Abstract
Islet-associated protein 2 (IA-2) and IA-2β are major autoantigens in type 1 diabetes and transmembrane proteins in dense core secretory vesicles (DCV) of neuroendocrine cells. The deletion of these genes results in a decrease in insulin secretion. The present study was initiated to test the hypothesis that this deletion not only affects the secretion of insulin, but has a more global effect on neuroendocrine secretion that leads to disturbances in behavior and learning. Measurement of neurotransmitters showed that norepinephrine, dopamine and serotonin were significantly decreased in the brain of double knockout (DKO) mice (P< 0.05 to <0.001). In tests evaluating anxiety-like behavior and conditioned-learning, the DKO mice showed a highly significant increase in anxiety-like behavior (P<0.01 to <0.001) and impairment of conditioned learning (P<0.01) as compared to WT mice. The DKO mice also displayed an increase in spontaneous and induced seizures (P<0.01) and age-related death. Contrary to the generally held view that IA-2 and IA-2β are expressed exclusively in DCV, subcellular fractionation studies revealed that IA-2β, but not IA-2, co-purifies with fractions rich in synaptic vesicles (SV), and that the secretion of dopamine, GABA and glutamate from the synaptosomes of the DKO mice was significantly decreased as was the number of SV (P<0.01). Taken together, these findings show that IA-2β is present in both DCV and SV, and that the deletion of IA-2/IA-2β has a global effect on the secretion of neurotransmitters. The impairment of secretion leads to behavioral and learning disturbances, seizures and reduced life span.
Keywords: IA-2, IA-2β, secretory vesicles, behavior
Introduction
IA-2 and IA-2β are major autoantigens in type 1 diabetes (Lan et al., 1994, Lan et al., 1996, Lu et al., 1996). About 70% of newly diagnosed patients have autoantibodies to IA-2 and about 50% have autoantibodies to IA-2β. These autoantibodies appear years before the onset of clinical disease and in combination with autoantibodies to two of the other major type 1 diabetes autoantigens, glutamic acid decarboxylase and insulin, have become predictive markers for this disease (Notkins and Lernmark, 2001, Notkins, 2007). Population screening studies have shown that subjects with autoantibodies to two or more of these major autoantigens are at a 50% or greater risk of developing type 1 diabetes within 5 year and this information is being used to enter subjects into therapeutic intervention trials (Notkins and Lernmark, 2001, Notkins, 2007, Achenbach et al., 2008).
Based on sequence, IA-2 and IA-2β, also known as ICA512 and phogrin, respectively, are members of the transmembrane protein tyrosine phosphatase (PTP) family, but are enzymatically inactive on standard PTP substrates because of two critical amino acid substitutions in the PTP domain (Magistrelli et al., 1996). Both proteins consist of an intracellular, transmembrane and luminal domain. IA-2 is 979 and IA-2β is 986 amino acids in length. Their intracellular domains are 74% identical, whereas their luminal domains are only 26% identical. IA-2 and IA-2β are located, respectively, on chromosomes 2q35 and 7q36, are encoded by 23 exons (Saeki et al., 2000, Kubosaki et al., 2004) and are expressed in neuroendocrine cells (e.g., pancreatic islets, brain) throughout the body (Solimena et al., 1996, Wasmeier and Hutton, 1996, Roberts et al., 2001). Electron microscopic studies revealed that both proteins are transmembrane proteins in DCV.
The mouse homologs of the IA-2 and IA-2β are similar in almost all respects to their human counterparts. At the protein level their intracellular domains are 98% to 99% identical to human IA-2 and IA-2β and in the mouse, the genes are located on chromosomes 1 and 12, respectively (Leiter et al., 1997). Recently, we succeeded in knocking out the individual IA-2 and IA-2β genes and generated double knockout (DKO) mice (Saeki et al., 2002, Kubosaki et al., 2004, Kubosaki et al., 2005). These DKO mice did not develop diabetes but showed abnormal glucose tolerance tests, impaired insulin secretion and a reduction in the insulin content of beta cells. Further studies revealed that the DKO females were infertile due to a decrease in the concentration of lutenizing hormone (LH) and follicular stimulating hormone (FSH) in the pituitary and a decrease in the LH surge that is required for ovulation (Kubosaki et al., 2006). Since IA-2 and IA-2β are present in neuroendocrine cells in the brain and the knockout of these two genes resulted in a decrease in the content and secretion of hormones (i.e., insulin, FSH and LH), the present experiments were initiated to determine whether the deletion of IA-2 and IA-2β also would affect the content and secretion of neurotransmitters and, in turn, result in behavioral disturbances.
