Abstract
PTH-stimulated intracellular signaling is regulated by the cytoplasmic adaptor molecule β-arrestin. We reported that the response of cancellous bone to intermittent PTH is reduced in β-arrestin2−/− mice and suggested that β-arrestins could influence the bone mineral balance by controlling RANKL and osteoprotegerin (OPG) gene expression. Here, we study the role of β-arrestin2 on the in vitro development and activity of bone marrow (BM) osteoclasts (OCs) and Ephrins ligand (Efn), and receptor (Eph) mRNA levels in bone in response to PTH and the changes of bone microarchitecture in wildtype (WT) and β-arrestin2−/− mice in models of bone remodeling: a low calcium diet (LoCa) and ovariectomy (OVX). The number of PTH-stimulated OCs was higher in BM cultures from β-arrestin2−/− compared with WT, because of a higher RANKL/OPG mRNA and protein ratio, without directly influencing osteoclast activity. In vivo, high PTH levels induced by LoCa led to greater changes in TRACP5b levels in β-arrestin2−/− compared with WT. LoCa caused a loss of BMD and bone microarchitecture, which was most prominent in β-arrestin2−/−. PTH downregulated Efn and Eph genes in β-arrestin2−/−, but not WT. After OVX, vertebral trabecular bone volume fraction and trabecular number were lower in β-arrestin2−/− compared with WT. Histomorphometry showed that OC number was higher in OVX-β-arrestin2−/− compared with WT. These results indicate that β-arrestin2 inhibits osteoclastogenesis in vitro, which resulted in decreased bone resorption in vivo by regulating RANKL/OPG production and ephrins mRNAs. As such, β-arrestins should be considered an important mechanism for the control of bone remodeling in response to PTH and estrogen deprivation.
Key words: β-arrestin2, RANKL, ephrin, low calcium diet, bone remodeling
INTRODUCTION
Bone remodeling is a process that occurs continuously throughout life to normally maintain bone structure and calcium homeostasis. Osteoclasts initiate the remodeling cycle by resorbing a portion of bone, and this is followed by new bone formation by osteoblasts. In postmenopausal women, declining estrogen and concomitantly increasing follicle-stimulating hormone (FSH) levels result in an expanded remodeling space and in a greater bone resorption than bone formation activity therein, leading to rapid bone loss.(1) PTH and vitamin D metabolites also play important roles in the regulation of bone remodeling. PTH influences bone formation and resorption with net effects depending on the mode of administration and/or pharmacodynamic profile of the hormone analog.(2,3) Secondary hyperparathyroidism after vitamin D insufficiency contributes to a negative bone remodeling balance with aging.(4) Hormonal effects on bone remodeling are locally mediated by a number of growth factors and cytokines. Among them, RANKL, produced by osteoblasts and T lymphocytes, binds to RANK at the surface of the osteoclast and is an indispensable factor for osteoclastogenesis and osteoclast activation. Osteoprotegerin (OPG), produced by cells of the osteoblastic lineage, acts as a decoy receptor and inhibits RANKL effects on osteoclasts.(5) Increased RANKL expression is the common determinant of increased bone turnover in both estrogen depletion(6) and high PTH.(7–9) Other mediators recently described are the ephrins (Efn), expressed at the surface of the osteoclasts, and the ephrin receptors (Eph), present on osteoblasts. The specific binding of EfnB2 to EphB4 induced osteoblast differentiation and reciprocal inhibition of osteoclast differentiation.(10) Recently, it has been shown that EphB2 expression was regulated by PTH in vitro.(11) Mediation of hormonal effects on bone turnover by local factors implies that molecular mechanisms regulating downstream signaling of hormone receptors in bone cells are also crucial to maintain the bone mineral balance.
β-arrestins are adaptor/scaffold proteins that regulate intracellular signaling by G-protein–coupled receptors (GPCRs), including the PTH/PTH-related protein (PTHrP) receptor,(12–15) a typical seven-transmembrane receptors (7TMRs) such as Frizzled-4, and smoothened (Smo),(16,17) and by non-7TMRs such as IGF-1 receptor tyrosine kinase and TGFβ receptors.(18) Accordingly β-arrestins are important in mediating a variety of physiological and pathophysiological processes, including cell proliferation, differentiation or survival, chemotaxis, and metastasization.(19) Moreover, the absence of β-arrestin2 enhances morphine analgesia in mice,(20,21) whereas overexpression of β-arrestin2 produces the opposite,(22) and β-arrestin2−/− mice develop allergic asthma.(23)
We previously reported that β-arrestin2−/− mice have a decreased trabecular bone response to intermittent PTH in males (i.e., in the presence of relatively low estrogen levels).(24) In contrast, in intact female β-arrestin2−/− mice, the trabecular bone response was maintained, whereas the cortical bone response to intermittent PTH was enhanced.(25) These findings are consistent with a sustained cAMP signaling and PTH activity observed in the absence of PTH/PTHrP receptor interaction with β-arrestins.(12,24) Hence, lack of β-arrestins resulted in an alteration of PTH-stimulated osteoblasts activity, characterized by a lower OPG/RANKL mRNA expression ratio, leading to endosteal bone resorption,(24) but also by a higher bone formation at the periosteum.(25) To further elucidate the role of β-arrestins on the bone remodeling process, here we directly investigate the influence of β-arrestins on osteoclastogenesis in vitro. Furthermore, we evaluated the changes of bone microarchitecture in wildtype (WT) and β-arrestin2−/− mice using three models of PTH-dependent and -independent bone remodeling (i.e., a low calcium diet, PTH, and ovariectomy). Of note, bone remodeling in these conditions results in somewhat differentiated effects on trabecular and cortical bone compartments, with a prominent loss of trabeculae in the absence of estrogens,(26) whereas secondary hyperparathyroidism predominantly affects cortical bone.(27) We further show that these compartment-specific effects are enhanced in the absence of β-arrestin2 and may be explained by excessive osteoclastogenesis and bone resorption, involving increased RANKL/OPG production and decreased ephrins gene expression.
MATERIALS and METHODS
Animals and experimental procedures
β-arrestin2−/− and wildtype (WT) mice were kindly provided by Dr. R. Lefkovitz(20) and were subsequently backcrossed for six generations onto a C57Bl/6J background. Mice had access to mouse chow (RM3; SDS) and water ad libitum, unless noted otherwise. All animal protocols were approved by the competent Ethical Committees of the University of L'Aquila (primary cell preparations), the University of Geneva School of Medicine and State of Geneva Veterinarian Office (ovariectomy and continuous PTH experiments), and the IACUC of Beth Israel Deaconess Medical Center (low calcium diet experiments).
