Abstract
The Arabidopsis thaliana CBF cold response pathway plays a central role in cold acclimation. It is characterized by rapid cold induction of genes encoding the CBF1-3 transcription factors, followed by expression of the CBF gene regulon, which imparts freezing tolerance. Our goal was to further the understanding of the cis-acting elements and trans-acting factors involved in expression of CBF2. We identified seven conserved DNA motifs (CM), CM1 to 7, that are present in the promoters of CBF2 and another rapidly cold-induced gene encoding a transcription factor, ZAT12. The results presented indicate that in the CBF2 promoter, CM4 and CM6 have negative regulatory activity and that CM2 has both negative and positive activity. A Myc binding site in the CBF2 promoter was also found to have positive regulatory effects. Moreover, our results indicate that members of the calmodulin binding transcription activator (CAMTA) family of transcription factors bind to the CM2 motif, that CAMTA3 is a positive regulator of CBF2 expression, and that double camta1 camta3 mutant plants are impaired in freezing tolerance. These results establish a role for CAMTA proteins in cold acclimation and provide a possible point of integrating low-temperature calcium and calmodulin signaling with cold-regulated gene expression.
INTRODUCTION
Most plants from temperate regions increase in freezing tolerance upon exposure to low nonfreezing temperatures, a phenomenon known as cold acclimation (Levitt, 1980). It is well established that cold acclimation involves changes in cell physiology and biochemistry, including extensive alterations in lipid, protein, and metabolome composition, many of which are brought about by changes in gene expression (Thomashow, 1999; Chinnusamy et al., 2007). Indeed, hundreds of genes are either induced or repressed in response to low temperature (Fowler and Thomashow, 2002; Kreps et al., 2002; Maruyama et al., 2004; Vogel et al., 2005). A fundamental goal of cold acclimation research is to determine how plants sense low temperature and bring about the changes in gene expression that increase plant freezing tolerance.
At present, the CBF cold response pathway is the best-understood regulatory pathway involved in cold acclimation. In Arabidopsis thaliana, three CBF genes, CBF1, CBF2, and CBF3 (also known as DREB1b, DREB1c, and DREB1a, respectively), are induced within 15 min of exposing plants to low temperature (Gilmour et al., 1998; Liu et al., 1998). CBF1-3 encode closely related members of the AP2/ERF family of transcription factors (Riechmann et al., 2000), which bind to the CRT/DRE (for C-repeat/dehydration responsive element) DNA regulatory elements found in the promoters of CBF-targeted genes (Stockinger et al., 1997; Liu et al., 1998). The CBF proteins induce the expression of ∼100 genes, termed the CBF regulon (Maruyama et al., 2004; Vogel et al., 2005), which, together, bring about an increase in freezing tolerance (Jaglo-Ottosen et al., 1998; Liu et al., 1998). This process involves the accumulation of low molecular weight cryoprotective metabolites, such as raffinose, sucrose, and proline (Cook et al., 2004; Kaplan et al., 2004), and the production of cryoprotective polypeptides, such as COR15a (Steponkus et al., 1998).
There is considerable information about the changes in gene expression that occur downstream of CBF1-3 induction, but little is known about how the CBF1-3 genes themselves are induced in response to low temperature. Zhu and colleagues (Chinnusamy et al., 2003; Agarwal et al., 2006) have identified two transcription factors that are involved: MYB15, a negative regulator of CBF1-3; and ICE1, which is a positive regulator of CBF3 but has little effect on CBF1 and CBF2 expression (Chinnusamy et al., 2003; Agarwal et al., 2006). Zarka et al. (2003) identified a 155-bp region of the CBF2 promoter that is capable of driving cold-induced transcription and found, within this promoter fragment, two short sequences, ICEr1 and ICEr2 (for inducer of CBF expression region 1 and 2), that contribute to cold induction. However, the transcription factors that act through these sequences remain to be determined.
There is also little information about the cold signaling pathways upstream of CBF and other first-wave cold-induced genes. However, a role for calcium has been suggested. Exposure of Arabidopsis plants to low temperature results in a rapid increase in cytosolic calcium levels that involves release of calcium from both extracellular and vacuolar stores (Knight et al., 1996). In addition, it is known that this rapid calcium influx is required for proper cold induction of KIN1, a member of the CBF regulon (Knight et al., 1996). Similarly, in alfalfa (Medicago sativa), cold induction of two genes, cas15 and cas18, involves calcium influx (Monroy and Dhindsa, 1995). These results point to the importance of calcium in the response of plants to low temperature. However, the molecular connection between calcium increase and cold-regulated gene expression is not well characterized.
Here, our goal was to define in more detail the cis-acting DNA regulatory elements and trans-acting factors involved in the regulation of CBF2. Seven motifs of 6 to 9 bp in length were identified as being conserved in regions of the CBF2 and ZAT12 promoters that impart cold-responsive transcription. Two of these motifs were found to have negative regulatory effects, and one had both positive and negative effects. We also show that members of the calmodulin binding transcription activator (CAMTA) family of transcription factors (Bouché et al., 2002) can bind to one of the conserved motifs, that CAMTA3 is a positive regulator of CBF2 expression, and that plants carrying mutations in both CAMTA1 and CAMTA3 are impaired in freezing tolerance. These results identify new cis-acting cold regulatory elements, establish a role for CAMTA proteins in cold acclimation, and provide a possible point of integration of calcium signaling and cold-regulated gene expression.
RESULTS
Cold Regulation of the CBF2 Promoter Involves Multiple DNA Motifs
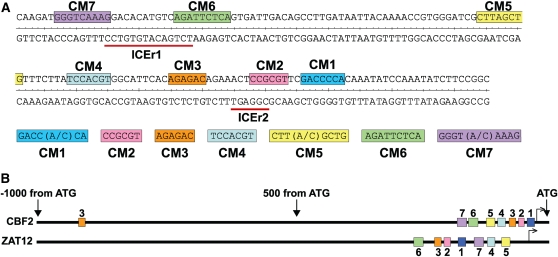
A previous study identified a 155-bp region of the CBF2 promoter that imparts cold-induced gene expression and two elements within this region, ICEr1 and ICEr2, that contribute to this regulation (Zarka et al., 2003). It has also been shown that the ZAT12 gene has very similar kinetics of cold induction to that of CBF2 and that both genes are responsive to mechanical agitation and treatment with cycloheximide (Vogel et al., 2005). A question raised was whether the promoters of these two genes share common DNA sequence motifs that might have roles in cold, mechanical, and cycloheximide regulation. To address this question, we conducted a mutational analysis of the ZAT12 promoter and identified a 225-bp fragment that was sufficient for cold induction (see Supplemental Figure 1 online). Computational analysis revealed that this region of the ZAT12 promoter included seven short DNA motifs that were also present in the 155-bp cold-responsive region of the CBF2 promoter. These sequences were designated Conserved Motif (CM) 1 to 7 (Figure 1). The CM sequences are clustered into short regions of the CBF2 and ZAT12 promoters but occur in a different order in each promoter. The previously identified ICEr1 element overlaps CM6 and CM7, and ICEr2 overlaps CM2 in CBF2. The CBF1 and CBF3 promoters (1-kb upstream region) only had two of the CM sequences: the CBF1 promoter had a CM3 and CM7 sequence at positions −99 and −177 from the start of transcription, respectively, and the CBF3 promoter had a CM3 and CM5 sequence at positions −111 and −142, respectively.
Figure 1.
CM DNA Motifs Present in the Promoter Regions of both CBF2 and ZAT12.
(A) DNA sequences and location of CM1-7 in the −189 to −35 promoter region of CBF2 (Zarka et al., 2003). ICEr1 and ICEr2: inducer of CBF expression region 1 and 2, respectively (Zarka et al., 2003).
(B) Positions of CM1-7 in the 1-kb upstream promoter regions of CBF2 and ZAT12. Putative start of transcription is indicated by the bent arrow.
