Abstract
Clostridium difficile is a nosocomial pathogen whose incidence and importance are on the rise. Previous work in our laboratory characterized the central role of selenoenzyme dependent Stickland reactions in C. difficile metabolism. In this work we have identified, using mass spectrometry, a stable complex formed upon reaction of auranofin (a gold containing drug) with selenide in vitro. X-ray absorption spectroscopy supports the structure that we proposed based on mass spectrometric data. Auranofin potently inhibits the growth of C. difficile but does not similarly affect other clostridia that do not utilize selenoproteins to obtain energy. Moreover, auranofin inhibits the incorporation of radioisotope selenium (75Se) in selenoproteins in both E. coli, the prokaryotic model for selenoprotein synthesis, and C. difficile without impacting total protein synthesis. Auranofin blocks the uptake of selenium and results in the accumulation of the auranofin-selenide adduct in the culture medium. Addition of selenium in the form of selenite or L-selenocysteine to the growth media significantly reduces the inhibitory action of auranofin on the growth of C. difficile. Based on these results, we propose that formation of this complex and the subsequent deficiency in available selenium for selenoprotein synthesis is the mechanism by which auranofin inhibits C. difficile growth. This study demonstrates that targeting selenium metabolism provides a new avenue for antimicrobial development against C. difficile and other selenium-dependent pathogens.
Keywords: Auranofin, selenium, EXAFS, Clostridium difficile, antimicrobial
Introduction
Auranofin [2,3,4,6-tetra-o-acetyl-1-thio-β-D-glucopyranosato-S-(triethyl-phosphine) gold] is a Au(I) complex containing a Au-S bond stabilized by a triethyl phosphine group [1]. It is used clinically to treat rheumatoid arthritis [2]. It is a potent inhibitor of the mammalian selenoenzyme thioredoxin reductase (TrxR) and it is proposed that the mechanism of action in arthritis treatment is, in part, due to its activity against this and other selenoenzymes [3, 4]. Recently, auranofin has been shown to inhibit growth of the parasite Trypanosoma brucei [5]. It has also exhibited activity against Schistosoma mansoni in a mammalian host [6]. In both reports the proposed mechanism of action was the inhibition of selenoenzymes critical for survival. It is hypothesized that auranofin inhibits selenoenzymes through interactions with the reduced selenocysteine residues at the active sites [4].
Recently we found that auranofin blocks the incorporation of selenium into selenoproteins in mammalian cells in culture [7], however the mechanism of this inhibition has not yet been determined. Selenoprotein synthesis is well defined in both prokaryotic and eukaryotic systems. It should be noted, however, that transport and metabolism of selenium upstream of the specific selenoprotein synthesis machinery is not well understood. In the first step of selenoprotein synthesis, the highly reactive, reduced form of selenium, hydrogen selenide (HSe−), serves as the substrate for selenophosphate synthetase (SPS) [8-12]. SPS produces selenophosphate in an ATP dependent manner. Selenocysteine synthase subsequently catalyzes the reaction of selenophosphate with a serine charged tRNA to form selenocysteine [13, 14]. Specialized translation factors and a stem-loop structure within the mRNA (selenocysteine insertion sequence or SECIS) then direct the insertion of selenocysteine into the polypeptide chain [15]. Given its reactivity with active site selenols, the possibility exists that auranofin could interact with reactive selenium metabolites upstream of SPS, such as HSe−, thus blocking selenoprotein synthesis entirely.
The role of selenium and selenoproteins in human health has been studied extensively. At least 25 human selenoproteins, have been identified [16]. In recent years studies have focused on the role of human selenoproteins as catalytic antioxidants and the impact of selenium supplementation on cancer incidence. In addition to humans, several pathogens, including, but not limited to, Clostridium difficile, Treponema denticola and Plasmodium falciparum also produce selenoproteins [17-19]. The importance of these selenoproteins and how they impact pathogenesis has yet to be fully elucidated. These unique enzymes and their specialized assembly machinery present an intriguing target for antimicrobial development.
Clostridium difficile is a gram positive, anaerobic, spore forming bacillus that has emerged as a significant nosocomial pathogen. Pathogenesis is mediated by two large clostridial cytotoxins, toxins A and B, and symptoms typically range from mild to severe diarrhea. In more severe infections, patients develop pseudomembranous colitis [20]. C. difficile associated disease (CDAD) contributes an estimated $1 billion in excess health care costs annually [21]. Recently the emergence of an epidemic strain (NAP1/O27) that exhibits increased virulence, and increasing mortality rates in the United States has been of particular concern [22, 23]. In addition, isolates of this strain have exhibited a wide array of antibiotic resistance [22, 24]. Analysis of data collected before and after the emergence of NAP1/O27 indicated a reduction in the effectiveness of vancomycin over metronidazole in treating C. difficile infection [25]. A recent update of the literature regarding this epidemic has suggested that C. difficile now rivals methicillin resistant Staphylococcus aureus (MRSA) as a significant clinical pathogen [26]. Given the increased incidence in the clinic and emerging resistant strains, new approaches to target this pathogen are certainly justified.
In this report we demonstrate that auranofin reacts with HSe− to form a stable complex. Subsequently, we show that auranofin blocks selenium utilization by both Escherichia coli, a model organism for prokaryotic selenoprotein synthesis, and C. difficile, a significant human pathogen. In addition, auranofin exhibits antimicrobial activity against C. difficile. We propose that the molecular mechanism of this growth inhibition is the formation of the complex with HSe− which prevents uptake and nutritional utilization of selenium by C. difficile.
Materials and Methods
Reaction of Hydrogen Selenide with Auranofin
Hydrogen selenide (HSe−) was synthesized by reaction of elemental selenium with sodium borohydride as previously described [27]. For experiments examining the oxidation of hydrogen selenide to elemental selenium, equal volumes (50μL) of varying concentrations of auranofin (in DMSO) and hydrogen selenide were added to the wells of a 96 well plate, mixed thoroughly by pipetting and incubated for 30 minutes under anaerobic conditions. The reactions were then exposed to ambient atmospheric conditions for 30 minutes followed by visual examination. Under these conditions hydrogen selenide oxidizes to Se0 and forms a red precipitate. Similarly, equal volumes of varying concentrations of auranofin and hydrogen selenide were reacted anaerobically before analysis by mass spectrometry or high performance liquid chromatography (HPLC).