Primary osteoclastic cell cultures
Differentiated primary osteoclasts were obtained from the bone marrow (BM) of 5- to 7-day-old β-arrestin2−/− and WT mice by a modification of the method described by David et al.(28,29) Pups were killed by cervical dislocation, and long bones were dissected free from soft tissues and cut into small fragments. BM cells were released by gently pipetting the fragments in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 10% FBS (Invitrogen). Cells were plated in culture dishes and allowed to attach for 24 h before nonadherent cells were removed by aspiration and extensive washing. In parallel, cells were plated on bone slices (Pantec, Torino, Italy). The total adherent cell fraction was cultured up to 7 days in the presence of 10−8 M 1,25(OH)2D3 (Roche Diagnostics, Basel, Switzerland). A subgroup of cultures was not cultured in the presence of 10−8 M 1,25(OH)2D3 but was instead treated with 10−7 M PTH(1-34) (Sigma-Aldrich, St. Louis, MO, USA), and osteoclastogenesis proceeded as described.
For selected experiments, macrophages were purified from the BM by centrifugation on Ficoll and differentiated into osteoclasts by incubation for 10 days with 50 ng/ml recombinant human macrophage-colony stimulating factor (M-CSF; Preprotech, Rocky Hill, NJ, USA) and 1–120 ng/ml recombinant human RANKL (Preprotech).
Cultures were fixed with 3% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.2), and TRACP activity was detected histochemically using Sigma-Aldrich kit no. 387A (Sigma-Aldrich), according to the manufacturer's instructions. Osteoclasts were also differentiated as described on bone slices and fixed in 3% paraformaldehyde in 0.1 M cacodylate buffer. Cells were removed by ultrasonication in 1% sodium hypochlorite, and slices were stained with 0.1% toluidine blue. Pits were counted and grouped in three visual categories: small (diameter < 10 μm), medium (diameter, 10–30 μm), and large (diameter > 30 μm) with the aid of a graduated eyepiece. The number of pits per each category was scored by multiplying by 0.3 for the small pits, by 1 for the medium pits, and by 3 for the large pits. The sum of the three scores gave the pit index.(30)
Primary osteoblast cell cultures
Calvariae were removed from 5- to 7-day-old β-arrestin2−/− and WT mice, cleaned free of soft tissues, and digested three times with 1 mg/ml Clostridium histolyticum type IV collagenase (Sigma-Aldrich) and 0.25% trypsin (Beckton-Dickinson, Sparks, MD, USA), for 20 min at 37°C with gentle agitation. Cells from the second and third digestions were plated and grown in standard conditions, in DMEM plus 10% FBS. At confluence, cells were trypsinized by standard procedures and plated according to the experimental protocol. These cells expressed the osteoblast markers alkaline phosphatase, Runx-2, PTH/PTHrP receptor, type I collagen, and osteocalcin.(31)
β-arrestin2 and RANK-L RNA silencing
Osteoblasts obtained as above were grown in standard conditions (DMEM + 10% FBS) until confluence, and then cells were trypsinized and transfected with 2 μg siRNAs for β-arrestin 2 or for RANKL (M-041022–00 and M-050280–00; Thermo Scientific Dharmacon, Chicago, IL, USA) or with control siRNA (nontargeting pool, D-001206-13; Thermo Scientific Dharmacon). Transfections were carried out using a “nucleofector kit” (Basic Nucleofector kit, VPI-1003; Amaxa Biosystems, Gaitherburg, MD, USA) in the “AMAXA nucleofector device” according to the manufacturer's instructions (model II S; Amaxa Biosystems).
RT-PCR and real-time RT-PCR
From cells, total RNA was extracted using the Trizol procedure (Invitrogen). One microgram of RNA was reverse transcribed using M-MLV reverse transcriptase, and the equivalent of 0.1 μg was used for the PCR reactions. For the comparative real-time PCR, the Brilliant SYBR green QPCR master mix (Stratagene, Cedar Creek, TX, USA) was used. PCR conditions and primer pairs (Invitrogen) used were as follows:
rankl: Fw 5′-CCAAGATCTCTAACATGACG-3′; Rv 5′-CACCATCAGCTGAAGATAGT-3′; 40 cycles: 95°C for 45 s, 58°C for 45 s, 72°C for 45 s
opg: Fw 5′-AGTCCGTGAAGCAGGAGT-3′; Rv 5′-CCATCTGGACATTTTTTGCAAA-3′; 40 cycles: 95°C for 45 s, 55°C for 45 s, 72°C for 45 s
GAPDH: Fw 5′-CACCATGGAGAAGGCCGGGG-3′; Rv 5′-GACGGACACATTGGGGGTAG-3′ ; 40 cycles: 95°C for 45 s, 60°C for 45 s, 72°C for 45 s
β-arrestin2: Fw 5′-GCACCATGGGAGAAAAACC-3′; Rv 5′-CTGGTAGGTGGCGATGAAC-3′; 40 cycles: 95°C for 45 s, 60°C for 45 s, 72°C for 45 s
Analysis of the real-time data was performed using MxProQPCR software (Stratagene). Briefly, mRNA expression from each gene of interest was normalized for the housekeeping gene Gapdh using CT (cycle threshold) values. Normalized data were expressed as fold increase or decrease versus the control, whose value was fixed at 1.
From bone tissue, total RNA was extracted from the femur diaphysis, after flushing of the BM. The diaphysis was homogenized in pegGOLD Trifast (peQLab Biotechnologies, Erlangen, Germany) and purified on mini-columns (RNeasy Mini kit; Qiagen, Hombrechtikon, Switzerland) in combination with a deoxyribonuclease treatment (RNase-free DNase Set; Qiagen). Single-stranded cDNA templates for quantitative real-time PCR analyses were carried out using SuperScript II Reverse Transcriptase (Invitrogen, Basel, Switzerland) following the manufacturer's instructions. Quantitative RT-PCR was performed using predesigned TaqMan Gene Expression Assays, made of two unlabeled primers and a FAM dye-labeled TaqMan MGB probe, and the corresponding TaqMan Universal PCR Master MIX (Applied Biosystems, Rotkreuz, Switzerland). cDNA was PCR amplified in a 7900HT SDS System, and raw threshold-cycle (Ct) values were obtained from SDS 2.0 software (Applied Biosystems). Relative quantities (RQs) were calculated with the formula RQ = E − Ct using an efficiency (E) of 2 by default. For each gene, the highest quantity was arbitrarily designed a value of 1.0. The mean quantity was calculated from triplicates for each sample, and this quantity was normalized to the similarly measured mean quantity of the β2-microglobulin gene. Normalized quantities were averaged from three to four animals.
Determination of soluble RANKL, OPG, and interleukin-6 in conditioned media by ELISA
Soluble RANKL, OPG, and interleukin (IL)-6 were quantified in conditioned media using the R&D System ELISA kits MTR00, MOP00, and M6000B, respectively, according to the manufacturer's instructions (R&D System, Minneapolis, MN, USA).
