Abstract
Using a novel pressure membrane (PM) apparatus for the extraction of apoplastic fluid from field-grown grape (Vitis vinifera L.) berries, our hypothesis that significant apoplast solutes accumulate at the beginning of the ripening process (i.e. veraison), and that this accumulation might contribute to progressive berry softening due to a progressive loss of mesocarp cell turgor pressure (P) was tested. It was necessary to correct the solute potential (Ψs) of fluid collected with the PM for dilution due to the presence of a dead volume in the apparatus, but after correction, the Ψs obtained with the PM agreed with that obtained by low speed centrifugation. A clear decline in fruit apoplastic solute potential () began approximately 10 d prior to fruit coloration, and it was found to be coincident with a decline in mesocarp cell P and fruit elasticity (E). By late in fruit development when berry growth ceased (90 d after anthesis), both apoplast and fruit Ψs reached almost –4 MPa. These results support the hypothesis that a decrease in is responsible for the observed loss in mesocarp cell P, and is the mechanistic cause of berry softening.
Keywords: Apoplast, elasticity, matric potential, pressure-membrane, softening, symplast, veraison
Introduction
In fleshy fruit, the onset of ripening is often characterized by softening (Coombe, 1976). Grape (Vitis vinifera L.) berries exhibit three growth stages, with growth mainly occurring in the first and third stages, and these are separated by a lag stage. Ripening is considered to begin with the transition (called ‘veraison’) between the second and third stages. Berry softening marks the beginning of this transition (Coombe and Bishop, 1980; Coombe, 1992), followed by the resumption of secondary expansive growth, sugar accumulation, and coloration in pigmented varieties (Coombe, 1992). However, since the onset of pigment accumulation can readily be observed, it is often regarded as the approximate date of veraison (Coombe, 1992).
The onset of ripening is a genetically programmed process in which a series of genes involved in sugar transport, cell wall modification, and anthocyanin biosynthesis are up-regulated at the translational level (Davies and Robinson, 2000; Goes da Silva et al., 2005). Clearly, however, increased phloem transport to the berries (Greenspan et al., 1994, 1996) and changes in cellular water relations (Matthews and Shackel, 2005; Wada et al., 2008) are also involved. Recent in situ measurements of turgor (P) in the mesocarp cells of developing grape berries (Matthews and Shackel, 2005; Thomas et al., 2006, 2008), have indicated that preveraison (PreV) berry softening, which essentially precedes all other physiological events that occur at or near veraison, is associated with reductions in P. At veraison, P reached approximately 0.05 MPa and was stable at this low level post-veraison (PostV) (Matthews and Shackel, 2005; Thomas et al, 2006). In a previous study, Wada et al. (2008) showed that low PostV P was associated with the presence of substantially increased concentrations of fructose and glucose in the berry apoplast, but only three distinct stages of berry development were studied using greenhouse-grown fruit, and there were no data presented related to berry softening. In this study, both a low speed centrifuge technique (Wada et al., 2008) as well as a novel pressure membrane (PM) technique were used to extract apoplastic fluid from developing grape berries, and our hypothesis that an accumulation of apoplastic solutes was synchronous with P loss and berry softening in field-grown fruit was tested.
Materials and methods
Plant materials
Grape berries (Vitis vinifera L. cv. Cabernet Sauvignon) were sampled from field-grown vines located in the Variety Collection Block of the Department of Viticulture and Enology facility at the University of California, Davis, CA, USA (38°32’ N latitude and 121°46’ W longitude, elevation 18 m above sea level). The anthesis date was noted as the day on which 50% of the cluster was flowering, with time measured as days after anthesis (DAA). All flowering within a cluster occurred within 2–3 d. To document the seasonal pattern of berry growth, six berries were tagged on DAA 30, and their diameter was measured between 07.30 h and 09.00 h periodically during development with a digital caliper. Individual berries and entire clusters were also sampled between 07.30 h and 09.00 h for destructive measurements. For P, 2–3 typical berries from each of 2–3 clusters were gently excised at the pedicel and immediately placed into small aluminium-foil covered mylar zip-top bags that excluded light and prevented water loss. It was only possible to complete P measurements on a total of 2–3 berries from each harvest (see below), but following P measurement, the accumulation of soluble solids was measured on small aliquots of juice from each P measured berry with a hand held refractometer (Reichert A2R200, Reichert GmbH, Seefeld Germany) and reported as °Brix. For firmness and all solute potential measurements, the same clusters as were sampled for P were excised, immediately placed into large ziptop bags covered with aluminium foil, stored in a Styrofoam box at ambient temperature, and transported to the laboratory. From these clusters, a total of about 12 berries were sampled and used for berry firmness measurements, and an additional 12 berries were used for tissue and apoplast osmotic potential measurements (see below).
