Abstract
Anabolic androgenic steroids (AAS) are often misused by adolescents and athletes. Their effects vary according to chemical structure and metabolism, route of administration, and AAS regimen. In this study, adult C57Bl/6 male mice were systemically exposed to testosterone propionate (TP), nandrolone or 17α-methyltestosterone (17α-meT), type I, type II and type III AAS, respectively, in order to determine the hedonic or aversive properties of each drug. For this purpose, the conditioned place preference (CPP) test was employed at three different AAS doses (0.075, 0.75 and 7.5 mg/kg). Other behavioral domains monitored were light-dark transitions (side changes) and general activity. TP shifted place preference at all doses tested, and nandrolone shifted place preference at 0.75 and 7.5mg/kg, but not at 0.075 mg/kg, the lower dose tested. Conversely, mice receiving 17α-meT did not show alteration in the preference score. The lower dose of nandrolone did modify exploratory based-anxiety showing a decrease in light-dark transitions if compared to vehicle-treated animals, while mice treated with TP or 17α-meT were not affected. Our data suggest that when studying hedonic and rewarding properties of synthetic androgens, distinction has to be made based on type of AAS and metabolism.
Keywords: conditioned place preference, anabolic androgenic steroids, reward, mice
1. INTRODUCTION
The synthetic derivatives of testosterone, known as anabolic androgenic steroids (AAS), are considered drugs of abuse and are misused in supraphysiological doses by an increasing number of athletes and adolescents (Bahrke et al., 1998; Hartgens & Kuipers, 2004). It has been argued that motivation for AAS misuse includes improvement of physical appearance, muscular strength, and/or peer approval (Schwerin et al., 1996). A growing body of work in rodents shows that androgen compounds have hedonic (Frye et al., 2001; Arnedo et al., 2002; Packard et al., 1997) and reinforcing effects (Johnson & Wood, 2001; Wood et al., 2004; Frye 2007; Frye et al., 2007). These effects have been shown with a variety of experimental paradigms including conditioned place preference (CPP) (Frye et al., 2001; Arnedo et al., 2002), oral self-administration (Johnson & Wood, 2001), jugular self-administration (Jorge et al., 2005), and intracerebral self-administration (Rosellini et al., 2001; Wood et al., 2004). However, rewarding properties of AAS have received relatively little attention.
There are approximately 60 different AAS compounds that can be classified in three classes (for review see Clark & Henderson, 2003) based upon their chemical structure and metabolism. Class I AAS are injectable compounds derived from esterification of the 17β-hydroxyl group of testosterone, which can be aromatized to estrogens, reduced to dihydrotestosterone (DHT), or hydrolyzed into testosterone. Class II AAS are comprised of injectable 19-nortestosterone derivatives, which have a longer half-life and less androgenic activity than Class I AAS. Similar to class I, Class II AAS can be aromatized to estrogens or reduced to DHT. The alkylation at carbon 17 distinguishes Class III AAS; this modification retards the metabolism of this class of AAS by the liver, making them orally active (Kuhn, 2002), and with restricted potential for aromatization (Andersen et al., 2006; Mor et al., 2001; Zhang et al., 2007). Class II and III are probably the most abused by adolescents and athletes (for review see Clark & Henderson, 2003; Pope & Brower, 2000). In this study we sought to investigate and compare rewarding effects of testosterone propionate (TP), nandrolone and 17α-methyltestosterone (17α-meT), representatives of type I, type II and type III AAS, respectively. Since differences in chemical structure and metabolism are present between these three drugs, we hypothesized that a differential behavioral response will be found using CPP, a paradigm that examines positive hedonic and reinforcement effects of drugs (Reid et al., 1989; Tzschentke, 1998). Specifically, TP has been modified adding a 3 carbon (3C) ester; nandrolone (19-nortestosterone) is very similar to testosterone, but a carbon (C19) is missing; and 17α-meT has a methyl group at carbon 17 (C17). Differences in metabolism between the three AAS are also present, being one of the most significant, the ability to be aromatized to estrogen by the aromatase enzyme. Anxiety-related behaviors and general activity were monitored as well, to better understand specific patterns of behavioral alteration. Our data suggest that different classes of anabolic steroids elicit differential modulation in hedonia and reward that could reflect the complex AAS metabolism, and consequently, their interaction with cellular substrates.
2. METHODS
2.1 Subjects
Adult (PN-90) C57B1/6J-NHsd mice male mice were purchased from Charles River and housed individually in the Animal Resources Center of the Medical Sciences Campus of the University of Puerto Rico (MSC-UPR) with food and water available ad libitum. They were kept on a 12:12 hour light/dark cycle. The temperature and relative humidity in the housing facility is 64 °F and 30%, respectively. The experiments were performed during the dark portion of the cycle, following the guidelines and approval of the Institutional Animal Care and Use Committee (IACUC) of the MSC-UPR and in accordance with USDA, National Institutes of Health, and American Veterinary Medical Association regulations.