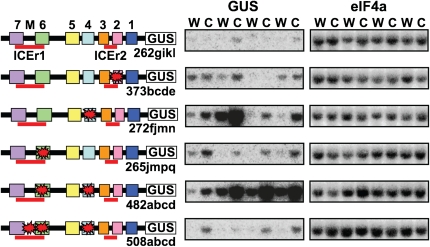
Promoter fusion studies were conducted to determine whether the CM sequences have roles in cold-regulated gene expression. The region of the CBF2 promoter from −189 to the ATG start codon (this includes the CBF2 promoter region shown in Figure 1) was fused to the β-glucuronidase (GUS) reporter gene, and the conserved motifs were mutagenized either singly or in combination and assayed for expression after transformation into Arabidopsis. Ten or more transgenic lines were obtained for each construct, and four representing the range of responses are presented (Figure 2). The results indicate that mutation of CM2, CM4, or CM6 resulted in higher levels of GUS transcripts at cold temperatures and that mutating both CM4/CM6 resulted in higher GUS levels of transcripts at both warm and cold temperature. These findings indicate that each of these motifs have repressor activity. This result was unexpected for CM6 as this sequence overlaps the ICEr1 region, which acts as a positive regulatory sequence (Zarka et al., 2003). These results suggested that there might be both a positive and a negative regulatory element within the ICEr1 region. Indeed, within the region between CM6 and CM7 is a Myc core recognition sequence, CANNTG, which binds basic helix-loop-helix (bHLH) transcription factors (Bailey et al., 2003). To test whether this sequence acts as a positive regulatory element, we added a mutation in this sequence to the CM4/CM6 double mutation and determined the expression of the GUS reporter gene. The results indicate that addition of the Myc mutation greatly reduced cold-induced expression of the reporter gene (Figure 2). Thus, there are at least two cold-responsive elements within the ICEr1 region, with CM6 acting as a negative regulator and the Myc element acting as a positive regulator.
Figure 2.
Roles for CM sequences in Cold Regulation of CBF2.
RNA gel blot analysis of GUS transcript levels in plants containing mutations in the −189 to −35 promoter region of CBF2. Boxes indicate CM1-7 sequences as described in Figure 1A. ICEr1 and ICEr2 regions are indicated by red lines. The core Myc binding site between CM6 and CM7 is indicated by the letter M. Mutations are indicated by a red star. Ten or more transgenic lines were obtained for each construct and four representing the range of responses are presented (the different promoter constructs are indicated by numbers and the transgenic lines by letters). Plants were grown at 24°C (w) and cold treated for 2 h at 4°C (c). eIF4a (eukaryotic initiation factor 4a) was the normalization control.
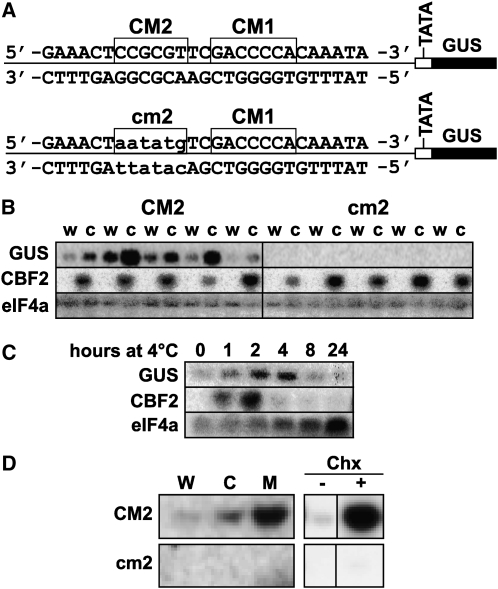
A 27-bp Region of the CBF2 Promoter Including CM1 and CM2 Is Sufficient to Impart Cold-Induced Transcription
Promoter fusion analysis indicated that trimers of CM1, CM2, CM4, CM5, CM6, or CM7 fused to the GUS reporter gene were unable to drive cold-induced expression (see Supplemental Figure 2 online). Also, tetramers formed by combining CM6 with CM7, or CM2 with CM3 were inactive (see Supplemental Figure 2 online). However, a 27-bp sequence containing CM1 and CM2 was sufficient to impart cold-induced transcription when fused as a tetramer to the GUS reporter gene (Figures 3A and 3B). Mutation of the CM2 sequence eliminated the ability of the fragment to induce cold-regulated gene expression, indicating that it was a positive regulatory element necessary for cold induction. Like endogenous CBF2 transcripts, the 27-bp:GUS transcript levels increase within 1 h of exposure to low temperature, peaked shortly after that and then decreased to low levels (Figure 3C). The difference in the kinetics of reduction of the CBF2 and GUS transcripts likely reflects the fact that CBF2 transcripts have an exceptionally short half-life of ∼7.5 min (Zarka et al., 2003).
Figure 3.
Cold, Mechanical, and Cycloheximide Regulation of the 27-bp CBF2 Promoter Fragment and Role of the CM2 Sequence.
(A) The 27-bp:GUS reporter gene sequences with intact CM2 sequence (CM2) and mutated CM2 sequence (cm2). In both cases, the reporter sequences were tetramers of the 27-bp sequence.
(B) RNA gel blot analysis of GUS and CBF2 transcript levels in plants containing the 27-bp:GUS reporter with a wild-type (CM2) or mutant (cm2) CM2 sequence. Plants tested were grown at 24°C and then either transferred to 4°C for 2 h (c) or kept at 24°C (w). Five independent transgenic lines were tested for the two reporter constructs. eIF4a was the normalization control.
(C) RNA gel blot analysis of GUS and CBF2 transcript levels in plants containing the 27-bp:GUS reporter with the wild-type CM2 sequence. Plants tested were grown at 24°C and then treated at 4°C for the indicated time periods. eIF4a was the normalization control.
(D) RNA gel blot analysis of GUS transcript levels in plants containing the 27-bp:GUS reporter with a wild-type (CM2) or mutant (cm2) CM2 sequence. Plants grown at 24°C (W) were treated for 2 h at 4°C (C), subjected to mechanical agitation for 15 min (M), or treated with (Chx+) or without (Chx−) cycloheximide for 2 h (10 μg mL−1).
As the CBF2 promoter is induced in response to both mechanical agitation and treatment with cycloheximide, we tested whether the 27-bp:GUS reporter was also responsive to these conditions. The results indicated that it was and that the CM2 sequence was required for these responses (Figure 3D). Thus, the CM2 element was involved in cold, mechanical, and cycloheximide induction of the 27-bp fragment.
CAMTA3 Has a Role in Cold-Induced Expression of CBF2
The CAMTA family of calmodulin binding transcription factors comprises six members in Arabidopsis (Bouché et al., 2002; Finkler et al., 2007). Each protein includes an IQ domain for calmodulin binding; ankyrin repeats for protein interactions; the TIG domain, which has nonspecific DNA binding activity; and the CG-1 domain, which has specific DNA binding activity. The CG-1 domain recognizes the core consensus sequence, vCGCGb, referred to as the CG-1 element (da Costa e Silva, 1994). This sequence matches the CM2 sequence, CCGCGT, and overlaps the ICEr1 element (Figure 1). Thus, the possibility was raised that one or more of the CAMTA proteins might have a role in regulating CBF2 expression.
As a first test of this possibility, we determined whether the CG-1 domain, which is at the N-terminal end of the CAMTA proteins, could bind to the CM2 sequence. The CG-1 DNA binding domains of CAMTA1, -2, -3, and -5 (CAMTA4 and -6 were not tested) were fused to glutathione S-transferase and expressed in Escherichia coli, and soluble protein extracts were assayed for DNA binding using the electromobility shift assay. The results indicated that the four CAMTA proteins tested did indeed bind to the CM2 sequence (see Supplemental Figure 3 online).
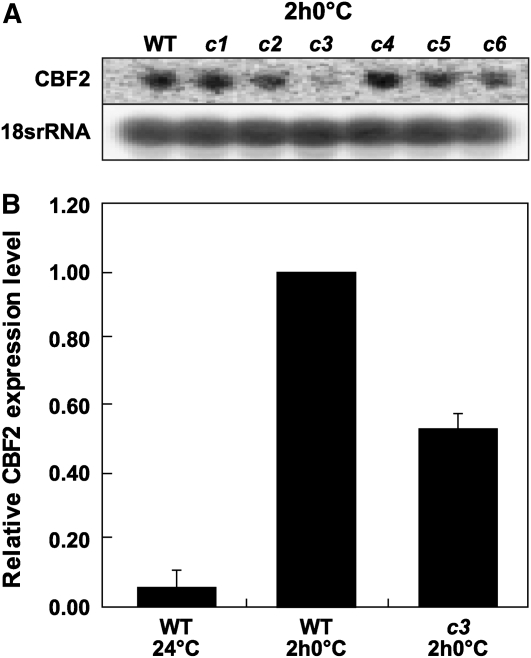
To directly test the possibility that one or more of the CAMTA proteins have a role in CBF2 expression, we identified homozygous T-DNA insertion lines for each CAMTA gene that resulted in undetectable transcripts for each corresponding gene (see Supplemental Figure 4 online) and tested these camta mutant plants for CBF2 expression. Initial RNA gel blot analysis indicated that the camta3 mutation resulted in a reduction of cold-induced accumulation of CBF2 transcripts (Figure 4A). This was confirmed by quantitative RT-PCR (qRT-PCR) analysis; CBF2 transcript levels in cold-treated camta3 plants were reduced to ∼50% of the levels found in cold-treated wild-type plants (Figure 4B). A statistically significant reduction in CBF2 expression was not observed in plants carrying the camta1, -2, -4, -5, or -6 mutations (P value < 0.05).