Apoplast and tissue solute potential (Ψs)
All Ψs measurements for the apoplast () and the tissue () were determined using dewpoint osmometry (5500 vapour pressure osmometer, Wescor Inc.) calibrated with NaCl standards, as described elsewhere (Wada et al., 2008). Briefly, for , tissues were frozen at –90 °C, stored at –20 °C, thawed at 25 °C for 10 min, centrifuged at 2000 g and Ψs of the supernatant was measured. Apoplastic fluid was extracted from developing berry mesocarp tissues using both low speed centrifugation (Pomper and Breen, 1995; Welbaum and Meinzer, 1990; Wada et al., 2008) and pressure membrane (PM) techniques. For the centrifuge technique, the extraction was conducted as described previously for Chardonnay berries (Wada et al., 2008). Briefly, each berry was sectioned at the stylar (distal) end with a razor blade, removing approximately 2 mm of the pericarp, which was used to determine tissue solute potential. The cut surface of the berry was carefully blotted and immediately placed on a circular support screen set at 35 mm from the bottom of a 12 ml glass centrifuge tube. The top of the tube was sealed with paraffin film to prevent water loss from the samples, which were centrifuged at either 350 g (PreV) or 100 g (PostV) at 4 oC. The extracted fluid was collected from the bottom of the centrifuge tube and its volume and Ψs measured. The extraction was completed within 4 h of harvest.
The PM apparatus which was used by Bondada et al. (2005) to establish a matric potential gradient and cause a flow of dye solution across the berry apoplast, was modified (Fig. 1) by adding a water-filled pressure vessel between the gas source and the apparatus to ensure 100% RH in the apparatus during pressurization. This method was then used to extract apoplastic fluid from the berry mesocarp tissue from 4–8 berries. As described previously (Bondada et al., 2005), the apparatus was similarly constructed with high-pressure brass pipe fittings, and a hydrophilic cellulose ester membrane (0.05 μm pore size, air entry pressure 2.4 MPa, MWP02500, Millipore, Billerica, MA) supported by a screen made of stainless steel and a custom-fabricated support and outlet. The internal dimensions of the cylindrical pressure vessel were 25 mm in diameter and 20 mm in height. Before tissue extraction, the membrane was saturated with distilled water, assembled in the device, and exposed to a pressure equivalent to the extraction pressure until water flow from the outlet had ceased (20–25 min). After this preconditioning, the cut surface of the berry was carefully blotted as for the centrifuge technique, and immediately and directly placed on the membrane. The berry tissue and membrane were gently assembled into the apparatus. PreV and PostV samples were exposed to 1.5 MPa and 0.8 MPa, respectively, but during the PreV to PostV transition (DAA 63–70, see Results), both 0.8 MPa and 1.5 MPa pressures were used. Apoplastic fluid was recovered in a vial attached to the outlet.
Fig. 1.
Schematic diagram of the pressure membrane apparatus (left). Expanded scale (right) shows the details of the PM apparatus composed of a membrane, support screen, and an outlet attached to the sample stage in the pressure vessel. A water-filled pressure vessel was placed between the gas source and the PM apparatus to ensure an RH of 100% in the vessel (see Materials and methods). When the berry tissue is placed on the membrane and a pressure is applied, apoplastic fluid from the tissue will move to the membrane, and ultimately through the outlet to the vial at atmospheric pressure, under the influence of the hydrostatic potential difference (i.e. matric potential difference). The dead volume in the apparatus was estimated to be 51.4 μl (sum of 26.8 μl for the membrane filter, 20.4 μl for the support screen, and 4.2 μl for the outlet).
The physical basis for water extraction by the PM apparatus is the same as that for the pressure plate apparatus widely used for soils (Or and Wraith, 2002), and a possible advantage of the PM approach over the low-speed centrifuge approach is that in the PM, all of the berry tissue, as well as the upper surface of the membrane, are at the same pressure, and hence there is no net load applied to the tissue. In the centrifuge technique, the tissue is physically compressed by centrifugal force, especially the tissue closest to the support screen. No negative biological effects of applying relatively high pressures to root systems for extended periods of time (days) have been reported (Passioura, 1988), and hence the PM approach, which was completed within 8 h of harvest, may be a physically and physiologically less disruptive method for obtaining apoplastic fluid than the low-speed centrifuge approach. Since both methods are designed to extract water from the tissue apoplast without damaging cells however, it is expected that at least some of the extracted apoplastic water will be replaced by symplastic water during the extraction process, leading to a dilution of the apoplastic solution. For the centrifuge approach, the degree of dilution will, in part, depend on the quantity of symplastic water that results from tissue compression, and for the PM approach, the degree of dilution will depend in part on the size distribution of fluid-filled pores in the apoplast, with less dilution as the frequency of larger pores (intercellular spaces) increases. Neither of these effects can be estimated with any certainty, but to the extent that the results of centrifugation and PM techniques are found to agree, the simplest explanation would be that neither effect is large.