Material and Methods
Mice
Targeted disruption of the individual IA-2 and IA-2β genes was described previously (Saeki et al., 2002, Kubosaki et al., 2004, Kubosaki et al., 2006). In the current paper, 8th generation C57BL/6 IA-2+/− mice were mated with 8th generation C57BL/6 IA-2β+/− mice, and the double heterozygous outcomes were interbred to generate: IA-2+/+/ IA-2β+/+; IA-2−/−/IA-2β+/−; and IA-2−/−/ IA-2β−/− mice. IA-2+/+/IA-2β+/+ (WT) mice served as controls. Because female IA-2−/−/IA-2β−/− mice are infertile (Kubosaki et al., 2006), male IA-2−/−/IA-2β−/− mice were bred to female IA-2−/−/IA-2β+/− mice to generate IA-2−/−/IA-2β−/− (DKO) mice. All mice were genotyped before use and each mouse was bred, aged and analyzed in the same facilities according to National Institutes of Health guidelines. Lights were on from 6:00 AM to 6:00 PM and food and water were available ad libitum. Behavioral experiments were generally conducted between 1:00 PM and 5:00 PM and each animal was used only for one experiment. Approximately the same number of male and female was used in each of the behavioral experiments with little or no difference based on gender. Predominantly male mice were used in the learning experiments to avoid endocrine changes related to the estrous cycle. All experimental procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Measurement of neurotransmitters
Whole mouse brains (12-weeks old and 30-weeks old) (except for the cerebellum) were removed, rapidly frozen on dry ice, weighed, and homogenized in a 5 ml of 0.1M perchloric acid. Homogenates were centrifuged for 10 min at 1500 × g, supernatants were filtered through a YM-10 micron centrifugal filter (Millipore, Bedford, MA) and analyzed for norepinephrine (NE), dopamine (DA), serotonin (5-hydroxytryptamine, 5-HT) by HPLC (high-performance liquid chromatography) with electrochemical detection (Ito, 2000, Chen et al., 2005). The HPLC system consisted of a gradient pump (Shimazu SCL-10A and LC-10AD, Shimadzu Scientific Co., Columbia, MD), a detector (SPD-6AV, Shimadzu Scientific Co.) and a Shimazu data processor (CR501, Shimadzu Scientific Co.). Detector potential at the analytical cell was set at +0.4V. HPLC analysis was performed on a C18 Spherisorb reverse phase column (5 μm pore size, 4.6 mm i.d., 25 cm long). The mobile phase consisted of 0.1M 1-octanesulfonic acid sodium salt and 10% acetnitrile. The flow rate was 1 ml/min.
Open field test
An individual mouse (12-weeks old and 30-weeks old) was placed in a novel environment (i.e., a square box measuring 30 × 40 × 30 cm), the floor of which was divided into squares (3 × 4 cm). Animal behavior in the open field (12-16 mice per group) was recorded by videotaping for 5 min and subsequently analyzed. Measurements included exploratory activity (i.e., the time required for a mouse to walk 30 cm), total distance traveled, the frequency of visits to central area of the box, the frequency of rearings and walking speed. For meaningful walking-speed measurements, only uninterrupted walking for >25 cm was used and 3 to 12 measurements were averaged.
The tail suspension test (TST)
The TST test (8-10 mice per group, 4-6 months old) was carried out essentially as described (Steru et al., 1985, Cryan et al., 2004). Pargyline HCl, bupropion HCl and fluoxetine HCl were obtained from Sigma (St. Louis, MO), dissolved in saline and injected intreperitoneally in a volume at 0.2 ml (i.e., fluoxetine 20mg/kg, bupropion 20 mg/kg, pargylene 75 mg/kg). Thirty minutes later after injection, mice were suspended by their tail from a metal rod fixed about 50 cm above the surface of a table covered with soft cloth in a sound-proof room. The tip of the tail was fixed using adhesive tape. The duration of the test was 6 min. The immobility time was determined using a stopwatch and video recorded.
Height-fear task
The height fear task test (12 mice per group, 4-6 months old) was carried out as reported previously, with slight modifications (Dere et al., 2004). This test is based on mice having a natural fear of heights. The natural response of mice placed on an elevated platform is to step down. The latency of this response is positively correlated with the height of the platform. White rectangle platforms of different heights in an open field were used and mice were placed on the platform. The mice received four trials (1.5cm, 3.0cm, 6.0cm and 7.5cm) with a 1 hour interval between the trials. The step-down latency in seconds was measured with a cut-off at 120 sec. Animals were immediately removed after they stepped down and returned to their home cages until the next trials.
Conditioned-taste aversion (CTA)
The experiment was carried out as described (Pennanen et al., 2004). Animals, water-deprived in their home cage, were allowed to drink water at a set time for a period of 20 minutes each day for 1 week. Animals (10 male mice per group, 8 weeks of age) then were trained for 20 minutes daily for 4 days to drink plain water from 2 pipettes (Med Associates, St. Albans, VT), each containing 10 ml of water. On day 5 (conditioning day), the animals were allowed to drink water containing saccharine (0.1% w/v, sodium salt) instead of plain water for 20 minutes. Fifteen minutes later the mice were subjected to an aversive visceral event (i.p. injection of 0.15M LiCl; 250 mg/Kg body weight; control mice were given 0.15M NaCl). This aversive event (LiCl) gives a short period of nausea and discomfort similar to a bad stomachache. One, three, and seven days later the animals were presented with a choice of two pipettes, one containing saccharine-flavored water and the other containing plain water. The amount of each liquid consumed in 20 minutes was recorded.
Olfactory Conditioning Test
The experiment was carried out as described (Brennan et al., 1998). During the test period (5 days) mice (8 male mice per group), 8 weeks of age, were placed on a restricted feeding regime in which a rodent diet was given once a day at a set time for a period of 15 min. The “reinforcer” was a sugar cube. The conditioned stimulus was an odorant, lemon or peppermint. The trials consisted of placing a Petri dish containing either the lemon odorant or peppermint odorant with a sugar cube in the center of the cage for a period of 10 min. Each mouse received four conditioning trails with lemon odorant and four with peppermint odorant over three days. The order of the trials was random and separated by approximately 25 min. One day after the last trial, each mouse was placed in the middle compartment of a three-compartment chamber (30 × 30 × 90 cm) with transparent plexiglass walls. The middle compartment was linked to the two end compartments by a 2 × 4 cm opening in the center of each wall. A dish containing the lemon odorant, but without sugar, was placed in one of the side compartment and a dish containing the peppermint odorant, but without sugar, was placed in the other side compartment. Total time spent in the compartment containing the previous conditioning odorant (e.g., peppermint) and reinforcer (sugar) was compared to the total time spent in the compartment containing the previous non-conditioning odorant (e.g. lemon) without reinforcer. Experiments were recorded by a hand-held video recorder, and time spent in each compartment was calculated.