Figure 4.
Cold-Induced Accumulation of CBF2 Transcripts in camta3 Plants.
(A) RNA gel blot analysis of CBF2 transcript levels in wild-type and camta mutant plants (c1-6) grown at 24°C and treated for 2 h at 0°C. The same blot was probed for 18s rRNA as a loading control.
(B) qRT-PCR analysis. Relative expression levels of CBF2 transcripts in wild-type and camta3 plants (c3) grown at 24°C and then cold treated for 2 h at 0°C (2 h0°C). The CBF2 transcript levels in cold-treated wild-type plants were set at 1. Error bars indicate se. The averages for the wild type at 24°C and camta3 at 0°C for 2 h are significantly different from the wild type at 0°C for 2 h (P value < 0.0001; n = 6 for the wild type at 24°C, n = 12 for the wild type at 0°C for 2 h, and n = 12 for camta3 at 0°C for 2 h).
Plants carrying a camta1, -2, -3, -4, or -5 mutation showed no obvious abnormalities in growth and development when their life cycles were performed at either warm (24°C) or cold (4°C) temperatures. The camta6 mutant plants displayed yellowing of the veins and midrib at warm temperature (see Supplemental Figure 5 online), an effect that was suppressed in new leaves produced at low temperatures. It remains to be determined whether this phenotype was due to the T-DNA insertion or a second site mutation.
CAMTA3 Functions through the CM2 Sequence
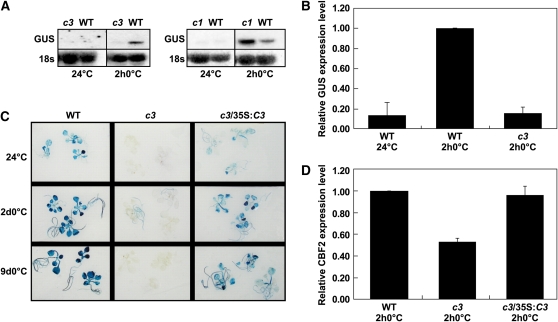
As shown above, the 27-bp CBF2 promoter fragment containing CM1 and CM2 imparted cold-induced transcription. Furthermore, this activity required the CM2 sequence to which the CAMTA3 protein binds. If the CAMTA3 protein contributed to CBF2 cold induction through binding to CM2 in planta, then the camta3 mutation would be expected to affect cold induction of the GUS reporter gene driven by the 27-bp promoter fragment. This was the case. RNA gel blot analysis indicated that whereas the camta1 mutation had no detectable effect on cold induction of the 27-bp:GUS reporter gene, the camta3 mutation greatly diminished it (Figure 5A). Further analysis by qRT-PCR indicated that the camta3 mutation essentially eliminated cold-induced expression of the 27-bp:GUS reporter gene (Figure 5B).
Figure 5.
Effect of the camta3 Mutation on Cold-Induced Expression of the 27-bp:GUS Reporter Gene.
(A) RNA gel blot analysis determining the transcript levels of GUS in wild-type plants, camta3 plants (c3), or camta1 plants (c1) transformed with the 27-bp:GUS reporter gene, grown at 24°C, and then cold treated for 2 h at 0°C. The same blots were probed for 18s rRNA as a loading control.
(B) qRT-PCR analysis determining the transcript levels of the 27-bp:GUS reporter gene in either wild-type plants or camta3 plants (c3) grown at 24°C and then cold treated for 2 h at 0°C (2 h0°C). Relative expression level of GUS transcripts with the wild-type cold-treated plants was set to 1. Error bars indicate se. The average GUS transcript levels in the camta3 background at 0°C for 2 h were significantly different from wild-type plants at 0°C for 2 h (P value < 0.0001; n = 2 for the wild type at 24°C, n = 6 for the wild type at 0°C for 2 h, and n = 6 for camta3 at 0°C for 2 h).
(C) Histochemical staining determining GUS activity in plants carrying the 27-bp:GUS reporter gene. Seedlings tested were wild-type, camta3 (c3) plants, and camta3 plants transformed with 35S:CAMTA3 (c3/35S:C3). Plants were grown at 24°C and then cold treated at 0°C for 2 d (2d0°C) or 9 d (9d0°C).
(D) qRT-PCR analysis of CBF2 transcript levels in wild-type plants, camta3 plants (c3), and camta3 plants transformed with the 35S:CAMTA3 transgene (c3/35S:C3). Plants were grown at 24°C and then cold treated at 0°C for 2 h (2 h0°C). Error bars indicate se (n = 3).
Histochemical staining of lines carrying the 27-bp:GUS reporter also indicated that GUS activity was greatly reduced in plants carrying the camta3 mutation whether they were grown at warm temperature or transferred or cold temperature for 2 or 9 d (Figure 5C). The small amount of GUS staining that was observed in the camta3 plants was limited to the roots. Staining of the wild-type plants grown under warm conditions was presumably due to the low-level expression of the reporter gene observed at warm temperature (Figure 3B). Transformation of these plants with the wild-type CAMTA3 gene under control of the cauliflower mosaic virus (CaMV) 35S promoter resulted in recovery of staining, confirming that the camta3 gene was responsible for the lack of reporter gene expression (Figure 5C). Recovery of CBF2 expression levels was also observed in the camta3 plants transformed with the wild-type CAMTA3 gene (Figure 5D). The expression level of the transgenic CAMTA3 gene used in these particular experiments was approximately equal to the level of the endogenous CAMTA3 gene in wild-type plants (i.e., it was not in great excess of the normal levels of expression) (see Supplemental Figure 6 online). Similar results were obtained with other transgenic lines expressing CAMTA3 at near endogenous levels.
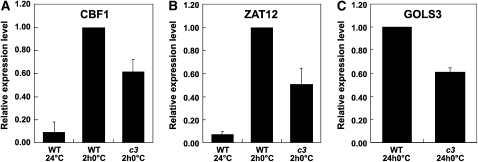
A Role for CAMTA3 in Cold Induction of CBF1, ZAT12, and GOLS3
Like CBF2, the promoter regions of CBF1 and ZAT12 include a CG-1 sequence within 1 kb upstream of the start codon. Therefore, it was of interest to determine whether any of the camta mutations affected cold-induced expression of these genes. The results indicated that the camta3 mutation reduced cold-induced accumulation of transcripts for CBF1 and ZAT12 by ∼40 and 50%, respectively (Figures 6A and 6B). None of the camta mutations had a statistically significant (P value < 0.05) effect on expression of CBF3, which does not have a CG-1 element in the 1-kb upstream region of its promoter. The camta3 mutation also affected expression of the CBF-targeted gene, GOLS3; transcript levels for GOLS3 were reduced ∼40% in the camta3 plants (Figure 6C). As GOLS3 does not have a CG-1 element in the 1-kb upstream promoter region, it is likely that the decrease in expression of this gene was due to the decrease in CBF1 and CBF2 expression.
Figure 6.
Effect of camta3 Mutation on Cold-Induced Accumulation of CBF1, ZAT12, and GOLS3 Transcripts.
(A) qRT-PCR analysis of CBF1 transcript levels in wild-type and camta3 plants (c3) grown at 24°C and then treated for 2 h at 0°C (2 h0°C), the maximum time of CBF1 expression. The levels in wild-type plants treated at low temperature were set to 1. Error bars indicate se. The averages for the wild type at 24°C and camta3 at 0°C for 2 h are significantly different from the wild type at 0°C for 2 h (Student's t test P value < 0.01; n = 10 for the wild type at 24°C and n = 12 for the wild type and camta3 treated at 0°C for 2 h).