Correction of dilution in the PM apparatus
The calculated water-filled volume on the atmospheric pressure side of the membrane (dead volume, VD) was 51.4 μl (Fig. 1, inset), and hence it is expected that the solute concentration of any solution extracted through the apparatus will be diluted by the presence of this dead volume. Given that the volume of the fluid extracted from the sample is VS, containing NS moles of solute and hence an undiluted solute concentration of CS ( = NS/VS), then complete mixing with VD will give the relation:
| (1) |
where CA is the concentration of the mixed fluid. Equation (1) shows that the relationship between CS/CA and 1/VS is expected to be a linear function with a y-intercept of 1 (CS=CA when VS>>VD) and a slope of VD. Hence, VD can be determined experimentally by measuring the CS of different extracted volumes of solutions with known CA. For this purpose, 20 mm high cylindrical sections of cellulose sponge material (general purpose sponge, R70ACER, ACE Hardware) were made with a cork borer at diameters of 11.5 mm and 13.5 mm (corresponding to the mean berry diameter of field-grown Cabernet Sauvignon berries at 60 DAA and 90 DAA, respectively), washed with distilled water thoroughly, stirred in distilled water (two times at least for 2 h) to remove solutes, squeezed dry, and oven-dried at 70 oC for 24 h (final dry weight of 57.02±7.16 g (n=40) and 82.58±5.10 g (n=36) for the two contrasting diameters) to remove water, and only used once. Dry sponges were placed on a membrane which had been conditioned as described above, a measured quantity of NaCl solution (0.13–0.5 ml) was added to the sponge, and the sponge extracted in the PM apparatus as for the tissue.
Berry cell P measurements and estimation of apoplast matric potential ()
The modified cell pressure probe technique (Hüsken et al., 1978) as described previously (Shackel et al., 1987; Thomas et al., 2006) was used to measure P of individual cells in the berry mesocarp ranging from 100 μm to 2000 μm below the surface of the epidermis. Briefly, microcapillary tips were prepared by a Koph 750 micropipette puller and were bevelled in a jet-stream of bevelling solution (Ogden et al., 1978) modified as described previously (Shackel et al., 1987). Micropipettes were inserted perpendicularly to the surface along the equatorial plane of the berries. All measurements were performed under laboratory conditions (diffuse fluorescent light, 25 °C air temperature, and localized 100% RH obtained with a humidifier) and were generally completed within 12 h of detachment from the cluster depending on the sample size. Previous work has shown that the berry P does not significantly change for up to 48 h after being excised from the vine if water loss from the berry is prevented (Thomas et al., 2006).
Many parenchymatous tissues have relatively thin cell walls and a consequently low fraction of cell wall space. Cosgrove and Cleland (1983) reported values of about 4% in a number of parenchymatous hypocotyl tissues. Although there are no measurements of this fraction specifically for grape, it is estimated from the cell and wall dimensions reported in Hardie et al. (1996) that the mesocarp cells of the grape berry are similar, with about 5% cell wall space. Based on this low fraction, it is assumed that tissue Ψs () can be used as a measure of cell Ψs, and hence that cell total water potential can be estimated as . Since the cell symplast and apoplast water potentials should be in equilibrium, this is also an estimate of apoplast total water potential , and hence apoplast matric potential () can be estimated as ().
Berry firmness (E) measurement
Berry firmness was determined non-destructively using a custom fabricated instrument which measured force and displacement during berry compression (Weis, 2006). As described in Thomas et al. (2008), the observed force/displacement relation was fit using SAS PROC NLIN (version 8.2; SAS Institute) to that expected for compression of a perfectly elastic sphere (Hertz equation, rearranged from equation (2) of Ravi et al., 2006).
| (2) |
where F, ν, RO, and D indicate the external force applied, Poisson ratio (ν, assumed=0.5), the undeformed radius, and the deformation during compression, respectively. F is measured in Newtons and RO and D in mm; the units of E are MPa.
Results
Dead volume and extraction pressure in the PM apparatus
In addition to the two contrasting diameters of sponge material, dilution experiments were performed using two contrasting extraction pressures and two contrasting solute concentrations, and in all cases dilution increased exponentially as the sample volume decreased (Fig. 2A–C). The relation between CS/CA and 1/VS was also linear (Fig. 2D–F), and there was no significant effect (at P <0.05) on the slope of the relationship for any factor studied. Based on this result, an overall regression was fit, and a value of 4.54 μl for the effective dead volume (VD) was obtained. This value was used to correct all of the values for .
Fig. 2.
Experimental determination of an effective dead volume in the pressure membrane with sponge columns instead of berry samples (see Results). Dilution rate (A–C) plotted against the volume of fluid collected from the outlet of PM devices, and CS/CA (D–F) plotted against 1/Vs. Effects of dilution due to each of the differences in concentration (with –1.00 MPa and –1.95 MPa of NaCl solutions) (A, D), sample size (with 11.5 mm and 13.5 mm diameters of sponge columns) (B, E), and applied pressures (with 0.80 MPa and 1.50 MPa) (C, F) were tested. Each dead volume was determined from the slope given by fitting at the y-intercept=1 in regression lines of CS/CA plotted against 1/Vs.