Rotarod test
Motor coordination was evaluated using the accelerating rotarod test (Model 7650; Ugo Basile, Comerio, Italy) (Lalonde et al., 1995). The rotarod test was performed by placing a mouse on a rotating drum and measuring the time each mouse was able to maintain its balance on the rod. The speed of the rotarod accelerated from 4 to 40 rpm over a 5 min period. 20 male mice (11-weeks old) were tested 10 times (5 minutes each) over the course of one week.
Seizure
Handling-induced seizure was observed using 20-30 DKO mice per group. Each mouse was lifted from its cage and moved to a new cage. The mice were observed for 2 min. Each mouse was tested twice about one week apart. A mouse was considered to have handling-induced seizures if it responded with at least one generalized seizure during the two tests. For drug induced seizure, 10 mice per group were used. Pentylenetetrazol (PTZ) induced seizures were evaluated in 4-8 month old mice that were injected intraperitoneally with PTZ at 40 mg/kg body weight and observed for 10 minutes. Kainic acid (KA) induced seizures were evaluated in 4-8 month old mice that were injected intraperitoneally with KA at 20 mg/kg body weight and observed for 60 min. PTZ and KA were obtained from Sigma. Saline injected animals served as controls. Observers were blind to genotype. Seizure severity was determined according to a previously defined 1 to 5 scale ranging from immobility and rigid posture to generalized seizures and death (Otani et al., 2006).
Western Blotting
Mouse brains were homogeniged in ice-cold lysis buffer containing 10mM Tris-HCL, pH 7.4, 150mM NaCl, 1mM EDTA, 0.1% SDS, 0.5% Nonidet P-40, and protease inhibitor mixture. Lysates were centrifuged at 3,000g for 10 min to remove insoluble debris. Protein concentrations in the supernatants were determined using Bradford protein assay. The proteins in the extract (50 μg) were separated by 10% SDS-PAGE and transferred to nitrocellulose membrane. Mouse anti-IA-2 (SK-1, 1:5000) and IA-2β (WT-4, 1:5000) monoclonal antibodies provided by Dr. Takashi Onodera were used as primary antibodies.
Subcellular Fractionation
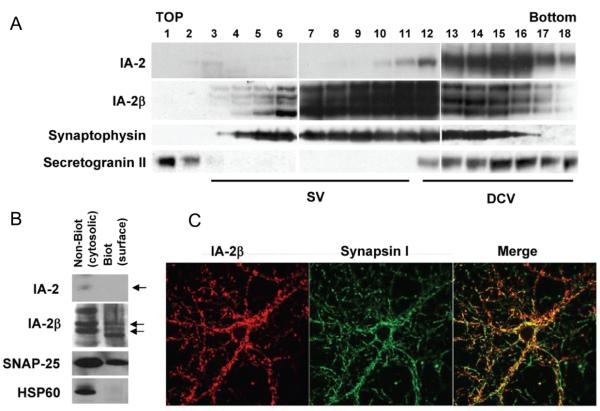
Subcellular fractionation of mouse forebrain was carried out as previously described with slight modifications (Walch-Solimena et al., 1993). Mouse brain was homogenized in 1 ml of 0.3 M sucrose, 5 mM EDTA, and 5mM HEPES-KOH, pH 7.2, in a glass-Teflon homogenizer with 12 strokes. Homogenates were centrifuged at 10,000 × g for 10 min at 4°C to remove debris and the supernatants were loaded on a linear sucrose gradient (0.6–1.8 M) and centrifuged at 100,000 × g for 6 h in a SW41Ti rotor (Beckman Coulter, Fullerton, CA). Eighteen fractions of 0.75 ml each were collected from the top of the gradient and analyzed by immunoblotting. Rabbit anti-secretogranin II polyclonal antibody (1:1000) (Santa Cruze Biotechnology, Santa Cruz, CA) and mouse anti-synaptophysin monoclonal antibody (1:1000) (Chemicon, Temecula, CA) were used as organelle markers.
Synaptosome fractionation and synaptic vesicle purification
The purification of synaptic vesicles from mouse forebrain was carried out as described previously (Huttner et al., 1983). In brief, 10 mouse forebrains were homogenized in a glass-teflon homogenizer in ice-cold buffered sucrose (320mM sucrose, 5mM HEPES-NaOH buffer, pH 7.4). The homogenates underwent three differential centrifugation steps and the final supernatant designated LS1 was centrifuged at 165,000 g for 2 h and the pellet (LP2) resuspended in 40 mM sucrose and layered on top of a linear sucrose gradient (50-800 mM sucrose). Sucrose gradient centrifugation was performed for 5 h at 65,000 g and the material banding at 200-400 mM sucrose was collected (SG-V).
Biotinylation of synatosome surface proteins
Synaptosomes were prepared as described by Huttner et al. (Huttner et al., 1983). Approximately 200 μg of the synaptosome preparations were pelleted and resuspended in Krebs-Ringer Bicarbonate (KRB) buffer. For biotinylation, samples were incubated at 4°C with 1 mg/ml biotin-EZ link (Pierce Biotechnology, Rockfold, IL) for 20 min. To remove and inactivate biotin residues, synaptosomes were centrifuged and washed in phosphate-buffered saline containing 50 mM glycine. Proteins were extracted in 140 mM KCl, 20 mM HEPES-KOH, (pH 7.4), 2 mM EDTA, and 1% (v/v) Triton X-100 for 1 h. Triton insoluble material was removed by centrifugation at 700 g for 3 min. The isolation of biotinylated proteins was performed with Streptavidin-Sepharose (Pierce) at 4°C overnight. Biotinylated proteins were subsequently analyzed by SDS electrophoresis and Western blotting. Rabbit anti-HSP60 polyclonal antibody (1:500) was obtained from Santa Cruz; mouse anti-SNAP-25 monoclonal antibody (1:1000) was obtained from Covance (Berkeley, CA).