HPLC-MS data were collected on an Agilent 1100 HPLC and Agilent 1969A time of flight mass spectrometer. The capillary voltage was 5,000 V, and the fragmentor voltage was 100 V. The nitrogen gas temperature was 300° C. The range scanned was from 145 to 2000 amu at 10,000 transients/scan in positive ion mode. The HPLC column was a Vydac C18 (218TP5105) with flow at 20 μL/min and temperature held at 30° C. Solvent A was 0.05% trifluoroacetic acid and solvent B was 0.05% trifluoroacetic acid in acetonitrile. The gradient ran from 0 to 95% B at 2%/min. The 0.05% TFA is added to the HPLC solvents to improve chromatographic separation and to increase the solubility of eluted compounds in acetonitrile [28]. However, TFA can severely suppress ionization of proteins, so 20 μL/min neat acetic acid is mixed in a tee placed between the HPLC UV detector and the electrospray needle. The resulting 50% acetic acid displaces TFA from the protein [29]. Some spectra were obtained by direct injection of the sample into the mass spectrometer, without HPLC separation, as shown in Figure 2. The mass of the Auranofin-selenide adduct was the same whether identified by direct injection or after separation by LC.
Figure 2. Mass spectrometry reveals major reaction products of auranofin and hydrogen selenide.
The predominant product from direct injection of an equimolar reaction of auranofin and hydrogen selenide has a mass of 1025.10 atomic mass units and exhibits a selenium isotope signature [51]. The inset plot shows all of the products obtained across the entire spectrum. The predominant compound was also obtained after liquid chromatography as described in the methods section, and its mass was confirmed (data not shown).
HPLC analysis (UV-visible detection) was performed using a Hewlett Packard 1050 system (diode array detection). 20 μL samples were loaded onto a C18 column at a flow rate of 0.5 mL per minute. The starting solvent (used for injection) was 0.05% trifluoroacetic (TFA) acid in H2O. A linear gradient (50 minutes) was developed to 100% acetonitrile, 0.05% TFA. Eluting compounds were monitored spectrophotometrically at 254 nm.
X-Ray Absorption Spectroscopy
We used Se K edge and Au L3 edge XAS to characterize the major product of reaction of hydrogen selenide with auranofin. The samples were prepared as described above, resulting in solutions containing either 3.0 mM auranofin + 2.0 mM hydrogen selenide in 60% DMSO (used for Se XAS) or 2.0 mM auranofin + 3.0 mM hydrogen selenide in 40% DMSO (used for Au XAS). As controls, we also measured Au XAS of a solution of 2.5 mM auranofin in 50% DMSO and Se XAS of a solution of 2.5 mM hydrogen selenide in 50% DMSO. Each sample was loaded into one 8 μL well of a polycarbonate cuvet with an X-ray transparent Kapton window on one side and quickly frozen in LN2 for shipment on dry ice. XAS data collection was performed on beam line 9-3 of the Stanford Synchrotron Radiation Laboratory with the SPEAR3 ring operating at 3.0 GeV and 85-100 mA. Beam line mirrors were configured for a nominal 15 keV cutoff for harmonic rejection and the focused beam passed through the LN2-cooled double crystal monochromator using Si[220] crystals at full tuning. The monochromatic x-ray beam was apertured to 1 × 1 mm to illuminate the sample in each well of a 5-well cuvet suspended in an Oxford continuous-flow LHe cryostat; all data were collected with the sample maintained at 10 K. Fluorescence excitation data were collected using a Canberra 30-element intrinsic Ge solid state detector, using Soller slits and fluorescence filters consisting of 6 absorption lengths of As (for Se K edge XAS) or of Ga (for Au L3 edge XAS). Three 22 min spectra were collected for each sample and the averaged data were used for analysis. Residual x-ray intensity that penetrated the sample was used to collect transmission data on an elemental standard (either Au foil or powdered elemental Se) positioned between two ionization chamber detectors downstream of the sample cryostat. We also collected Au L3 XAS data by transmission on three structurally characterized (“model”) complexes presented as finely ground powders diluted with BN in the same LHe cryostat: KAuBr4 [30, 31], KAuCl4 [32] and Na3Au(S2O3)2 [33, 34]. These were used to learn about scattering characteristics of Au-Br (similar to Au-Se), Au-Cl, and Au-S (the latter also similar to Au-P). Data reduction was accomplished by standard techniques using the EXAFSPAK program suite (http://ssrl.slac.stanford.edu/exafspak.html). Energy calibration using the internal standard spectra relied on assuming the first inflection point of the L3 edge of Au foil and the K edge of elemental Se occur at 11920.0 and 12658.0 eV, respectively. Pre-edge subtraction used a Gaussian model and fit the data through 11885 eV for Au L3 and through 12620 eV for Se K edge data. Extended X-ray absorption fine structure (EXAFS) data were extracted using a third-order three-region spline function with spline points of 11930, 12190, 12450, and 12710 eV for Au L3 and 12665, 12928, 13191, and 13454 eV for Se K edge data. E0 (k = 0) values were assumed to be 11930 and 12665 eV, respectively. EXAFS data of model compounds were analyzed by constructing a molecular model of the known structures, using the software package feff version 7 (feff v7, [35]) to calculate multiple scattering paths, then importing these into the OPT program of EXAFSPAK. OPT was used to optimize ΔE0 as well as fine tune the values of other parameters including first-shell distances (Ras) and Debye-Waller factors (σas2). The amplitude reduction factor (S02) was fixed at 0.9 in all fits. In all cases, optimized distances were within ± 0.02 Å of crystallographic distances, if they were available. Debye-Waller factors from the fits of Au model compounds were used to judge the validity of coordination numbers resulting from fits of Au L3 EXAFS of auranofin-containing samples, which were carried out in a similar fashion, starting with a hypothetical structure. Se K EXAFS were analyzed using the same molecular models, again using feff v7 to generate multiple-scattering paths for Se as absorber.
Growth of Escherichia coli
Wild type E. coli (MC4100) and a selenoprotein deficient strain (WL400, ΔselD) were cultured in modified Luria broth (10 gm/L tryptone, 5 gm/L torula yeast extract, 5 gm/L NaCl, 1% dextrose) at 37°C in a Coy anaerobic chamber. A 1% inoculum of an overnight culture was used in each experiment. Optical density measurements were taken 24 hours after inoculation and hydrogen production was assessed by Durham tubes and/or bubbling of cultures upon vigorous shaking. For experiments utilizing E. coli, auranofin was dissolved in ethanol rather than DMSO because the latter inhibits gas production in this organism.