In order to obtain a sufficient quantity of apoplastic fluid for analysis from the PM method, PostV berries only required a pressure of 0.8 MPa for a short time (<1 h) pressurization, whereas PreV berries required a higher pressure (1.5 MPa) for a longer time (1.5–2.5 h), depending on sample (berry) size. The volume of PM-recovered fluid, i.e. VS in equation (1) was 7.2±2.2 μl (mean of n=32±1 SD), ranging from 2.0–11.5 μl. The PM yield volume was slightly larger than that obtained using centrifugation (5.3±4.4 μl, mean of n=95±1 SD, ranging from 1–21 μl), but not significantly so (at P <0.05). Since there was no significant difference (at P <0.05) for samples extracted using a pressure of either 0.8 or 1.5 MPa and collected between 63 DAA and 70 DAA in terms of yield volume, , and (data not shown), data for both extraction pressures were pooled.
measurements
In order to test whether the residual (unused) tissue from a particular berry could be used to determine the of the tissue that was sampled (used) for centrifugation or in the PM apparatus, the values of for these tissues were compared. The regression line between Ψs of sampled (x) and residual tissues (y) was y=0.98x–0.12 with r2=0.97 for PM samples (Fig. 3A). Thus, the relationship was linear and essentially equivalent across a broad range of sampled , ranging from –0.62 to –4.03 MPa (Fig. 3). A similar regression line was obtained for centrifugation samples (y=0.99x–0.10 with r2=0.96) (Fig. 3B). Each equipotential line was within the 95% confidence bands of both regression lines (Fig. 3A, B), suggesting that neither extraction significantly affected . Based on this result, residual tissue was used for the measurement of .
Fig. 3.
Residual tissue cell solute potentials as a function of sampled tissue cell solute potentials from individual berries sampled from field-grown Cabernet Sauvignon vines at various days after anthesis (DAA). All solute potentials were measured at the extractions with either the PM technique (A) or the centrifuge technique (B). The solid line indicates the regression line. Working–Hotelling hyperbolic confidence bands for the regression lines were calculated from data points at P=0.95 for each case. Hyperbolic confidence curves for regression line are drawn with dashed lines. The dotted line indicates the equipotential line.
Apoplastic solute accumulation was coincident with P and E losses
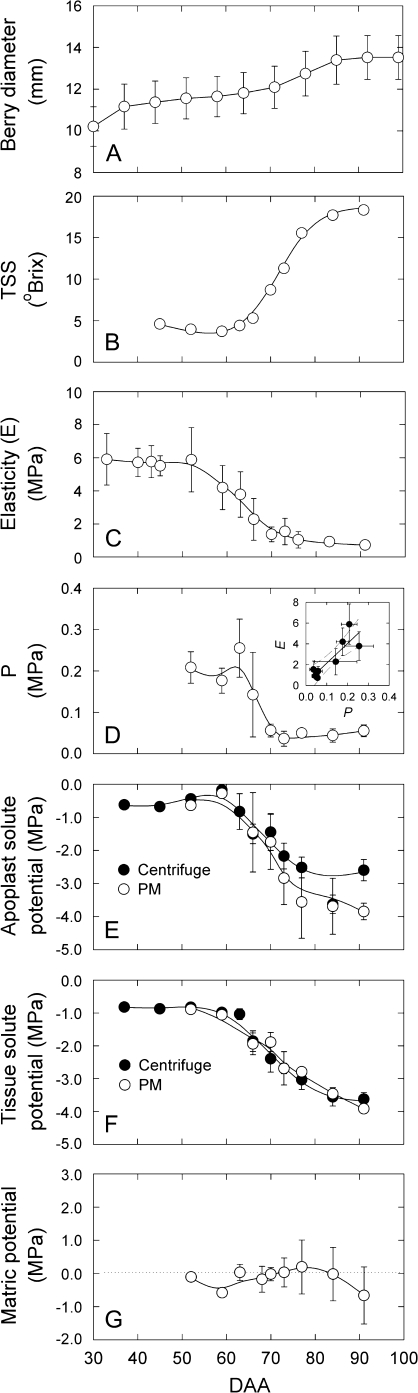
For Cabernet Sauvignon berries growing under field conditions, the typical double-sigmoid pattern of berry enlargement was evident from measurements of berry diameter (Fig. 4A). After berry growth slowed at the beginning of Stage II, soluble solids were steady at about 4 oBrix and fruit E was high (about 6 MPa; Fig. 4A–C). Fruit E and P both decreased between about 52 DAA and 70 DAA (Fig. 4C, D), with a linear positive correlation (see inset in Fig. 4D), and during this time there were relatively small changes in oBrix (Fig. 4B). The determined with both extraction techniques was about –0.60 MPa on 52 DAA, increased slightly to –0.20 MPa on 59 DAA and showed a progressive decline starting at 59 DAA, about 12 d prior to the appearance of red colour (Fig. 4E). The reduction in coincided with losses in fruit cell P and fruit E. The agreement between the two extraction techniques was good, despite large PostV sample-to-sample variation (e.g. 92 DAA; Fig. 4E). The regression line between obtained by centrifugation (x) and obtained by the PM (y) was y=1.17x–0.15 with r2=0.90 (not shown). Based on a linear regression of Ψs over time between 59 DAA and 70 DAA, the accumulation rate for solutes in the apoplast was found to be about 1.5-fold greater than for solutes in the bulk tissue (–0.14 MPa d−1 for apoplast and –0.09 MPa d−1 for tissue, Fig. 4E, F). Fruit E and fruit cell P reached a low level after veraison, which was maintained throughout the rest of development (Fig. 4C, D). Both and reached almost –4.0 MPa by late in development (Fig. 4E, F). The Ψm that was indirectly calculated according to Wada et al. (2008) was negative (–0.16 MPa on average); however, only the first two points (52 DAA and 59 DAA) were significantly different (lower) than zero (Fig. 4G).
