Abstract
Whereas the effects of water and nitrogen (N) on plant Δ13C have been reported previously, these factors have scarcely been studied for Δ18O. Here the combined effect of different water and N regimes on Δ13C, Δ18O, gas exchange, water-use efficiency (WUE), and growth of four genotypes of durum wheat [Triticum turgidum L. ssp. durum (Desf.) Husn.] cultured in pots was studied. Water and N supply significantly increased plant growth. However, a reduction in water supply did not lead to a significant decrease in gas exchange parameters, and consequently Δ13C was only slightly modified by water input. Conversely, N fertilizer significantly decreased Δ13C. On the other hand, water supply decreased Δ18O values, whereas N did not affect this parameter. Δ18O variation was mainly determined by the amount of transpired water throughout plant growth (Tcum), whereas Δ13C variation was explained in part by a combination of leaf N and stomatal conductance (gs). Even though the four genotypes showed significant differences in cumulative transpiration rates and biomass, this was not translated into significant differences in Δ18Os. However, genotypic differences in Δ13C were observed. Moreover, ∼80% of the variation in biomass across growing conditions and genotypes was explained by a combination of both isotopes, with Δ18O alone accounting for ∼50%. This illustrates the usefulness of combining Δ18O and Δ13C in order to assess differences in plant growth and total transpiration, and also to provide a time-integrated record of the photosynthetic and evaporative performance of the plant during the course of crop growth.
Keywords: Δ13C and Δ18O, leaf gas exchange, water and nitrogen limitation, wheat, WUE
Introduction
In recent decades, stable carbon and oxygen isotopes have been shown to be powerful non-invasive probes for characterizing photosynthetic metabolism in plants. It has been known for some time that the carbon isotope composition of plant dry matter (δ13C), which is frequently expressed as the discrimination value (Δ13C), provides a time-integrated measurement of the plant's transpiration efficiency (i.e. the ratio of carbon gain to water transpired) over the period during which dry matter is assimilated. Indeed, it is >20 years since Δ13C was first proposed as a potential tool for screening wheat genotypes with higher transpiration efficiency (Farquhar and Richards, 1984; Rebetzke et al., 2002), and the first genotypes selected using this approach were subsequently released in Australia (Rebetzke et al., 2002; Condon et al., 2004). Whereas increased water input has been widely reported to have a positive effect on Δ13C (Araus et al., 2003; Condon et al., 2004), there are contradictory reports as to how the amount of nitrogen (N) fertilization affects Δ13C. Thus, for wheat and other cereals, Δ13C has been reported to increase (Shangguan et al., 2000), decrease (Choi et al., 2005; Cabrera-Bosquet et al., 2007; Zhao et al., 2007; Serret et al., 2008), or not be affected (Hubick, 1990; White et al., 1990) as a result of N supply.
The oxygen isotope composition (δ18O) of organic matter is known to reflect variation in: (i) the isotopic composition of source water; (ii) evaporative enrichment in leaves due to transpiration; and (iii) biochemical fractionation during synthesis of organic matter (Craig and Gordon, 1965; Dongmann et al., 1974; Yakir, 1992; Farquhar and Lloyd, 1993). Hence, the oxygen isotope signature of plant matter, expressed either as composition (δ18O) or as enrichment above source water (Δ18O), has been used to assess the leaf evaporative conditions at the time the material was formed (Yakir et al., 1990; Yakir, 1992; Saurer et al., 1997; Barbour et al., 2000a, b; Barbour, 2007). The leaf oxygen isotope signature has been negatively correlated with relative humidity (Saurer et al., 1997; Barbour et al., 2000b; Ferrio et al., 2007) and also with transpiration rate (Barbour and Farquhar, 2000; Barbour et al., 2000a). Conversely, Sheshshayee et al. (2005) reported a positive relationship between Δ18O and the transpiration rate in groundnut and rice genotypes that contrasts with the present theory. Moreover, Barbour et al. (2000a) have proposed the use of the stable oxygen isotope signature (in their study expressed as δ18O) measured in plant matter as an integrative indicator of genetic differences in stomatal conductance (gs), as well as a predictor of grain yield in field-grown wheat. Therefore, the 18O signature of plant tissue is of interest in terms of breeding for improved water use, and genotypic variability for this trait has been identified in bread wheat (Barbour et al., 2000a; Farquhar et al., 2007; Ferrio et al., 2007). As plant material has been shown to record leaf evaporative conditions, measurement of the 18O signature might provide a powerful tool for plant breeders (Barbour, 2007), to track genotypic differences not only in stomatal conductance but also in yield. However, up to now, most studies dealing with the use of 18O for breeding have been conducted under well-watered conditions (Barbour et al., 2000 a, b; Bindumadhava et al., 2005; Sheshshayee et al., 2005), even if the plants in question have been grown under different evaporative demand (Barbour and Farquhar, 2000; Bindumadhava et al., 2006). Research involving the 18O signature under water-limited conditions is far scarcer (Yakir et al., 1990; Ferrio et al., 2007), although genotypic differences have been identified. Furthermore, the combined effect of N fertilizer and water regime on the δ18O oxygen isotope composition of plant material has not been assessed, despite the fact that the amount of N fertilization may affect the photosynthetic (Lawlor, 2001) and transpiration rates (Claus et al., 1993) in wheat and other cereals.
In terms of surface area, cereals are the main crops in the Mediterranean basin, occupying preferably the dry lands. Among cereals, durum wheat is the most widely grown in the South Mediterranean basin and is a very important crop in the European agro-food industry. In these environments, water limitation, which is frequently accompanied by low N availability, is the main constraint on wheat yield (Oweis et al., 1998; Araus et al., 2002; Passioura, 2002). Selecting genotypes better suited to the lack of water (Condon et al., 2004) and N (Hirel et al., 2007) is therefore one of the main targets for increasing yield in these environments. The use of physiological traits such as phenotypical criteria for selecting genotypes better adapted to such growing conditions might help plant breeders (Bänziger et al., 2000; Araus et al., 2002; Lafitte et al., 2003). The best potential traits to use provide time-integrated (i.e. through the crop cycle) information on plant performance (Araus et al., 2002). Among these traits the signature of the stable carbon and oxygen isotopes in plant matter reflects the photosynthetic (Farquhar et al., 1989) and transpirative (Barbour 2007; Farquhar et al., 2007) conditions in which the plants were grown (Araus et al., 1998, 2003).
The aim of the present research was to study the combined effect of different water and N regimes on Δ13C, Δ18O, water-use efficiency (WUE), and growth of four contrasting genotypes of durum wheat. The usefulness of combining Δ13C and Δ18O measurements in shoot dry matter to track differences in WUE, leaf gas exchange, cumulative transpiration, and growth in these plants was also tested.
Materials and methods
Plant material and growth conditions
Three durum wheat [Triticum turgidum L. ssp. durum (Desf.) Husn.] genotypes (Bicrecham-1, Lahn/Haucan, and Omrabi-3) released by the CIMMYT/ICARDA durum wheat breeding programme, plus one of the most cultivated Spanish varieties (Mexa) were grown in a greenhouse at the University of Barcelona, from December 2004 to April 2005. These four genotypes were chosen on the basis of their contrasting yield performance under Mediterranean conditions (Villegas et al., 2001; Ferrio et al., 2005). Fourteen seeds of each genotype were planted per pot (5.0 L, filled with washed sand). After germination, they were thinned to seven plants per pot, representing a plant density of ∼223 plants m−2, and watered daily with deionized water for 2 weeks. After this, three different water regimes and two N levels were imposed, by applying nutrient solution every 2 d. The water levels were achieved by weighing the pots prior to watering, and then adding the amount of water needed to reach 40, 70, and 100% of their container capacity (CC). The N supply was controlled using two different nutrient solutions: complete Hoagland solution (Hoagland and Arnon, 1950) and the same solution with N diluted four times (i.e. 0.06725 g N L−1 and 0.27 g N L−1 for the low and high N, respectively). Pots with different treatments were displayed in a randomized complete block design. Each treatment was replicated four times and the whole experimental set-up accounted for a total of 96 pots. Plants were grown in the greenhouse under mean day/night temperatures of ∼25/15 °C, a maximum photosynthetic photon flux density (PPFD) of ∼1000 μmol m−2 s−1, and a mean vapour pressure deficit (VPD) of 0.75 kPa.