(B) Same as in (A) except ZAT12 transcript levels were determined. Error bars indicate se. The averages for the wild type at 24°C and camta3 at 0°C for 2 h are significantly different from the wild type at 0°C for 2 h (Student's t test P value < 0.01; n = 10 for the wild type at 24°C and n = 12 for the wild type and camta3 treated at 0°C for 2 h).
(C) Same as in (A) except GOLS3 transcript levels were determined on wild-type and camta3 plants grown at 24°C and then treated for 24 h at 0°C (24 h0°C). Error bars indicate se. The GOLS3 transcript levels were significantly reduced in camta3 plants treated at 0°C for 24 h compared with wild-type plants treated at 0°C for 24 h (Student's t test P value < 0.01, n = 4).
The transcript levels of CBF1 and ZAT12 were determined at 2 h and those of GOLS3 at 24 h as these are the times at which these respective genes are expressed at high levels; CBF1 and ZAT12, like CBF2 (see Figure 3C), are rapidly induced, whereas CBF target genes, including GOLS3, are delayed, reaching maximum levels at ∼24 h.
CG-1 Binding Sites Are Enriched in Promoter Regions of Early Cold-Responsive Genes
The results above raised the question of whether the CAMTA proteins might have a broad role in regulating gene expression in response to low temperature. Computational analysis provided evidence that supported this idea. To test whether the CG-1 sequence was overrepresented in early cold-induced genes, transcripts were selected that are upregulated rapidly in response to low temperature similarly to CBF2. Thirty such genes were identified after examining the cold-regulated gene expression experiments described by Vogel et al. (2005) and Kilian et al. (2007) (Table 1); in both data sets, the genes were upregulated at least twofold at 1 or 3 h, or both. Of these 30 genes, 13 contained at least one CG-1 element in the promoter region 500 bp upstream (Table 1) of the start site (from TAIR7 database). Hypergeometric testing (Rosner, 2006) showed that the occurrence of the element in these genes was enriched compared with the rest of the genome (P < 0.001). This result indicated that the CG-1 sequence was overrepresented in the first wave of cold-induced genes, giving evidence of a significant role for CAMTA proteins in the early response to low temperature.
Table 1.
Genes Producing Transcripts That Are Induced Rapidly in Response to Low Temperature
| AGI | Description |
|---|---|
| At1g19050 | ARR7 |
| At1g21050 | Unknown protein |
| At1g21910 | Member DREB subfamily A-5 of ERF/AP2 transcription factor family |
| At1g27730 | ZAT10 |
| At1g68840 | RAV2 |
| At1g74890 | ARR15 |
| At1g76600 | Unknown protein |
| At1g80440 | Kelch repeat-containing F-box family protein |
| At1g80840 | WRKY40 |
| At2g25900 | CTH |
| At2g26530 | Unknown protein |
| At2g38470 | WRKY33 |
| At3g15450 | Unknown protein |
| At3g48100 | ARR5 |
| At3g48360 | BT2 |
| At3g55980 | Zinc finger (CCCH-type) family protein |
| At4g01250 | WRKY22 |
| At4g24570 | Mitochondrial substrate carrier family protein |
| At4g25470 | CBF2 |
| At4g25480 | CBF3 |
| At4g29780 | Unknown protein |
| At4g34150 | C2 domain-containing protein |
| At4g36040 | DNAJ heat shock N-terminal domain-containing protein |
| At4g37610 | BT5 |
| At5g20230 | Blue copper binding protein |
| At5g28770 | bZIP 63 |
| At5g37260 | CIR1 |
| At5g57560 | Cell wall–modifying enzyme |
| At5g59820 | ZAT12 |
| At5g62920 | ARR6 |
Thirty transcripts identified as upregulated early in response to treatment at 4°C. Those in bold contain the CAMTA binding sequence vCGCGb in the region 500 bp upstream of the 5′ untranslated region (TAIR7). AGI, Arabidopsis Genome Initiative.
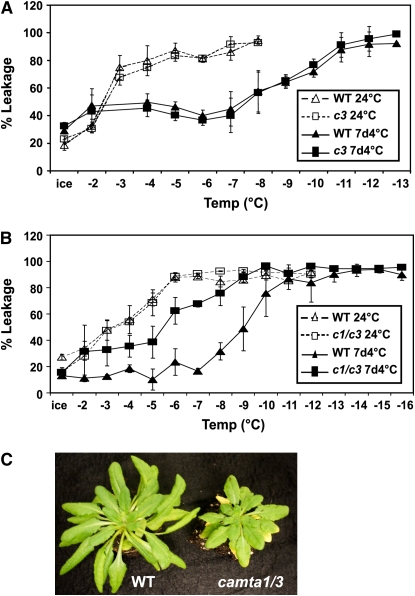
Full Levels of Freezing Tolerance Require Concerted Action of Both CAMTA1 and CAMTA3
The results presented above established that the camta3 mutation caused about a 40 to 50% decrease in cold-induced accumulation of transcripts for CBF1 and -2, as well as for ZAT12, which controls a small regulon of genes involved in freezing tolerance (Vogel et al., 2005). Thus, it seemed possible that the camta3 mutation might have a negative effect on freezing tolerance. However, this was not observed; no difference in freezing tolerance was detected between wild-type and camta3 mutant plants grown at warm temperature or cold acclimated for 7 d (Figure 7A). Additional testing indicated that the camta1, -2, -4, -5, and -6 mutations alone did not affect freezing tolerance either. However, as the CAMTA proteins, particularly CAMTA1, -2, and -3, have very similar amino acid sequences (Finkler et al., 2007), they might have overlapping functions. Analysis of publicly available microarray data (Vogel et al., 2005; Kilian et al., 2007) indicated that the expression pattern of CAMTA3 in response to low temperature was most similar to that of CAMTA1 (Pearson correlation of 0.56). Thus, we crossed the camta1 and camta3 mutants to obtain a camta1 camta3 double mutant, which we then tested for freezing tolerance (Figure 7B). The results indicated that there was no significant difference in freezing tolerance between wild-type and camta1 camta3 mutant plants when grown at 24°C but that there was considerable difference after cold acclimation. Whereas a 7-d period of cold acclimation resulted in about a 5°C increase in freezing tolerance of wild-type plants, the camta1 camta3 plants increased only ∼2°C. Thus, for Arabidopsis to attain full levels of freezing tolerance, the concerted action of both CAMTA1 and CAMTA3 was required.
Figure 7.
Effects of camta3 and camta1 camta3 Mutations on Plant Freezing Tolerance.
(A) Electrolyte leakage assays for nonacclimated (open symbols) wild-type and camta3 (c3) plants grown at 24°C and cold-acclimated plants (closed symbols) treated for 7 d at 4°C. Error bars show sd of three technical replicates.
(B) Electrolyte leakage assays of nonacclimated (open symbols) wild-type and camta1 camta3 (c1/c3) plants grown at 24°C and cold-acclimated plants (closed symbols) treated for 7 d at 4°C. Error bars show sd of three technical replicates.
(C) Wild-type and camta1 camta3 (c1/c3) double mutant plants grown at 24°C.
The basis for the requirement of CAMTA1 and -3 is not known. There is a morphological difference between wild-type and camta1 camta3 mutant plants grown at either warm (Figure 7C) or cold temperature (see Supplemental Figure 7 online); in both cases, the camta1 camta3 plants were smaller than the wild-type plants and the older leaves were more chlorotic (as much as possible, yellow tissue was avoided in the electrolyte leakage assays) (Figure 7C). However, the freezing tolerance of the wild-type and mutant plants was the same at warm temperature. Thus, the camta1 camta3 double mutation did not result in impaired freezing tolerance per se; the effect was on the ability of the plants to cold acclimate (i.e., increase in freezing tolerance in response to low temperature).
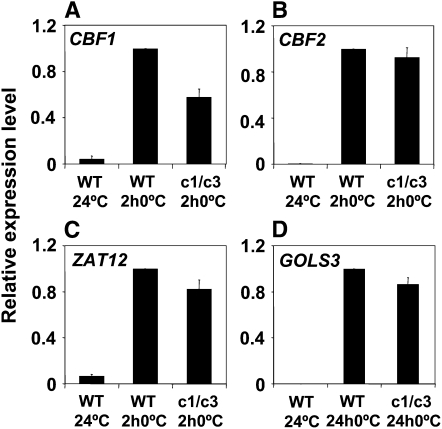
As shown above (Figures 4A, 4B, and 5D), the camta3 mutation resulted in a decrease in the cold-induced transcript levels for CBF1, CBF2, ZAT12, and GOLS3; in each case, expression was reduced by 40 to 50%. However, in the camta1 camta3 double mutant, only the CBF1 transcript levels were reduced in cold-treated plants (Figure 8); no significant difference was observed with CBF2, ZAT12, or GOLS3 (the cold-induced transcript levels of COR15a and CBF3 in the camta1 camta3 double mutant were also not statistically different from those in wild-type plants). These results indicated an interplay between the CAMTA1 and CAMTA3 genes in cold-regulated gene expression.