Fig. 4.
Changes in berry size (A), soluble solids (B), fruit E (C), mesocarp cell turgor (D), apoplastic solute potential (E), tissue solute potential (F), and calculated apoplastic matric potential (G) for field-grown Cabernet Sauvignon berries. With the exception of (B), all error bars are ±1 SD and are smaller than the symbol if not present. In (A) data are means of six tagged berries measured over the season. In (B) data are the mean of the 2–3 berries used for P measurements, and in (D), data are the mean of 7–18 cells in 2–3 berries. A positive correlation between E and P was observed (inset in D: E=18.93P + 0.26, r2=0.76, and Working–Hotelling hyperbolic confidence bands at P=0.95). (C, E–G) Data are means of about 12 values with the exception of the last two values (85 DAA and 92 DAA) for which n=6 and n=3, respectively. In (G) the weight-averaged matric potential is shown (see Results). Solid lines in all graphs are 65% smoothed cubic splines (version 8.2; SAS Institute) fitted to the raw data.
Discussion
These results obtained for Cabernet Sauvignon under field conditions have confirmed our earlier observations, limited to three stages of Chardonnay berry development under controlled environment conditions (Wada et al., 2008), that significant concentrations of apoplastic solutes occur in grape berries as part of the ripening process, as also suggested by Matthews and Shackel (2005) and Thomas et al. (2006). Given the difficulty of obtaining direct measurements of cell P in intact grape berries and the resulting small sample size, the decline in E, P, , and all began about 60 DAA, more than 10 d prior to skin coloration. Based on the correlation between P and fruit firmness observed in this and other studies (Miganani et al., 1995; Tong et al., 1999; Thomas et al., 2008), it is suggested that softening of the grape berry, which is considered to be the first indication of ripening (Coombe, 1992) is due to a loss of cellular P, and, further, that most of this loss in P can be attributed to the appearance of solutes in the fruit apoplast. Fruit obtained by both PM and low speed centrifugation techniques exhibited a wide range of values (4 MPa), a range which is consistent with the progressive lowering of berry Ψw over development reported by Matthews et al. (1987). Indirect estimates of apoplastic matric potential (Fig. 4G) also indicated that post-veraison reductions in berry Ψw could be entirely attributed to reductions in , consistent with previous results (Wada et al., 2008), and with the post-veraison drop in xylem tension reported by Tyerman et al. (2004).
Recently, Krasnow et al. (2008) reported a loss of cell viability in the locular region of normally developing grape berries, and some research (Lang and During, 1991) has suggested that a loss of membrane integrity and compartmentation, presumably leading to a loss of cell viability as well as a loss in firmness (E) and P, occurs as a normal part of berry development. However, our results show that P and E both reach low and relatively stable values about the time that fruit growth resumes and fruit coloration reaches about 50% (Fig. 4), which is long before any loss in cell viability was observed by Krasnow et al. (2008; about 115 DAA) in the same variety, also under field conditions. Hence, it is suggested here that the presence of solutes in the fruit apoplast may be a mechanism to down-regulate P, and that the level of P could play an important role at the cellular level in berry developmental/ripening processes. An active down-regulation of P, rather than a general loss in compartmentation, is also more consistent with numerous lines of evidence for the continued expression of membrane intrinsic protein genes after veraison (sugar transporters: Davies et al., 1999; Fillion et al., 1999; aquaporins: Delrot et al., 2001; Picaud et al., 2003). In tomato, Mignani et al. (1995) showed an association of low P to increased rates of a number of ripening-related processes (colour development, polygalacturonase activity, pectin solubilization), and Saladie et al. (2007) have reported a decline in P associated with ripening, as well as a maintenance of P that was associated with a maintenance of whole fruit integrity post-harvest. While Saladie et al. (2007) primarily attributed the differential maintenance of P to differences in cuticle properties and whole fruit water loss, a role of symplast/apoplast distribution of solutes was also recognized as a possible mechanism to explain P differences.