Leaf gas exchange measurements
Gas exchange of the flag leaf blade was measured ∼2 weeks after anthesis, just before harvesting, using an open IRGA LI-COR 6400 system (LI-COR Inc., Lincoln, NE, USA). Photosynthetic measurements were performed under light-saturated conditions (1000 μmol photon m−2 s−1 of PPFD), at 25 °C and 400 μmol mol−1 of CO2. The measured gas exchange parameters were: light-saturated net CO2 assimilation rate (Asat); transpiration rate (E); and stomatal conductance (gs). Then the ratio of intercellular to ambient CO2 concentration (Ci/Ca) was calculated according to Sharkey and Raschke (1981).
Determination of cumulative transpiration and water status
The amount of water evapotranspired was monitored throughout the experiment by weighing each pot just prior to watering. The pots were then adjusted to their water regime (40, 70, and 100% CC) by adding nutrient solution to maintain the experimental design. Simultaneously, empty pots without plants were also weighed to record direct evaporation from the soil. Then, the cumulative transpiration (Tcum) was calculated as the difference between evapotranspiration and evaporation.
Water status was determined by means of measurements on relative water content (RWC) in the flag leaf blades 1 d after the last watering and just before harvesting. Leaf blade segments were weighed (fresh weight; FW), floated on distilled water at 4 °C overnight, and weighed again (TW). They were then dried at 80 °C for 48 h. After this, the dry weight (DW) was determined. RWC was then calculated as:
| (1) |
Water-use efficiency
WUE was calculated using both instantaneous and time-integrated measurements. The time-integrated WUE, WUEbiomass, was calculated as the ratio of total aboveground biomass (AB) to evapotranspired water throughout plant growth. Moreover, instantaneous WUE (WUEinstantaneous) and intrinsic WUE (WUEintrinsic) were calculated as the ratio of Asat to E and gs, respectively.
Total nitrogen content and carbon isotope analyses
For each analysis, 1 mg of fine powdered shoot, spike, flag leaf, or root tissue was weighed in tin cups. The total N content of samples was analysed at the Colorado Plateau Stable Isotope Laboratory (CPSIL) using an Elemental Analyser (EA) (Carlo Erba 2100, Milan, Italy). The same EA interfaced with an isotope ratio mass spectrometer (IRMS) (Thermo-Finnigan Deltaplus Advantage, Bremen, Germany) was also used to analyse 13C/12C ratios (R) of plant material. Results were expressed as δ13C values, using a secondary standard calibrated against Vienna Pee Dee Belemnite calcium carbonate (VPDB), and the analytical precision was ∼0.1‰.
| (2) |
The carbon isotope discrimination (Δ13C) of plant parts was then calculated from δa and δp (Farquhar et al., 1989) as:
| (3) |
where a and p refer to air and the plant, respectively. Air samples inside the greenhouse were taken and analysed by the gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS) technique as previously described (Nogués et al., 2008). This analysis was undertaken at the Scientific Facilities of the University of Barcelona. The carbon isotope composition of the air measured within the greenhouse was δ13C=–11.3‰.
Oxygen isotope analyses
The oxygen isotope composition was determined in shoots (including leaves and stems), since this plant part was considered as the most representative because it makes up a significant proportion of the dry weight in the plant. In addition, Δ13C of shoots integrates the photosynthetic performance and the water status of the plant during their growth better than other plant parts (Tambussi et al., 2007a).
The 18O/16O ratios of irrigation water were determined by the CO2:H2O equilibration technique and using an IRMS (Delta S Finnigan MAT, Bremen, Germany). The 18O/16O ratios of shoot dry matter were determined by an on-line pyrolysis technique using a thermo-chemical elemental analyser (TC/EA Thermo Quest Finnigan, Bremen, Germany) coupled with an IRMS (Delta C Finnigan MAT, Bremen, Germany). Results were expressed as δ18O values, using a secondary standard calibrated against the Vienna standard mean oceanic water (VSMOW), and the analytical precision was ∼0.2‰.
| (4) |
Then, following the same notation used for carbon isotope discrimination, the 18O enrichment in shoot parts (Δ18Os) was calculated as follows:
| (5) |
where δ18Os and δ18Oiw refer to the oxygen isotope compositions of shoots and irrigation water, respectively (δ18Oiw was approximately –6.5‰.). The 18O/16O ratios of both irrigation water and shoot dry matter were calculated at the Scientific Facilities of the University of Barcelona.
Biomass determination
Total biomass was collected ∼2 weeks after anthesis. The spike, flag leaf, the rest of the shoot (including leaves and stems), and the root were separated and oven-dried at 80 °C for 48 h, before being weighed and powdered. The leaf dry mass per unit leaf area (LDM) of the flag leaf blades was calculated as the ratio between dry weight and leaf surface (g m−2). Moreover, the specific leaf nitrogen (SLN) of the flag leaf blades was calculated as the ratio between dry N content in dry matter and the leaf surface (g m−2).
Statistical analysis
Analysis of variance (ANOVA) was performed using the General Linear Model procedure to calculate the effects of water and genotype within each N treatment. Means were compared using a Tukey-b multiple comparison test (P <0.05). The bivariate correlation procedure was used to calculate the Pearson correlation coefficients. Multiple linear regression analysis (stepwise) was used to analyse the relationship between the variables studied. Linear stepwise models were built from water and genotype means within each N treatment. Data were analysed using the SPSS statistical package (SPSS Inc., Chicago, IL, USA).
Results
Plant growth and N content
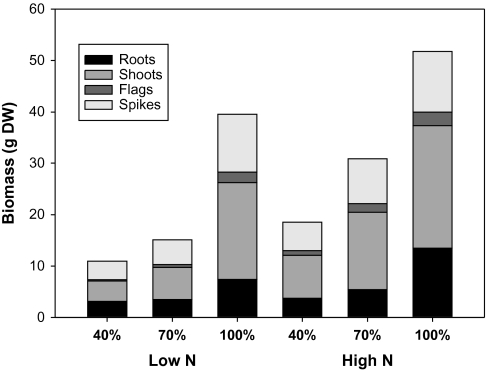
Large differences in total AB and root biomass, as well as in the different components of AB, were observed in response to the different levels of water supply and N fertilization studied (Table 1, Fig. 1). Accumulated transpiration (Tcum) also changed greatly in response to both N and water treatments (Table 1). Thus, AB, spike biomass (SB), and Tcum in control plants (i.e. high N and 100% CC) were five, three, and three times higher, respectively, than in plants grown under the most limiting conditions (i.e. low N and 40% CC, Table 1). However, the effect of water regime was greater in the low than in the high N regime; in fact, there were two-way significant interactions between water regime and N levels for total AB, SB, and Tcum. In addition, large genotypic differences in these three traits were also observed. Omrabi-3 was the genotype with highest AB and Tcum in both N treatments, whilst Mexa had the lowest values. Bicrecham-1 and Lahn/Haucan had intermediate values (Table 1). However, whereas interactions of genotypes with both N and water conditions were not significant for Tcum, these interactions did reach significance for total biomass, although only between genotypes and N regime. LDM values were affected by genotype but not by water regime and N, and the only significant interaction was between genotype and water regime.
Table 1.