2.2 Androgen administration
Control groups received intraperitoneal (i.p.) injections of vehicle, which consisted of 0.9% NaCl and 30% cyclodextrin (0.02cc/10g of body weight). Experimental groups received i.p. injections of TP, nandrolone or 17α-meT at doses of 0.075, 0.75 and 7.5mg/kg (0.02cc/10g of body weight). TP, nandrolone (19-nortestosterone) and 17α-meT were purchased from Sigma Chemical Co. (St. Louis, MO). As 1 mg/kg is sufficient to restore endocrine function and reproductive behaviors in rodents (Clark & Barber, 1994), the dosage used in this experiment reflects a low, intermediate and high dose, respectively.
2.3 Behavioral assessment
2.3.1 Behavioral paradigm 1
Conditioned place preference (CPP)
This paradigm has been used typically to measure hedonic properties of drugs of abuse as well as natural reinforces (Packard et al., 1997; Tzschentke, 1998). Acrylic chambers (42cm long × 30cm high × 42cm high) were used. A black acrylic box (40.6 cm long × 20.3 cm high × 12.7 cm wide) with an entry opening (10.2 cm × 6.4 cm) was placed alongside the activity chamber. Chambers were cleaned between animals with a mild detergent to minimize olfactory cues between sessions. Sessions were videotaped for subsequent analysis and interpretation.
CPP was performed with the lights ‘on’ and lasted 13 days (days 0–12), Fig. 1. On day 0, animals were habituated to the recording chambers without the black acrylic box for 20 min. On day 1 (baseline), individual animals were allowed to move freely between the light and the dark compartments of the chamber for 15 min. The time spent (in sec) in a given compartment was recorded. The preference score (PS) for a given compartment was calculated as follows: PS = (% time in the non-preferred side/% time in the non-preferred + preferred side) × 100. Mice that demonstrated strong preference for a given compartment (> 90 %) during the baseline were excluded from the analysis. Animals were randomly assigned to control or experimental groups. Experimental groups received either intraperitoneal (i.p.) injections of a given AAS (TP, nandrolone or 17α-meT) and were restricted to the non-preferred (NP) side of the chamber, or they received i.p. vehicle injections and were restricted to the preferred side (P). Control groups received daily vehicle injections (i.p.) and were restricted to a given compartment (P vs. NP) of the chamber for 30 min. AAS versus vehicle i.p. injections in the experimental groups occurred on alternate days for a total of 5 injections of vehicle and 5 injections of a given AAS in the experimental group. On day 13 (test day), injection-free animals were allowed to move freely between the light and the dark compartments of the chambers, and the PS was recalculated.
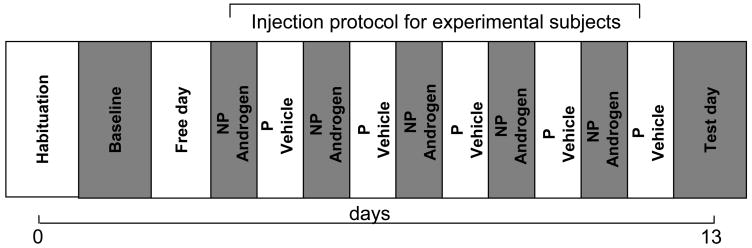
Figure 1. Experimental design and injection protocol for the conditioned place preference (CPP) in AAS treated animals.
CPP was performed for 13 days (days 0–12). On day 0 animals were habituated to the recording chambers for 20 min. On day 1 (baseline), animals were allowed to move freely between the dark and light compartments of the chamber for 15 min. Preference score for a given compartment was immediately calculated. After a free day (day 2), alternate injections (i.p.) of androgen or vehicle were started (days 3–12) in the non-preferred (NP) and preferred side (PS) of the chamber, respectively. For each injection, animals spent 30 min in the chamber. On day 13 (test day), injection free animals were allowed to move freely between the light and dark compartments, and the preference score was recalculated. Control animals received daily injections of vehicle and were restricted to a given compartment of the chamber for 30 min each.
2.3.2 Behavioral paradigm 2
Light-dark transitions (side changes)
To measure exploratory-based anxiety, the number of transitions between the dark and illuminated side of the chamber were measured during the baseline and test day (30 min each). This behavior is optimized for mice, titrating the tendency of mice to explore a novel environment versus the aversive properties of a brightly lit open field (Crawley, 2000). Classic anxiolytics can be detected using this paradigm (Bourin and Hascoët, 2003). Anxiogenic animals will display reduced transitions between the dark and light chambers (Zhang et al., 2007).