Figure 8.
Cold-Induced Accumulation of CBF1, CBF2, ZAT12, and GOLS3 Transcripts in camta1 camta3 Plants.
qRT-PCR analysis of transcript levels in wild-type and camta1 camta3 double mutant (c1/c3) plants grown at 24°C or cold treated at 0°C for 2 h ([A] to [C]) or 24 h (D). Relative expression levels of cold-treated wild-type samples were set to 1. Error bars indicate se and n = 5.
(A) CBF1. Averages for the wild type and camta1 camta3 at 0°C were significantly different; Student's t test P value < 0.01.
(B) CBF2. Averages for the wild type and camta1 camta3 at 0°C were not significantly different at a P value cutoff of <0.05.
(C) ZAT12. Averages for the wild type and camta1 camta3 at 0°C were not significantly different at a P value cutoff of <0.05.
(D) GOLS3. Averages for the wild type and camta1 camta3 at 0°C were not significantly different at a P value cutoff of <0.05.
DISCUSSION
A fundamental goal of cold acclimation research is to determine how plants sense low temperature and process this information to bring about changes in gene expression that increase freezing tolerance. One approach to address this issue is to identify cis-acting elements and trans-acting factors that work upstream of genes that comprise the first wave of cold-responsive genes. Such information serves as a starting point from which to work back into the mechanism of the low-temperature “thermometer(s).” Here, we focused on identifying DNA regulatory elements and transcription factors that function upstream of CBF2. The results indicate that the CBF2 promoter is under complex regulation involving both positive and negative DNA regulatory elements. In addition, these results establish roles for CAMTA proteins in both regulating CBF2 transcription and imparting freezing tolerance. As CAMTA proteins bind calmodulin, the results also suggest a possible mechanism governing integration of low-temperature calcium signaling with cold-regulated gene expression.
Multiple DNA Motifs Participate in Regulation of CBF2
The results presented indicate that there are seven DNA motifs, CM1 to CM7, present in the promoters of both CBF2 and ZAT12. From the data presented, we conclude that at least three of these, CM2, CM4, and CM6, participate in the regulation of CBF2. All three elements have a negative regulatory effect (Figure 2). In addition, the CM2 motif was found to function as a positive cold regulatory element, a finding that is consistent with its overlapping the ICEr2 positive regulatory element (Zarka et al., 2003). A mechanistic explanation of how the CM2 element might impart both negative and positive regulation will require additional experimentation. However, what the data clearly establish is that the 27-bp promoter fragment containing CM1 and CM2 is sufficient to impart cold-inducible transcription and that CM2 was required for this activity. Finally, a subregion of ICEr1 containing a Myc DNA binding site was found to serve as a positive regulator (Figure 2). The negative regulatory effect of CM6 was not anticipated as it overlapped the ICEr1 region, which Zarka et al. (2003) had reported as having positive regulatory activity. Thus, the ICEr1 region appears to have at least two regulatory motifs: Myc having positive effects and CM6 having negative effects.
Computational searches indicated that CM6, AGATTCTCA, does not correspond to any known regulatory motif. However, the CM4 sequence, TCCACGT, has the core binding site for βZIP transcription factors, ACGT (Jakoby et al., 2002). In the CBF2 promoter, the residue immediately after the CM4 motif is a G (in ZAT12 it is an A). This extended sequence in CBF2 corresponds to both a G-box binding site, CACGTG (Jakoby et al., 2002), and a Myc binding site, CANNTG (Bailey et al., 2003). The Myc site is of particular interest, as it binds bHLH proteins, and the ICE1 transcription factor is a bHLH protein that acts as a positive regulator of CBF3 (Chinnusamy et al., 2003). Perhaps a bHLH transcription factor related to ICE1 binds to the CM4 sequence of CBF2 but acts as a negative regulator. It is also of interest that the ICEr1 region includes a Myc binding site to which the ICE1 protein binds strongly. However, the ICE1 protein appears not to work through this element, or the element at CM4, because the dominant ice1 mutation has very little, if any, effect on CBF2 expression (Chinnusamy et al., 2003). In this case, perhaps a homolog of ICE1 binds to the Myc sequence and, like ICE1, acts as a positive regulator. Finally, the CM2 sequence, CCGCGT, is a specific version of the CG-1 element, vCGCGb, the binding site of CAMTA transcription factors.
A Role for CAMTA Proteins in Cold Acclimation
Given that the CM2 sequence was a potential CAMTA binding site, we hypothesized that one or more of the six Arabidopsis CAMTA proteins might have a role in CBF2 expression and, furthermore, might have a role in cold acclimation. Both hypotheses proved true. The DNA binding regions of CAMTA1, -2, -3, and -5 were found to bind the CM2 motif, and the camta3 mutation was found to cause a 50% decrease in the cold-induced levels of CBF2 transcripts. The ability of plants to cold acclimate was not affected by any of the single CAMTA T-DNA insertion mutants, but plants carrying a double camta1 camta3 mutation were greatly impaired in freezing tolerance. Whereas wild-type plants increased in freezing tolerance by ∼5°C upon cold acclimation, the camta1 camta3 mutant plants increased only ∼2 to 3°C degrees, a 50 to 70% reduction in freezing tolerance (Figure 7).
It is clear that CAMTA proteins have a role in CBF2 induction and the ability of plants to cold acclimate, yet a number of the experimental observations remain to be explained. The results establish that CAMTA3 has a role in CBF2 induction, but the camta3 mutation resulted in only a 50% decrease in CBF2 transcript levels. The residual level of CBF2 expression might be due to other positive regulatory elements in the CBF2 promoter contributing to cold induction. This interpretation is compatible with the Myc sequence within ICEr1 having a positive regulatory effect on CBF2 expression (Figure 2) (Zarka et al., 2003). However, the quantitative nature of the decrease in expression might have also been due to overlapping functions of the CAMTA proteins. The CAMTA proteins are closely related, especially CAMTA1, -2, and -3 (Finkler et al., 2007). CAMTA1 and CAMTA2 have a sequence identity of 71% and sequence similarity of 82%; CAMTA3 has a sequence identity of 44% with both CAMTA1 and -2 and a sequence similarity to both of ∼64%. However, this explanation would not seem to be the case: the camta3 mutation virtually eliminated cold-induced expression of the 27-bp CBF2 promoter fragment, which requires the CM2 element for activity. This finding suggests that CAMTA3 is the primary CAMTA protein contributing to the positive regulation imparted by the CM2 element in the CBF2 promoter. Thus, other positive regulatory elements, such as the ICEr1 Myc sequence, are probably functional in the camta3 mutant and account for the observed CBF2 induction.
Although CAMTA3 may be the sole CAMTA protein that functions at the CM2 element of the CBF2 promoter, there are indications that the CAMTA proteins interact in regulating CBF2 expression. Specifically, the camta1 mutation is epistatic to the camta3 mutation; that is, combining the camta1 mutation, which has little if any effect on CBF2 expression, with the camta3 mutation, which decreases CBF2 expression by ∼50%, resulted in camta1 camta3 double mutant plants having nearly wild-type levels of CBF2 expression. The same situation held for ZAT12 and GOLS3. The decrease in expression of ZAT12 and GOLS3 observed in the camta3 mutant was eliminated in the camta1 camta3 mutant. The underlying mechanism responsible for this apparent epistasis remains to be determined.