Growth of Clostridium difficile, C. perfringens and C. tetani
Four strains of C. difficile were used in this study, ATCC 9689, VPI 10463, NAP1/O27 and strain 630. C. difficile 630 was kindly provided by Peter Mullany (Eastman Dental Institute, London, United Kingdom), and NAPI/027 was provided by Dr. Michel Warny (Acambis, Inc., Cambridge, MA). Two pathogenic clostridia that do not produce selenoproteins, C. perfringens (ATCC 19406) and C. tetani (ATCC 10543), were used as experimental controls. Cultures were grown in brain heart infusion (BHI; Oxoid) supplemented with 0.5 g/L L-cysteine to pre-reduce the culture media. All cultures were grown at 37°C in a Coy Laboratories anaerobic chamber under an atmosphere of 98% nitrogen, 2% hydrogen. A 1% inoculum of an overnight culture was used in each experiment. Auranofin (Alexis Biochemicals, San Diego, CA) was diluted in DMSO before addition to the culture media. Equal volumes of the resulting auranofin dilutions, or DMSO as a vehicle control, were added to each culture. To examine the role of selenium metabolism in the toxicity of auranofin the growth medium was supplemented with selenium (sodium selenite or L-selenocysteine) as indicated. Optical density measurements of cultures at 600 nm were determined using a Spectramax multiwell plate reader (Molecular Devices, Sunnyvale, CA) from 200 μL of culture after 24 hours of growth in each experiment.
75Se Incorporation into selenoproteins
One mL cultures of E. coli (MC4100) were prepared in modified Luria both as described above in the presence of increasing concentrations of auranofin (5, 10, and 50 μM). For the identification of selenoproteins, 6 μCi of 75Se (University of Missouri, Columbia), in the form of sodium selenite (100 nM), was added to each culture. To examine total protein synthesis, 20 μCi of 35S (methionine/cysteine mixture) was added to replicate cultures. After 24 hours of growth under anaerobic conditions, cells were harvested by centrifugation for 5 min at 5,000 × g and resuspended in lysis buffer (25 mM Tris, pH 8.8, 1 mM DTT, 0.5 mM EDTA, 0.1 mM benzamidine). Cells were lysed by sonication using a sonic dismembranator, model 100 (Fisher Scientific), for 10 seconds at a power output of 12 W, and the resultant crude cell extracts were clarified by centrifugation at 13,500 × g for 10 minutes. Protein concentration was determined by the Bradford assay using bovine serum albumin (Pierce) as a standard [36]. Selenoproteins and total protein synthesis were analyzed by separating 25 μg of cell extracts using a 12% SDS-polyacrylamide gel, and radioisotope-labeled proteins were detected by PhosphorImager analysis (Molecular Dynamics).
The impact of auranofin on selenoprotein synthesis in C. difficile (NAP1/O27) was determined in 1 mL mid-logarithmic phase cultures. A 20% inoculum of an overnight culture was used in each experiment. C. difficile was cultivated in BHI + cysteine for 4 hours before addition of auranofin and 6 μCi of 75Se in the form of sodium selenite (100 nM) or 20 μCi of 35S (methionine/cysteine mixture). The cultures were incubated for an additional 4 hours before harvesting. Cell extracts were prepared and analyzed as described for E. coli.
75Se Uptake studies
The impact of auranofin on selenite uptake in E. coli and C. difficile (NAP1/O27) was determined in 1 mL mid-logarithmic phase cultures. A 20% inoculum of an overnight culture was used in each experiment. Both organisms were cultivated in BHI + cysteine for 2 hours before the addition of chloramphenicol (30 μg/mL) to inhibit protein synthesis during uptake analysis in response to drug treatment. Various concentrations of auranofin (0 to 10 μM) were added to the cultures immediately followed by 4 μCi of 75Se in the form of sodium selenite (2 nM selenium). Cultures were incubated for 20 minutes at 37°C. Cells were harvested by centrifugation (1 minute at 16200 × g). Culture media supernatants were placed on ice for further analysis by HPLC. Cells were washed once in 500 μL PBS and harvested by centrifugation (1 minute at 16,200 × g). The supernatant was discarded and the cells were resuspended in 500 μL PBS before measuring the total uptake of 75Se using a Model 1470 Gamma counter (Perkin–Elmer, Wellesley, ME).
HPLC analysis of 75Se in C. difficile growth media
20 μL samples of reserved growth medium from the 75Se uptake study were separated by HPLC as described above for the mixtures of auranofin and selenide (above). Fractions were collected every minute and the distribution of 75Se (counts per minute) was measured using a gamma counter (Perkin-Elmer).
Results
Auranofin reacts with HSe− to form an adduct
HSe− is highly reactive and exquisitely sensitive to oxidation under aerobic conditions. When it is exposed to oxygen it is rapidly oxidized to elemental selenium forming a red precipitate. We took advantage of this simple oxidation reaction to determine if HSe− interacts directly with auranofin. Reactions of HSe− and auranofin were prepared anaerobically and subsequently exposed to ambient conditions. HSe− alone formed the expected red precipitate; however, reaction with auranofin protected it from oxidation to elemental selenium, suggesting formation of an oxygen-resistant complex (data not shown).
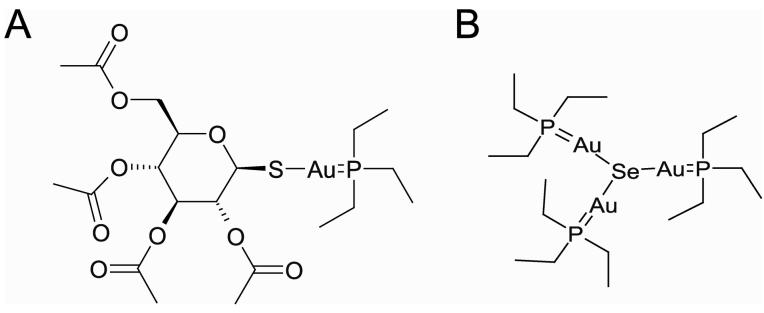
To isolate and identify this apparently stable complex, mixtures of auranofin and selenide were separated by HPLC using reverse phase chromatography. Auranofin alone elutes as a single peak (denoted as peak 1 in Figure 1). When HSe− is added to auranofin, this results in the disappearance of peak 1 and coincides with the appearance of an earlier selenium-dependent peak (Peak 2, Figure 1). Similar reactions containing sodium selenite or selenocysteine did not alter the elution profile of auranofin, indicating that these forms of selenium are not reactive (data not shown). Reaction of auranofin with 75Se labeled selenide (formed by reaction of 75Se labeled selenite with DTT) confirmed that the earlier peak (peak 2) contained selenium (data not shown). Mass spectrometry revealed that the major product of an equal molar reaction of auranofin and HSe− exhibits a selenium isotope signature and has a mass of 1025.10 atomic mass units (amu) (Figure 2). Based upon the mass obtained, we hypothesize that hydrogen selenide displaces the sulfur atoms in auranofin (Figure 3a) to form a stable complex (Figure 3b).