2.3.3 Behavioral paradigm 3
General activity
Jumping and climbing activities were scored on baseline and test day during the CPP. The door between the dark and light chambers has a thin rim above its aperture and animals showed a tendency to climb over it. Thus, we scored jumping when animals attained vertical locomotion (jumping) without making contact with the door rim, and climbing was scored when at least two animal’s paws contacted the door rim.
2.4 Neuroendocrine effects
Gonadal weight- After the CPP on day 13 (test day), animals were sacrificed to analyze testicular weight between vehicle and AAS-treated animals.
2.5 Statistical analysis
Data are presented as mean ± standard error of the mean (S.E.M.). One-way analysis of variance followed by Tukey Test post hoc analysis was employed. Statistical significance was established at p ≤ 0.05
3. RESULTS
3.1 Behavioral paradigm 1: CPP
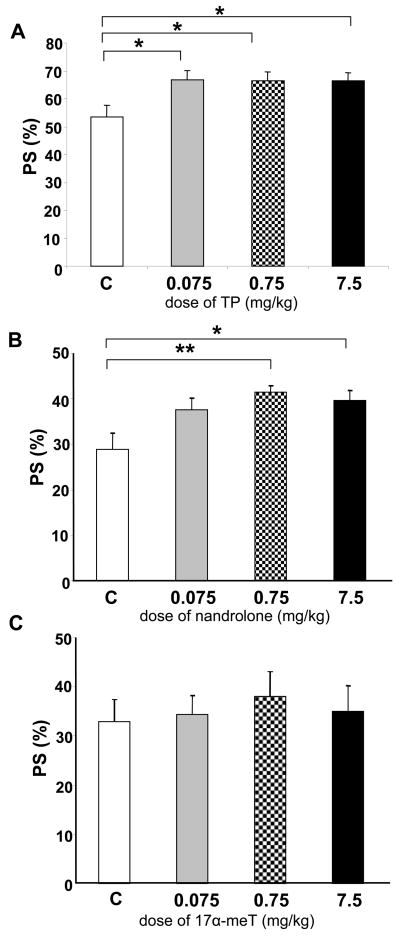
We tested the effect of three different classes of AAS compounds, TP, nandrolone and 17α-meT in the CPP task after five alternating days of systemic exposure. No statistically significant differences were found in the preference score between baseline and the test day. Indeed, none of the tested behaviors showed significant differences between baseline and test day (data not shown). Thus, we proceeded to analyze the vehicle and androgen-treated animals during the test day. TP at all doses tested shifted the place preference in the CPP paradigm, F(3, 36) =, p < 0.05. Post hoc analysis showed significant differences between the vehicle-treated group and each of the three doses (Fig. 2A). In particular, the preference score for control animals was (53.38 ± 4.38). This value was shifted to (66.80 ± 3.23), (66.49 ± 2.26), and (66.56 ± 2.85) for animals treated with TP at 0.075, 0.75, and 7.5 mg/kg, respectively, (p < 0.05).
Figure 2. Effect of TP, nandrolone and 17α-meT on conditioned place preference (CPP) test.
(A) Animals receiving TP at all doses tested showed a significant increase in the preference score for a given compartment of the testing arena. (B) Similarly, nandrolone at 0.75 and 7.5 mg/kg showed a significant increase in the preference score. (C) The AAS 17α-meT did not modify the place preference at any of the dose levels tested. All bars represent the data of the test day. For TP, vehicle: n= 10; 0.075 mg/kg: n= 8; 0.75 mg/kg: n= 10; 7.5 mg/kg: n= 9. * p ≤ 0.05 vs. vehicle. For nandrolone, vehicle: n = 9; 0.075 mg/kg: n= 8; 0.75 mg/kg: n= 9; 7.5 mg/kg: n= 8, *p ≤ 0.05 vs. vehicle, **p ≤ 0.01 vs. vehicle. For 17α-meT, vehicle: n= 7; 0.075 mg/kg: n= 6; 0.75 mg/kg: n= 8; 7.5 mg/kg: n= 8. Error bars represent standard error of the mean.