In vivo studies
Ovariectomy experiment:
Fifteen-week-old WT and β-arrestin2−/− mice were ovariectomized (OVX) or not (Sham) under ketamine (120 mg/kg)/xylazine (16 mg/kg) anesthesia (Ketasol 50; Dr Graub, Bern, Switzerland and Rompun 2%; Provet, Lyssach, Switzerland, respectively). OVX mice were allowed to lose bone for 8 wk and were pair-fed to the sham group.
Low Ca diet experiment:
Sixteen-week-old male WT and β-arrestin2−/− mice, which had been raised on identical diets, were switched to one of two experimental diets that differed only in calcium content (Harlan Teklad, Madison, WI, USA): low calcium (0.02% Ca) or control (0.6% Ca). Mice were maintained on the diet for 4 wk.
PTH treatment:
Sixteen-week-old female WT and β-arrestin2−/− mice were treated for 7 days with hPTH(1-34) (80 μg/kg/d; Bachem, Bubendorf, Switzerland) continuously by osmotic minipumps (Alzet pump 1007D; Charles River Laboratories, L'Arbresle, France), intermittently with daily subcutaneous injections of hPTH(1-34) (80 μg/kg/d) or vehicle.
BMD and bone morphology
We evaluated total body, spine, and femoral BMD (g/cm2) in vivo using DXA (PIXImus; GE-Lunar, Madison, WI, USA). We assessed trabecular and cortical bone architecture using μCT (μCT40; Scanco Medical, Basserdorf, Switzerland), using a 12-μm isotropic voxel size. Specifically, trabecular bone architecture was evaluated at the fifth lumbar vertebra and distal femoral metaphysis, whereas cortical bone morphology was evaluated at the femoral midshaft, as previously described.(24,25) Morphometric parameters, including bone volume fraction (BV/TV, %), trabecular number (TbN, mm−1), trabecular thickness (TbTh, μm), and trabecular separation (TbSp, μm) were computed without assumptions regarding the underlying bone architecture.(32) At the femoral midshaft, 50 transverse CT slices were obtained and used to compute the total area (TA, mm2), cortical bone area (BA, mm2), medullary area (MA, mm2), cortical thickness (CortTh, μm), and bone area fraction (BA/TA, %).
Biochemical determinations
Serum TRACP5b, osteocalcin, and PTH were measured according to manufacturers' instructions (SBA Sciences, Turku, Finland; Biomedical Technologies, Stoughton, MA, USA; and Immunotopics, San Clemente, CA, USA, respectively).
Histology
Femurs were embedded in methylmethacrylate (Merck), and 5-μm-thick sagittal sections were cut with a Polycut E microtome (Leica Microsystems, Glattbrugg, Switzerland) and were stained with modified Goldner's trichrome. Histomorphometry measurements were performed on the secondary spongiosa of the distal femur metaphysis using a Leica Q image analyzer at ×40 magnification, where osteoclast and osteoblast numbers were counted and expressed according to standard formulas and nomenclature.(33)
Statistical analysis
A two-factor ANOVA was used to assess the effect of low calcium diet, OVX, or PTH and β-arrestin2 deficiency on skeletal morphology or gene expression. As appropriate, posthoc testing was performed using Fisher's protected least squares difference (PLSD). All tests were two-tailed, with differences considered significant at p < 0.05. Data are presented as mean ± SE, unless otherwise noted.
RESULTS
Increased osteoclastogenesis from BM cultures of β-arrestin2−/− mice
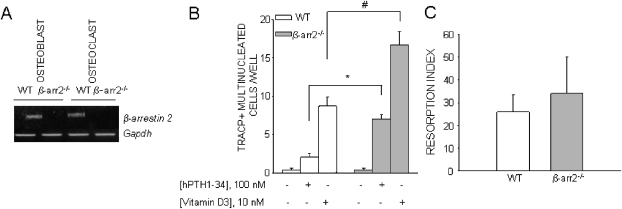
We first studied the role of β-arrestin2 on osteoclast development by comparing in vitro BM cultures of β-arrestin2−/− and WT mice in plastic dishes. We have previously reported that β-arrestin1 gene expression was not overexpressed in β-arrestin2−/− cells.(24) We first confirmed that β-arrestin2 was expressed in WT osteoclasts (Fig. 1A). Whereas osteoclastogenesis was similar in cultures from β-arrestin2−/− compared with WT mice, as judged by the number of TRACP+ multinucleated cells, stimulation by PTH and vitamin D3 led to significantly more osteoclasts in β-arrestin2−/− compared with WT, vitamin D3 being more potent than PTH (Fig. 1B). Of note, a nonsignificant trend for larger β-arrestin2−/− osteoclasts compared with WT was noticed (nuclei/cell in WT: 6 ± 0.8, nuclei/cell in β-arrestin2−/−: 9 ± 0.9, number of evaluated cells/genotype = 10, p = nonsignificant). Furthermore, bone resorption assays showed no change in bone resorption index in β-arrestin2−/− osteoclast cultures relative to WT cultures treated with PTH (Fig. 1C), indicating that the major effect of PTH is on osteoclast differentiation rather than on osteoclast activity. Similar results were obtained from marrow cultures of β-arrestin2−/− and WT mice cultured on bone slices (data not shown).
FIG. 1.
Osteoclastogenesis in WT and β-arrestin2−/− BM co-cultures. (A) RNA was extracted from WT and β-arrestin2−/− calvarial osteoblasts and osteoclasts differentiated from purified BM macrophages in the presence of M-CSF and RANKL and subjected to RT-PCR. (B) BM cells from WT and β-arrestin2−/− mice were cultured for 7 days in 96-well culture dishes in the presence of hPTH(1-34) or 1,25(OH)2D3. At the end of incubation, cultures were fixed and histochemically stained to detect TRACP activity, and TRACP+ multinucleated cells were counted. Results are the mean ± SE of three independent experiments. *p = 0.004; # p = 0.02. (C) BM cells from WT and β-arrestin2−/− mice were differentiated for 7 days plated in the presence of 100 nM PTH. At the end of incubation, cells were trypsinized and replated in equal number on bone slices to allow osteoclast adhesion and resorption. Cells were removed and stained with toluidine blue. Resorption pits were counted, and the pit index was evaluated. Results are the mean ± SE of three independent experiments.