Mean values for plant growth and gas exchange parameters of four wheat genotypes (G) subjected to different water regimes (WR) and grown under low and high nitrogen
Data are the mean of 16 (WR) or 12 (G) replicates. Within each nitrogen treatment and within each water regime (40, 70. and 100% CC) or genotype (Mexa, Bicrecham-1, Lahn/Haucan. and Omrabi-3); values with different letters are significantly different according to the Tukey-b test (P <0.05).
| AB | SB | RWC | LDM | SLN | LN | SN | Tcum | Asat | E | gs | Ci/Ca | |
| Low N | ||||||||||||
| WR | ||||||||||||
| 40% | 7.9 c | 3.6 c | 85.1 b | 31.7 a | 1.1 b | 3.6 b | 1.8 a | 2.58 c | 16.2 b | 3.1 a | 0.32 a | 0.72 a |
| 70% | 11.6 b | 4.8 b | 90.5 a | 34.3 a | 1.4 a | 4.1 a | 1.9 a | 3.67 b | 18.6 a | 3.7 a | 0.37 a | 0.72 a |
| 100% | 32.2 a | 11.2 a | 91.3 a | 33.2 a | 1.4 a | 4.1 a | 1.9 a | 8.21 a | 17.4 a,b | 3.0 a | 0.30 a | 0.68 a |
| G | ||||||||||||
| Mexa | 14.9 b | 7.5 a | 87.7 a | 30.8 b | 1.0 c | 3.4 c | 1.9 a | 4.48 b | 15.4 b | 2.9 a | 0.30 a | 0.72 a |
| Bic-1 | 16.1 b | 6.4a | 87.9 a | 32.9 a.b | 1.3 b | 3.9 b | 1.9 a | 4.63 b | 16.9 b | 3.3 a | 0.34 a | 0.72 a |
| L/H | 17.2 b | 7.1 a | 89.8 a | 33.4 a,b | 1.3 b | 4.0 b | 1.9 a | 4.65 b | 16.4 b | 3.4 a | 0.30 a | 0.70 a |
| Omr-3 | 20.7 a | 5.2 b | 90.4 a | 35.2 a | 1.6 a | 4.5 a | 1.8 a | 5.51 a | 21.0 a | 3.5 a | 0.38 a | 0.69 a |
| High N | ||||||||||||
| WR | ||||||||||||
| 40% | 14.8 c | 5.6 c | 87.5 a | 32.1 a | 1.4 b | 4.3 a | 2.8 c | 3.25 c | 14.1 b | 2.1 b | 0.19 b | 0.62 a |
| 70% | 25.5 b | 8.7 b | 88.7 a | 34.7 a | 1.5 a,b | 4.4 a | 3.0 b | 5.56 b | 15.6 a,b | 2.3 b | 0.23 a,b | 0.64 a |
| 100% | 38.3 a | 11.8 a | 90.7 a | 34.9 a | 1.6 a | 4.5 a | 3.2 a | 7.85 a | 18.1 a | 2.9 a | 0.29 a | 0.66 a |
| G | ||||||||||||
| Mexa | 20.1 c | 10.0 a | 86.0 b | 31.1 b | 1.2 c | 4.0 c | 2.5 b | 5.03 b | 16.1 a | 2.8 a | 0.28 a | 0.69 a |
| Bic-1 | 26.9 b | 9.7 a | 91.2 a,b | 36.2 a | 1.6 a,b | 4.4 b | 3.2 a | 5.80 a,b | 15.8 a | 2.4 a | 0.24 a | 0.64 a |
| L/H | 24.5 b | 8.3 b | 88.5 a,b | 33.5 a,b | 1.4 b | 4.3 b | 3.0 a | 5.29 a,b | 15.4 a | 2.3 a | 0.22 a | 0.63 a |
| Omr-3 | 33.3 a | 6.9 c | 90.2 a | 34.7 a,b | 1.7 a | 4.9 a | 3.2 a | 6.10 a | 16.4 a | 2.2 a | 0.20 a | 0.58 b |
AB, aboveground biomass (g dry weight); SB, spike biomass (g dry weight); RWC, relative water content (%); SLN, specific leaf N (g m−2); LDM, leaf dry mass per unit leaf area (g m−2); LN, flag leaf nitrogen content (%); SN, shoot nitrogen content (%); Tcum, cumulative transpiration (L); Asat, light-saturated net CO2 assimilation rate (μmol CO2 m−2 s−1); gs, stomatal conductance (mol CO2 m−2 s−1); E, transpiration rate (mmol H2O m−2 s−1); Ci and Ca, intercellular and ambient CO2 concentrations.
Fig. 1.
Effect of water and nitrogen treatments on root, shoot, flag, and spike biomass. Values are the mean of 16 replicates.
The results also showed that the N content of shoots on a dry matter basis (SN), as well as that of the flag leaf per unit leaf area (SLN) and dry weight (LN), increased in response to N supply and, to a lesser extent, as water increased (Table 1). In addition, genotypic differences in the N content of shoots and the flag leaf were found. In both N treatments, Omrabi-3 was the genotype with the highest SLN and LN in the flag leaf, whereas Mexa had the lowest values. SN was also the highest and the lowest in Omrabi-3 and Mexa, respectively, but only under high N conditions. In fact, SN showed a genotypic interaction between genotype and both water and N treatments, whereas LN did not show any interactions between genotypes and growing conditions; for SLN, only interactions between genotype and water regime were observed (Table 1).
Leaf gas exchange
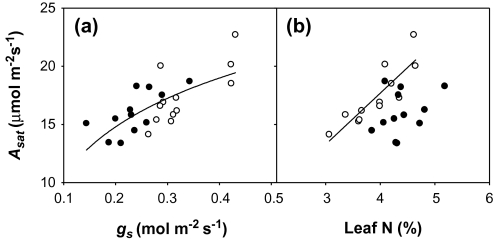
Water supply had a significant positive effect on light-saturated net CO2 assimilation (Asat) but not on stomatal conductance (gs) or transpiration (E). However, for all three parameters there was a significant interaction between water regime and N levels. Thus, Asat, gs, and E increased (28, 52, and 38%, respectively) with water supply under high N treatments, whereas no differences were found in plants grown with a low N supply (Table 1). The Ci/Ca ratio did not change significantly in response to water treatment or according to the N regime. The N regime did have a significant effect on the four gas exchange parameters shown in Table 1. Hence, higher values of Asat, gs, Ci/Ca, and E were found in the low N compared with the high N treatment. Genotypic differences for Asat and Ci/Ca were observed, but, whereas the interaction between genotype and water regime was not significant, that between genotype and N regime was. Thus, at low N, Omrabi-3 showed higher values of Asat compared with the other three genotypes, while at high N no differences between the four genotypes were observed. Conversely, for Ci/Ca, Omrabi-3 showed lower values than the other genotypes at high N, while no differences were observed at low N. Differences across treatments and genotypes in net photosynthesis were mediated mostly by differences in stomatal conductance, as shown by the relationship between Asat and gs when all the treatments were plotted together (Fig. 2a). In contrast, Asat and LN were only significantly correlated at low N (Fig. 2b).
Fig. 2.
(a) Relationship between Asat and gs, y=21.3(1–e–6.03x); r=0.76, P <0.0001. (b) Relationship between Asat and leaf N; low N treatment, y=3.74xLN–2.68; r=0.66, P <0.01. Open and filled circles represent, respectively, the low and high N treatment means for each genotype and water condition. Each value is the mean of four replicates.
Water status and WUE
Water supply increased the RWC values in both N treatments, although significant differences between water treatments were only found in plants grown at low N (Table 1). However, these values remained relatively high (>85%) even in the lower water regime. The N regime did not affect RWC. WUE, measured both gravimetrically (WUEbiomass) and using gas exchange measurements (WUEintrinsic and WUEinstantaneous), increased in response to N fertilization (Table 2). However, the effect of water deficit on these parameters was not clear. While at low N the three traits showed the same pattern of increase as the water supply increased, only the response of WUEbiomass reached significance. At high N, an increase in water supply only led to a significant decrease in WUEintrinsic, and, in fact, significant interactions between water and N conditions were observed for WUEbiomass and WUEintrinsic. Furthermore, significant differences between genotypes were also observed for all three WUEs, particularly in plants grown at high N. Nevertheless, Omrabi-3 was the most efficient genotype in terms of water use, regardless of the N regime.
Table 2.