Figure 1. HPLC analysis of products formed during reaction of auranofin and hydrogen selenide.

Auranofin (10 mM in DMSO) was mixed anaerobically with equal volumes of H2O (a) or 20 mM hydrogen selenide (b). Reactions were incubated for 20 minutes before injection. 20 μL samples were loaded onto a C18 column at a flow rate of 0.5 mL per minute. The starting solvent (used for injection) was 0.05% trifluoroacetic (TFA) acid in H2O. A linear gradient (50 minutes) was developed to 100% acetonitrile, 0.05% TFA. Products were identified at 254 nm using a diode-array detector.
Figure 3.
Structure of auranofin (A) and the putative structure of the stable product formed upon reaction of auranofin and selenide (B), based on mass spectrometry
Characterization of Au-Se adduct using XAS
We used both the Au L3 edge and Au L3 and Se K EXAFS of the product of this reaction to evaluate the hypothesized molecular structure of this compound, referred to as the auranofin-selenide adduct (Figure 3b). We prepared and analyzed two reaction mixtures with either HSe− or auranofin in substoichiometric amounts to assure that all the Se or Au, respectively, was in a single species (the auranofin-selenide adduct). Figure 4 compares the Au L3 edge spectra of this adduct with that of the auranofin reactant and three structurally characterized compounds, two containing Au(III) (KAuBr4, KAuCl4) and one containing Au(I) (Na3Au(S2O3)2). The electronic environment of Au dominates these edges and the auranofin-selenide adduct has a similar electronic environment to the parent auranofin, containing predominantly Au(I). Au L3 EXAFS provides information about the local structural environment around the Au including precise metric details and Table 1 summarizes the detailed curve-fitting optimization results for the auranofin-selenide adduct, compared to structural models and to the parent auranofin (Figure 3a). We were also able to investigate local structural details around the Se in the adduct using Se K EXAFS; these results are also summarized in Table 1. Graphical depiction of the observed EXAFS and FT data compared with the best-fit simulations is provided in Figures 5 (auranofin-selenide adduct) and Supplementary Figures S1 and S2 (model compounds, auranofin).
Figure 4. Au L3 XAS Spectra.

Au L3 edge XAS spectra for Au(III) compounds (KAuBr4, dashed black; KAuCl4, dashed red), Au(I) compound (Na3Au(S2O3)2, dashed green), compared to that of auranofin (solid black) and the product of reaction of auranofin with hydrogen selenide (solid red).
Table 1.
Curve fitting results for Au L3 (Fits 1-5) and Se K (Fit 6) EXAFSa
| Sample, filename (k range) Δk3χ | Fit | Shell | Ras (Å) |
σas2 (Å2) |
ΔE0 (eV) |
f′b |
|---|---|---|---|---|---|---|
| KAuBr4, AUBRB (k = 2 – 14 Å−1) Δk3χ = 38.74 |
1 | Au-Br4 | 2.41 | 0.0012 | −5.11 | 0.029 |
| KAuCl4, AUCLA (k = 2 – 14 Å−1) Δk3χ = 19.06 |
2 | Au-Cl4 | 2.28 | 0.0015 | −2.45 | 0.051 |
| Na3Au(S2O3)2, AUTSA (k = 2 – 14 Å−1) Δk3χ = 11.03 |
3 | Au-S2 Au…S2 |
2.27 [3.36]c |
0.0015 [0.0022] |
−6.54 [−6.54] |
0.048 |
| Auranofin, A105B (k = 2 – 13 Å−1) Δk3χ = 9.92 |
4 | Au-P2 | 2.28 | 0.0022 | −11.2 | 0.087 |
| 2:3 Auranofin:selenide, A462B (k = 2 – 13 Å−1) Δk3χ = 14.57 |
5 | Au-P1 Au-Se1 |
[2.28] 2.40 |
[0.0022] 0.0014 |
−7.05 [−7.05] |
0.066 |
| 3:2 Auranofin:selenide, E643B (k = 2 – 14 Å−1) Δk3χ = 17.96 |
6 | Se-Au3 Se…P3 |
2.42 [4.70] |
0.0045 0.0162 |
−0.72 [−0.72] |
0.077 |
Shell is the chemical unit defined for the multiple scattering calculation. Subscripts denote the number of scatterers per metal. Ras is the metal-scatterer distance. σas2 is a mean square deviation in Ras. ΔE0 is the shift in E0 for the theoretical scattering functions.
Numbers in square brackets were constrained to be either a multiple of the value in the row above (σas2) or to maintain a constant difference from the value in the row above (Ras, ΔE0). In Fit 5, square brackets for Au-P indicates that this value was fixed to the value obtained in Fit 4
Figure 5. Curve-fitting analysis of Au L3 (top) and Se K (bottom) EXAFS data for the product of the reaction of auranofin with hydrogen selenide.

In all figures, thin black lines represent the observed data (Fourier transforms, FT, in full frames, EXAFS in insets) and bold red lines represent the best-fit simulation using feff v7 calculations based on molecular models derived from the hypothesized structure in Figure 3B. The model used for Au EXAFS consisted of a P–Au–Se fragment with a P-Au-Se angle of 180°; the simulation was generated by the parameters summarized in Fit 5 of Table 1. The model use for Se EXAFS consisted of a Se surrounded by three Au–P fragments with Au directly bonded to Se, Se-Au-P angles of 180° and Au-Se-Au angles of 120°; the simulation was generated by the parameters summarized in Fit 6 of Table R. Fourier transforms were k3 weighted over the k = 2.0 – 13.0 Å−1 range (Au L3) or the k = 2.0 – 14.0 Å−1 (Se K) range and were phase-corrected based on P.
Au L3 EXAFS analysis of the structure of the auranofin-selenide adduct depended on scattering parameter values obtained from the parent auranofin and model compound data. Fit 4 of Table 1 describes a multiple scattering feff fit to a model of the Au environment of auranofin that assumes two identical P ligands (P and S have very similar EXAFS scattering characteristics and display nearly identical Au-(P,S) distances of 2.28 Å in many structurally characterized compounds). This model provides an excellent fit (Figure S2, bottom) even reproducing the minor FT peak at 4.3 Å (associated with the P-Au-(P,S) angle of 180°) and defines the auranofin Au-P interaction that we assumed was present in the Au EXAFS of the auranofin-selenide adduct (Fit 5, Table 1) as hypothesized in the structure of Figure 3b. The Au EXAFS data for the adduct requires the presence of another first-shell ligand and an excellent fit results (Fit 5 of Table 1, Figure 5) by assuming this is a single Se bound 180° from the P at a Au-Se distance of 2.40 Å. The Au-Se coordination number is validated by the value of σas2, which is nearly identical to that for the Au-Br scattering in KAuBr4 (Fit 1, Table 1); Au-Se and Au-Br scattering are expected to be nearly indistinguishable.