Also to be determined is why neither the camta1 nor camta3 mutation alone had an effect on freezing tolerance, but plants carrying the camta1 camta3 double mutation did. One possible explanation is that the impaired freezing tolerance of the double mutant was a secondary effect of the phenotypic abnormalities observed with the camta1 camta3 plants at both warm (Figure 7) and cold temperature (see Supplemental Figure 7 online). However, if the effects on freezing tolerance were only secondary, one might expect that freezing tolerance would be affected in plants grown at warm nonacclimating temperatures. This effect was not observed; a difference in freezing tolerance was found only with the cold-treated plants. Thus, the double camta1 camta3 mutation affects the cold acclimation process, not freezing tolerance per se. A possible explanation for these results is that CAMTA1 and CAMTA3 participate in the regulation of overlapping cold response pathways and that the portion that comprises the overlap has an important role in freezing tolerance. Determining the effects of individual and combined camta mutations on global gene expression at warm and cold temperature should aid in constructing a CAMTA gene interaction map and provide further insight into the role of CAMTA regulons in cold acclimation.
A final point regards a general role for CAMTA proteins in cold-regulated gene expression. Computational analysis identified 30 genes as being members of the first wave of cold-induced genes with induction kinetics similar to CBF2 and ZAT12. Of these 30 genes, 13 had a CG-1 sequence within 500 bp of the ATG translational start site. Hypergeometric testing indicated that the CG-1 element is enriched (P > 0.001) in the genes. Thus, the CAMTA proteins may have an important role in orchestrating the first rapid-response changes in gene expression that occur upon exposure to low temperature.
Linking Calcium Signaling to Cold-Regulated Gene Expression
It is well established that a spike in cytosolic calcium levels is rapidly elicited in plants upon exposure to low temperature (Minorsky, 1989; Knight et al., 1991). In Arabidopsis, the increase in cytosolic calcium comes from rapid cold-induced release of calcium from both extracellular and vacuolar stores (Knight et al., 1996). A connection between the calcium spikes and cold-regulated gene expression has been demonstrated in both Arabidopsis and alfalfa. In Arabidopsis, the rapid influx of calcium into the cytosol is required for normal cold induction of the CBF target genes KIN1 and KIN2 (Knight et al., 1996; Tahtiharju et al., 1997). Similarly, in alfalfa, cold-induced accumulation of transcripts for two cold-responsive genes, cas15 and cas18, is dependent upon calcium (Monroy et al., 1993; Monroy and Dhindsa, 1995).
A role for calcium in cold-regulated gene expression has been established, but the molecular link between the two is unknown. Our finding that CAMTA3 has a role in cold-induced expression of CBF1, CBF2, and ZAT12 and that the CG-1 element is enriched in genes that are rapidly induced in response to low temperature provides a potential link between calcium and cold-regulated gene expression. A simple model is that the cold-induced increase in calcium levels is sensed by the CAMTA proteins through their interactions with one or more of the six Arabidopsis calmodulin proteins (McCormack et al., 2005). When calcium levels increase, one or more of the calmodulins would bind the calcium, thereby altering its interactions with one or more of the CAMTA proteins. This would result in modification of the CAMTA protein activity (e.g., DNA binding, interaction with transcriptional coactivators, etc.) that, in turn, would result in induction of CBF2 and other cold-responsive genes having the CG-1 element in their promoters. Future experiments will be directed at determining the validity of this model.
CAMTA Proteins as Early Sensors of Stress
The CBF1-3 genes and ZAT12 are induced rapidly not only in response to low temperature, but also in response to stress caused by mechanical agitation and treatment with cycloheximide (Vogel et al., 2005). Here, we show that the 27-bp fragment of CBF2 is sufficient not only to impart cold-induced transcription, but also to induce transcription in response to both mechanical agitation and cycloheximide treatment. Moreover, in each of these cases, the CM2 sequence is required for the observed induction (Figure 3). These results suggest that CAMTA proteins may be a common integrator of stress responses. Two observations support this idea: the finding that the camta3 mutation reduces the increase in CBF2 transcript levels occurring in response to mechanical agitation (C.J. Doherty, unpublished data) and a spike of cytosolic calcium that occurs in response to mechanical stress (Knight et al., 1991).
The results of Walley et al. (2007) also suggest a wide range of roles for CAMTA proteins in plant stress responses. In their study, this group reported the identification of a novel cis-element, CGCGTT, which is overrepresented in the promoters of genes that are rapidly induced (≤5 min) in response to wounding. Many of these genes were also induced in response to a number of abiotic and biotic stresses, including low temperature, drought, insects, and bacterial pathogens. Thus, the element was designated the rapid stress response element (RSRE). It was postulated that plants might have a transcriptional network similar to the general stress-signaling pathway of yeast (Chatterjee et al., 2000; Kultz, 2005) and that the RSRE might have a key role in the coordinate regulation of the genes that comprise the proposed stress network. An inspection of the RSRE reveals that it matches the consensus CG-1 core element. Thus, CAMTA proteins may be involved in the expression of many abiotic and biotic stress genes. Consistent with this idea is the recent finding of Galon et al. (2008) indicating that CAMTA3 is a suppressor of defense responses in Arabidopsis.
CAMTA proteins, and the CG-1 element to which they bind, are highly conserved in multicellular eukaryotic organisms ranging from insects to mammals and plants (Finkler et al., 2007). They are not, however, present in single-celled eukaryotic organisms such as yeast. Thus, this cis-element/trans-acting factor pair would appear to have evolved early in the lineage of multicellular eukaryotic organisms. In plants, evidence is emerging that the CAMTA/CG-1 system has a fundamental role in rapid responses to a variety of abiotic and biotic stresses. Also, in mammals, CAMTA proteins have been linked to hypertrophic cardiac growth induced by stress. These results lead us to suggest that the CAMTA/CG-1 system may have arisen early in the evolution of multicellular organisms as an early warning system to cope with a variety of conditions that cause stress.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana ecotype Wassilewskija-2 was used in all of the promoter analysis experiments and ecotype Columbia-0 was used in all the CAMTA experiments. Except for the freezing tolerance tests, plants were grown at 24°C under sterile conditions on Gamborg's B5 medium (Caisson Laboratories) for 10 d at 100 μmol m−2 s−1 constant light, as previously described (Gilmour et al., 2004; Vogel et al., 2005). Plants containing the camta T-DNA insertion mutations and their complemented lines were grown without sucrose in the medium. For electrolyte leakage experiments, plants were grown on soil as described (Gilmour et al., 2004; Vogel et al., 2005) under an 8-h photoperiod with light levels of ∼100 μmol m−2 s−1. All seeds were stratified for 3 to 5 d in the dark at 4°C prior to growth. Low-temperature treatment for plants grown on plates was at 4°C or 0°C with constant light of ∼35 μmol m−2 s−1. Cold treatment of soil-grown plants was at 4°C under an 8-h photoperiod with light at 35 μmol m−2 s−1. Cycloheximide (10 μg mL−1 for 2 h) and mechanical treatment (15 min) were as previously described (Zarka et al., 2003). T-DNA insertion mutants were identified using the SIGnAL database (Alonso et al., 2003) and obtained from ABRC. These were camta1 (Salk_008187), camta2 (Salk_007027), camta3 (Salk_001152), camta4 (Salk_013723), and camta6 (Salk_078900). The camta5 mutant was obtained from GABI-Kat Line ID 815B08 (Rosso et al., 2003). Homozygous lines were identified using a combination of antibiotic selection and PCR screening of genomic DNA using gene-specific primers. RT-PCR and qRT-PCR (see section on RNA analysis) was used to determine CAMTA transcript levels in RNA from the Salk lines. Primer sequences used for PCR and qRT-PCR are shown in Supplemental Table 1 online. Arabidopsis transformation was performed using the floral dip method (Clough and Bent, 1998).
Constructs
ZAT12 Promoter Deletion Constructs
A ZAT12 promoter fragment from −912 to +81 from the start of transcription was obtained by PCR of genomic DNA and cloned into pGEM-T easy (Promega) (D. Zarka, unpublished data). This promoter fragment was inserted into vector pBI101.1-plus in front of the GUS reporter gene. pBI101.1-plus was made by fusing the CaMV 35S −46 minimal promoter in front of the GUS gene of pBI101.1. 5′ and 3′ deletions of the ZAT12 promoter fragment were made by PCR (see Supplemental Table 2 online for primer sequences), cloned into pGEM-T-easy (Promega), and then cloned into pBI101.1-plus.
CBF2 Promoter Constructs
The CBF2 promoter:GUS fusion consisted of the −189 to the ATG sequence of the CBF2 gene fused to GUS in pBI101.2 as described (Zarka et al., 2003). Site-directed mutagenesis of this promoter fragment was performed with the Quick Change kit (Stratagene) according to the manufacturer's protocol (see Supplemental Table 3 for mutant and wild-type CM sequences).