Mean values for water-use efficiency (WUE): (i) measured from the biomass accumulated and total water transpired (WUEbiomass); and (ii) calculated from the gas exchange measurements (WUEintrinsic and WUEinstantaneous), carbon isotope discrimination (Δ13C) of the spike (Δ13Csp), flag (Δ13Cf), root (Δ13Cr), and shoot (Δ13Cs), and the oxygen isotope enrichment above source water in shoots (Δ18Os) of four wheat genotypes (G) subjected to different water regimes (WR) and grown under low and high N
| WUEbiomass | WUEintrinsic | WUEinstantaneous | Δ13Csp | Δ13Cf | Δ13Cr | Δ13Cs | Δ18Os | Δ13Cs/Δ18Os | |
| Low N | |||||||||
| WR | |||||||||
| 40% | 2.2 b | 53.9 a | 5.3 a | 18.0 a | 20.4 a | 18.9 a | 19.6 a,b | 35.8 a | 0.55 a |
| 70% | 2.4 b | 52.5 a | 5.3 a | 18.2 a | 20.7 a | 19.1 a | 19.7 a | 35.1 a,b | 0.56 a |
| 100% | 2.9 a | 60.3 a | 5.9 a | 17.5 b | 19.9 b | 18.9 a | 19.2 c | 34.5 b | 0.56 a |
| G | |||||||||
| Mexa | 2.3 b | 54.5 a | 5.4 a,b | 18.5 a | 21.2 a | 19.5 a | 20.0 a | 35.2 a | 0.57 a |
| Bic-1 | 2.3 b | 52.7 a | 5.1 b | 17.9 b | 20.2 b | 19.0 b | 19.5 b | 35.2 a | 0.56 a |
| L/H | 2.5 a | 57.0 a | 5.1 b | 17.6 b | 20.0 b | 18.5 c | 19.1 b | 35.0 a | 0.55 a |
| Omr-3 | 2.6 a | 58.0 a | 6.4 a | 17.6 b | 19.9 b | 18.8 a,b | 19.3 b | 35.3 a | 0.55 a |
| High N | |||||||||
| WR | |||||||||
| 40% | 3.5 a | 78.7 a | 6.9 a | 16.1 b | 18.3 b | 18.0 a,b | 17.8 b | 35.6 a | 0.50 b |
| 70% | 3.6 a | 74.2 a,b | 6.9 a | 16.1 b | 18.4 b | 17.9 b | 17.8 b | 34.9 a | 0.52 b |
| 100% | 3.6 a | 66.0 b | 6.5 a | 16.7 a | 18.9 a | 18.3 a | 18.6 a | 33.8 b | 0.55 a |
| G | |||||||||
| Mexa | 3.0 c | 60.6 b | 6.0 a | 17.6 a | 20.3 a | 19.0 a | 19.3 a | 34.6 a | 0.56 a |
| Bic-1 | 3.6 b | 71.2 b,c | 6.8 a | 16.2 b | 18.1 c | 18.1 b | 18.1 b | 34.6 a | 0.52 b |
| L/H | 3.5 b | 75.3 a,b | 6.9 a | 16.6 b | 18.8 b | 18.1 b | 18.1 b | 35.1 a | 0.52 b |
| Omr-3 | 4.3 a | 86.6 a | 7.5 a | 14.8 c | 16.9 d | 17.3 c | 16.8 c | 34.7 a | 0.48 c |
Data are the mean of 16 (WR) or 12 (G) replicates. Within each nitrogen treatment and within each water regime (40, 70, and 100% CC) or genotype (Mexa, Bicrecham-1, Lahn/Haucan, and Omrabi-3), values with different letters are significantly different according to the Tukey-b test (P <0.05).
Effects of water and nitrogen on Δ13C and Δ18Os
N conditions and genotype had a significant effect on Δ13C, whereas water had no effect (except for Δ13C of flag leaves). When the two N treatments were compared, higher Δ13C values in plant dry matter were found in low N plants (Table 2). Moreover, a slight but significant increase in Δ13C of all plant parts was observed with increasing water supply from 70% to 100% CC in plants grown under high N, while, under low N, Δ13C values from 70% to 100% CC followed the opposite pattern. In fact, the two-way interactions between growing conditions were significant for all the Δ13C. In addition, significant genotypic differences in Δ13C were found in all plant organs within each N and water treatment. The Mexa genotype showed the highest values of Δ13C, regardless of the N regime and the tissue considered, whereas Omrabi-3 showed the lowest values but only at high N. In fact, there was a significant interaction between genotype and N regime for the four different Δ13C analysed. In addition, differences in Δ13C between plant parts were also found (Table 2).
The δ18O of shoots was enriched compared with the δ18O of irrigation water (–6.5‰) and, therefore, Δ18Os was positive in all cases (Table 2). The water regime had a significant effect on Δ18Os, whilst no differences in Δ18Os values were found when comparing genotypes or N treatments. Thus, within each N treatment, Δ18Os decreased as the water supply increased. Moreover, two-way interactions were not significant in any case.
Relationships between different traits
When data for all the water regimes and genotypes grown together at high N were combined, Δ13C of shoots, flag leaves, spikes, and roots correlated strongly and negatively with time-integrated WUE, and also negatively with instantaneous measurements of WUE (Table 3). Under low N conditions, correlations of different Δ13C only reached significance against WUEintrinsic, with Δ13C of spikes being the best correlated. In contrast, the results showed no significant correlations between Δ18O of shoots and either instantaneous or time-integrated measurements of WUE in either of the N treatments (Table 3). In addition, Δ18O of shoots did not significantly correlate with Δ13C of shoots, regardless of the N regime considered (data not shown).
Table 3.
Correlation coefficients of the linear relationships between Δ13C of the different plant organs and Δ18O of shoots against WUEs for all the treatments and genotypes together
| WUEinstantaneous | WUEintrinsic | WUEbiomass | ||
| Low N | Δ13Csp | –0.406 | –0.851** | –0.626* |
| Δ13Cf | –0.378 | –0.757** | –0.510 | |
| Δ13Cs | –0.293 | –0.778** | –0.491 | |
| Δ13Cr | –0.123 | –0.689* | –0.450 | |
| Δ18Os | –0.278 | –0.291 | –0.145 | |
| High N | Δ13Csp | –0.736** | –0.708* | –0.958** |
| Δ13Cf | –0.729** | –0.704* | –0.924** | |
| Δ13Cs | –0.734** | –0.746** | –0.928** | |
| Δ13Cr | –0.823** | –0.758** | –0.948** | |
| Δ18Os | 0.124 | 0.273 | 0.131 |
Linear correlations were calculated within each N treatment using water and genotype means (n=12), *P <0.05, **P <0.01.
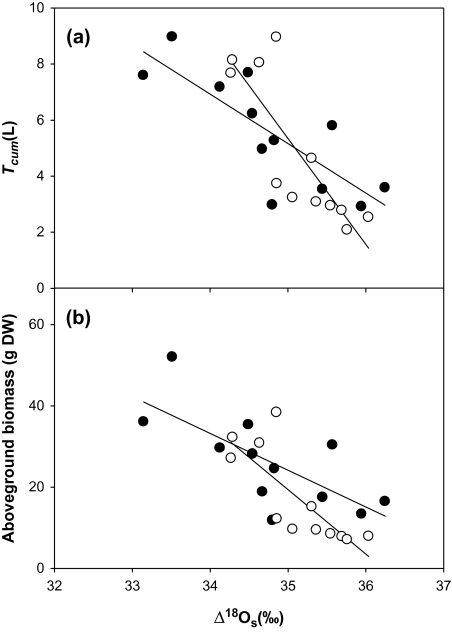
Due to the water regime increasing biomass and decreasing Δ18Os values, significant negative correlations were found between Δ18Os and either AB, SB, or Tcum in both N treatments (Table 4; Fig. 4). Conversely, Δ13Cs did not correlate with either Tcum or AB (Table 4). However, it did correlate positively with SB, although only in the high N treatment.
Table 4.
Correlation coefficients of the stable isotopes with biomass and gas exchange parameters for all the treatments and genotypes together.
| AB | SB | gs | E | Tcum | ||
| Low N | Δ18Os | –0.79** | –0.89** | 0.15 | 0.18 | –0.84** |
| Δ13Cs | –0.48 | –0.13 | 0.29 | –0.02 | –0.38 | |
| Δ13Cs /Δ18Os | –0.02 | 0.34 | 0.17 | –0.11 | 0.09 | |
| High N | Δ18Os | –0.72** | –0.80** | –0.58* | –0.71* | –0.80** |
| Δ13Cs | 0.11 | 0.63* | 0.74** | 0.67* | 0.13 | |
| Δ13Cs /Δ18Os | 0.18 | 0.80** | 0.80** | 0.80** | 0.40 |
Linear correlations were calculated within each N treatment using water and genotype means (n=12), *P<0.05, **P<0.01.
AB, aboveground biomass; SB, spike biomass; gs, stomatal conductance; E, transpiration rate; Tcum, cumulative transpiration.