Since the grape berry as a whole is accumulating solutes (mainly sugars) during the time that P is declining (Fig. 4), and the main contributors to are sugars (Wada et al., 2008) it is not clear whether the decrease in is a consequence of solute release, or simply reduced solute uptake, by mesocarp cells. The transcription level of cell wall invertase in grape was elevated prior to veraison (Hayes et al., 2007), and this enzyme has been reported in tissue surrounding the fruit vasculature (Famiani et al., 2000), both consistent with a role of invertase in the hydrolysis of sucrose unloaded from phloem (Patrick, 1997) and a decrease in the solute potential of the fruit apoplast. However, changes in solute transport to and within the grape berry may only be one of a number of events that occur prior to veraison.
One difficulty in hypothesizing a generalized sequence of events for grape berry development using veraison as the developmental reference point is that the term ‘veraison’ has not been used consistently in the literature. Historically, this term primarily referred to colour change (Coombe, 1992; Mullins et al., 1992). The growth habit of the berry, typically designated as Stage I, II, and III (Winkler et al., 1972; Coombe, 1976), has been used to assign veraison as the transition from Stage II to Stage III (Matthews et al., 1987). Coombe and Bishop (1980) used berry deformation under an applied load as a measure of softening, and found a gradual increase in deformation from 0.1 mm to 0.2 mm over about 6 d, followed by a rather sudden (1 d) increase to 0.4 mm. The occurrence of this sudden increase was identified as the day of veraison, and, later, Coombe (1992) proposed simply that a berry deformation of 0.4 mm indicated veraison. One difficulty with this approach is that deformation is inversely related to berry elasticity (E), such that the apparently small initial increase in deformation from 0.1 mm to 0.2 mm actually corresponded to the largest and most rapid decrease in E (Thomas et al., 2008). Hence, small increases in deformability, rather than a fixed threshold deformability, may be most indicative of softening. For instance, Nunan et al. (1998) measured a small increase in deformability (to about 0.2 mm) between 44 DAA and 58 DAA, but following Coombe's criteria did not consider this as veraison, even though they also reported the largest change in linkage composition for the wall polysaccharides that were considered to be the most significant (1–4 linked Galp residues) at this time. Our data (Fig. 4) suggest that P and E are closely linked, and that softening, as measured by a decrease in P or E, is an earlier event in ripening than the 0.4 mm increase in deformation proposed by Coombe (1992).
Implicit with each use of veraison has been the understanding that a number of other changes, such as an increased rate of sugar accumulation, softening, and berry growth, also occur around this time, and most uses parenthetically define veraison as ‘the onset of ripening’. However, for a process as complex as ripening, ‘onset’ may be difficult to define. Hence, we suggest that the term veraison only be used to refer to a general period of time during which a number of processes are occurring (softening, renewed growth, sugar accumulation, loss of green colour, and synthesis of red colour in pigmented varieties), and that terms and objective methods of measurement that are specific to each of these processes be adopted for describing berry development.
Essentially all of the sugar that is accumulated by the developing grape berry must ultimately reside in the vacuoles of the mesocarp cells, and so the similarity in symplast and apoplast Ψs during this accumulation (Fig. 4E, F), indicates that sugar transport rates across the plasmalemma and tonoplast membranes are highly co-ordinated in the intact fruit. If similar processes occur in other fruits, then experiments which expose excised fruit tissue to water or dilute nutrient solutions to measure apparent membrane integrity by the loss of solutes (Azevedo et al., 2008) must be interpreted with caution. For instance, solute loss under these conditions may be the result of selective membrane transport to down-regulate P, rather than an indication of a loss in membrane integrity. In an early study on cell water relations and fruit development in apple, Steudle and Wieneke (1985) were able to manipulate P in excised tissue over a relatively wide range (0.2–1.0 MPa) by manipulating the Ψs of the incubation media, but acknowledged that intact fruit P could not be inferred from their values. Interestingly, Steudle and Wieneke (1985) found the expected increase in cell E with P, but also found that cell E increased as fruit size and cell size increased, contrary to their expectations that apple fruit growth and softening would be associated with a decrease in cell E. This underscores the critical need for measurements of P and other cell water relations properties in intact fruits, in order to develop a better understanding of the processes that contribute to fruit ripening and softening.
Acknowledgments
This work was supported in part by USDA/CSREES Award No. 2006-35100-17440 and grants from the American Vineyard Foundation. We thank Dr Kentaro Inoue of the Department of Plant Sciences, University of California, Davis, for use of his centrifuge.