Conserved Motif Reporter Constructs
The CBF2 promoter CM reporter constructs were made by annealing the pairs of oligonucleotides shown in Supplemental Table 4 online, directionally cloning them into pBluescript SK−, and then cloning them into the GUS reporter vector pBI101.1-plus. For tetramers of two conserved motifs, dimers were annealed, directionally cloned into pBluescript SK−, and then a second ligation was performed to directionally clone in the second copy. The whole tetramer was then cloned into pBI101.1-plus. The 27-bp:GUS and mutant 27-bp:GUS constructs were tetramers of wild-type CM1 and CM2 sequences and tetramers of CM1 and mutant CM2 sequences, respectively (see Supplemental Table 4 online).
CAMTA Constructs
The CAMTA3 coding sequence was obtained by PCR of CAMTA3 cDNA (using the CAMTA3 open reading frame primers shown in Supplemental Table 5 online) and cloned into the Gateway entry vector pDONR221 (Invitrogen). These coding sequences were then cloned into the plant expression vector NTAPi (which had been modified by removal of the TAP tag) under control of the CaMV 35S promoter. The recombination reactions were performed according to the Gateway user manual.
Electrophoretic Mobility Shift Assays
CAMTA proteins used in the DNA binding assays were made by fusing the CG-1 DNA binding domains of CAMTA1, -2, -3, and -5 to an N-terminal GST tag using the Gateway expression vector pDEST15 (Invitrogen). Primer sequences used in preparation of the constructs are shown in Supplemental Table 5 online. The amino acids included in the constructs were: CAMTA1, Met1-Leu173; CAMTA2, Met1-Leu171; CAMTA3, Met1-Gly170; CAMTA5, Met1-Ala150; CAMTA5*, Met1-Gly116-Leu117-Arg118-Ser119-Thr120-STOP. CAMTA5* is a truncated version of CAMTA5 with amino acids 117 to 120 changed from their original sequence as indicated above and a stop codon added. This polypeptide was insoluble in Escherichia coli lysates and used as a negative control. The proteins were expressed in E. coli and the cells lysed by sonication. Crude lysates (0.1 ng total protein) were incubated at room temperature for 20 min in binding buffer [25 mM HEPES, pH 8.0, 1 mM EDTA, 40 mM KCl, 5 mM MgCl2, 5% glycerol, 1 mM DTT, and 50 μg/mL poly(dI-dC)]. The CM1/CM2-containing dimerized 27-bp CBF2 promoter fragment (see Supplemental Table 4 online) used as a probe was end-labeled with 32P, then 6 fmol of labeled probe was added to the binding reaction with or without a 100-fold molar excess of unlabeled competitor DNA as indicated This was incubated for 20 min at room temperature. Samples were resolved on a 4% polyacrylamide gel in 0.5× TBE, dried, and exposed overnight to a phosphor-imager screen.
Effect of camta Mutations on Expression of the 27-bp:GUS Reporter Construct
Homozygous lines containing the 27-bp:GUS tetramer reporter fusion in Wassilewskija-2 were selected and crossed into either camta1 or camta3 mutant plants. Seven independent T4 lines homozygous for the reporter construct and either the camta1 or camta2 mutation were identified and analyzed for GUS transcript levels (one representative line is shown in the Results).
Complementation of the camta3 mutation was tested using the CaMV 35S:CAMTA3 construct. Two lines carrying the homozygous camta3 mutation were transformed with 35S:CAMTA3. T2 lines were analyzed by histochemical staining for GUS activity as previously described (Zarka et al., 2003). Selected T3 homozygous lines were analyzed for GUS, CBF2, and CAMTA3 mRNA levels by real-time qRT-PCR (one representative line is shown in the Results).
Freezing Tolerance Tests
Electrolyte leakage assays for freezing tolerance and whole-plant freeze tests of plate-grown seedlings were performed as described (Gilmour et al., 2004; Vogel et al., 2005) except that plants were grown individually in pots under an 8-h photoperiod and cold treated under an 8-h photoperiod.
RNA Analysis
Whole seedlings were harvested from plates, frozen in liquid nitrogen, and stored at −80°C, except for experiments on complementation of the camta3 mutation, in which case only aerial parts of the plants were used. Total RNA was extracted from plant material using RNeasy plant mini kits (Qiagen) with modifications as described (Zarka et al., 2003). RNA gel blot transfers were prepared and hybridized as described (Hajela et al., 1990) and washed under high-stringency conditions (Stockinger et al., 1997). Probes were made by labeling the coding region of each gene with 32P using the Random Primer DNA Labeling System (Invitrogen) using the manufacturer's protocol.
For real-time qRT-PCR, first-strand cDNA synthesis was performed using the Reverse Transcription System (Promega) with random primers according to the manufacturer's instructions using a 40-μL reaction volume and an incubation time of 1 h at 42°C with total RNA of either 0.1 or 0.01 μg. The cDNA reaction mixture was diluted fivefold with water, and 3 μL was used as a template in a 30-μL PCR reaction using the Applied Biosystems FAST 7500 real-time PCR system in standard mode with SYBR Green PCR Core Reagents Mix (Applied Biosystems) according to manufacturer's protocols. For CBF2 transcript analysis using primer set A in Supplemental Table 1 online, the annealing/extension temperature was 62°C rather than 60°C. Reactions were performed in triplicate and products checked by melting curve analysis. The abundance of transcripts was analyzed with the relative standard curve method normalizing to the reference transcript Actin3 (At3g53750). The primers used for amplification are shown in Supplemental Table 1 online. In some experiments, the Applied Biosystems FAST 7500 real-time PCR system was used in FAST mode. Here, the PCR reactions were in 10 μL and performed in triplicate using 1 μL of 10-fold diluted cDNA from a 20-μL reverse transcription reaction. In these experiments, CBF2 (B) and CBF3 (B) primer pair sets were used, whereas CBF2 (A) and CBF3 (A) primer pair sets were used for all other PCR and RT-PCR reactions (see Supplemental Table 1 online). Unless indicated otherwise, all qRT-PCR experiments replicates (n) refer to biological replicates.
Motif Discovery
The ZAT12 promoter from −355 to −109 and CBF2 promoter from −189 to −35 were analyzed for regions of similarity using MEME version 30 (release date 2001/03/05 14:24:28), minimum width = 6, maximum width= 25, minimum sites = 2, and maximum sites = 2 (Bailey and Elkan, 1995).
Statistical Analysis
Experiments consisting of three or more conditions were tested for statistical significance using one-way analysis of variance followed by a protected t test. Experiments with only two comparisons were tested for significance using Student's t test.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL database under the following accession numbers: CAMTA1 (At5g09410), AK228740; CAMTA2 (At5g64220), BAB09853; CAMTA3 (At2g22300), AAD23613; CAMTA4 (At1g67310), NP_176899; CAMTA5 (At4g16150), NM_117710; CAMTA6 (At3g16940), BAA94977; CBF1 (At4g25490), AB013816; CBF2 (At4g25470), AB013817; CBF3 (At4g25480), AB013815; ZAT12 (At5g59820), NM_125374; COR15a (At2g42540), NM_129815; GOLS3 (At1g09350), NM_100805; ACT3 (At3g53750); and eIF4a (At3g13920), NM_112246. T-DNA insertion lines used were as follows: camta1 (Salk_008187, germplasm 4518335), camta2 (Salk_007027, germplasm 4517175), camta3 (Salk_001152, germplasm 4511300), camta4 (Salk_013723, germplasm 4523871), camta5 (GABI-Kat Line GK-815B08), and camta6 (Salk_078900, germplasm 4681488).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Identification of a 225-bp Region of the ZAT12 Promoter That Is Sufficient to Impart Cold-Regulated Gene Expression.
Supplemental Figure 2. CM Multimer Constructs.
Supplemental Figure 3. CAMTA Proteins 1, 2, 3, and 5 Bind to the CM2 Sequence Present in the 27-bp CBF2 Promoter Fragment Containing CM1 and CM2.
Supplemental Figure 4. T-DNA Insertions in CAMTA Genes Result in Greatly Reduced Levels of Corresponding CAMTA Transcripts.
Supplemental Figure 5. Yellowing of Leaf Veins in camta6 Plants.
Supplemental Figure 6. Ectopic Expression of CAMTA3 in the camta3 Mutant.
Supplemental Figure 7. Effect of camta1 camta3 Double Mutation on Plant Growth.