Fig. 4.
(a) Relationship between the 18O enrichment in shoots and cumulative transpiration, Tcum. Low N treatment, y= –3.78x+137.58; r=0.84, P <0.01, n=12; high N treatment, y= –1.76x+66.74; r=0.80, P <0.01, n=12; (b) Relationship between the 18O enrichment in shoots and aboveground biomass. Low N treatment y= –15.87x+574.96; r=0.79, P <0.01, n=12; high N treatment, y= –9.05x+341.1; r=0.72, P <0.01, n=12. Open and filled circles represent, respectively, the low and high N treatment means for each genotype and water condition. Each value is the mean of four replicates.
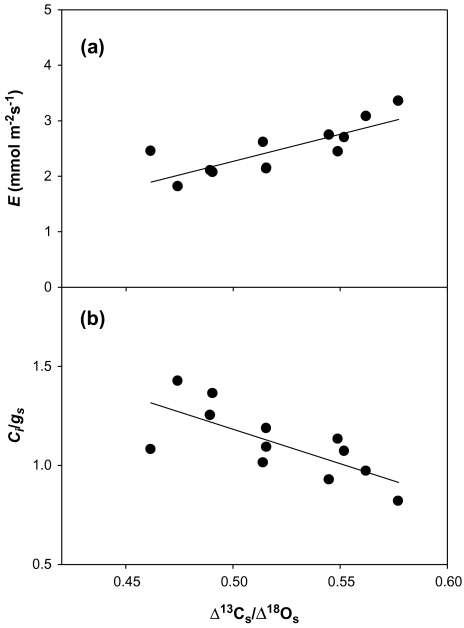
Furthermore, whereas at high N Δ13Cs correlated positively with gs and E, Δ18Os correlated negatively with these same gas exchange parameters; these correlations were not found in the low N treatments. Moreover, in the high N treatments, strong correlations were found between the ratio of carbon and oxygen isotopes (Δ13Cs/Δ18Os) and gs and E (Table 4; Fig. 5).
Fig. 5.
Relationship between the ratio Δ13Cs/Δ18Os and transpiration rate, E (a), y=9.77x–2.62; r=0.80, P <0.01, n=12; and the ratio Ci/gs (b), y= –3.47x+2.92; r= –0.73, P<0.01, n=12. Symbols represent the water and genotype means for the high N treatment. Each value is the mean of four replicates.
In addition, a stepwise analysis was performed to explain the causes of variation of the different parameters studied (Table 5). Thus, the variation in the total AB was explained in both N treatments by the combination of Δ18Os and Δ13Cs. Δ18Os and Δ13C explained, respectively, 59% and 17% of the variation in AB at low N, while under high N Δ18Os and Δ13C explained 48% and 36% of AB variation, respectively. Moreover, in both N treatments, variation in Δ18Os was mainly explained by changes in Tcum. On the other hand, Δ13Cs and Asat variations were explained in both N treatments by a combination of gs and leaf N (expressed as LN or SLN). Differences in the ratio Ci/Ca were explained in the high N treatments by changes in gs and leaf N, whereas no explanation was found for low N treatments when these variables were entered into the model.
Table 5.
Multiple linear regressions (stepwise) explaining biomass variation from stable isotopes (Δ13Cs and Δ18Os); Δ18Os and Δ13Cs variations from gas exchange parameters (Asat, E, gs, Ci/Ca, Tcum) plus SLN, SN, and LN; and Ci/Ca and Asat variations (gs, SLN, SN, and LN) derived from water and genotype means within each N treatment.
Initial variable, first variable entering the model; initial r2 and mean square error (MSE), adjusted coefficient of determination (r2) and MSE after including the first variable in the model; final r2 and MSE, adjusted r2, and MSE obtained with the final stepwise model.
| N treatment | Trait | Initial variable | Initial r2 | Initial MSE | Final stepwise model | Final r2 | Final MSE |
| Low N | AB | Δ18Os | 0.59** | 7.36 | AB= –15.1×Δ18Os–8.5×Δ13Cs+716.8 | 0.76*** | 5.70 |
| Δ18Os | Tcum | 0.67** | 0.33 | Δ18Os= –0.19×Tcum+36.3 | 0.67** | 0.33 | |
| Δ13Cs | SLN | 0.37* | 0.45 | Δ13Cs= –1.8×SLN+5.6×gs+20.0 | 0.68** | 0.32 | |
| Ci/Ca | – | – | – | – | – | – | |
| Asat | LN | 0.72*** | 1.32 | Asat=3.3×LN+17.4×gs–1.4 | 0.85*** | 0.96 | |
| High N | AB | Δ18Os | 0.48** | 8.4 | AB= –11.6×Δ18Os–5.8×Δ13Cs+538.5 | 0.84*** | 6.64 |
| Δ18Os | Tcum | 0.61** | 0.58 | Δ18Os= –0.4×Tcum+36.8 | 0.61** | 0.58 | |
| Δ13Cs | LN | 0.59** | 0.65 | Δ13Cs= –1.7×LN+10.7×gs+23.1 | 0.87*** | 0.36 | |
| Ci/Ca | gs | 0.59** | 0.03 | Ci/Ca=0.68×gs–0.06×LN+0.74 | 0.74*** | 0.03 | |
| Asat | gs | 0.43** | 1.39 | Asat=31.5×gs+2.9×LN–4.5 | 0.74*** | 0.93 |
AB, aboveground biomass; Δ13Cs, shoot carbon isotope discrimination; Δ18Os, 18O enrichment in shoots; E, transpiration rate; gs, stomatal conductance; Ci and Ca, intercellular and ambient CO2 concentrations; SLN, specific leaf nitrogen; Tcum, cumulative transpiration.
*P <0.05; **P <0.01; ***P <0.001.
Discussion
Water and nitrogen effects on plant growth and water status
Although the different water regimes produced large differences in biomass in both N treatments (Fig. 1), the relatively small decreases in gas exchange parameters, Δ13C and RWC, suggest that mild water stress was experienced even with the most water-limited treatments. This can be explained in part by a combination of different factors, including the fact that plant growth, more than gas exchange, is affected by moderate water stress (Hsiao, 1973; Jones, 1980; McCree, 1986; Kramer and Boyer, 1995). Hence, both the way in which water regimes were imposed (i.e. sustained water limitation during the course of crop growth) and the growing conditions in pots led plants to acclimatize, with an adjustment of the total leaf area to the available water conditions in the pot and, therefore, with water status (e.g. gs and RWC) remaining at moderate or non-water stress levels. Moreover, the lack of differences in LDM between water treatments (and therefore the absence of differences in leaf structure) reinforces the idea that plants adjust their total leaf area to water availability.
In addition, when both N treatments were compared, a negative effect of N supply on gas exchange parameters was observed. High N supply clearly increased the total plant biomass (and therefore the total leaf area), thereby causing leaves to compete for the available water in the pot. This resulted in a negative effect on gas exchange parameters (mainly in terms of decreasing gs) in both well-watered and water-limited plants (in which the effect was greater, Table 1). Genotypic differences may also support this situation, as in the case of Omrabi-3, where the large increase in biomass reached in the high N treatment resulted in a clear decrease in gs and E values. Similar results were found in field-grown rice in aerobic soils (Kondo et al., 2004), where a large biomass production due to high N fertilization exacerbated water stress, which resulted in lower stomatal conductance and Δ13C.
Source of variation on plant isotope signatures
Carbon isotope discrimination (Δ13C) varied extensively between the different analysed plant parts (shoots, flags, spikes, and roots), as predicted by both theory (Hubick and Farquhar, 1989; Badeck et al., 2005) and previous results reported in wheat grown under Mediterranean conditions (Araus et al., 1997; Merah et al., 2002). Lower Δ13C values in spikes compared with the shoot or flag leaf may reflect changes in soil water availability, as well as the increase in evaporative demand occurring during the final stages of crop growth (Condon and Richards, 1992); however, the lower gs of the spike compared with that of leaves (Araus et al., 1993; Tambussi et al., 2005, 2007b) may also be involved.