Se EXAFS of the auranofin-selenide adduct was used to provide an alternate view of local structure, this time around the Se atom. Fit 6 of Table 1 (and Figure 5) shows that the hypothesized Se environment of the adduct, containing three Au-PEt3 auranofin fragments as required by the MS-determined molecular weight, provides a very good fit to the Se EXAFS, although the higher values for the σas2 parameters may suggest some disorder in Se-Au distances and more disorder in the outer-shell phosphine environments. The resulting Se-Au distance is within 0.02 Å of the Au-Se distance determined from Au EXAFS. Generally speaking, the results from the Au L3 and Se K EXAFS analyses show that these data are fully consistent with the hypothesized structure of the auranofin-selenide adduct shown in Figure 3b and provide metric details for the structure: Se-Au distances of 2.41 ± 0.02 Å, Au-P distances of 2.28 ± 0.02 Å, Se-Au-P angles of ca. 180°.
Auranofin impacts the growth of anaerobically grown E. coli
Given that auranofin reacts with HSe− in vitro, we next examined whether formation of this adduct impacts microbial growth in culture. Although E. coli does not require selenium under normal laboratory conditions, selenoproteins play an important role in mixed acid fermentation during anaerobic growth. The most predominant selenoprotein in anaerobically grown E. coli is formate dehydrogenase (FDHH). FDHH production is required for activity of the formate hydrogen lyase complex (FHL) and the production of hydrogen gas [37]. Thus, E. coli can be used to study dynamically the impact of auranofin on prokaryotic selenoprotein synthesis.
We tested the effect of auranofin on growth and gas production in wild type E. coli (MC4100). Auranofin reduced the growth yield of MC4100 at 24 hours when present at concentrations of 25 and 50 μM (Supplementary figure S3). The growth inhibition observed under these conditions was similar to that seen with the deletion of selD and may be attributed to build-up of formic acid in the growth medium. In addition, growth of the isogenic selD deletion mutant (WL400) was not affected by auranofin. Further, gas production was significantly reduced in MC4100 cultures containing 10 and 20 μM auranofin (as assessed by Durham tubes) and was completely absent in those grown with 50 μM auranofin (data not shown). The effect of auranofin on MC4100 could be due interruption of selenoprotein synthesis, or possibly due to direct inhibition of FDHH.
Auranofin inhibits growth of C. difficile
Recent work in our laboratory demonstrated the central role of Stickland reactions in the growth of C. difficile [17]. Stickland reactions are described as the coupled fermentation of amino acids in which one, the Stickland donor, is oxidized and the other, the Stickland acceptor, is reduced [38, 39]. Glycine reduction results in production of acetyl phosphate, and thus ATP synthesis via substrate-level phosphorylation [40, 41]. Reduction of proline has been tied to membrane gradients [42]. The enzymes that catalyze these reactions in C. difficile are glycine reductase and D-proline reductase respectively. Both are selenoproteins [17, 43].
Based upon the knowledge that C. difficile utilizes Stickland reactions for energy metabolism and the enzymes that catalyze these reactions are selenoproteins, we decided to determine the impact of auranofin on the growth of the organism. To determine the antimicrobial activity of auranofin, it was tested against four strains of C. difficile. As with E. coli, variable concentrations of auranofin (0.25 to 2 μM) were added to rich culture medium (BHI) before inoculation. The turbidity of C. difficile cultures was measured spectrophotometrically (at 600 nm) following 24 hours of anaerobic growth. Growth of C. difficile is potently inhibited by auranofin and this growth inhibition is consistent among all four strains (Figure 6). At 2 μM auranofin, no appreciable growth was observed. A sharp decrease in growth occurred between 750 nM and 1 μM auranofin in all strains tested. The estimated IC50 values are as follows: NAP1/O27, 775 nM; VPI 10463, 1000 nM; 630, 800 nM; ATCC 9689, 750 nM. Thus all strains were significantly inhibited by concentrations of auranofin in the high nanomolar range. In order to insure that vegetative growth rather than spore germination was examined, a 1% inoculum of mid-exponential phase cells was used in these experiments. Moreover we followed the inhibition of growth of one strain for the entire batch growth period and found concentration dependent inhibition of growth at each time point (Supplementary figure S4).
Figure 6. Auranofin inhibits growth of C. difficile.
C. difficile cultures (NAP1/O27, VPI10463, 630, and ATCC 9689) were grown anaerobically in BHI + cysteine. Auranofin in DMSO (0.25, 0.5, 0.75, 0.8, 0.85, 0.9, 0.95, 1.0, 1.5, and 2.0 μM) was added to the growth medium prior to inoculation. Growth was measured as optical density at 600 nm after 24 hours at 37°C. Percent growth (growth yield of inhibited cultures versus control) is plotted at the indicated concentrations of auranofin. Data shown is from at least three independent cultures. Error bars indicate standard deviation.
C. tetani and C. perfringens are human pathogens that are classified with C. difficile in a group of organisms known as the toxigenic clostridia. We therefore tested the growth of these organisms in the presence of auranofin to determine the relative specificity within this class. No significant inhibition of growth was observed at concentrations of auranofin up to 10 μM for C. tetani or C. perfringens (Supplementary figure S5). A similar pattern of sensitivity of C. difficile relative to other toxigenic species was observed in cultures grown in reinforced clostridial medium (data not shown). Based on genomic DNA sequence, neither C. tetani nor C. perfringens carry genes encoding glycine or D-proline reductase. There is also no evidence presented in the literature that these strains can catalyze Stickland reactions. In addition, neither strain carries genes encoding the needed components for selenoprotein synthesis (selA, selB, selC or selD) [44]. We experimentally confirmed that no specific selenoproteins were expressed in C. perfringens using 75Se radiolabeling (data not shown). The inhibitory action of auranofin in C. difficile can be attributed to the organism's reliance upon selenium and selenoproteins for growth.
Auranofin inhibits selenoprotein synthesis in E. coli model and C. difficile
Previous work in our laboratory has demonstrated that auranofin prevents the incorporation of selenium into selenoproteins in mammalian cells. To determine the impact of auranofin on overall selenoprotein synthesis in E. coli, we cultivated MC4100 in the presence of several concentrations of auranofin with the addition of radiolabeled selenium (75Se) in the form of selenite. Cell extracts were prepared after 24 hours of anaerobic growth. The results clearly demonstrate a concentration dependent decrease of selenium incorporation into FDHH(Figure 7a). Total protein synthesis was not impaired by auranofin as indicated by 35S labeling (Figure 7b).