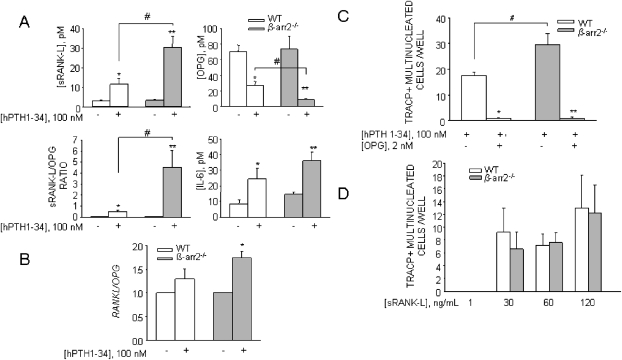
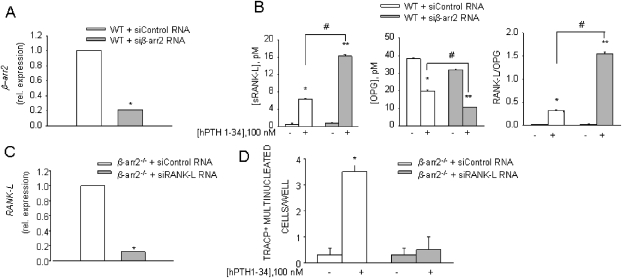
BM cultures contain osteoclast precursors and stromal cells as a source of osteoclastogenic cytokines. To assess whether the enhanced osteoclastogenesis from β-arrestin2−/− mice was secondary to an increased expression or production of critical cytokines or resulted from some intrinsic property of the osteoclast precursors themselves, we measured RANKL in the conditioned media of PTH-treated cultures. In these conditions, significantly more RANKL protein was released in β-arrestin2−/− compared with WT BM cultures, whereas OPG level was decreased, thus resulting in a higher RANKL/OPG ratio (Fig. 2A). IL-6, another important mediator of bone turnover, was also increased by PTH, although not significantly in both β-arrestin2−/− and WT osteoclast cultures (Fig. 2A). Real-time RT-PCR of RNAs from primary osteoblasts further showed a significant increase of RANKL mRNA in β-arrestin2−/− cultures, leading to a significant increment of the RANKL/OPG mRNA ratio in β-arrestin2−/− mice compared with WT (Fig. 2B). To further confirm that these effects were caused by the absence of β-arrestin2, we silenced β-arrestin2 in WT osteoblast cultures. Real-time PCR showed a decrease of β-arrestin2 mRNA by 80% relative to osteoblasts treated with control siRNA (Fig. 3A). In these conditions, sRANKL release was increased, whereas OPG release was decreased, resulting in a higher RANKL/OPG ratio. These data reproduced the results observed in β-arrestin2−/− mice (Fig. 3B).
FIG. 2.
(A) BM cells from WT and β-arrestin2−/− mice were cultured for 7 days in the presence of 100 nM hPTH(1-34). Conditioned media were harvested, and RANKL, OPG, and IL-6 proteins were detected by ELISA. *p < 0.05, **p < 0.001, # p < 0.005. (B) Osteoblasts from WT mice were cultured in standard conditions and treated with 100 nM PTH. After 7 days of treatment, RNA was extracted, reverse transcribed, and subjected to real-time RT-PCR for the indicated genes. Results were expressed as fold-increase of β-arrestin2−/− vs. WT. *p < 0.05. (C) Osteoclastogenesis in WT and β-arrestin2−/− BM co-cultures with or without OPG. BM cells from WT and β-arrestin2−/− mice were cultured for 7 days in 96-well cultures dishes in the presence of 100 nM PTH with or without excess (2 nM) OPG. At the end of the incubation, cultures were fixed and histochemically stained to detect TRACP activity, and TRACP+ multinucleated cells were counted. *p = 0.0001 compared with WT without OPG, **p = 0.0007 compared with β-arrestin2−/− without OPG, # p = 0.04. (D) BM macrophages from WT and β-arrestin2−/− mice were purified by centrifugation on Ficoll and treated with 50 ng/ml M-CSF and the indicated concentrations of RANKL for 10 days. At the end of incubation, osteoclast formation was assessed as described in Fig. 1B. Results are the mean ± SE of three independent experiments.
FIG. 3.
Silencing of β-arrestin2 in WT osteoblast and RANKL in β-arrestin2−/− osteoblast cultures. (A) Osteoblasts from WT mice were cultured in standard conditions until confluence, trypsinized, and nucleofected with siRNA for β-arrestin2 or with control siRNA. After 48 h, RNA was extracted, reverse transcribed, and subjected to real-time RT-PCR. Results are the mean ± SE of three independent experiments. *p = 0.0001. (B) Conditioned media were harvested from siRNA-treated WT osteoblasts, treated or not with PTH, and RANKL and OPG proteins were detected by ELISA. *p = 0.001 compared with WT + siControl RNA without PTH, **p = 0.001 compared with WT + siβarr RNA without PTH, #p = 0.005. (C) Osteoblasts from β-arrestin2−/− mice were cultured in standard conditions until confluence, trypsinized, and nucleofected with siRNA for RANKL or with control siRNA. After 48 h, RNA was extracted, reverse transcribed, and subjected to real-time RT-PCR. Results are the mean ± SE of three independent experiments. *p = 0.0001. (D) Osteoblasts from β-arrestin2−/− mice were cultured in standard conditions until confluence, trypsinized, and nucleofected with siRNA for RANKL or with control siRNA, and then co-cultured with WT monocytes (previously treated with 50 ng/ml M-CSF for 4 days) in the presence of 100 nM hPTH(1-34). After 48 h of co-culture, osteoclast formation was assessed. Results are the mean ± SE of three independent experiments. *p = 0.0001.
To show that increased RANKL/OPG ratio in the β-arrestin2−/− cultures was responsible for increased osteoclastogenesis, PTH-stimulated BM cultures were further treated with an excess of OPG. Figure 2C shows a decrease in osteoclast formation in β-arrestin2−/− cultures in these conditions, which became not different from WT, indicating that osteoclastogenesis in β-arrestin2−/− BM cultures was indeed secondary to an increase in RANKL availability. To confirm these data, β-arrestin2−/− cultures were transfected with siRNA against RANKL, which produced a decrease of 90% of RANKL mRNA (Fig. 3C). Stimulation by PTH did not increase osteoclastogenesis in these cells (Fig. 3D), replicating the data obtained with an excess of OPG. Recently, it has been shown that β-arrestin2 inhibits NF-κB.(34) Because RANK is the receptor activator of NF-κB, and β-arrestin2 was expressed in WT osteoclasts (Fig. 1A), this raised the possibility that RANK activity in osteoclasts could be altered in β-arrestin2−/− cells. To test this hypothesis, BM macrophages were purified and induced to differentiate into osteoclasts by treatment with M-CSF and increasing concentrations of RANKL. As shown in Fig. 2D, β-arrestin2−/− and WT cultures produced similar numbers of osteoclasts at all RANKL concentrations tested. Altogether, these data indicate that increased osteoclastogenesis is caused by increased RANKL gene expression and altered RANKL versus OPG concentrations in the BM environment in β-arrestin2−/− mice but not to an altered response to RANKL.