Moreover, when N treatments were compared, a significant decrease in Δ13C values in plants grown under high N supply was found. According to Farquhar et al. (1989), a decrease in Δ13C can be explained by a reduction in the ratio Ci/Ca (Table 1). This reduction in Ci/Ca may be due to either greater photosynthetic capacity or lower gs, or to both factors (Farquhar and Richards, 1984; Condon et al., 2004). Although many reports have suggested that N fertilization may decrease Δ13C by lowering Ci/Ca, mainly as a result of improved carboxylation efficiency (Livingstone et al., 1999; Ripullone et al., 2004), a significant decrease in Asat in response to N fertilization was found. This can be explained by the lower gs values found in the high N treatments, which resulted in a significant decrease in both Asat and Δ13C as compared with the low N treatments.
However, within each N treatment, and regardless of differences found between N treatments, the increase in Asat was associated with increases in gs and LN (as revealed by stepwise analysis, Table 5). Nevertheless, the effect of LN was higher under low N treatments. Hence, at low N, where the effect of gs was lower, a higher N content in leaves (SLN and LN) associated with more water input and/or N application was probably involved in lowering Ci/Ca and Δ13C. Conversely, at high N, differences in Asat were mainly associated with changes in gs. This is supported by the close relationships found between Asat and gs and LN (Fig. 2).
It was also observed that Δ18Os increased significantly in the water-limited treatments, while no differences in Δ18Os were found between N treatments. A similar pattern in response to differences in water status was found in bread wheat kernels (Ferrio et al., 2007), where plants with the wettest conditions had the lower δ18O values. Similarly, Saurer et al. (1997) reported a variation in δ18O in the cellulose of trees grown under different soil moisture conditions, with the lowest values in those species growing with the highest moisture soil index.
Even though the four genotypes showed significant differences in cumulative transpiration rates, this was not translated into significant differences in Δ18Os between genotypes. Nevertheless, there are reports showing genotypic variability for this trait in bread wheat (Barbour et al., 2000a; Ferrio et al., 2007). However, a negative trend was observed, with higher 18O enrichment in those genotypes with lower transpiration rates when grown at high N.
Stepwise analysis revealed that variation in Δ18Os was explained in both N treatments as a response to Tcum. In accordance with the theory (Barbour and Farquhar, 2000), where Δ18O in plant organic material may provide an integrated measurement of water loss, it was found that under both N treatments Δ18Os was able to differentiate between water treatments, becoming enriched in 18O as water supply decreased. This was also the case under low N levels, where differences between water regimes in terms of leaf gas exchange were less evident.
Although Δ18O clearly showed differences between water treatments, the 18O signature could be altered as shoots including leaves and stems were measured. It is well known that during cellulose formation in newly developing stems (sink tissues), the cleavage of the sucrose formed in leaves allows re-equilibration of some or all of the oxygen with the surrounding stem water (Barbour and Farquhar, 2000), altering the 18O enrichment which has already occurred in the leaves (sucrose source). However, the shoot samples contained not only stems but also a large proportion of leaves. Nevertheless, a simplified Péclet model developed by Barbour and co-workers (http://www.ecophys.biology.utah.edu/) was used to validate the results. The model reasonably predicted the measured Δ18Os values (r=0.69 and slope 1.1) although the range of prediction was lower than that observed (Supplementary Fig. S1 available at JXB online).
Correlation between traits
The negative correlations observed between Δ18Os and both gs and E are consistent with the strong relationships found between Δ18O and gs and E in bread wheat (Barbour et al., 2000a) and in cotton (Barbour and Farquhar, 2000). However, these results contrast with the positive relationships between Δ18O of leaf biomass and mean transpiration rate reported in groundnut and rice (Sheshshayee et al., 2005) and, more recently, in the tropical tree, Ficus insipida (Cernusak et al., 2007). Farquhar et al. (2007) have recently discussed the results of Sheshshayee et al. (2005) stating that when variation in E is caused by changes in the evaporative demand a positive relationship between E and isotopic enrichment can be maintained. However, when variation in E is driven by changes in gs a negative relationship between E and isotope enrichment is expected. This is the case in the present study where, at a given VPD, a lower stomatal conductance caused 18O enrichment in plant dry matter, and a negative correlation between Δ18Os and E was observed (Table 4). A nice example is shown in Barbour and Farquhar (2000) where at a given relative humidity cotton leaves with lower gs, and therefore lower E and higher leaf temperature, exhibited higher 18O enrichment.
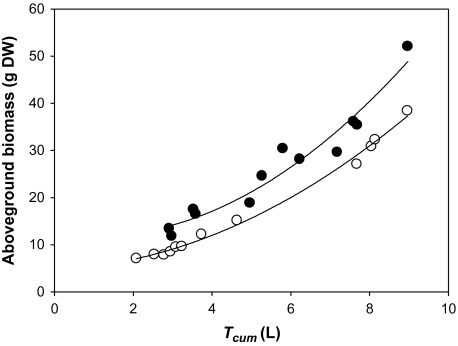
Regardless of the correlations between Δ18O and gas exchange parameters, under the particular growing conditions of the present research, where water regimes were maintained at a constant and steady level through watering, it was found that cumulative transpiration (Tcum) played a pivotal role in Δ18O. Moreover, Tcum also strongly determined the amount of biomass accumulated, and this was supported by the strong positive correlations found between AB and Tcum (r=0.99, P <0.001 and r=0.99, P <0.001 for the low and high N treatments, respectively; Fig. 3). Therefore, a negative relationship between Δ18O and AB in both N treatments was also found (Fig. 4b, Table 4).
Fig. 3.
Relationship between cumulative transpiration (Tcum) and aboveground biomass. Low N treatment y=4.35e0.25x; r=0.99, P <0.001, n=12; high N treatment, y=7.34e0.22x; r=0.99, P <0.001, n=12. Open and filled circles represent, respectively, the low and high N treatment means for each genotype and water condition. Each value is the mean of four replicates.
In addition, and as suggested by Barbour et al. (2000a), under well-watered to mild water-limited conditions, and when grain yield and stomatal conductance are positively correlated, the 18O signature would be a good predictor of yield. In agreement with this, a positive correlation between gs and SB (r=0.87, P <0.01, data not shown) was found in the high N treatments, and therefore the expected correlation between SB and Δ18Os was also found (r= –0.80, P <0.01, Table 4).
Furthermore, Barbour et al. (2000a) reported that when variation in Δ13C is mainly driven by changes in gs, a negative correlation between Δ13C and Δ18O (or positive between δ13C and δ18O) is expected. Saurer et al. (1997) also reported a linear positive relationship between δ13C and δ18O cellulose of trees grown under different soil moisture conditions, stating the linear dependence of the ratio Ci/Ca on the ratio ea/ei. In the present results, although a negative relationship was found between Δ13Cs and Δ18Os (r= –0.42), this was not statistically significant. This can be explained by the fact that plants grown at high N supply displayed a Δ13C variation that was not completely attributable to changes in gs but was also influenced by intrinsic photosynthetic capacity (Table 5). As a consequence, the increase in Ci/Ca caused by increased gs will be partially offset by a decrease in Ci/Ca associated with an increased photosynthetic capacity (Barbour et al., 2000a), and therefore the expected correlation between Δ13C and Δ18O is reduced. In addition, at low N, no relationship was found between the two isotopes.
Bindumadhava et al. (2005) reported that the ratio Ci/gs can be used as a rapid estimate of ‘instantaneous’ carbon assimilatory capacity, since at a given gs the variation in Ci is mainly a function of photosynthetic capacity. Moreover, in their study, variation in Δ13C and Δ18O was mainly driven by changes in photosynthetic capacity and stomatal conductance, respectively, and therefore they reported that the ratio Δ13C/Δ18O would represent a time-averaged estimate of Ci/gs, and hence an integrated estimation of the photosynthetic capacity during the course of crop growth. Conversely, the present results showed the inverse trend, with a negative correlation between the ratios Ci/gs and Δ13C/Δ18O (r= –0.73, P <0.01, Fig. 5b) in the high N treatments. These opposing results can be explained through consideration of the causes affecting variation in 13C and 18O signatures. Whereas the work of Bindumadhava et al. (2005) indicated that differences in Δ13C were mainly driven by changes in photosynthetic capacity, the present results showed that Δ13Cs was associated with changes in both gs and intrinsic photosynthetic capacity. This could also explain why gs and E correlated better with Δ13Cs/Δ18Os than with each isotope alone (Table 4).