Figure 7. Auranofin prevents incorporation of 75Se in anaerobically grown E. coli and C. difficile.
Soluble extracts of MC4100 treated with varying concentrations of auranofin (0, 5, 10, 50 μM lanes 1-4 respectively) were prepared following anaerobic growth overnight in the presence of 6 μCi of 75Se (0.1 μM Na2SeO3) (a) or 20 μCi of 35S (b). Twenty-five micrograms of protein from crude cell extracts were separated by a 12% SDS-polyacrylamide gel, and the radioactive bands were visualized using a phosphoimager. (c). Soluble extracts of C. difficile (NAPI/O27 strain) were prepared after treatment mid-logarithmic phase with auranofin (0, 5, 10 μM lanes 1-3 respectively) in the presence of 6 μCi of 75Se (0.1 μM Na2SeO3) (a) or 20 μCi of 35S (d). Twenty-five micrograms of protein from crude cell extracts were separated by a 12% SDS-polyacrylamide gel, and the radioactive bands were visualized using a phosphoimager. The predicted selenoproteins are indicated by arrows based on the molecular weights of the genes as described previously [17].
Because auranofin potently inhibits the growth of C. difficile we treated exponentially growing cells with several concentrations of auranofin plus 75Se and prepared cell extracts after four hours of anaerobic growth. We have previously used these same techniques to describe the presence of and expression of both glycine and D-proline reductase in C. difficile [17]. This allowed us to examine if auranofin also interferes with the incorporation of selenium into selenoproteins in this organism. As was the case with E. coli, a clear decrease in selenoprotein synthesis was observed (Figure 7c), but there was no impact on total protein synthesis (Figure 7c). A slight increase in the selenoprotein component of D-proline reductase (PrdB, Figure 7c) is also observed. The cause for this increase is not yet known. Nonetheless, these results clearly demonstrate that auranofin prevents selenoprotein synthesis in both organisms. The mechanism of C. difficile growth inhibition is not likely due to direct inhibition of glycine or D-proline reductase. Rather, auranofin does not allow the production of these critical selenoenzymes.
Auranofin prevents the uptake of selenium by E. coli and C. difficile
The mechanisms of selenium uptake in both prokaryotes and eukaryotes are undefined. Thus far we have shown that auranofin forms a complex with HSe− in vitro and auranofin inhibits selenoprotein synthesis in both E. coli and C. difficile. We examined whether the formation of the auranofin-selenide adduct prevents the uptake of selenium from the growth medium. Uptake of radiolabeled selenite (75Se) occurs rapidly in mid-logarithmic cultures of both organisms in rich media (BHI) and is easily quantified using a gamma counter. For this study, actively growing cultures were treated with varying concentrations of auranofin immediately followed by the addition of 75Se (2 μCi). The uptake of selenium was followed kinetically for a period of 60 minutes and was found to be essentially linear over this period (data not shown). Using a fixed time point (20 minutes) we observed a clear inhibition in the amount of radiolabel transported into the cells (Figure 8) when auranofin was added. This inhibition was also concentration dependent. For C. difficile, 500 nM auranofin reduced the uptake of 75Se by approximately 50%. The slight variation in the effect of auranofin on 75Se uptake between E. coli and C. difficile may be attributed to differences in the cell membranes of these organisms (E. coli is gram negative whereas C. difficile is gram positive).
Figure 8. Auranofin prevents uptake of 75Se by E. coli and C. difficile.

Actively growing cultures of E. coli (A) and C. difficile(B) were treated with auranofin (0 to 25 μM as indicated) as well as 75Se. Cultures were incubated for 20 minutes at 37°C. Cells were harvested by centrifugation and washed with PBS. Total uptake of 75Se was analyzed using a Model 1470 Gamma counter (Perkin–Elmer, Wellesley, ME) and is reported as counts per minute (CPM). Data shown is from at least three independent cultures. Error bars indicate standard dEviation.
Auranofin forms a complex with selenium in C. difficile growth media
Media supernatants from the uptake study with C. difficile were fractionated by HPLC as described for the analysis of the mixtures of auranofin and selenide above. In media treated with DMSO alone (vehicle control) a small peak of radioactivity was observed at the beginning of the trace with no other distinguishable peaks (Figure 9a). In contrast, media treated with 10 μM auranofin exhibited a clearly defined peak of 75Se at approximately 22 minutes (Figure 9b). These data indicate that the earlier identified auranofin-selenium complex is formed in the growth media preventing uptake and nutritional utilization of selenium by C. difficile.
Figure 9. Auranofin forms a complex with radiolabeled selenium in C. difficile growth medium.

Growth media from the uptake experiments (0 and 10μM auranofin, a and b respectively) were separated by HPLC as described for the analysis of the mixtures of auranofin and hydrogen selenide. Fractions were collected every minute and analyzed using the gamma counter
Selenium supplementation prevents auranofin dependent growth inhibition
C. difficile expresses at least three major selenoproteins that could be directly inhibited by auranofin (glycine reductase, D-proline reductase, and selenophosphate synthetase). If the formation of the auranofin-selenide adduct is indeed blocking the metabolic use of selenium in bacterial cultures, then supplementation would alleviate this inhibition. Conversely, if auranofin is directly inhibiting one or more selenoproteins, then supplementation would be unlikely to affect the action of the drug. Thus, we evaluated the impact of selenium supplementation on growth of the NAP1/O27 strain in the presence of inhibitory concentrations of auranofin.
The addition of 5 μM sodium selenite to the growth medium significantly reduces the impact of auranofin on C. difficile, with lower concentrations of selenite also exhibiting a protective effect (Figure 10a). Selenocysteine was not as potent, but the addition of 5 μM selenocysteine to the growth medium was also protective (Figure 10b). This disparity may be due to differences in the ability of the organism to utilize selenium from selenite versus selenocysteine. It should be noted that supplementation of BHI with selenite or selenocysteine did not significantly increase growth yield alone.
Figure 10. Selenium supplementation prevents auranofin-dependent growth inhibition of C. difficile.

C. difficile (NAPI/O27) was cultivated in BHI + cysteine. The culture medium was supplemented with either sodium selenite (0.5, 1.0, 5.0 μM) (a) or L-selenocysteine (0.5, 1.0, 5.0 μM) (b). Percent growth (growth yield of inhibited cultures versus control) is plotted at the indicated concentrations of auranofin after 24 hours at 37°C. Data shown is from at least three independent cultures. Error bars indicate standard deviation.