Effects of low calcium diet on bone microarchitecture and bone turnover in β-arrestin2−/− mice
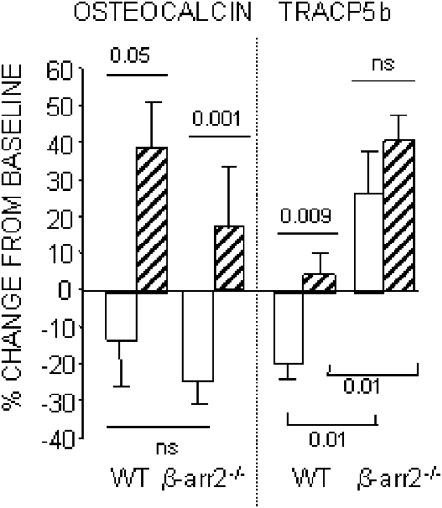
To assess the implication of an increase in RANKL-mediated osteoclastic response in the absence of β-arrestin2 in vivo, we placed adult male β-arrestin2−/− and WT mice on a low calcium diet (Ca 0.02%) for 4 wk. Compared with regular diet (Ca 0.6%), low calcium diet dramatically increased PTH levels in both WT and β-arrestin2−/− (p diet = 0.017; Table 1). In WT, serum osteocalcin and TRACP5b did not change (−13% and −19%, respectively) with regular diet during the 4 wk of the experiment, whereas in β-arrestin2−/− mice, osteocalcin significantly decreased (−24%), but TRACP5b significantly increased (+41%; Table 1). A low calcium diet significantly increased osteocalcin in WT and β-arrestin2−/− (p diet < 0.001; Table 1) and maintained TRACP5b baseline levels in the former. In β-arrestin2−/−, however, low calcium diet increased TRACP5b significantly more than in WT (Table 1; Fig. 4).
Table 1.
Effect of Low Calcium Diet on Bone Biochemical Markers in WT and β-Arrestin2−/− Mice
|
WT |
β-arrestin2−/− |
|||
| Control (n = 8) | Low Ca (n = 8) | Control (n = 9) | Low Ca (n = 9) | |
| Osteocalcin (ng/ml) | ||||
| Baseline | 140 ± 12 | 145 ± 8 | 138 ± 11 | 102 ± 9 |
| 4 wk | 114 ± 8 | 198 ± 15* | 100 ± 4* | 118 ± 10* |
| TRACP5b (U/liter) | ||||
| Baseline | 2.83 ± 0.16 | 2.95 ± 0.19 | 2.14 ± 0.18 | 1.79 ± 0.08 |
| 4 wk | 2.32 ± 0.13 | 3.05 ± 0.15 | 2.59 ± 0.12* | 2.50 ± 0.13* |
| PTH (pg/ml) | ||||
| Baseline | ND | 19.6 ± 8.9 | ND | 22.5 ± 9.9 |
| 4 wk | ND | 367.1 ± 160.8 | ND | 150.9 ± 74.6 |
* p < 0.05 by paired t-test.
ND, not determined.
FIG. 4.
Effects of low calcium diet on bone biochemical markers in WT and β-arrestin2−/− mice. Mice were fed either a control diet (plain bar) or a low calcium diet (hatched bar) for 4 wk.
In these conditions of secondary hyperparathyroidism and increased bone turnover, total body BMD decreased in β-arrestin2−/− and WT, but the difference in response between WT and β-arrestin2−/− did not reach statistical significance (Table 2). Similar patterns were seen for changes in spine and femur BMD by DXA. Low Ca diet deteriorated trabecular architecture so that bone volume fraction (BV/TV) and TbN were significantly decreased and TbSp was significantly increased in the distal femur of WT and β-arrestin2−/−mice. However, at the lumbar spine, loss of TbN and increase in TbSp reached significance in β-arrestin2−/− but not WT mice. At the femoral midshaft, cortical bone area, bone area fraction (BA/TA), and cortical thickness were significantly decreased in β-arrestin2−/− fed with the low calcium diet but not in WT mice (Table 2). However, there was no difference between the two genotypes fed the low calcium diet.
Table 2.
Effect of Low Calcium Diet on Skeletal Characteristics of WT and β-Arrestin2−/− Mice
|
WT |
β-arrestin2−/− |
|||
| Control (n = 9) | Low Ca (n = 8) | Control (n = 8) | Low Ca (n = 9)* | |
| BMD change (%) | ||||
| Total body | 4.0 ± 1.4 | −3.3 ± 1.2† | 2.7 ± 0.8 | −6.0 ± 1.1† |
| Lumbar spine | −2.1 ± 3.3 | −23.3 ± 4.9† | −2.9 ± 3.0 | −24.9 ± 3.3† |
| Femoral shaft | 3.8 ± 1.6 | −4.8 ± 2.7‡ | 2.9 ± 1.2 | −3.2 ± 1.2† |
| Vertebral trabecular bone | ||||
| BV/TV (%) | 30.3 ± 0.9 | 26.0 ± 1.3‡ | 29.4 ± 0.9 | 23.9 ± 1.3§ |
| TbTh (μm) | 56.2 ± 1.7 | 55.8 ± 2.2 | 58.1 ± 1.7 | 53.6 ± 1.7 |
| TbN (mm−1) | 5.30 ± 0.08 | 4.97 ± 0.14 | 4.96 ± 0.10¶ | 4.47 ± 0.13‡¶ |
| TbSp (μm) | 175 ± 3 | 190 ± 7 | 188 ± 5¶ | 214 ± 8‡¶ |
| Distal femur trabecular bone | ||||
| BV/TV (%) | 19.7 ± 1.1 | 14.3 ± 1.0† | 18.7 ± 1.2 | 14.2 ± 1.5‡ |
| TbTh (μm) | 56.4 ± 1.7 | 57.5 ± 2.7 | 58.4 ± 1.5 | 56.2 ± 2.0 |
| TbN (mm−1) | 4.70 ± 0.09 | 3.99 ± 0.11† | 4.20 ± 0.08¶ | 3.78 ± 0.08† |
| TbSp (μm) | 200 ± 6 | 243 ± 9† | 228 ± 5¶ | 256 ± 6† |
| Cortical bone (diaphysis) | ||||
| Total area (mm2) | 2.13 ± 0.07 | 2.03 ± 0.08 | 2.07 ± 0.08 | 1.98 ± 0.07 |
| Bone area (mm2) | 0.92 ± 0.05 | 0.85 ± 0.03 | 0.93 ± 0.04 | 0.80 ± 0.04‡ |
| Medullary area (mm2) | 1.22 ± 0.04 | 1.18 ± 0.06 | 1.14 ± 0.05 | 1.18 ± 0.04 |
| BA/TA (%) | 42.8 ± 1.5 | 42.1 ± 1.1 | 45.1 ± 0.5 | 40.5 ± 0.7† |
| Cortical thickness (μm) | 181 ± 7 | 178 ± 4 | 196 ± 4 | 170 ± 4‡ |
* n = 7 for β-arrestin2−/− vertebral specimens.