In the present study, the differences in gas exchange parameters (gs and E), as well as their correlation with stable isotopes found in the high N treatments, contrast with the lack of such results in the low N treatments. In the case of the correlation between Δ13C and gas exchange parameters, this might be explained by Δ13C dependence on Ci/Ca. Thus, at low N, changes in Ci/Ca across water regimes are not as dependent on gs as at high N (see Table 5), and, in fact, increasing water input did not produce a parallel increase in gs and E or Asat. In the case of Δ18O, instantaneous transpiration at low N is also less clearly affected by the water regime than at high N. Conversely, cumulative transpiration is highly dependent on the total leaf area, which in turn is strongly affected by the water regime in both N treatments. Thus, at high N, more water implies not just more photosynthesis and a better water status but, probably (and more importantly), higher levels of accumulated transpiration and, therefore, higher rates of plant growth. In contrast, at low N, the effect of higher quantities of irrigation leading to increased plant growth and increased cumulative transpiration is indirect, and this is mediated by greater availability of N with increasing fertirrigation. Hence, differences obtained in terms of biomass were not paralleled by significant differences in gas exchange parameters.
In addition, the strong correlation found between Δ18Os and AB in both N treatments, along with the lack of correlation of AB with Δ13Cs, emphazise the fact that plant growth (and thus total water transpired by the plant) more than gas exchange per unit leaf area is affected by moderate and steady water stress.
Conclusion
In accordance with the current theory of Barbour and Farquhar (2000), this study shows an increase in the 18O enrichment of plant matter in water-limited plants as a consequence of a decrease in transpiration. Thus, under the particular growing conditions studied here, Δ18O was strongly and negatively associated with AB regardless of the N regime. In fact, Δ18O was the only trait among the stable isotope and gas exchange traits analysed that did not show an interaction between growing conditions and which was only affected by the water regime. This illustrates the usefulness of measuring Δ18O to assess differences in plant growth and total transpiration. In addition, this study showed that the two isotopes, 13C and 18O, are not mutually exclusive and that the combined measurement of both at the plant matter level may provide a time-integrated record of the photosynthetic and evaporative performance of the plant during the course of crop growth, thus helping plant breeders to select genotypes that are better adapted to water limitation, regardless of the N status.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Material
Acknowledgments
This study was supported in part by the European research project WatNitMed (INCO CT-2004-509107) and the Spanish Ministry of Education and Science project (AGL2006-13541-C02). LC-B and GM were the recipients of a doctoral fellowship (Programa Nacional de Formación de Personal Universitario, Spanish Ministry of Education and Science).
Glossary
Abbreviations
- AB
aboveground biomass
- Asat
light-saturated net CO2 assimilation rate
- Ci and Ca
intercellular and ambient CO2 concentrations
- δ13C
carbon isotope composition
- Δ13C
carbon isotope discrimination
- δ18O
oxygen isotope composition
- Δ18O
oxygen isotope enrichment
- E
transpiration rate
- gs
stomatal conductance
- LDM
leaf dry mass per unit leaf area
- RWC
relative water content
- SB
spike biomass
- WUEbiomass
time-integrated water-use efficiency
- WUEinstantaneous
instantaneous water-use efficiency
- WUEintrinsic
intrinsic water-use efficiency
References
- Araus JL, Amaro T, Casadesús J, Asbati A, Nachit MM. Relationships between ash content, carbon isotope discrimination and yield in durum wheat. Australian Journal of Plant Physiology. 1998;25:835–842. [Google Scholar]
- Araus JL, Amaro T, Zuhair Y, Nachit MM. Effect of leaf structure and water status on carbon isotope discrimination in field-grown durum wheat. Plant, Cell and Environment. 1997;20:1484–1494. [Google Scholar]
- Araus JL, Brown HR, Febrero A, Bort J, Serret MD. Ear photosynthesis, carbon isotope discrimination, and the respiratory CO2 to differences in grain mass in durum wheat. Plant, Cell and Environment. 1993;16:383–392. [Google Scholar]
- Araus JL, Slafer GA, Reynolds MP, Royo C. Plant breeding and drought in C3 cereals: what should we breed for? Annals of Botany. 2002;89:925–940. doi: 10.1093/aob/mcf049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus JL, Villegas D, Aparicio N, del Moral LFG, El Hani S, Rharrabti Y, Ferrio JP, Royo C. Environmental factors determining carbon isotope discrimination and yield in durum wheat under Mediterranean conditions. Crop Science. 2003;43:170–180. [Google Scholar]
- Badeck F-W, Tcherkez G, Nogués S, Piel C, Ghashgaie J. Post-photosynthetic fractionation of carbon stable isotopes between plant organs—a widespread phenomenon. Rapid Communication in Mass Spectrometry. 2005;19:1381–1391. doi: 10.1002/rcm.1912. [DOI] [PubMed] [Google Scholar]
- Bänziger M, Edmeades GO, Beck D, Bellon M. Breeding for drought and nitrogen stress tolerance in maize: from theory to practice. Mexico, D.F.: CIMMYT; 2000. [Google Scholar]
- Barbour MM. Stable oxygen isotope composition of plant tissue: a review. Functional Plant Biology. 2007;34:83–94. doi: 10.1071/FP06228. [DOI] [PubMed] [Google Scholar]
- Barbour MM, Farquhar GD. Relative humidity- and ABA-induced variation in carbon and oxygen isotope ratios of cotton leaves. Plant, Cell and Environment. 2000;23:473–485. [Google Scholar]
- Barbour MM, Fischer RA, Sayre KD, Farquhar GD. Oxygen isotope ratio of leaf and grain material correlates with stomatal conductance and grain yield in irrigated wheat. Australian Journal of Plant Physiology. 2000a;27:625–637. [Google Scholar]
- Barbour MM, Schurr U, Beverley KH, Chin Wong S, Farquhar GD. Variation in the oxygen isotope ratio of phloem sap sucrose from castor bean. evidence in support of the Péclet effect. Plant Physiology. 2000b;123:671–679. doi: 10.1104/pp.123.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindumadhava H, Prasad TG, Joshi MK, Sharma N. Oxygen isotope enrichment (Δ18O) is a potential screening approach for higher leaf yield in tea (Camillia sinesis) accessions. Current Science. 2006;91:956–960. [Google Scholar]
- Bindumadhava H, Sheshshayee MS, Shashidhar G, Prasad TG, Udayakumar M. Ratio of stable carbon and oxygen isotope discrimination (Δ13C/Δ18O) reflects variability in leaf intrinsic carboxylation efficiency in plants. Current Science. 2005;89:1256–1258. [Google Scholar]
- Cabrera-Bosquet L, Molero G, Bort J, Nogués S, Araus JL. The combined effect of constant water deficit and nitrogen supply on WUE, NUE and Δ13C in durum wheat potted plants. Annals of Applied Biology. 2007;151:277–289. [Google Scholar]
- Cernusak LA, Winter K, Aranda J, Turner BL, Marshall JD. Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility. Journal of Experimental Botany. 2007;58:3549–3566. doi: 10.1093/jxb/erm201. [DOI] [PubMed] [Google Scholar]
- Choi WJ, Chang SX, Allen HL, Kelting DL, Ro HM. Irrigation and fertilization effects on foliar and soil carbon and nitrogen isotope ratios in a loblolly pine stand. Forest Ecology and Management. 2005;213:90–101. [Google Scholar]
- Claus S, Wemecke P, Pigla U, Dubsky G. A dynamic model describing leaf temperature and transpiration of wheat plants. Ecological Modelling. 1993;81:31–40. [Google Scholar]
- Condon AG, Richards RA. Broad sense heritability and genotypes–environment interaction for carbon isotope discrimination in field-grown wheat. Australian Journal of Agricultural Research. 1992;43:921–934. [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. Breeding for high water-use efficiency. Journal of Experimental Botany. 2004;55:2447–2460. doi: 10.1093/jxb/erh277. [DOI] [PubMed] [Google Scholar]