A statistically significant difference was observed in the preference score between nandrolone and vehicle treated animals, F(3, 33) = 4.79, p < 0.01. Post hoc analysis showed significant differences between groups of animals treated with the higher doses of nandrolone (Fig. 2B) when compared with the vehicle group. Vehicle-treated animals exhibited a preference score of 28.84 ± 3.62, whereas those treated with nandrolone at 0.75 and 7.5 mg/kg showed significant increases in the preference score to 41.47 ± 1.46 (p < 0.01) and 39.66 ± 2.14 (p < 0.05), respectively. Preference score was not affected in animals treated with nandrolone at 0.075 mg/kg. Thus, animals treated with nandrolone at higher doses displayed an increase in the preference for the non-preferred side of the chamber. In contrast, no statistical differences were found between 17α-meT and vehicle groups, F(3, 28) = 0.209, p = 0.889, during the same experimental conditions in the CPP (Fig. 2C). Specifically, vehicle treated animals showed a preference score of 32.86 ± 4.54 which is not statistically different from 17α-meT preference scores at 0.075 mg/kg (34.34 ± 3.96), 0.75 mg/kg (37.91 ± 5.23) and 7.5 mg/kg (34.90 ± 5.23).
3.2 Behavioral paradigm 2: light-dark transitions
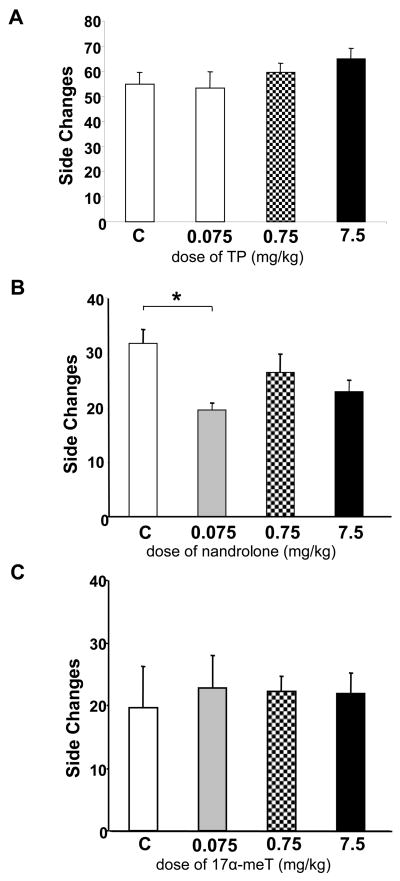
Figure 3 shows the effect of AAS in exploratory-based anxiety as measured by light-dark transitions (side changes). A statistical difference in the frequency of transitions between nandrolone and vehicle treated animals was observed, F(3, 17) = 3.91, p < 0.05. Post hoc analysis revealed that mice receiving nandrolone 0.075 mg/kg showed a significant reduction in exploration (Fig. 3B) between the light and dark compartments (vehicle: 32 ± 2.52; nandrolone 0.075 mg/kg: 19.60 ± 1.33), p < 0.05. No differences were found at higher doses of nandrolone. On the other hand, TP (Fig. 3A) nor 17α-meT (Fig. 3C) produced differences in the frequency of light-dark transitions.
Figure 3. Effect of TP, nandrolone and 17α-meT on light-dark transitions.
Animals receiving nandrolone at 0.075 mg/kg throughout the CPP task (B) showed a reduction in the frequency of light-dark transitions (side changes). Animals treated with higher doses were not affected. TP (A) or 17α-meT (C) do not produced changes in light-dark transitions at any of the dose levels tested. All bars represent the data of the test day. For TP, vehicle: n= 10; 0.075 mg/kg: n= 8; 0.75 mg/kg: n= 10; 7.5 mg/kg: n= 9. For nandrolone, vehicle: n = 3; 0.075 mg/kg: n= 5; 0.75 mg/kg: n= 5; 7.5 mg/kg: n= 5 *p < 0.05 vs. vehicle. For 17α-meT, vehicle: n= 5; 0.075 mg/kg: n= 6; 0.75 mg/kg: n= 12; 7.5 mg/kg: n= 8. Error bars represent standard error of the mean.
3.3 Behavioral paradigm 3: general activity
General activity as assessed by jumping/climbing activity was only affected by TP at 0.75 mg/kg. However, we have found that TP at this dose did not affect locomotor activity as assessed by the elevated plus maze (data not shown).
3.4. Neuroendocrine effects
Finally, the selected dose levels, the duration, and the pattern of exposure to TP, nandrolone and 17α-meT did not show any effect in gonadal weight as assessed by the analysis of testicular weight between control and AAS-treated animals (data not shown).
4. Discussion
We have found that TP and nandrolone, but not 17α-meT, produces a dose-dependent shift in place preference after 5 systemic AAS injections. Only the higher doses of 0.75 and 7.5 mg/kg shifted preference to the non-preferred compartment of the chamber in nandrolone-treated animals, while TP was effective at all doses tested. These effects in CPP cannot be attributed to changes in general activity of the animals. The lack of alteration of general locomotor activity after TP and nandrolone treatment was previously reported. In particular, this study demonstrated that several nandrolone doses ranging from 3.75 to 30 mg/kg given to male mice had no effect in locomotion (Salvador et al., 1999). In our study, although TP (0.75 mg/kg) displayed an increase in jumping/climbing (vertical activity), we have found no effect when animals were tested in the elevated plus maze (data not shown).