† p < 0.005, ‡ p < 0.05, and § p < 0.01 vs. control diet within genotype.
¶ p < 0.05 vs. WT within diet group.
BV/TV, bone volume fraction; TbTh, trabecular thickness; TbN, trabecular number; TbSp, trabecular separation; BA/TA, bone area fraction.
These findings indicate that the absence of β-arrestin2 exaggerated the bone remodeling effects of a low calcium diet, and these effects were most prominent in cortical bone, in keeping with known effects of continuous high PTH on this compartment.
Effects of PTH on Eph and Efn gene expression in bone
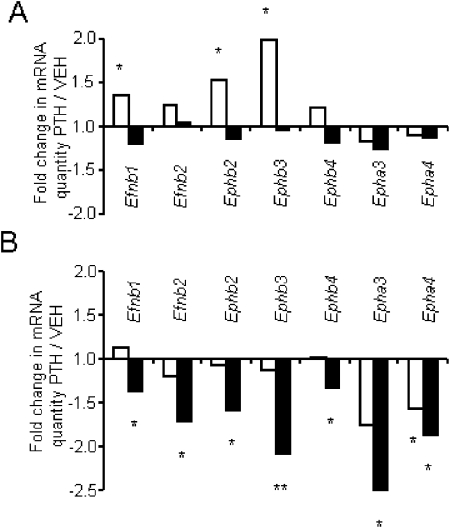
We next hypothesized whether the increased bone remodeling observed in β-arrestin2−/− mice exposed to the low calcium diet/high levels of PTH could be associated with dysregulations of Eph and Efn gene expression. For this purpose, WT and β-arrestin2−/− mice were continuously or intermittently treated for 7 days with PTH at 80 μg/kg/d, and gene expression was assessed on the femur diaphysis. Compared with vehicle, continuous PTH did not modify the gene expression of any Eph and Efn tested in WT mice. In contrast, in β-arrestin2−/− mice, PTH significantly downregulated the expression of all the Eph and Efn measured (Fig. 5). Intermittent PTH significantly increased the gene expression of EfnB1, EphB2, and EphB3 compared with vehicle in WT mice, whereas in β-arrestin2−/− mice, only EphA4 gene expression was decreased by intermittent PTH. These data indicate that the absence of β-arrestin2 decreased the gene expression of Eph and Efn induced by continuous PTH in cortical bone, consistent with a reduction of the bidirectional signaling between osteoblasts and osteoclasts favoring osteoclastogenesis on osteoblastogenesis.
FIG. 5.
Effects of continuous and intermittent PTH on ephrins and ephrin receptors gene expression in bone. The expression of ephrin ligand (Efn) and ephrin receptors (Eph) was determined by quantitative real-time PCR in femur diaphysis of WT (A) and β-arrestin2−/− mice (B), treated with intermittent (open bars) or continuous PTH (plain bars) at 80 μg/kg/d for 7 days. Values are expressed as fold changes mRNA quantity in PTH-treated relative to vehicle-treated mice, normalized for β2-microglobulin expression levels. Bars represent mean ± SE of three to four animals with quantification of each animal performed in triplicate. *p < 0.05; **p < 0.001 by Fisher's PLSD for posthoc analysis.
Effects of ovariectomy on bone morphology and bone turnover in β-arrestin2−/− mice
To test whether the increase in bone remodeling observed in β-arrestin2−/− mice was specifically caused by a low calcium/high PTH or could be induced by other proresorptive conditions, β-arrestin2−/− and WT mice were OVX. After 8 wk, OVX caused significant BMD loss at the total body in WT and at spine in β-arrestin2−/− compared with sham (Table 3).
Table 3.
Bone Mass and Cortical and Trabecular Microarchitecture in OVX β-Arrestin2−/− Mice
|
WT |
β-arrestin2−/− |
|||
| Sham (n = 8) | OVX (n = 7) | Sham (n = 8) | OVX (n = 9) | |
| BMD change (%) | ||||
| Total body | 3.4 ± 1.5 | −0.8 ± 1.1 | 4.6 ± 2.2 | −1.3 ± 1.4* |
| Lumbar spine | 3.3 ± 2.2 | −13.1 ± 2.9† | 2.7 ± 6.7 | −9.0 ± 2.3 |
| Femoral shaft | 11.0 ± 3.4 | 1.5 ± 3.4 | 7.5 ± 1.5 | 2.8 ± 2.2 |
| Vertebral trabecular bone | ||||
| BV/TV (%) | 25.4 ± 0.9 | 23.0 ± 1.3 | 27.0 ± 1.6 | 20.7 ± 1.6* |
| TbTh (μm) | 57.29 ± 1.19 | 53.47 ± 1.01* | 63.6 ± 1.82# | 55.11 ± 2.12* |
| TbN (/mm) | 4.19 ± 0.11 | 4.05 ± 0.10 | 4.07 ± 0.12 | 3.72 ± 0.10*‡ |
| TbSp (μm) | 236.9 ± 7.2 | 246.6 ± 6.6 | 238.5 ± 7.0 | 267.2 ± 7.9* |
| Distal femur trabecular bone | ||||
| BV/TV (%) | 5.17 ± 0.44 | 4.09 ± 0.59 | 4.51 ± 0.40 | 3.00 ± 0.53* |
| TbTh (μm) | 44.78 ± 1.13 | 40.17 ± 1.50* | 45.76 ± 1.18 | 43.96 ± 2.49 |
| TbN (/mm) | 3.19 ± 0.10 | 3.01 ± 0.11 | 3.00 ± 0.13 | 2.31 ± 0.16*§ |
| TbSp (μm) | 312.1 ± 10.6 | 335.5 ± 14.0 | 339.4 ± 19.0 | 462.2 ± 48.4*‡ |
| Cortical bone (diaphysis) | ||||
| Total area (mm2) | 1.62 ± 0.04 | 1.64 ± 0.04 | 1.56 ± 0.03 | 1.56 ± 0.03 |
| Bone area (mm2) | 0.79 ± 0.02 | 0.77 ± 0.01 | 0.78 ± 0.02 | 0.74 ± 0.01 |
| Medullary area (mm2) | 0.83 ± 0.03 | 0.88 ± 0.03 | 0.78 ± 0.02 | 0.82 ± 0.02 |
| BA/TA (%) | 48.8 ± 0.5 | 46.7 ± 0.6* | 49.8 ± 0.7 | 47.5 ± 0.4* |
| Cortical thickness (μm) | 200 ± 2 | 193 ± 2* | 203 ± 4 | 191 ± 2* |
* p < 0.05 and † p < 0.005 vs. Sham within genotype.
‡ p < 0.05 and § p < 0.005 vs. WT within treatment group.
BV/TV, bone volume fraction; TbN, trabecular number; TbTh, trabecular thickness; TbSp, trabecular separation; BA/TA, bone area fraction.