Uptake of neurotransmitters by synaptosomes
The uptake of neurotransmitters by synaptosomes was determined as described with slight modifications (Fykse and Fonnum, 1988). Synaptosomes (80 ug protein in 100ul) were pre-incubated at 30°C for 15 min in oxygenated KRB buffer. For the uptake of neurotransmitters, 120 nM L-[H3] glutamate (0.5 μCi), or 45 nM [H3] GABA (1 μCi) or 100 nM [H3] dopamine (0.5 μCi) were obtained from Amersham Bioscience (Piscataway, NJ) and were added to the synaptosomes. After incubation at 30°C for 3 min, the reactions were terminated by dilution with an ice-cold solution of 0.15 M KCl and filtered rapidly using a GF/C glass membrane. After washing 3 times in KRB buffer, the radioactivity remaining on the filters was determined in a liquid scintillation counter.
Release of neurotransmitters from synaptosomes
The release of neurotransmitters from synaptosomes was determined as described previously with slight modifications (Turner and Goldin, 1989, Linetska et al., 2003). Synaptosomes were diluted with oxygenated KRB buffer (50 ug protein in 100 ul) and pre-incubated at at 30°C for 15 min. Then radiolabelled glutamate (0.5 μCi) or GABA (1 μCi) or dopamine (0.5 μCi) was added for 10 min. The loading reaction was stopped by a centrifugation at 10,000 × g for 1 min and the pellet resuspended with ice-cold oxygenated KRB. The suspension was immediately used to study the release of neurotrasmitters. Samples were stimulated without (basal) or with (evoked) 25mM KCl for 6 min at 37°C and rapidly centrifuged at 10,000 × g for 20 sec at 4°C. Aliquots of supernatants and SDS-solubilized pellet were diluted with scintillation liquid ACS (1.5 ml) and counted. Total radiolabelled GABA, glutamine or dopamine in the synaptosomes was calculated as counts in the supernatant plus counts in the pellet. The percentage of neurotransmitter released was expressed as the amount in the supernatant divided by the total amount in the synaptosomes (i.e., supernatant plus pellet) in the presence and absence of stimulating agent, 25 mM potassium chloride.
Hippocampal cell cultures
Primary neuronal cultures were prepared from the hippocampus of 0-1 day old mice. The dissociated cells were plated on glass coverslips coated with poly-L-lysine at a density of about 50,000 cells per well in 12-well Falcon plate (Becton Dickinson, Sparks, MD). The cells were maintained in Neurobasal medium (Gibco Invitrogen Corporation, Carlsbad, CA) supplemented with B27 (Gibco Invitrogen), and 2 mM glutamine for 2 weeks. Samples were fixed with 4% paraformaldehyde in PBS, pre-treated with 10% normal goat serum in PBS and then incubated with primary and secondary antibodies. Rabbit anti-synapsin-I polyclonal antibody (1:1000) was obtained from Abcam (Cambridge, MA), and goat anti-mouse IgG secondary antibody (1:500) (Alexa Fluor 488) and goat anti-rabbit IgG secondary antibody (1:500) (Alexa Fluor 568) from Invitrogen. After washing, samples were preserved by mounting coverslips on slides with Prolong Gold antifade reagent (Invitrogen).
Immunohistochemistry
Ten week old WT and DKO mice were anesthetized and transcardially perfused with PBS and then with 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were removed, postfixed with the same buffer at 4°C for 5 h, and cryoprotected by immersion in 15% sucrose in PBS overnight at 4°C. After embedding in Tissue-Tek OCT compound (Sakura Finetechnical, Tokyo, Japan), the brains were frozen and sectioned sagittally or coronally at a thickness of 14 um. The sections were air dried for 1 h and rinsed in PBS three times. Samples were treated with 10% normal goat serum in PBS and then incubated with primary and secondary antibodies in the same buffer and rinsed in PBS. The sections were then mounted with Prolong Gold antifade reagent (Invitrogen).
Electron microscopy
WT and DKO mice were deeply anesthetized and perfused for 15 min with freshly prepared 2% glutaraldehyde and 2% paraformaldehyde in 100 mM phosphate buffer, pH 7.4. Brains were removed and placed in 2% glutaraldehyde overnight at 4°C. Following dissection and postfixation for 1 hr in 1% osmium tetroxide, 0.1 M phosphate buffer, the hippocampi were dehydrated using a series of ethanol dilutions and were embedded in an Epon-Araldite mixture. Ultrathin sections were cut and stained with uranyl and lead citrate. Ten separate electron micrographs (area of 50 μm2) from each animal, prepared by Paragen Bioservices, Baltimore, MD, were analyzed without knowledge of genotype and the number of synaptic vesicles was counted.
Statistical analysis
Data were analyzed by Student's t-test and two-way analysis of variance (ANOVA) with repeated measures in rotarod test. All data are expressed as the mean ± S.E.M. A P-value of <0.05 was considered statistically significant.
Results
Expression of IA-2, IA-2β and neurotransmitters in brain of WT and DKO mice
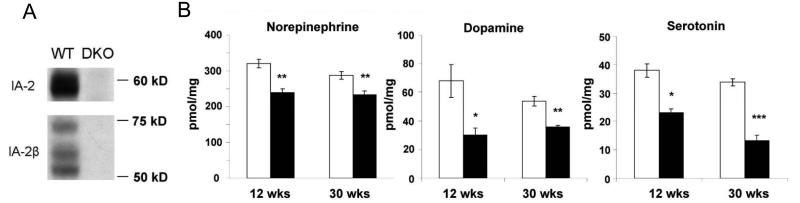
IA-2 and IA-2ß are widely distributed in cells throughout the brain. By immunofluorescence, both of these proteins are found in the cerebral cortex, hippocampus, thalamus and hypothalamus with somewhat wider expression of IA-2ß as compared to IA-2 in the cerebral cortex and hippocampus (see Supplementary Fig.1). In contrast to WT mice, DKO mice do not express either of these proteins as determined by immunofluorescence (see Supplementary Fig.1) or Western blot (Figure 1A).