Supplemental Table 1. Primer Sequences Used for PCR and qRT-PCR.
Supplemental Table 2. Primer Sequences Used for ZAT12 Promoter Constructs.
Supplemental Table 3. Sequences of Wild-Type and Mutant CMs Used in the CBF2 Promoter Constructs.
Supplemental Table 4. Oligonucleotide Sequences Used in Making the CBF2 Promoter CM Constructs.
Supplemental Table 5. Primer Sequences Used for CAMTA Constructs.
Supplementary Material
Acknowledgments
We thank Sarah Gilmour, Marlene Cameron, and Karen Bird for their help in preparing the manuscript and Daniel Zarka for making the ZAT12 full-promoter construct. This research was supported in part by grants to M.F.T. from the National Science Foundation Plant Genome Project (DBI 0110124 and DBI 0701709), the Department of Energy (DE-FG02-91ER20021), and the Michigan Agricultural Experiment Station.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Michael F. Thomashow (thomash6@msu.edu).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Agarwal, M., Hao, Y., Kapoor, A., Dong, C.-H., Fujii, H., Zheng, X., and Zhu, J.-K. (2006). A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 281 37636–37645. [DOI] [PubMed] [Google Scholar]
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Bailey, P.C., Martin, C., Toledo-Ortiz, G., Quail, P.H., Huq, E., Heim, M.A., Jakoby, M., Werber, M., and Weisshaar, B. (2003). Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell 15 2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, T.L., and Elkan, C. (1995). Unsupervised learning of multiple motifs in biopolymers using expectation maximization. Mach. Learn. 21 51–80. [Google Scholar]
- Bouché, N., Scharlat, A., Snedden, W., Bouchez, D., and Fromm, H. (2002). A novel family of calmodulin-binding transcription activators in multicellular organisms. J. Biol. Chem. 277 21851–21861. [DOI] [PubMed] [Google Scholar]
- Chatterjee, M.T., Khalawan, S.A., and Curran, B.P.G. (2000). Cellular lipid composition influences stress activation of the yeast general stress response element (STRE). Microbiology 146 877–884. [DOI] [PubMed] [Google Scholar]
- Chinnusamy, V., Ohta, M., Kanrar, S., Lee, B.-h., Hong, X., Agarwal, M., and Zhu, J.-K. (2003). ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 17 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy, V., Zhu, J., and Zhu, J.K. (2007). Cold stress regulation of gene expression in plants. Trends Plant Sci. 12 444–451. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Cook, D., Fowler, S., Fiehn, O., and Thomashow, M.F. (2004). A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc. Natl. Acad. Sci. USA 101 15243–15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa e Silva, O. (1994). CG-1, a parsley light-induced DNA binding protein. Plant Mol. Biol. 25 921–924. [DOI] [PubMed] [Google Scholar]
- Finkler, A., Ashery-Padan, R., and Fromm, H. (2007). CAMTAs: Calmodulin-binding transcription activators from plants to human. FEBS Lett. 581 3893–3898. [DOI] [PubMed] [Google Scholar]
- Fowler, S., and Thomashow, M.F. (2002). Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14 1675–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon, Y., Nave, R., Boyce, J.M., Nachmias, D., Knight, M.R., and Fromm, H. (2008). Calmodulin-binding transcription activator (CAMTA) 3 mediates biotic defense responses in Arabidopsis. FEBS Lett. 582 943–948. [DOI] [PubMed] [Google Scholar]
- Gilmour, S.J., Fowler, S.G., and Thomashow, M.F. (2004). Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol. Biol. 54 767–781. [DOI] [PubMed] [Google Scholar]
- Gilmour, S.J., Zarka, D.G., Stockinger, E.J., Salazar, M.P., Houghton, J.M., and Thomashow, M.F. (1998). Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 16 433–442. [DOI] [PubMed] [Google Scholar]
- Hajela, R.K., Horvath, D.P., Gilmour, S.J., and Thomashow, M.F. (1990). Molecular cloning and expression of cor (cold-regulated) genes in Arabidopsis thaliana. Plant Physiol. 93 1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen, K.R., Gilmour, S.J., Zarka, D.G., Schabenberger, O., and Thomashow, M.F. (1998). Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280 104–106. [DOI] [PubMed] [Google Scholar]
- Jakoby, M., Weisshaar, B., Droge-Laser, W., Vicente-Carbajosa, J., Tiedemann, J., Kroj, T., and Parcy, F. (2002). bZIP transcription factors in Arabidopsis. Trends Plant Sci. 7 106–111. [DOI] [PubMed] [Google Scholar]
- Kaplan, F., Kopka, J., Haskell, D.W., Zhao, W., Schiller, K.C., Gatzke, N., Sung, D.Y., and Guy, C.L. (2004). Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 136 4159–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian, J., Whitehead, D., Horak, J., Wanke, D., Weinl, S., Batistic, O., D'Angelo, C., Bornberg-Bauer, E., Kudla, J., and Harter, K. (2007). The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 50 347–363. [DOI] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1996). Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, M.R., Campbell, A.K., Smith, S.M., and Trewavas, A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352 524–526. [DOI] [PubMed] [Google Scholar]
- Kreps, J.A., Wu, Y., Chang, H.S., Zhu, T., Wang, X., and Harper, J.F. (2002). Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 130 2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kultz, D. (2005). Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 67 225–257. [DOI] [PubMed] [Google Scholar]
- Levitt, J. (1980). Responses of Plants to Environmental Stress: Chilling, Freezing and High Temperature Stresses. (New York: Academic Press).
- Liu, Q., Kasuga, M., Sakuma, Y., Abe, H., Miura, S., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1998). Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama, K., Sakuma, Y., Kasuga, M., Ito, Y., Seki, M., Goda, H., Shimada, Y., Yoshida, S., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2004). Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J. 38 982–993. [DOI] [PubMed] [Google Scholar]
- McCormack, E., Tsai, Y.-C., and Braam, J. (2005). Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 10 383–389. [DOI] [PubMed] [Google Scholar]
- Minorsky, P.V. (1989). Temperature sensing by plants: A review and hypothesis. Plant Cell Environ. 12 119–135. [Google Scholar]
- Monroy, A.F., and Dhindsa, R.S. (1995). Low-temperature signal transduction: Induction of cold acclimation-specific genes of alfalfa by calcium at 25°C. Plant Cell 7 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy, A.F., Sarhan, F., and Dhindsa, R.S. (1993). Cold-Induced changes in freezing tolerance, protein phosphorylation, and gene expression (evidence for a role of calcium). Plant Physiol. 102 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann, J.L., et al. (2000). Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290 2105–2110. [DOI] [PubMed] [Google Scholar]
- Rosner, B. (2006). Fundamentals of Biostatistics, Edition 6. (Belmont, CA: Thomson Brooks/Cole).
- Rosso, M.G., Li, Y., Strizhov, N., Reiss, B., Dekker, K., and Weisshaar, B. (2003). An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol. Biol. 53 247–259. [DOI] [PubMed] [Google Scholar]
- Steponkus, P.L., Uemura, M., Joseph, R.A., Gilmour, S.J., and Thomashow, M.F. (1998). Mode of action of the COR15a gene on the freezing tolerance of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 95 14570–14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger, E.J., Gilmour, S.J., and Thomashow, M.F. (1997). Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 94 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahtiharju, S., Sangwan, V., Monroy, A.F., Dhindsa, R.S., and Borg, M. (1997). The induction of kin genes in cold-acclimating Arabidopsis thaliana. Evidence of a role for calcium. Planta 203 442–447. [DOI] [PubMed] [Google Scholar]
- Thomashow, M.F. (1999). Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 571–599. [DOI] [PubMed] [Google Scholar]
- Vogel, J.T., Zarka, D.G., Van Buskirk, H.A., Fowler, S.G., and Thomashow, M.F. (2005). Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 41 195–211. [DOI] [PubMed] [Google Scholar]
- Walley, J.W., Coughlan, S., Hudson, M.E., Covington, M.F., Kaspi, R., Banu, G., Harmer, S.L., and Dehesh, K. (2007). Mechanical stress induces biotic and abiotic stress responses via a novel cis-element. PLoS Genetics 3 e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarka, D.G., Vogel, J.T., Cook, D., and Thomashow, M.F. (2003). Cold induction of Arabidopsis CBF genes involves multiple ICE (inducer of CBF expression) promoter elements and a cold-regulatory circuit that is desensitized by low temperature. Plant Physiol. 133 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.