The rewarding effect of naturally-occurring androgens (testosterone) according to the CPP task has been previously demonstrated in male mice (Arnedo et al., 2002) and male rats (Frye et al., 2001). In addition, it has been shown that male rodents self-administer naturally-occurring androgens (Frye et al., 2007; Johnson and Wood, 2001; Rosellini et al., 2001; Wood et al., 2004). However, limited studies have been done to assess the rewarding properties of AAS. Whenever present, these studies preclude the effects of anabolic steroids in determining the modulatory effect in the rewarding properties of other drugs of abuse (Célérier et al., 2003; 2006). Now, we present for the first time a study that contrast the rewarding effects of three different types of synthetic androgens that differ in chemical structure and metabolism using the conditioned place preference test.
No studies have been found in the literature showing the effect of nandrolone or 17α-meT in CPP, although Gans and Erskine (2003) demonstrated that neonatal TP treatment did not disrupt or enhance the CPP induced by paced mating. The differential modulation we observed in the CPP ie., TP and nandrolone vs. 17α-meT might reflect a distinct ability for each class of AAS to act in estrogenic receptors. It is well known that unlike TP and nandrolone, 17α-meT decrease the aromatase mRNA expression, or inhibit its activity acting as a competitive aromatase inhibitor (Mor et al., 2001; Zhang et al., 2007). Furthermore, a decrease in endogenous E2 levels have been reported after exposure to 17α-meT (Andersen et al., 2006). On the contrary, aromatase has been shown to be upregulated in a study using nandrolone as the AAS source (Roselli, 1998; Takahashi et al., 2007; 2008), while a decrease was observed after exposure to 17-alpha alkylated compounds (Roselli, 1998). Thus, the product metabolism of aromatase activity ie., estrogen, seems to be a key candidate for the differential modulation observed in the CPP. Estradiol subcutaneous injections induced CPP in ovariectomized rats (Frye and Rhodes, 2006) and may involve actions at estrogen receptors in the nucleus accumbens (Walf et al., 2007). Moreover, DiMeo and Wood (2006) have shown that estradiol is reinforcing in male hamster. Similar differential modulation has been previously shown when studying other behavioral paradigms. For instance, differences in male aggression between 17α-meT, testosterone propionate, stanozolol and nandrolone decanoate may reflect differences in the abilities to act at androgen versus estrogenic receptors (for review see Clark and Henderson, 2003; McGinnis et al., 2002).
AAS compounds can target a variety of neurochemical substrates in the brain including the GABAergic, dopaminergic and peptidergic systems (for review see Clark and Henderson, 2003). It is well known from previous work that steroids interact with the benzodiazepine/GABA receptor complex (Bitran, 1993; Majewska, 1992; Gee et al., 1988) and that AAS can modulate several subunits of the GABAA receptor (Jones et al., 2006; Yang et al., 2002; 2005). Moreover, it is now clear that the testosterone metabolite 3α-androstane-3α, 17βdiol (3α-diol) acts through this GABAergic complex, and is one of the most potent modulators of this system (for review see Frye, 2007). 3α-diol is converted from dihydrotestosterone (DHT) by the action of the 3α-hydroxysteroid dehydrogenase (3α-HSD). The fact that TP and nandrolone can be metabolized to 3α-diol and produced a shift in place preference might be in agreement with Frye (2007) who had demonstrated that this metabolite elicited the most rewarding effects of testosterone. This contrast with the knowledge that 17α-alkylated compounds can not be reduced to DHT nor converted to 3α-diol (for review see Clark and Henderson, 2003; Sundaram et al., 1995). Correspondingly, 17α-meT proved to be a stronger inhibitor of 5α-reductase, the enzyme responsible for the irreversible conversion of testosterone to DHT (Lo et al., 2007). Therefore, if the cellular concentration of DHT is depleted by 17α-alkylated compounds, a decrease in the rewarding metabolite 3α-diol could be observed.
A connection between AAS, the mesocorticolimbic reward system and the central dopaminergic activity have been study for many years. Thus, dopamine and its metabolites have been studied in mesolimbic structures such as the nucleus accumbens and the striatum. A study where comparison of the dopaminergic metabolism between class I (TP), class II (nandrolone) and class III (methandrostenolone) was assessed in the striatum, found no differences in dopamine (DA) metabolites between AAS treatment, although all of the AAS tested increase their levels if compare to control animals (Thiblin et al., 1999). Based in this study, a differential modulation of DA levels or its metabolites by different classes of AAS, could not be a plausible explanation for the differences we observed in CPP.