Discussion
Recently, targeting selenoproteins has become an interesting avenue for the development of anticancer therapies [45, 46]. These strategies provide a new angle to combating an immensely complex human health problem. In addition to their role in mammalian cells, selenoproteins are necessary for the growth of several significant human pathogens. It is becoming clear that the potential of selenoenzyme inhibition and interruption of selenoprotein synthesis as a means for antimicrobial development cannot be overlooked. The unique enzymatic characteristics of selenoproteins and their complex assembly machinery provide several prospective antimicrobial targets.
C. difficile continues to be a major cause of hospital acquired infection that warrants further attention. With the mortality rates from C. difficile increasing by 35% per year from 1999 to 2004 [47], and an increasingly poor response to metronidazole, the preferred treatment for CDAD, [48] new tactics for combating this disease must be developed. In this study we capitalized on C. difficile's unique reliance on selenoenzymes for energy metabolism. We demonstrated that auranofin diminishes the growth of C. difficile at nanomolar concentrations.
Based upon the data that we have gathered so far, we cannot eliminate the possibility that auranofin may directly inhibit one or more of the selenoenzymes in C. difficile. Preliminary experiments did not indicate that D-proline reductase was inhibited by auranofin (data not shown), but C. difficile expresses at least two other selenoproteins, glycine reductase and selenophosphate synthetase. The observed effect of selenium supplementation on auranofin toxicity may be due to relief of enzyme inhibition through competitive binding to free hydrogen selenide. Figures 9 and 10, however, clearly show a reduction in the incorporation of radiolabeled selenium into selenoproteins with the addition of auranofin to the growth medium. In this context it appears that potential direct inhibition of glycine reductase, or D-proline reductase, is irrelevant.
Previous studies suggest that Au(I) containing compounds, such as auranofin, inhibit selenoenzymes by binding to the reduced selenocysteine at the active site. Although there is substantial literature examining the chemical interactions between Au and Se, little research has focused on the biological implications. The alteration of mammalian selenium metabolism by Au(I) containing drugs has been demonstrated suggesting that covalent reactions between Au(I) and the nucleophilic metabolites of selenium could limit the nutritional availability of selenium for the production of selenoproteins [49]. In this report we have clearly shown that auranofin reacts with HSe− in vitro to form a stable complex. This is consistent with other chemical studies utilizing organic selenolate compounds to demonstrate similar Au-Se interactions [50]. If auranofin reacts with hydrogen selenide in vivo then the pool of available selenium for selenoprotein synthesis would be reduced. We have shown that this complex occurs in bacterial culture to prevent the uptake and nutritional utilization of selenium by both E. coli and C. difficile. The implications of these results for mammalian systems must be further studied.
Recently there have been several studies that have demonstrated the activity of auranofin against significant eukaryotic human pathogens [5, 6]. They examined inhibition of selenoenzymes in these organisms, but did not consider the possibility that auranofin could inhibit overall selenoprotein synthesis. In light of our results, this warrants further examination.
The mechanisms of selenium transport and reduction to hydrogen selenide remain enigmatic. Our results provide some insight into the metabolism of selenium upstream of selenophosphate synthetase, suggesting that the reduction of selenite to HSe− occurs before it is taken up by the bacterial cell. In addition to its potential implications for human health, auranofin may be used as a tool to study selenium metabolism. We can take advantage of the ability of auranofin to form a complex with hydrogen selenide to further elucidate prokaryotic selenium metabolism upstream of selenophosphate synthetase.
Finally, given that auranofin appears to block the metabolic use of an essential nutrient, rather than acting upon a single enzyme, the development of resistant strains is improbable. Studies are underway to determine whether strains can be isolated that are resistant to auranofin. Given its importance in energy metabolism, and the fact that multiple enzymes require selenium, resistance is unlikely to occur by point mutation.
Supplementary Material
Acknowledgements
The authors thank Michel Warny (Acambis, Inc., Cambridge, MA) for providing C. difficile NAPI/027. We also thank August Böck (University of Munich, Munich, Germany) for providing the E. coli strain WL400 (selD). The gold compounds used as models were generous gifts from Susan Miller, UCSF. XAS work in the Scott group is supported by a grant from NIH (GM042025). Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program. This work was supported in part by the Intramural Research Program of the NIH (NHLBI). This work was also supported in part by grants to WTS from the Florida Department of Health (05-NIR-10) and the National Institutes of Health (ES01434).
Abbreviations
- SPS
selenophosphate synthetase
- CDAD
Clostridium difficile associated diarrhea
- XAS
X-ray absorption spectroscopy
Extended Fine structure
REFERENCES
- 1.Kean WF, Hart L, Buchanan WW. British journal of rheumatology. 1997;36:560–572. doi: 10.1093/rheumatology/36.5.560. [DOI] [PubMed] [Google Scholar]
- 2.Messori L, Marcon G. Met Ions Biol Syst. 2004;41:279–304. [PubMed] [Google Scholar]
- 3.Becker K, Gromer S, Schirmer RH, Muller S. Eur J Biochem. 2000;267:6118–6125. doi: 10.1046/j.1432-1327.2000.01703.x. [DOI] [PubMed] [Google Scholar]
- 4.Gromer S, Arscott LD, Williams CH, Jr., Schirmer RH, Becker K. J Biol Chem. 1998;273:20096–20101. doi: 10.1074/jbc.273.32.20096. [DOI] [PubMed] [Google Scholar]