Fig. 1. Decreased neurotransmitters in brain of DKO mice.
(A) Western blot of whole brain showing expression of IA-2 and IA-2β in WT, but not DKO mice. (B) Concentration of norepinephrine, dopamine and serotonin in the brain of WT and DKO mice at 12 and 30 weeks of age as determined by HPLC. Each group represents 6 mice. Data are expressed as mean ± SEM. *P < 0.05, **P<0.01, ***P<0.001. WT mice (open bars); DKO mice (closed bars).
To see whether the deletion of IA-2/IA-2ß had any effect on neurotransmitters, brain homogenates were analyzed by HPLC (Figure 1B). At 12 weeks after birth, the level of norepinephrine, dopamine and serotonin in DKO as compared to WT mice was decreased by 23.5%, 50.5%, and 37.0%, respectively. At 30 weeks, the decrease was 19.6%, 34.2%, and 60.9%, respectively. Thus, the deletion of IA-2/IA-2ß has a substantial effect on the level of a number of neurotransmitters in the brain.
Physical examination of DKO mice
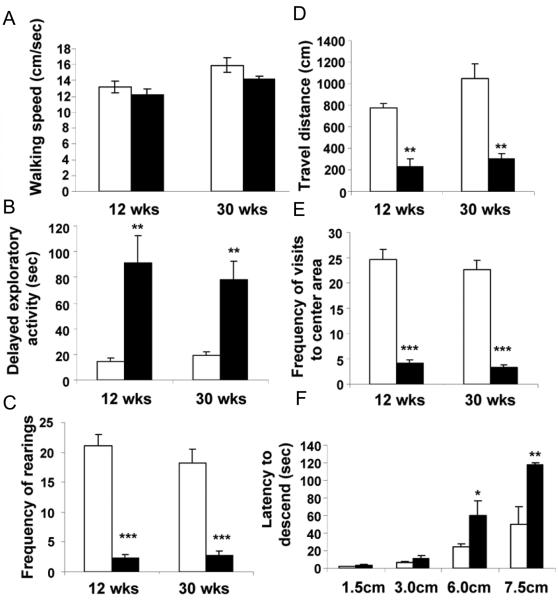
Physical examination (Crawley, 2000) of the DKO mice revealed normal appearing animals with no gross abnormality: body position and gait were normal as was walking speed (Figure 2A). Heart rate, respirations and body weight similarly were normal. In a battery of neurological reflex tests including righting and corneal reflexes no significant differences were found between the WT and DKO mice, except the DKO appeared somewhat less active than the WT mice.
Fig. 2. Behavioral changes in open field (A-E) and height-fear (F) tests in DKO mice.
(A) The average walking speed of WT and DKO mice was similar. (B) Exploratory activity was significantly delayed as determined by the time required to leave a fixed spot in the center of the cage. (C) DKO mice show reduced frequency of rearings. (D) DKO mice show reduced travel distance. (E) DKO mice show reduced number of visits to the center area of a cage. (F) Latency (seconds) to step down from elevated platforms of different heights in the height-fear test. Open and closed bars represent WT and DKO mice, respectively. Bars show mean ± SEM. 12 to 16 mice per group, *P < 0.05, **P<0.01, ***P<0.001 (DKO mice vs. WT mice).
Anxiety-like behavior in DKO mice
To see if the deletion of IA-2/IA-2ß had any effect on behavior, WT and DKO mice were evaluated in an open field test. Because this test places an animal in a novel environment imposing a conflict between an innate desire to explore versus safety, it is used as a surrogate for anxiety-like behavior. The DKO mice showed a highly significant increase in the time required for them to begin exploratory activity (Figures 2B) at both 12 and 30 weeks of age as compared to the WT mice. In addition the frequency of rearing (standing on two feet to look at the surroundings) (Figure 2C), total distance traveled (Figure 2D) and frequency of visits to central area of cage (Figure 2E) were all significantly reduced.
Further evaluation of anxiety-like behavior was obtained with the height-fear test, which measures the length of time (latency) to step down from increasingly high platforms on which the mouse is placed (Figure 2F). At 1.5 and 3.0 cm there was no significant difference between the DKO and WT mice, but at 6.0 and 7.5 cm there was a marked increase in the latency of descent, requiring two to three times as long for the DKO as the WT mice to step down.
Conditioned Learning in DKO mice
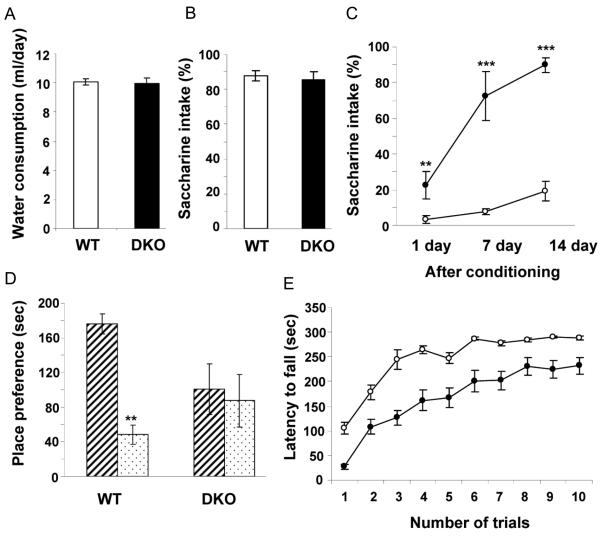
To evaluate the effect of the knockout of IA-2 and IA-2β, the conditioned taste aversion (CTA) test was used. During the training period DKO and WT consumed the same volume of water (Figure 3A) and both showed an equally strong preference for saccharine-flavored water (Figure 3B) indicating that taste and ability to consume fluid was not impaired in the DKO mice. In the conditioning phase, mice were given saccharine-flavored water, followed by an intraperitoneal injection of LiCl which causes abdominal discomfort. At different times thereafter the mice were given a choice of saccharine-flavored or plain water. As seen in Figure 3C, one day after exposure to the conditioning stimulus, less than 5% of the water consumed by the WT mice was saccharine-flavored as compared to 22.7% for the DKO. At 7 and 14 days after the conditioning stimulus 7.5% and 19.4%, respectively, of the water consumed by the WT mice was saccharine-flavored as compared to 72.4% and 89.9%, respectively, for the DKO mice indicating highly accelerated extinction of conditioned learning.