Several domains of anxiety have been typically shown to be affected by AAS in animal models (Barreto-Estrada et al., 2004; Bitran et al., 1993; Kouvelas et al., 2008; Rocha et al., 2007). In this study we observed a decrease in exploratory-based anxiety as assessed by light-dark transitions but only when animals were exposed to the lower dose (0.075 mg/kg) of nandrolone. This result can be interpreted as an increase in anxiety during exploratory behavior at this dose level. Nandrolone (5 mg/kg) has been shown to increase anxiety levels in rats when tested in the elevated plus maze (EPM), a paradigm that measure generalized anxiety (Rocha et al., 2007). The fact that we do not observed an anxiogenic response at a similar dose (7.5 mg/kg) might be due to our short-term steroid exposure (5 alternate days), if compared with the long-term administration of twice a week for six weeks (Rocha et al. 2007) and/or drug desensitization at higher doses. Interestingly, a more recent study that also administered nandrolone for 6 weeks, showed an anxiolytic effect at a dose of 15 mg/kg (Kouvelas et al., 2008), a two-fold increase in the nandrolone concentration if compared with the study of Rocha and colleagues (2007). These results argue in favor of nandrolone producing anxiogenic versus anxiolytic effects depending of the pharmacological dose and regime of administration. In our study it is important to highlight the dissociation between hedonic properties and anxiety. It seems that low levels of nandrolone are required to attain changes in anxiety status, while higher doses (0.75 and 7.5 mg/kg) elicit shifts in place preference.
Other behavioral studies (Barreto-Estrada et al., 2004) have shown no significant effects in the light-dark transitions or in locomotor activity after two weeks of systemic exposure to 17α-meT (7.5 mg/kg). In this previous study, 17α-meT decreased gonadal weight. However, in the present study five alternate injections of AAS were not enough to produce similar effects. These results interestingly suggest that it might takes longer to produce endocrine changes than to produce changes in hedonia and reward.
It is difficult to assess whether the improvement of physical appearance, the increase in muscular strength and performance, hedonia, and/or reinforcing effects is underlying the misuse of androgen compounds in the human population. However, anecdotal information from AAS users points towards an association between the misuse of anabolic steroids and mood disorders, depressive symptoms, euphoria (Bahrke et al., 1998; Perry et al., 1990; Wood, 2004) and positive (pleasure) emotions (Brower et al., 1991). Nevertheless, a growing body of experimental evidence with animal models clearly shows that androgen compounds possess rewarding and reinforcing properties. In conclusion our results suggest that: 1) not all AAS elicit hedonic effects; 2) the AAS-induced hedonic effects might be dose-dependent, 3) doses of AAS not producing hedonia might alter other behavioral domains such as anxiety-related behaviors and 4) the interaction of AAS and their metabolites with cellular substrates, more than the chemical structure, might ultimately predict their behavioral effects. Further basic and translational studies will be required to unravel the hedonic and rewarding properties of anabolic steroids in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen L, Goto-Kazeto R, Trant JM, Nash JP, Korsgaard B, Bjerregaard P. Short-term exposure to low concentrations of the synthetic androgen methyltestosterone affects vitellogenin and steroid levels in adult male zebrafish (Danio rerio) Aquat Toxicol. 2006;76(3–4):343–352. doi: 10.1016/j.aquatox.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Arnedo MT, Salvador A, Martínez-Sanchís S, Pellicer O. Similar rewarding effects of testosterone in mice rated as short and long attack latency individuals. Addict Biol. 2002;7(4):373–379. doi: 10.1080/1355621021000005955. [DOI] [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE, Brower KJ. Anabolic-androgenic steroid abuse and performance-enhancing drugs among adolescents. Child Adolesc Psychiatr Clin N Am. 1998;7(4):821–838. [PubMed] [Google Scholar]
- Barreto-Estrada JL, Barreto J, Fortis-Santiago Y, Rivera-Ramos I, Fortis-Santiago A, Jorge JC. Modulation of affect after chronic exposure to the anabolic steroid 17α-methyltestosterone in adult mice. Behav Neurosci. 2004;118(5):1071–1079. doi: 10.1037/0735-7044.118.5.1071. [DOI] [PubMed] [Google Scholar]
- Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463(1–3):55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Blow FC, Young JP, Hill EM. Symptoms and correlates of anabolic-androgenic steroid dependence. Br J Addict. 1991;86(6):759–768. doi: 10.1111/j.1360-0443.1991.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Célérier E, Ahdepil T, Wikander H, Berrendero F, Nyberg F, Maldonado R. Effects of the anabolic-androgenic steroid nandrolone in cannabinoid dependence. Neuropharmacol. 2006;50(7):788–806. doi: 10.1016/j.neuropharm.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Célérier E, Simmonnet G, Maldonado R. Effects of nandrolone on acute morphine responses, tolerante and dependence in mice. Eur J Pharmacol. 2003;465(1–2):69–81. doi: 10.1016/s0014-2999(03)01462-6. [DOI] [PubMed] [Google Scholar]
- Clark AS, Barber DM. Anabolic-androgenic steroids and aggression in castrated male rats. Physiol Behav. 1994;56:1107–1113. doi: 10.1016/0031-9384(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci and Biobehav Rev. 2003;27(5):413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. 1. New York: Wiley-Liss; 2000. [Google Scholar]
- DiMeo AN, Wood RI. Self-administration of estrogen and dihydrotestosterone in male hamsters. Horm Behav. 2006;49(4):519–526. doi: 10.1016/j.yhbeh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alpha-androstanediol. Pharmacol Biochem Behav. 2007;86(2):354–67. doi: 10.1016/j.pbb.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Babson A, Walf AA. Self-administration of 3alpha-androstanediol increases locomotion and analgesia and decreases aggressive behavior of male hamsters. Pharmacol Biochem Behav. 2007;86(2):415–21. doi: 10.1016/j.pbb.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Frye CA, Park D, Tanaka M, Rosellini R, Svare B. The testosterone metabolite and neurosteroid 3α-androstanediol may mediate the effects of testosterone on conditioned place preference. Psychoneuroendocrinology. 2001;26(7):731–750. doi: 10.1016/s0306-4530(01)00027-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Administration of estrogen to ovariectomized rats promotes conditioned place preference and produces moderate levels of estrogen in the nucleus accumbens. Brain Res. 2006;1067:209–215. doi: 10.1016/j.brainres.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Gans S, Erskine MS. Effects of testosterone treatment on pacing behaviors and development of a conditioned place preference. Horm & Behav. 2003:354–364. doi: 10.1016/s0018-506x(03)00157-0. [DOI] [PubMed] [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in the rat brain: structure-activity requirements, regional dependence and mechanisms of action. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34(8):513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Wood RI. Oral testosterone self-administration in male hamsters. Neuroendocrinology. 2001;73(4):285–292. doi: 10.1159/000054645. [DOI] [PubMed] [Google Scholar]
- Jones BL, Whiting PJ, Henderson LP. Mechanisms of anabolic androgenic steroid inhibition of mammalian epsilon-subunit containing GABAA receptors. J Physiol. 2006;573(Pt 3):571–593. doi: 10.1113/jphysiol.2006.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge JC, Velázquez KT, Ramos-Ortolaza DL, Lorenzini I, Marrero J, Maldonado-Vlaar CS. A testosterone metabolite is rewarding to female rats. Behav Neurosci. 2005;119(5):1222–1226. doi: 10.1037/0735-7044.119.5.1222. [DOI] [PubMed] [Google Scholar]
- Kouvelas D, Pourzitaki C, Papazisis G, Dagklis T, Dimou K, Kraus MM. Nandrolone abuse decreases anxiety and impairs memory in rats via central androgenic receptors. Int J Neuropsychopharmacol. 2008;14:1–10. doi: 10.1017/S1461145708008754. [DOI] [PubMed] [Google Scholar]
- Kuhn CM. Anabolic steroids. Rec Prog Horm Res. 2002;57:411–434. doi: 10.1210/rp.57.1.411. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38(4):379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Breuer ME, Possidente B. Physical provocation potentiates aggression in male rats receiving anabolic androgenic steroids. Horm Behav. 2002;41:101–110. doi: 10.1006/hbeh.2001.1742. [DOI] [PubMed] [Google Scholar]
- Mor G, Eliza M, Song J, Wiita B, Chen S, Naftolin F. 17α-methyltestosterone is a competitive inhibitor of aromatase activity in Jar choriocarcinoma cells and macrophage-like THP-1 cells in culture. J Ster Biochem Mol Biol. 2001;79:239–246. doi: 10.1016/s0960-0760(01)00162-5. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cornell AH, Alexander GM. Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behav Neurosci. 1997;111:219–224. doi: 10.1037//0735-7044.111.1.219. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Yates WR, Andersen KH. Psychiatric symptoms associated with anabolic steroids: a controlled, retrospective study. Ann Clin Psych. 1990;147:510–512. [Google Scholar]