- Craig H, Gordon LI. Deuterium and oxygen 18 variations in the ocean and marine atmosphere. In: Tongiorigi E, editor. Stable isotopes in oceanographic studies and paleotemperatures. Pisa, Italy: Consiglio Nazionale Delle Ricerche Laboratorio Di Geologia Nucleare; 1965. pp. 9–130. [Google Scholar]
- Dongmann G, Nurnberg HW, Forstel H, Wagener K. Enrichment of H218O in the leaves of transpiring plants. Radiation and Environmental Biophysics. 1974;11:41–52. doi: 10.1007/BF01323099. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Cernusak LA, Barnes B. Heavy water fractionation during transpiration. Plant Physiology. 2007;143:11–18. doi: 10.1104/pp.106.093278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology. 1989;40:503–537. [Google Scholar]
- Farquhar GD, Lloyd J. Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In: Ehleringer JR, Hall AE, Farquhar GD, editors. Stable isotopes and plant carbon–water relations. San Diego: Academic Press, Inc; 1993. pp. 47–70. [Google Scholar]
- Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology. 1984;11:539–552. [Google Scholar]
- Ferrio JP, Mateo MA, Bort J, Abdalla O, Voltas J, Araus JL. Relationships of grain delta C-13 and delta O-18 with wheat phenology and yield under water-limited conditions. Annals of Applied Biology. 2007;150:207–215. [Google Scholar]
- Ferrio JP, Villegas D, Zarco J, Aparicio N, Araus JL, Royo C. Assessment of durum wheat yield using visible and near-infrared reflectance spectra of canopies. Field Crops Research. 2005;94:126–148. [Google Scholar]
- Hirel B, Le Gouis J, Ney B, Gallais A. The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. Journal of Experimental Botany. 2007;58:2369–2387. doi: 10.1093/jxb/erm097. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular. 1950;347:1–32. [Google Scholar]
- Hsiao TC. Plant responses to water stress. Annual Review of Plant Physiology. 1973;24:519–570. [Google Scholar]
- Hubick KT. Effect of nitrogen source and water limitation on growth, transpiration efficiency and carbon isotope discrimination in peanut genotypes. Australian Journal of Plant Physiology. 1990;17:423–430. [Google Scholar]
- Hubick KT, Farquhar GD. Carbon isotope discrimination and the ratio of carbon gained to water lost in barley cultivars. Plant, Cell and Environment. 1989;12:795–804. [Google Scholar]
- Jones HG. Interaction and integration of adaptive responses to water stress: the implications of an unpredictable environment. In: Turner NC, Kramer PJ, editors. Adaptation of plants to water and high temperature stress. New York: Wiley; 1980. pp. 353–365. [Google Scholar]
- Kondo M, Pablico PP, Aragones DV, Agbisit R. Genotypic variations in carbon isotope discrimination, transpiration efficiency, and biomass production in rice as affected by soil water conditions and N. Plant and Soil. 2004;267:165–177. [Google Scholar]
- Kramer PJ, Boyer JS. Water relations of plants and soils. San Diego, USA: Academic Press Inc; 1995. Growth; pp. 313–342. [Google Scholar]
- Lafitte HR, Blum A, Atlin G. Using secondary traits to help identify drought-tolerant genotypes. In: Fischer KS, Lafitte RH, Fukai S, Atlin G, editors. Breeding rice for drought-prone environments. Los Baños, The Philippines: IRRI; 2003. pp. 37–48. [Google Scholar]
- Lawlor DW. Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. Journal of Experimental Botany. 2001;53:773–787. [PubMed] [Google Scholar]
- Livingston NJ, Guy RD, Sun ZJ, Ethier GJ. The effects of nitrogen stress on the stable carbon isotope composition, productivity and water use efficiency of white spruce (Picea glauca (Moench) Voss) seedlings. Plant, Cell and Environment. 1999;22:281–289. [Google Scholar]
- McCree KJ. Whole plant carbon balance during osmotic adjustment to drought and salinity stress. Australian Journal of Plant Physiology. 1986;13:33–44. [Google Scholar]
- Merah O, Deléens E, Teulat B, Monneveux P. Association between yield and carbon isotope discrimination value in different organs of durum wheat under drought. Journal of Agronomy and Crop Science. 2002;188:426–434. [Google Scholar]
- Nogués S, Aranjuelo I, Pardo T, Azcón-Bieto J. Assessing the stable-carbon isotopic composition of intercellular CO2 in a CAM plant at two CO2 levels. Rapid Communication in Mass Spectrometry. 2008;22:1017–1022. doi: 10.1002/rcm.3460. [DOI] [PubMed] [Google Scholar]
- Oweis T, Pala M, Ryan J. Stabilizing rainfed wheat yields with supplemental irrigation and nitrogen in a Mediterranean climate. Agronomy Journal. 1998;90:672–681. [Google Scholar]
- Passioura JB. Environmental biology and crop improvement. Functional Plant Biology. 2002;29:537–546. doi: 10.1071/FP02020. [DOI] [PubMed] [Google Scholar]
- Rebetzke GJ, Condon AG, Richards RA, Farquhar GD. Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Science. 2002;42:739–745. [Google Scholar]
- Ripullone F, Lauteri M, Grassi G, Amato M, Borghetti M. Variation in nitrogen supply changes water-use efficiency of Pseudotsuga menziesii and Populus×euroamericana; a comparison of three approaches to determine water-use efficiency. Tree Physiology. 2004;24:671–679. doi: 10.1093/treephys/24.6.671. [DOI] [PubMed] [Google Scholar]
- Saurer M, Aellen K, Siegwolf R. Correlating δ13C and δ18O in cellulose of trees. Plant, Cell and Environment. 1997;20:1543–1550. [Google Scholar]
- Serret MD, Ortiz-Monasterio I, Pardo A, Araus JL. The effect of urea fertilization and genotype on yield, NUE, δ15N and δ13C in wheat. Annals of Applied Biology. 2008;153:243–257. [Google Scholar]
- Shangguan ZP, Shao MA, Dyckmans J. Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environmental and Experimental Botany. 2000;44:141–149. doi: 10.1016/s0098-8472(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Raschke K. Separation and measurement of direct and indirect effects of light on stomata. Plant Physiology. 1981;68:33–40. doi: 10.1104/pp.68.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheshshayee MS, Bindumadhava H, Ramesh R, Prasad TG, Lakshminarayana MR, Udayakumar M. Oxygen isotope enrichment (Delta O-18) as a measure of time-averaged transpiration rate. Journal of Experimental Botany. 2005;56:3033–3039. doi: 10.1093/jxb/eri300. [DOI] [PubMed] [Google Scholar]
- Tambussi EA, Bort J, Araus JL. Water use efficiency in C-3 cereals under Mediterranean conditions: a review of physiological aspects. Annals of Applied Biology. 2007a;150:307–321. [Google Scholar]
- Tambussi EA, Bort J, Guiamet JJ, Nogués S, Araus JL. The photosynthetic role of ears in C3 cereals: metabolism, water use efficiency and contribution to grain yield. Critical Reviews in Plant Sciences. 2007b;26:1–16. [Google Scholar]
- Tambussi EA, Nogués S, Araus JL. Ear of durum wheat under water stress: water relations and photosynthetic metabolism. Planta. 2005;221:446–458. doi: 10.1007/s00425-004-1455-7. [DOI] [PubMed] [Google Scholar]
- Villegas D, Aparicio N, Blanco R, Royo C. Biomass accumulation and main stem elongation of durum wheat grown under Mediterranean conditions. Annals of Botany. 2001;88:617–627. [Google Scholar]
- White JW, Castille A, Ehleringer JR. Association between productivity, root growth and carbon isotope discrimination in Phaseolus vulgaris under water deficit. Australian Journal of Plant Physiology. 1990;17:189–198. [Google Scholar]
- Yakir D, Deniro MJ, Gat JR. Natural deuterium and O-18 enrichment in leaf water of cotton plants grown under wet and dry conditions—evidence for water compartmentation and its dynamics. Plant, Cell and Environment. 1990;13:49–56. [Google Scholar]
- Yakir D. Variations in the natural abundance of O-18 and deuterium in plant carbohydrates. Plant, Cell and Environment. 1992;15:1005–1020. [Google Scholar]
- Zhao LJ, Xiao HL, Liu XH. Relationships between carbon isotope discrimination and yield of spring wheat under different water and nitrogen levels. Journal of Plant Nutrition. 2007;30:947–963. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.