1815–1822. doi: 10.1086/345818. [DOI] [PubMed] [Google Scholar]
- Bleharski JR, Li H, Meinken C, Graeber TG, Ochoa MT, Yamamura M, Burdick A, Sarno EN, Wagner M, Rollinghoff M, Rea TH, Colonna M, Stenger S, Bloom BR, Eisenberg D, Modlin RL. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science. 2003;301:1527–1530. doi: 10.1126/science.1087785. [DOI] [PubMed] [Google Scholar]
- Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Alcais A, Alter A, Antoni G, Orlova M, Nguyen VT, Singh M, Vanderborght PR, Katoch K, Mira MT, Vu HT, Ngyuen TH, Nguyen NB, Moraes M, Mehra N, Schurr E, Abel L. Stepwise replication identifies a low-producing lymphotoxin-alpha allele as a major risk factor for early-onset leprosy. Nat Genet. 2007;39:517–522. doi: 10.1038/ng2000. [DOI] [PubMed] [Google Scholar]
- Korner H, Cook M, Riminton DS, Lemckert FA, Hoek RM, Ledermann B, Kontgen F, Fazekas de St Groth B, Sedgwick JD. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur J Immunol. 1997;27:2600–2609. doi: 10.1002/eji.1830271020. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Matsumoto M, Baranyay F, Nahm MH, Kanagawa O, Chaplin DD. Absence of lymph nodes in lymphotoxin-alpha (LT alpha)-deficient mice is due to abnormal organ development, not defective lymphocyte migration. J Inflamm. 1995;45:72–78. [PubMed] [Google Scholar]
- Riminton DS, Korner H, Strickland DH, Lemckert FA, Pollard JD, Sedgwick JD. Challenging cytokine redundancy: inflammatory cell movement and clinical course of experimental autoimmune encephalomyelitis are normal in lymphotoxin-deficient, but not tumor necrosis factor-deficient, mice. J Exp Med. 1998;187:1517–1528. doi: 10.1084/jem.187.9.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman RW, Krahenbuhl JL. Viable M. leprae as a research reagent. Int J Lepr Other Mycobact Dis. 2001;69:1–12. [PubMed] [Google Scholar]
- Lahiri R, Randhawa B, Krahenbuhl J. Application of a viability-staining method for Mycobacterium leprae derived from the athymic (nu/nu) mouse foot pad. J Med Microbiol. 2005;54:235–242. doi: 10.1099/jmm.0.45700-0. [DOI] [PubMed] [Google Scholar]
- Hagge DA, Marks VT, Ray NA, Dietrich MA, Kearney MT, Scollard DM, Krahenbuhl JL, Adams LB. Emergence of an effective adaptive cell mediated immune response to Mycobacterium leprae is not impaired in reactive oxygen intermediate-deficient mice. FEMS Immunol Med Microbiol. 2007;51:92–101. doi: 10.1111/j.1574-695X.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Ridley DS. A logarithmic index of bacilli in biopsies. 2. Evaluation Int J Lepr Other Mycobact Dis. 1967;35:187–193. [PubMed] [Google Scholar]
- Lockwood DN, Lucas SB, Desikan KV, Ebenezer G, Suneetha S, Nicholls P. The histological diagnosis of leprosy type 1 reactions: identification of key variables and an analysis of the process of histological diagnosis. J Clin Pathol. 2008;61:595–600. doi: 10.1136/jcp.2007.053389. [DOI] [PubMed] [Google Scholar]
- Adams LB, Job CK, Krahenbuhl JL. Role of inducible nitric oxide synthase in resistance to Mycobacterium leprae in mice. Infect Immun. 2000;68:5462–5465. doi: 10.1128/iai.68.9.5462-5465.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams LB, Scollard DM, Ray NA, Cooper AM, Frank AA, Orme IM, Krahenbuhl JL. The study of Mycobacterium leprae infection in interferon-gamma gene-disrupted mice as a model to explore the immunopathologic spectrum of leprosy. J Infect Dis. 2002;185 Suppl 1:S1–S8. doi: 10.1086/338002. [DOI] [PubMed] [Google Scholar]
- Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317:622–625. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- Dobrzanski MJ, Reome JB, Hylind JC, Rewers-Felkins KA. CD8-mediated type 1 antitumor responses selectively modulate endogenous differentiated and nondifferentiated T cell localization, activation, and function in progressive breast cancer. J Immunol. 2006;177:8191–8201. doi: 10.4049/jimmunol.177.11.8191. [DOI] [PubMed] [Google Scholar]
- Feng CG, Zheng L, Jankovic D, Bafica A, Cannons JL, Watford WT, Chaussabel D, Hieny S, Caspar P, Schwartzberg PL, Lenardo MJ, Sher A. The immunity-related GTPase Irgm1 promotes the expansion of activated CD4(+) T cell populations by preventing interferon-gamma-induced cell death. Nat Immunol. 2008;9:1279–1287. doi: 10.1038/ni.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemball CC, Harkins S, Whitton JL. Enumeration and functional evaluation of virus-specific CD4+ and CD8+ T cells in lymphoid and peripheral sites of coxsackievirus B3 infection. J Virol. 2008;82:4331–4342. doi: 10.1128/JVI.02639-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan L, Gurnani K, Dicaire CJ, van Faassen H, Zafer A, Kirschning CJ, Sad S, Sprott GD. Rapid clonal expansion and prolonged maintenance of memory CD8+ T cells of the effector (CD44highCD62Llow) and central (CD44highCD62Lhigh) phenotype by an archaeosome adjuvant independent of TLR2. J Immunol. 2007;178:2396–2406. doi: 10.4049/jimmunol.178.4.2396. [DOI] [PubMed] [Google Scholar]
- Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, Fountain JJ, Martino CA, Roberts AD, Cooper AM, Winslow GM, Woodland DL. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc Natl Acad Sci USA. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard CC. The experimental disease that follows the injection of human leprosy bacilli into foot-pads of mice. J Exp Med. 1960;112:445–454. doi: 10.1084/jem.112.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L, Ji B. The mouse foot-pad technique for cultivation of Mycobacterium leprae. Lepr Rev. 2006;77:5–24. [PubMed] [Google Scholar]
- Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41 Suppl 3:S189–S193. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- Smith S, Liggitt D, Jeromsky E, Tan X, Skerrett SJ, Wilson CB. Local role for tumor necrosis factor alpha in the pulmonary inflammatory response to Mycobacterium tuberculosis infection. Infect Immun. 2002;70:2082–2089. doi: 10.1128/IAI.70.4.2082-2089.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff CA, Sacca R, Ruddle NH. Differential induction of adhesion molecule and chemokine expression by LTalpha3 and LTalphabeta in inflammation elucidates potential mechanisms of mesenteric and peripheral lymph node development. J Immunol. 1999;162:5965–5972. [PubMed] [Google Scholar]
- Weiser S, Miu J, Ball HJ, Hunt NH. Interferon-gamma synergises with tumour necrosis factor and lymphotoxin-alpha to enhance the mRNA and protein expression of adhesion molecules in mouse brain endothelial cells. Cytokine. 2007;37:84–91. doi: 10.1016/j.cyto.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Hagge DA, Ray NA, Krahenbuhl JL, Adams LB. An in vitro model for the lepromatous leprosy granuloma: fate of Mycobacterium leprae from target macrophages after interaction with normal and activated effector macrophages. J Immunol. 2004;172:7771–7779. doi: 10.4049/jimmunol.172.12.7771. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Krahenbuhl JL. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma interferon. Infect Immun. 1987;55:446–450. doi: 10.1128/iai.55.2.446-450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter A, Alcais A, Abel L, Schurr E. Leprosy as a genetic model for susceptibility to common infectious diseases. Hum Genet. 2008;123:227–235. doi: 10.1007/s00439-008-0474-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.