Glossary
Abbreviations
- PostV
post-veraison
- PreV
pre-veraison
- E
berry firmness (apparent fruit elasticity)
- P
turgor
- Ψm
matric potential
- Ψs
solute potential
apoplast solute potential
tissue solute potential
apoplast total water potential
- ΔP
pressure gradient (hydrostatic pressure difference)
- PM
pressure membrane
- VS (l)
volume of the fluid extracted from the sample
- NS (mol)
number of moles of solute containing in fluid (sample) loaded
- CS (mol l−1)
undiluted solute concentration in fluid (sample)
- VD (l)
dead volume
- F (N)
external force applied
- ν (dimensionless)
Poisson ratio
- RO (mm)
undeformed radius
- y (mm)
deformation during compression
References
- Azevedo IG, Oliveira JG, daSilva MG, Pereira T, Corrêa SF, Vargas H, Façanha AR. P-type H+-ATPases activity, membrane integrity, and apoplastic pH during papaya fruit ripening. Postharvest Biology and Technology. 2008;48:242–247. [Google Scholar]
- Bondada BR, Matthews MA, Shackel KA. Functional xylem in the post-veraison grape berry. Journal of Experimental Botany. 2005;56:2949–2957. doi: 10.1093/jxb/eri291. [DOI] [PubMed] [Google Scholar]
- Coombe B. Development of fleshy fruits. Annual Review of Plant Physiology and Plant Molecular Biology. 1976;27:207–228. [Google Scholar]
- Coombe BG. Research on development and ripening of the grape berry. American Journal of Enology and Viticulture. 1992;43:101–110. [Google Scholar]
- Coombe BG, Bishop GR. Development of the grape berry. II. Changes in diameter and deformability during veraison. Australian Journal of Agricultural Research. 1980;31:499–509. [Google Scholar]
- Cosgrove DJ, Cleland RE. Solutes in the free space of growing stem tissues. Plant Physiology. 1983;72:326–331. doi: 10.1104/pp.72.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Robinson SP. Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of cDNAs encoding putative cell wall and stress response proteins. Plant Physiology. 2000;122:803–812. doi: 10.1104/pp.122.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Wolf T, Robinson SP. Three putative sucrose transporters are differentially expressed in grapevine tissues. Plant Science. 1999;147:93–100. [Google Scholar]
- Delrot S, Picaud S, Gaudillere JP. Water transport and aquaporins in grapevine. In: Roubelakis-Angelakis KA, editor. Molecular biology and biotechnology of the grapevine. London: Kluwer Academic Publishers; 2001. pp. 241–262. [Google Scholar]
- Famiani F, Walker RP, Tesci L, Chen Z, Proietti P, Leegood RC. An immunohistochemical study of the compartmentation of metabolism during the development of grape (Vitis vinifera L.) berries. Journal of Experimental Botany. 2000;51:675–683. [PubMed] [Google Scholar]
- Fillion L, Ageorges A, Picaud S, Coutos-Thevenot P, Lemoine R, Romieu C, Delrot S. Cloning and expression of a hexose transporter gene expressed during the ripening of grape berry. Plant Physiology. 1999;120:1083–1093. doi: 10.1104/pp.120.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goes da Silva F, Iandolino A, Al-Kayal F, et al. Characterizing the grape transcriptome. Analysis of expressed sequence tags from multiple Vitis species and development of a compendium of gene expression during berry development. Plant Physiology. 2005;139:574–597. doi: 10.1104/pp.105.065748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan MD, Schultz HR, Matthews MA. Field evaluation of water transport in grape berries during water deficits. Physiologia Plantarum. 1996;97:55–62. [Google Scholar]
- Greenspan MD, Shackel KA, Matthews MA. Developmental changes in the diurnal water budget of the grape berry exposed to water deficits. Plant, Cell and Environment. 1994;17:811–820. [Google Scholar]
- Hardie WJ, O'Brien TP, Jaudzems V. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Australian Journal of Grape Wine Research. 1996;2:97–142. [Google Scholar]
- Hayes MA, Davies C, Dry IB. Isolation, functional characterization, and expression analysis of grapevine (Vitis vinifera L.) hexose transporters: differential roles in sink and source tissues. Journal of Experimental Botany. 2007;58:1985–1997. doi: 10.1093/jxb/erm061. [DOI] [PubMed] [Google Scholar]
- Hüsken D, Steudle E, Zimmermann U. Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiology. 1978;61:158–163. doi: 10.1104/pp.61.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow M, Matthews M, Shackel K. Evidence for substantial maintenance of membrane integrity and cell viability in normally developing grape (Vitis vinifera L.) berries throughout development. Journal of Experimental Botany. 2008;59:849–859. doi: 10.1093/jxb/erm372. [DOI] [PubMed] [Google Scholar]
- Lang A, During H. Partitioning control by water potential gradient: Evidence for compartmentation breakdown in grape berries. Journal of Experimental Botany. 1991;42:1117–1122. [Google Scholar]