Fig. 3. Conditioned learning in DKO mice as evaluated by taste aversion, olfactory-place preference and rotarod-latency to fall.
(A) Prior to conditioning WT and DKO mice consumed the same amount of water (10 mice per group). (B) Both preferred saccharine-flavored over plain water (10 mice per group). (C) After conditioning (linking the saccharine-flavored water with LiCl which caused abdominal discomfort) the WT mice preferred plain water for the 14 days of the experiment, whereas the DKO mice showed accelerated extinction of learning and rapidly resumed their preference for saccharine-flavored water (10 mice per group). Open and closed circles represent WT and DKO mice, respectively. (D) Behavioral preference for conditioning versus non-conditioning odorant (8 mice per group). WT mice spent significantly more time than DKO mice in the compartment containing the conditioning odorant (stripped bar) as compared to the compartment containing the non-conditioning odorant (dotted bar). (E) Motor learning as evaluated by latency to fall in the rotarod test (20 mice per group). Both the WT and DKO mice improved performance with increase in training, but the response of the DKO mice never reached that of the WT mice. Rotarod data, analyzed with a two-way ANOVA, revealed a significant main effect of genotype (P<0.01) and a significant main effect of trial (P<0.0001), but no genotype effect by trial interaction (P = 0.33). Open and closed circles represent WT and DKO mice, respectively. Data points and bars represent mean ± SEM. **P< 0.01, ***P< 0.001.
In the olfactory conditioning test an odorant (lemon or peppermint) was placed in a dish in the conditioning cage with a sugar cube. After conditioning (a total of 8 trials), animals were placed in the central compartment of a 3 compartment interconnected cage in which the conditioning odorant (but without a sugar cube) was placed in one of the side-compartments and the non-conditioning odorant (also without a sugar cube) was placed in the other side-compartment. The amount of time that the mouse spent exploring each compartment was determined. As seen in Figure 3D, whereas the WT mouse spent nearly 4 times as long in the compartment with the odorant previously associated with the sugar cube, the DKO mouse spent an equal amount of time in the two side-compartments demonstrating a lack of place-preference learning for the conditioning stimulus.
Motor learning was evaluated by the rotarod test which measures the length of time that a mouse can remain on a rotating drum and its capacity to learn motor coordination upon repeated trials (Figure 3E). The latency to fall for DKO mice on the first trial was 27 ± 5 seconds, whereas for WT mice it was a 105 ± 12 seconds indicating a possible coordination problem. However, upon repeated trials, the DKO mice learned how to maintain their balance and the latency to fall was increased to approximately 232 ± 17 seconds nearly reaching the latency of fall (288 ± 4 seconds) observed in the WT mice. These findings argue that the DKO mice may suffer from some impairment of coordination, but maintain much of their motor learning ability.
Spontaneous and induced seizures and sudden death in DKO mice
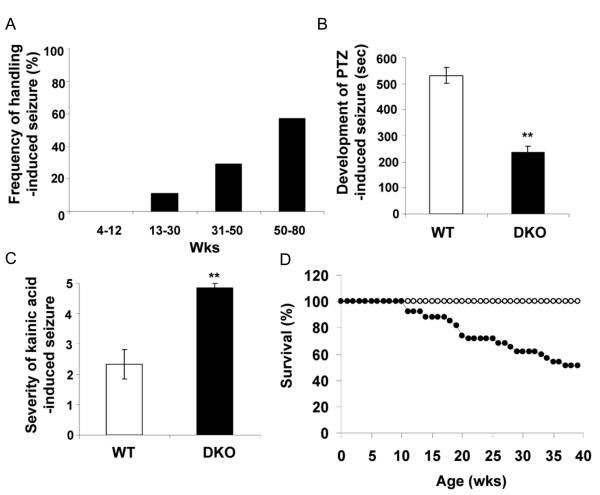
Up to about 12 weeks of age handling the DKO mice rarely induced seizures. However, with increasing age, handling-induced seizures increased from about 10% at 13 to 30 weeks of age to nearly 60% at 50 to 80 weeks of age (Figure 4A). The seizures were characterized by facial twitching and myoclonic jerks followed shortly thereafter by generalized clonic-tonic seizures and loss of posture. Most of the animals recovered within several minutes and resumed a standing position.
Fig. 4. Increase in spontaneous and induced seizures and shorten lifespan in DKO mice.
(A) Frequency of handling-induced seizure increases with age in DKO mice (20-30 animals per group). (B) Latency in seconds for the development of PTZ-induced seizures (40 mg/kg body weight) as evaluated by loss of posture (10 mice per group). (C) Severity of kainic acid induced seizures (20mg/kg body weight) (10 mice per group). (D) Survival of WT (28 untreated, open circles) and DKO (31 untreated, closed circles) mice. Bars represent mean ± SEM. **P< 0.01.
To see if the DKO mice were more susceptible to drug-induced seizures, 9 to 12 week old mice were injected with pentylenetetrazol (PTZ), a GABA receptor antagonist, before the onset of handling-induced seizures. DKO mice showed an increased susceptibility to PTZ-induced seizures as demonstrated by a shorter latency to first fall (225 seconds) as compared to the WT mice (525 seconds) (Figure 4B). Similarly, the DKO mice were more susceptible to kainic acid (KA) as shown by the severity of seizures based on a 1 to 5 scale (Otani et al., 2006) (Figure 4C). Approximately 80% of the DKO mice died within 2 hours after 20 mg/kg KA injection, whereas all the WT mice survived. Moreover, injections of KA, which is particularly toxic to neurons in the hippocampus, produced more severe neuronal degeneration in the DKO than in the WT mice as characterized by the loss of neurons and pyknotic nuclei (data not shown).