- 5.Lobanov AV, Gromer S, Salinas G, Gladyshev VN. Nucleic Acids Res. 2006;34:4012–4024. doi: 10.1093/nar/gkl541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuntz AN, Davioud-Charvet E, Sayed AA, Califf LL, Dessolin J, Arner ES, Williams DL. PLoS Med. 2007;4:e206. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talbot SaS WT. British Journal of Pharmacology. 2008 In press. [Google Scholar]
- 8.Leinfelder W, Forchhammer K, Veprek B, Zehelein E, Bock A. Proc Natl Acad Sci U S A. 1990;87:543–547. doi: 10.1073/pnas.87.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veres Z, Tsai L, Scholz TD, Politino M, Balaban RS, Stadtman TC. Proc Natl Acad Sci U S A. 1992;89:2975–2979. doi: 10.1073/pnas.89.7.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veres Z, Kim IY, Scholz TD, Stadtman TC. J Biol Chem. 1994;269:10597–10603. [PubMed] [Google Scholar]
- 11.Ehrenreich A, Forchhammer K, Tormay P, Veprek B, Bock A. Eur J Biochem. 1992;206:767–773. doi: 10.1111/j.1432-1033.1992.tb16983.x. [DOI] [PubMed] [Google Scholar]
- 12.Glass RS, Singh WP, Jung W, Veres Z, Scholz TD, Stadtman TC. Biochemistry. 1993;32:12555–12559. doi: 10.1021/bi00210a001. [DOI] [PubMed] [Google Scholar]
- 13.Forchhammer K, Leinfelder W, Boesmiller K, Veprek B, Bock A. The Journal of biological chemistry. 1991;266:6318–6323. [PubMed] [Google Scholar]
- 14.Forchhammer K, Bock A. The Journal of biological chemistry. 1991;266:6324–6328. [PubMed] [Google Scholar]
- 15.Forchhammer K, Leinfelder W, Bock A. Nature. 1989;342:453–456. doi: 10.1038/342453a0. [DOI] [PubMed] [Google Scholar]
- 16.Papp LV, Lu J, Holmgren A, Khanna KK. Antioxidants & redox signaling. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 17.Jackson S, Calos M, Myers A, Self WT. Journal of bacteriology. 2006;188:8487–8495. doi: 10.1128/JB.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rother M, Bock A, Wyss C. Archives of microbiology. 2001;177:113–116. doi: 10.1007/s002030100351. [DOI] [PubMed] [Google Scholar]
- 19.Lobanov AV, Delgado C, Rahlfs S, Novoselov SV, Kryukov GV, Gromer S, Hatfield DL, Becker K, Gladyshev VN. Nucleic acids research. 2006;34:496–505. doi: 10.1093/nar/gkj450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly CP, LaMont JT. Annual review of medicine. 1998;49:375–390. doi: 10.1146/annurev.med.49.1.375. [DOI] [PubMed] [Google Scholar]
- 21.Kyne L, Hamel MB, Polavaram R, Kelly CP. Clin Infect Dis. 2002;34:346–353. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 22.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr., Kazakova SV, Sambol SP, Johnson S, Gerding DN. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 23.Redelings MD, Sorvillo F, Mascola L. Emerging infectious diseases. 2007;13:1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourgault AM, Lamothe F, Loo VG, Poirier L. Antimicrob Agents Chemother. 2006;50:3473–3475. doi: 10.1128/AAC.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. The American journal of gastroenterology. 2007;102:2781–2788. doi: 10.1111/j.1572-0241.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 26.Gerding DN. Infect Control Hosp Epidemiol. 2007;28:113–115. doi: 10.1086/512550. [DOI] [PubMed] [Google Scholar]
- 27.Klayman DL, Griffin TS. 1973:197–199. [Google Scholar]
- 28.Mahoney WC, Hermodson MA. The Journal of biological chemistry. 1980;255:11199–11203. [PubMed] [Google Scholar]
- 29.Apffel A, Fischer S, Goldberg G, Goodley PC, Kuhlmann FE. Journal of chromatography. 1995;712:177–190. doi: 10.1016/0021-9673(95)00175-m. [DOI] [PubMed] [Google Scholar]
- 30.Schneider D, Schuster O, Schmidbaur H. Dalton Transactions. 2005:1940–1947. doi: 10.1039/b502861b. [DOI] [PubMed] [Google Scholar]
- 31.Yang GA, Raptis RG. Inorganica Chimica Acta. 2003;352:98–104. [Google Scholar]
- 32.Freisinger E, Schimanski A, Lippert B. Journal of Biological Inorganic Chemistry. 2001;6:378–389. doi: 10.1007/s007750100212. [DOI] [PubMed] [Google Scholar]
- 33.Bryce RA, Charnock JM, Pattrick RAD, Lennie AR. J. Phys. Chem. A. 2003;107:2516–2523. [Google Scholar]
- 34.Ruben HZ A, Faltens MO, Templeton DH. Inorganic Chemistry. 1974;13:1836. [Google Scholar]
- 35.Moreno MS, Jorissen K, Rehr JJ. Micron. 2007;38:1–11. doi: 10.1016/j.micron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Bradford MM. Analytical biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 37.Gladyshev VN, Khangulov SV, Axley MJ, Stadtman TC. Proc Natl Acad Sci U S A. 1994;91:7708–7711. doi: 10.1073/pnas.91.16.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stickland LH. The Biochemical journal. 1934;28:1746–1759. doi: 10.1042/bj0281746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stickland LH. The Biochemical journal. 1935;29:288–290. doi: 10.1042/bj0290288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stadtman TC. Methods Enzymol. 1978;53:373–382. doi: 10.1016/s0076-6879(78)53043-7. [DOI] [PubMed] [Google Scholar]
- 41.Sliwkowski MX, Stadtman TC. Proc Natl Acad Sci U S A. 1988;85:368–371. doi: 10.1073/pnas.85.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lovitt RW, Kell DD, Morris JG. FEMS Microbiol Lett. 1986;36:269–273. [Google Scholar]
- 43.Andreesen JR. Current opinion in chemical biology. 2004;8:454–461. doi: 10.1016/j.cbpa.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Stadtman TC. Annu Rev Biochem. 1996;65:83–100. doi: 10.1146/annurev.bi.65.070196.000503. [DOI] [PubMed] [Google Scholar]
- 45.Yoo MH, Xu XM, Carlson BA, Gladyshev VN, Hatfield DL. The Journal of biological chemistry. 2006;281:13005–13008. doi: 10.1074/jbc.C600012200. [DOI] [PubMed] [Google Scholar]
- 46.Yoo MH, Xu XM, Carlson BA, Patterson AD, Gladyshev VN, Hatfield DL. PLoS ONE. 2007;2:e1112. doi: 10.1371/journal.pone.0001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redelings MD, Sorvillo F, Mascola L. Emerging infectious diseases. 2007 doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, Hamill RJ. Clin Infect Dis. 2005;40:1586–1590. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 49.Gregus Z, Gyurasics A, Csanaky I. Toxicol Sci. 2000;57:22–31. doi: 10.1093/toxsci/57.1.22. [DOI] [PubMed] [Google Scholar]
- 50.Eikens W, Kienitz C, Jones PG, Thone C. Journal of the Chemical Society-Dalton Transactions. 1994:83–90. [Google Scholar]
- 51.Coplen TB, Bohlke JK, De Bievre P, Ding T, Holden NE, Hopple JA, Krouse HR, Lamberty A, Peiser HS, Revesz K, Rieder SE, Rosman KJR, Roth E, Taylor PDP, Vocke RD, Xiao YK. Pure and Applied Chemistry. 2002;74:1987–2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.