- Matthews MA, Shackel KA. Growth and water transport in fleshy fruit. In: Holbrook MN, Zwieniecki MA, editors. Vascular transport in plants. Boston: Elsevier Academic Press; 2005. pp. 189–197. [Google Scholar]
- Matthews MA, Cheng G, Weinbaum SA. Changes in water potential and dermal extensibility during grape berry development. Journal of the American Society for Horticultural Science. 1987;112:314–319. [Google Scholar]
- Mignani I, Greve LC, Benarie R, Stotz HU, Li CY, Shackel KA, Labavitch JM. The effects of GA3 and divalent-cations on aspects of pectin metabolism and tissue softening in ripening tomato pericarp. Physiologia Plantarum. 1995;93:108–115. [Google Scholar]
- Mullins MG, Bouquet A, Williams LE. Biology of the grapevine. Cambridge, UK: Cambridge University Press; 1992. [Google Scholar]
- Nunan KJ, Sims IM, Bacic A, Robinson SP, Fincher GB. Changes in cell wall composition during ripening of grape berries. Plant Physiology. 1998;118:783–792. doi: 10.1104/pp.118.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden TE, Citron MC, Pierantoni R. The jet stream microbeveler: an inexpensive way to bevel ultrafine glass micropipettes. Science. 1978;201:469–470. doi: 10.1126/science.663670. [DOI] [PubMed] [Google Scholar]
- Or D, Wraith J. Soil water content and water potential relationship. In: Warrick A, editor. Soil physics companion. LLC: CRC Press; 2002. pp. 49–84. [Google Scholar]
- Passioura JB. Root signals control leaf expansion in wheat seedlings growing in drying soil. Australian Journal of Plant Physiology. 1988;15:687–693. [Google Scholar]
- Patrick JW. Phloem unloading: sieve element unloading and post-sieve element transport. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:191–222. doi: 10.1146/annurev.arplant.48.1.191. [DOI] [PubMed] [Google Scholar]
- Picaud S, Becq F, Dedaldechamp F, Ageorges A, Delrot S. Cloning and expression of two plasma membrane aquaporins expressed during the ripening of grape berry. Functional Plant Biology. 2003;30:621–630. doi: 10.1071/FP02116. [DOI] [PubMed] [Google Scholar]
- Pomper KW, Breen PJ. Levels of apoplastic solutes in developing strawberry fruit. Journal of Experimental Botany. 1995;46:743–752. [Google Scholar]
- Ravi N, Wan KT, Swindle K, Hamilton PD, Duan G. Development of techniques to compare mechanical properties of reversible hydrogels with spherical, square columnar and ocular lens geometry. Polymer. 2006;47:4203–4209. [Google Scholar]
- Saladie M, Matas AJ, Isaacson T, et al. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiology. 2007;144:1012–1028. doi: 10.1104/pp.107.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackel KA, Matthews MA, Morrison JC. Dynamic relation between expansion and cellular turgor in growing grape (Vitis vinifera L) leaves. Plant Physiology. 1987;84:1166–1171. doi: 10.1104/pp.84.4.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E, Wieneke J. Changes in water relations and elastic properties of apple fruit cells during growth and development. Journal of the American Society for Horticultural Sciences. 1985;110:824–829. [Google Scholar]
- Thomas TR, Matthews MA, Shackel KA. Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant, Cell and Environment. 2006;29:993–1001. doi: 10.1111/j.1365-3040.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Thomas TR, Shackel KA, Matthews MA. Mesocarp cell turgor in Vitis vinifera L. berries throughout development and its relation to firmness, growth, and the onset of ripening. Planta. 2008;228:1067–1076. doi: 10.1007/s00425-008-0808-z. [DOI] [PubMed] [Google Scholar]
- Tong C, Krueger D, Vickers Z, Bedford D, Luby J, El-Shiekh A, Shackel K, Ahmadi H. Comparison of softening-related changes during storage of ‘Honeycrisp’ apple, its parents, and ‘Delicious’. Journal of the American Society for Horticultural Sciences. 1999;124:407–415. [Google Scholar]
- Tyerman SD, Tilbrook J, Pardo C, Kotula L, Sullivan W, Steudle E. Direct measurement of hydraulic properties in developing berries of Vitis vinifera L. cvs Shiraz and Chardonnay. Australian Journal of Grape Wine Research. 2004;10:170–181. [Google Scholar]
- Wada H, Shackel K, Matthews M. Fruit ripening in Vitis vinifera: apoplastic solute accumulation accounts for pre-veraison turgor loss in berries. Planta. 2008;227:1351–1361. doi: 10.1007/s00425-008-0707-3. [DOI] [PubMed] [Google Scholar]
- Weis N. Berry development in Vitis vinifera cultivar Cabernet Sauvignon: measuring berry firmness, altering the phenology of veraison, and characterizing berry shrivel, a veraison disorder. MS Thesis, University of California, Davis. 2006 [Google Scholar]
- Welbaum GE, Meinzer FC. Compartmentation of solutes and water in developing sugarcane stalk tissue. Plant Physiology. 1990;93:1147–1153. doi: 10.1104/pp.93.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AJ, Cook JA, Kliewer WM, Lider LA. General viticulture. Berkeley, CA: University of California Press; 1972. pp. 138–139. [Google Scholar]