The KO of IA-2/IA-2ß also resulted in a significantly shorter life span. Up to 40 weeks of age none of the WT mice died, whereas approximately 50 % of the DKO mice died during that time period (Figure 4D). The deaths were sudden. Animals appearing perfectly normal at the end of the day were found dead the next morning. Although death following spontaneous seizures was not observed, seizures appear to be a likely explanation for the sudden deaths.
IA-2ß colocalized with synaptic vesicles
IA-2 and IA-2ß are transmembrane proteins of DCV. There is very little information about the relationship of these two proteins to SV. The fact that deletion of IA-2/IA-2ß resulted in a decrease in the levels of norepinephrine, dopamine and serotonin (Figure 1B) and also resulted in behavioral, learning and neurological abnormalities suggested that some of these changes might be related to alterations in SV. To see if IA-2 or IA-2ß colocalized with SV, brain homogenates were subjected to sucrose gradient fractionation. Figure 5A shows a different distribution for IA-2 and IA-2ß. Both of these proteins were found in the fractions associated with the DCV marker, secretogranin II, whereas IA-2ß, but not IA-2, was found in fractions associated with the SV marker, synaptophysin. A similar distribution was found upon sucrose gradient fractionation of PC12 cells (data not shown).
Fig. 5. Distribution of IA-2 and IA-2β in SV and DCV.
(A) Homogenized brain lysates were fractionated on a 0.6-1.8 M sucrose gradient and analysed by immunoblotting with antibodies to IA-2, IA-2β, synaptophysin and secretogranin II. (B) Biotinylated labeling of cell surface proteins, followed by separation from the non-biotinylated cytosolic proteins by avidin-coated beads. Western blot shows that IA-2β, but not IA-2, is expressed on the surface of the synaptosomes. Surface biotinylation of HSP60 was not observed, indicating that synaptosomal damage and contamination of proteins from the cytoplasm did not occur. (C) Colocalization of IA-2β and synapsin 1 in cultured primary neurons from the hippocampus.
Subcellular fractions enriched for SV also showed that IA-2ß colocalizes with SV (see Supplementary Fig.2). Fractions sequentially enriched in SV (e.g. SG-V) contained primarily IA-2ß and little or only trace amounts of IA-2. Further evidence for the presence of IA-2ß in synaptic vesicles comes from biotinylating proteins on the surface of synaptosomes and from separating intracellular non-biotinylated proteins from biotinylated surface proteins. By Western blot, with antibodies to the luminal domain of IA-2ß and to SNAP-25 (Figure 5B), both IA-2ß and SNAP-25 were found both on the surface of the synaptosomes (biotinylated) and within synaptosomes (i.e., cytosolic, non-biotinylated). That the surface expression of IA-2ß was not the result of contamination with cytosolic proteins was demonstrated by the absence of HSP60 in the biotinylated surface fraction. Moreover, IA-2 was not found on the surface of the synaptosomes and only trace amounts were found within the cytosolic fraction of the synaptosomes. These finding suggest that when SV fuse with the surface of the synaptosome, the luminal domain of IA-2ß becomes exposed. In other experiments, primary neuronal cultures from the hippocampus were immunostained with antibodies to IA-2ß and synapsin I, a marker for SV (Figure 5C). Both antibodies produced a speckled pattern that colocalized when the images were merged.
Functional analysis of synaptosomes from DKO mice
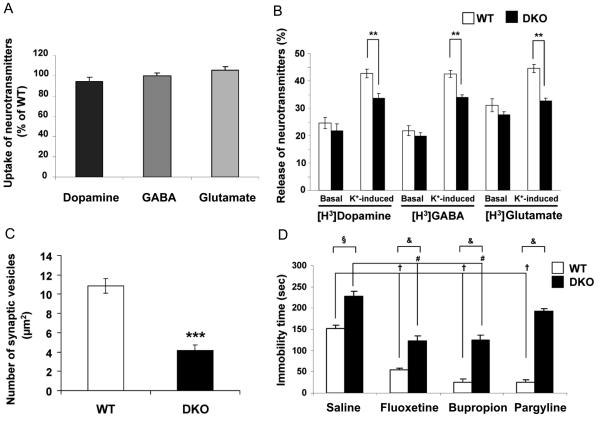
No difference was found in the uptake of radiolabelled dopamine, GABA or glutamate (Figure 6A) by synaptosomes prepared from DKO as compared to WT mice. Moreover, no difference was found in the release of dopamine, GABA or glutamate under basal conditions when the synaptosomes of the DKO mice were compared to those of the WT mice (Figure 6B). However, upon stimulation with K+ there was a significant decrease ranging from 20.3 %, to 26.7% in the fractional release of all three neurotransmitters from the synaptosomes of the DKO mice. Electron microscopic studies on tissue secretions from the hippocampus revealed normal appearing synapses (not shown), but approximately a 60% decrease in the number of SV (Figure 6C).
Fig. 6. Decreased secretion of neurotransmitters from synaptosomes of DKO mice.