- Pope HG, Jr, Brower KJ. In: Comprehensive Textbook of Psychiatry. 7. Sadock BJ, Sadock BA, editors. Lippincott William & Wilkins; Philadelphia: 2000. pp. 1085–1095. [Google Scholar]
- Reid LD, Marglin SH, Mattie ME, Hubbell CL. Measuring morphine’s capacity to establish a place preference. Pharmacol Biochem Behav. 1989;33:765–775. doi: 10.1016/0091-3057(89)90468-1. [DOI] [PubMed] [Google Scholar]
- Rocha VM, Calil CM, Ferreira R, Moura MJ, Marcondes FK. Influence of anabolic steroid on anxiety levels in sedentary male rats. Stress. 2007;10(4):326–331. doi: 10.1080/10253890701281344. [DOI] [PubMed] [Google Scholar]
- Roselli CE. The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area. Brain Res. 1998;792(2):271–276. doi: 10.1016/s0006-8993(98)00148-6. [DOI] [PubMed] [Google Scholar]
- Rosellini RA, Svare BB, Rhodes ME, Frye CA. The testosterone metabolite and neurosteroid 3α-androstanediol may mediate the effects of testosterone on conditioned place preference. Brain Res Brain Res Rev. 2001;37(1–3):162–171. doi: 10.1016/s0165-0173(01)00116-3. [DOI] [PubMed] [Google Scholar]
- Salvador A, Moya-Albiol L, Martinez-Sanchis S, Simon VM. Lack of effects of anabolic-androgenic steroids on locomotor activity in intact male mice. Percep Mot Skills. 1999;88(1):319–328. doi: 10.2466/pms.1999.88.1.319. [DOI] [PubMed] [Google Scholar]
- Schwerin MJ, Corcoran KJ, Fisher L, Patterson D, Askew W, Olrich T, Shanks S. Social physique anxiety, body esteem, and social anxiety in bodybuilders and self-reported anabolic steroid users. Addictive Behav. 1996;21(1):1–8. doi: 10.1016/0306-4603(95)00031-3. [DOI] [PubMed] [Google Scholar]
- Sundaram K, Kumar N, Monder C, Bardin CW. Different patterns of metabolism determine the relative anabolic activity of 19-norandrogens. J Steroid Biochem Mol Biol. 1995;53:253–257. doi: 10.1016/0960-0760(95)00056-6. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hallberg M, Magnusson K, Nyberg F, Watanabe Y, Långström B, Bergström M. Increase in [11C]vorozole binding to aromatase in the hypothalamus in rats treated with anabolic androgenic steroids. Neuroreport. 2007;18(2):171–174. doi: 10.1097/WNR.0b013e328010ff14. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tamura YM, Watanabe Y, Långström B, Bergström M. Alteration in [11C]vorozole binding to aromatase in neuronal cells of rat brain induced by anabolic androgenic steroids and flutamide. Neuroreport. 2008;19(2):431–435. doi: 10.1097/WNR.0b013e3282f7cdb7. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Finn A, Ross SB, Stenfors C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. British J Pharmacol. 1999:1301–1306. doi: 10.1038/sj.bjp.0702412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Progress Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Meade JR, Harney JP, Frye CA. Estradiol-induced conditioned place preference may require actions at estrogen receptors in the nucleus accumbens. Neuropsychopharmacol. 2007;32(3):522–530. doi: 10.1038/sj.npp.1301124. [DOI] [PubMed] [Google Scholar]
- Wood RI. Reinforcing aspects of androgens. Physiol Behav. 2004;83(2):279–289. doi: 10.1016/j.physbeh.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Wood RI, Johnson LR, Chu L, Schad C, Self DW. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacol (Berl) 2004;171(3):298–305. doi: 10.1007/s00213-003-1587-7. [DOI] [PubMed] [Google Scholar]
- Yang P, Jones BL, Henderson LP. Mechanisms of anabolic androgenic steroid modulation of alpha (1)beta(3) gamma(2L) GABA(A) receptors. Neuropharmacol. 2002;43(4):619–633. doi: 10.1016/s0028-3908(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Yang P, Jones BL, Henderson LP. Role of the alpha subunit in the modulation of GABA (A) receptors by anabolic androgenic steroids. Neuropharmacol. 2005;49(3):300–316. doi: 10.1016/j.neuropharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, Dlaboga D, Jin SL, Conti M, O’donnell JM. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B) Neuropsychopharmacol. 2008;33(7):1611–1623. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhang Y, Zhang L, Zhao H, Li X, Huang H, Lin H. The mRNA expression of P450 aromatase, gonadotropin beta subunits and FTZ-F1 in the orange-spotted grouper (Epinephelus Coioides) during 17alpha-methyltestosterone-induced precocious sex change. Mol Reprod Dev. 2007;74(6):665–73. doi: 10.1002/mrd.20642. [DOI] [PubMed] [Google Scholar]