Trabecular microarchitecture was more affected in β-arrestin2−/− than WT at lumbar spine and femoral metaphysis. After OVX, vertebral BV/TV, TbN, and TbTh were decreased and TbSp was increased compared with Sham in β-arrestin2−/− mice, whereas only TbTh was significantly decreased in WT. Furthermore, OVX β-arrestin2−/− mice had significantly less trabeculae (TbN) than OVX WT. Similar observations were made at the distal femur (Table 3). At the femoral midshaft, OVX induced a modest decrease of BA/TA and cortical thickness, but these changes were not different between WT and β-arrestin2−/− mice (Table 3).
Histomorphometry indicated that, after OVX, osteoclast number per bone area (OcN/BA) was significantly higher in β-arrestin2−/− compared with WT (OVX-β-arrestin2−/−: 856.6 ± 149.9; OVX-WT: 298.2 ± 38.8, p = 0.01), whereas no differences were observed in sham mice (β-arrestin2−/−: 541.9 ± 84.2; WT: 388.0 ± 51.9, p = nonsignificant). In contrast, osteoblast cell number per bone area (ObN/BA) was similar in WT and β-arrestin2−/− in the sham (WT: 1022.5 ± 149.1; β-arrestin2−/−: 1012.5 ± 254.4, p = nonsignificant). In the OVX mice, there was a nonsignificant trend for increased OcN/BA in β-arrestin2−/− mice (WT: 968.0 ± 112.2; β-arrestin2−/−: 1733.3 ± 395.5, p = 0.14).
DISCUSSION
By analyzing the skeletal response of β-arrestin2−/− mice to a low calcium diet and OVX, we confirmed and expand our previous findings for a role of β-arrestin2 in the regulation of trabecular and cortical bone remodeling.(24,25) Hence, a low calcium diet produced a more severe cortical bone phenotype in β-arrestin2−/− mice compared with their WT littermates (i.e., a significant decrease of midfemoral cortical bone area and cortical thickness). Estrogen deficiency, on the other hand, affected the cancellous bone of β-arrestin2−/− more prominently than WT mice, with decreased trabecular number and bone volume fraction in the vertebrae and distal femur. These data indicate that the microarchitecture of β-arrestin2−/− mice was more sensitive to hormonal influences and that the specific compartment where these differences become most prominent further depends on the bone remodeling stimulus. These findings may be relevant to the pathophysiology of bone loss in humans, where menopause first causes a disproportionate loss of cancellous bone,(35) whereas hyperparathyroidism causes mostly cortical porosity and thinning.(27)
We report here several lines of evidence to support a direct influence of β-arrestin2 on osteoclast development and bone resorption: (1) TRACP5b was higher in β-arrestin2−/− fed a low calcium diet compared with WT; (2) osteoclast number was increased post-OVX in β-arrestin2−/− versus WT; (3) Efn and Eph gene expression was decreased in cortical bone of β-arrestin2−/−mice; and (4) osteoclastogenesis was enhanced in BM cultures of β-arrestin2−/−mice. Furthermore, PTH-stimulated osteoclastic activity was increased in β-arrestin2−/− cells. Previous studies using mouse stromal cells, osteoblasts, and calvaria organ cultures have shown that bolus or continuous administration of PTH increase RANKL and decrease OPG expression,(7,36,37) whereas exposure to intermittent PTH has opposite effects.(24) In primary osteoblasts from mouse calvariae, moreover, we reported that short exposure to PTH increased OPG mRNA in WT cells but not in β-arrestin2−/− cells.(24,25) Now we show that not only the RANKL/OPG gene expression ratio but also their protein ratio was higher in the conditioned medium of β-arrestin2−/− BM cultures. In turn, prominent osteoclastogenesis in the latter was entirely dependent on this excess of RANKL, because it was completely inhibited by exogenous OPG. However, whether the cleavage of RANKL would be altered in β-arrestin2−/− mice is thus far unknown. RANKL is regulated at the transcriptional levels by protein kinase A (PKA) activators such as PTH. Furthermore, it has recently been shown that cAMP response element-binding proteins (CREB)—mediators of PKA—were present in upstream regulatory elements of the RANKL gene.(38) Thus, it is tempting to speculate that, in the absence of β-arrestin2, accumulating cAMP(14) in response to PTH directly stimulates RANKL gene expression, leading to increased RANKL secretion and increased bone remodeling in β-arrestin2−/− mice fed a low calcium diet.
In contrast, the response of osteoclasts to RANKL itself was not altered. This observation was slightly surprising because not only did we find β-arrestin2 to be expressed in osteoclasts, but it has also been reported that overexpression of β-arrestin2 inhibits NF-κB activity by binding the inhibitor IκBα, although β-arrestin2 deficiency did not increase NF-κB activity.(34) Similar to high PTH, estrogen depletion increases RANKL expression and stimulates RANKL-induced differentiation of osteoclast precursors through an estrogen receptor–dependent mechanism.(6) BM mononuclear cells from postmenopausal women have more RANKL at their surface compared with cells from premenopausal women, and RANKL level is correlated with markers of bone turnover.(9) It remains possible therefore that enhanced RANK-mediated NF-κB activity could partly explain the increased osteoclastic response to estrogen deficiency in β-arrestin2−/− mice.
In conclusion, we showed that β-arrestin2 controls RANKL-dependent osteoclastogenesis in vitro, which results in PTH-dependent and -independent bone resorption decreases in vivo. Furthermore, we provided data indicating that β-arrestin2 is involved in the regulation of the ephrins. These findings provide new insights into the molecular mechanisms underlying the regulation of bone remodeling by systemic hormones and their local mediators.
ACKNOWLEDGMENTS
The authors thank Madeleine Lachize for skillful technical expertise and Fanny Lin and Robert Lefkowitz for providing β-arrestin2–deficient mice. Funding of this project was provided by the EU-supported OSTEOGENE project (Contract LSHM-CT-2003-502941) and NIH Grant AR-049265.
Footnotes
Drs. Capulli, Rucci, Rufo, and Teti serve as consultants for Amgen. All other authors state that they have no conflicts of interest.
REFERENCES
- 1.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 2.Kostenuik PJ, Ferrari S, Pierroz D, Bouxsein M, Morony S, Warmington KS, Adamu S, Geng Z, Grisanti M, Shalhoub V, Martin S, Biddlecome G, Shimamoto G, Boone T, Shen V, Lacey D. Infrequent delivery of a long-acting PTH-Fc fusion protein has potent anabolic effects on cortical and cancellous bone. J Bone Miner Res. 2007;22:1534–1547. doi: 10.1359/jbmr.070616. [DOI] [PubMed] [Google Scholar]
- 3.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: A double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15:60–65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Ledger GA, Burritt MF, Kao PC, O'Fallon WM, Riggs BL, Khosla S. Abnormalities of parathyroid hormone secretion in elderly women that are reversible by short term therapy with 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1994;79:211–216. doi: 10.1210/jcem.79.1.8027229. [DOI] [PubMed] [Google Scholar]
- 5.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, Nissenson RA. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–244. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 8.Stilgren LS, Rettmer E, Eriksen EF, Hegedus L, Beck-Nielsen H, Abrahamsen B. Skeletal changes in osteoprotegerin and receptor activator of nuclear factor-kappab ligand mRNA levels in primary hyperparathyroidism: Effect of parathyroidectomy and association with bone metabolism. Bone. 2004;35:256–265. doi: 10.1016/j.bone.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Allan EH, Hausler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B, Pompolo S, Sims NA, Gillespie MT, Onyia JE, Martin TJ. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23:1170–1181. doi: 10.1359/jbmr.080324. [DOI] [PubMed] [Google Scholar]
- 12.Bisello A, Chorev M, Rosenblatt M, Monticelli L, Mierke DF, Ferrari SL. Selective ligand-induced stabilization of active and desensitized parathyroid hormone type 1 receptor conformations. J Biol Chem. 2002;277:38524–38530. doi: 10.1074/jbc.M202544200. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari SL, Behar V, Chorev M, Rosenblatt M, Bisello A. Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves beta-arrestin2. Real-time monitoring by fluorescence microscopy. J Biol Chem. 1999;274:29968–29975. doi: 10.1074/jbc.274.42.29968. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari SL, Bisello A. Cellular distribution of constitutively active mutant parathyroid hormone (PTH)/PTH-related protein receptors and regulation of cyclic adenosine 3′,5′-monophosphate signaling by beta-arrestin2. Mol Endocrinol. 2001;15:149–163. doi: 10.1210/mend.15.1.0587. [DOI] [PubMed] [Google Scholar]
- 15.Rey A, Manen D, Rizzoli R, Caverzasio J, Ferrari SL. Proline-rich motifs in the parathyroid hormone (PTH)/PTH-related protein receptor C terminus mediate scaffolding of c-Src with beta-arrestin2 for ERK1/2 activation. J Biol Chem. 2006;281:38181–38188. doi: 10.1074/jbc.M606762200. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, Lefkowitz RJ. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science. 2004;306:2257–2260. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Kirkbride KC, How T, Nelson CD, Mo J, Frederick JP, Wang XF, Lefkowitz RJ, Blobe GC. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 19.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: Not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 21.Bradaia A, Berton F, Ferrari S, Luscher C. Beta-arrestin2, interacting with phosphodiesterase 4, regulates synaptic release probability and presynaptic inhibition by opioids. Proc Natl Acad Sci USA. 2005;102:3034–3039. doi: 10.1073/pnas.0406632102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang B, Shi Y, Li H, Kang L, Ma L. Decreased morphine analgesia in rat overexpressing beta-arrestin 2 at periaqueductal gray. Neurosci Lett. 2006;400:150–153. doi: 10.1016/j.neulet.2006.02.071. [DOI] [PubMed] [Google Scholar]
- 23.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrari SL, Pierroz DD, Glatt V, Goddard DS, Bianchi EN, Lin FT, Manen D, Bouxsein ML. Bone response to intermittent parathyroid hormone is altered in mice null for {beta}-Arrestin2. Endocrinology. 2005;146:1854–1862. doi: 10.1210/en.2004-1282. [DOI] [PubMed] [Google Scholar]
- 25.Bouxsein ML, Pierroz DD, Glatt V, Goddard DS, Cavat F, Rizzoli R, Ferrari SL. beta-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J Bone Miner Res. 2005;20:635–643. doi: 10.1359/JBMR.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 27.Lotinun S, Sibonga JD, Turner RT. Differential effects of intermittent and continuous administration of parathyroid hormone on bone histomorphometry and gene expression. Endocrine. 2002;17:29–36. doi: 10.1385/ENDO:17:1:29. [DOI] [PubMed] [Google Scholar]
- 28.David JP, Neff L, Chen Y, Rincon M, Horne WC, Baron R. A new method to isolate large numbers of rabbit osteoclasts and osteoclast-like cells: Application to the characterization of serum response element binding proteins during osteoclast differentiation. J Bone Miner Res. 1998;13:1730–1738. doi: 10.1359/jbmr.1998.13.11.1730. [DOI] [PubMed] [Google Scholar]
- 29.Teti A, Taranta A, Villanova I, Recchia I, Migliaccio S. Osteoclast isolation: New developments and methods. J Bone Miner Res. 1999;14:1251–1252. doi: 10.1359/jbmr.1999.14.7.1251. [DOI] [PubMed] [Google Scholar]
- 30.Caselli G, Mantovanini M, Gandolfi CA, Allegretti M, Fiorentino S, Pellegrini L, Melillo G, Bertini R, Sabbatini W, Anacardio R, Clavenna G, Sciortino G, Teti A. Tartronates: A new generation of drugs affecting bone metabolism. J Bone Miner Res. 1997;12:972–981. doi: 10.1359/jbmr.1997.12.6.972. [DOI] [PubMed] [Google Scholar]
- 31.Marzia M, Sims NA, Voit S, Migliaccio S, Taranta A, Bernardini S, Faraggiana T, Yoneda T, Mundy GR, Boyce BF, Baron R, Teti A. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J Cell Biol. 2000;151:311–320. doi: 10.1083/jcb.151.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hildebrand T, Ruegsegger P. Quantification of Bone Microarchitecture with the Structure Model Index. Comput Methods Biomech Biomed Engin. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- 33.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 34.Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci USA. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borah B, Dufresne TE, Ritman EL, Jorgensen SM, Liu S, Chmielewski PA, Phipps RJ, Zhou X, Sibonga JD, Turner RT. Long-term risedronate treatment normalizes mineralization and continues to preserve trabecular architecture: Sequential triple biopsy studies with micro-computed tomography. Bone. 2006;39:345–352. doi: 10.1016/j.bone.2006.01.161. [DOI] [PubMed] [Google Scholar]
- 36.Fu Q, Jilka RL, Manolagas SC, O'Brien CA. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem. 2002;277:48868–48875. doi: 10.1074/jbc.M208494200. [DOI] [PubMed] [Google Scholar]
- 37.Kondo H, Guo J, Bringhurst FR. Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells. J Bone Miner Res. 2002;17:1667–1679. doi: 10.1359/jbmr.2002.17.9.1667. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Yamazaki M, Shevde NK, Pike JW. Transcriptional control of receptor activator of nuclear factor-kappaB ligand by the protein kinase A activator forskolin and the transmembrane glycoprotein 130-activating cytokine, oncostatin M, is exerted through multiple distal enhancers. Mol Endocrinol. 2007;21:197–214. doi: 10.1210/me.2006-0315. [DOI] [PubMed] [Google Scholar]