(A) Uptake of [H3]dopamine, [H3]GABA and [H3]glutamate by synaptosomes of DKO mice as percentage of uptake by WT mice. Data are mean ± SEM from three separate experiments performed in triplicate. (B) Basal and K+-induced (25 mM) release of [H3]dopamine, [H3]GABA, and [H3]glutamate from synaptosomes of WT and DKO mice. The amount of radiolabelled neurotransmitter released, expressed as percentage of total radiolabelled neurotransmitter, is shown. Values are mean ± SEM from three separate experiments performed in triplicate; ** P<0.01. Open and closed bars represent WT and DKO mice, respectively. (C) Quantification of synaptic vesicles from 10 areas of hippocampus each representing 50 μm2. *** P<0.001. (D) Effect of neuropharmacologic agents on reducing the increased anxiety-like behavior in DKO mice (saline) as compared to WT mice, as measured by the decrease in immobility time in the tail suspenstion test (TST) (8-10 mice per group). (§) Untreated (saline) DKO mice differ significantly from untreated (saline) WT mice (P<0.001). (†) Treated (i.e., fluoxetine 20 mg/kg, bupropion 20 mg/kg, pargylene 75 mg/kg) WT mice differ significantly from untreated (saline) WT mice (P<0.001). (#) Fluoxetine and bupropion-treated DKO mice differ significantly from untreated (saline) DKO mice (P<0.001). (&) Treated DKO mice differ significantly from treated WT mice (P<0.001).
Effect of neuropharmacologic agents on behavior
To determine whether neuropharmacologic agents could reverse some of the behavioral abnormalities found in the DKO mice, the tail suspension test (TST) was used. In this test rodents become immobile in aversive situations from which they cannot escape. As seen in Figure 6D, the DKO mice treated with saline remained immobile considerably longer (225 seconds) than the WT mice also treated with saline (150 seconds). Many anti-depression drugs are known to reduce immobility time in the TST test (Cryan et al., 2004). Treatment of WT mice with fluoxetine, bupropion or pargyline, as expected, markedly reduced immobility from 150 seconds (saline control) to between 25 and 50 seconds. Treatment of DKO mice with fluoxetine and bupropion, but not pargyline, reduced immobility from 225 seconds (saline control) to about 125 seconds. This is within the time range of the untreated WT saline controls, but does not approach the reduction in immobility seen when fluoxetine and bupropion were used to treat WT mice.
Discussion
Considerable new information has accumulated over the last few years on the function of IA-2 and IA-2ß (Harashima et al., 2005, Mziaut et al., 2006, Harashima et al., 2007). Both are transmembrane DCV proteins. The knockout of IA-2 results in a decrease in the number and content of DCV and the amount of insulin secreted (Saeki et al., 2002, Kubosaki et al., 2004, Harashima et al., 2005, Kubosaki et al., 2005, Mziaut et al., 2006, Harashima et al., 2007). The knockdown of IA-2 with siRNA in MIN-6 cells also results in a decrease in the secretion of insulin (Harashima et al., 2005). In contrast, overexpression of IA-2 increases by over two fold the number of DCV and by four-to-six fold the amount of insulin secreted. Overexpression also increases the half-life of DCV by almost two fold (i.e., from 22 to 40 hours) suggesting that the degree of expression of IA-2 influences the stability of DCV and this in turn modulates the number of DCV, their content and secretion (Harashima et al., 2005). Less is known about IA-2ß, but its knockout in mice also results in a decrease in the secretion of insulin (Kubosaki et al., 2004) and its knockdown with siRNA in MIN6 and PC12 cells decreases, respectively, the secretion of insulin and dopamine (unpublished data).
Earlier studies from our laboratory showed that abnormalities in secretion were more pronounced in DKO than single knockout (SKO) mice and that certain abnormalities not observed in SKO were observed in DKO mice such as female infertility (Kubosaki et al., 2006). Since our initial behavioral studies also revealed few if any abnormalities in IA-2 or IA-2ß SKO mice, but substantial changes in IA-2/IA-2ß DKO mice, we restricted our current studies to the DKO mice. Previously it was thought that IA-2 and IA-2ß were present only in DCV. The findings reported here show that IA-2ß, but not to any appreciable degree IA-2, also is present in SV and that the knockout of both of these genes results in a decrease in the concentration of neurotransmitters (e.g., norepinephrine, dopamine and serotonin) in the brain. The presence of IA-2ß in SV is further supported in the current study by the decrease in K+-induced secretion of radiolabelled dopamine, GABA and glutamate from the synaptosomes of DKO as compared to WT mice. From these and other studies we conclude that IA-2 and IA-2ß are widely distributed in neuroendocrine cells throughout the body, that they may have an additive, but not necessarily identical, function and that their deletion has a global effect on neuroendocrine secretion.
This global effect on secretion is by far the most likely explanation for the behavioral, learning and neurological disturbances observed in the DKO mice. Many different genes and neurotransmitters are involved in these complex functions. Because of the global effect of the knockout on neuroendocrine secretion, precisely which neurotransmitter or combination of neurotransmitters is responsible for each of the observed disturbances remains unresolved. Some answers, however, may come from treating DKO mice with neuropharmacological agents that have a known function. For example, in our study, bupropion (Martin et al., 1990), a monoamine reuptake inhibitor, and fluoxetine (Delgado et al., 1999), a selective serotonin reuptake inhibitor, but not pargyline, a monoamine oxidase inhibitor, were effective in reducing the increased anxiety-like behavior observed in the DKO mice. The fact that the reduction in anxiety-like behavior in the DKO mice was not as great as that observed in the WT mice is most likely due to the decrease in the number and cargo content of the DCV and SV in DKO mice. In the future it should be possible to obtain more precise information on these behavioral and neurological abnormalities by electrophysiological studies and by conditionally knocking out IA-2 and/or IA-2ß. It should be emphasized that at the present time there is no evidence that the autoantibodies to IA-2 and/or IA-2ß found in type 1 diabetes are responsible for any of the neuroendocrine or behavioral changes observed in the human disease.
The demonstration here that the K+ -induced secretion of neurotransmitters including GABA from the synaptosomes of the DKO mice is impaired could be at least one of explanations for the handling-induced seizures and the seizures induced by low doses of KA and PTZ. Targeted deletion of glutamic acid decarboxylase which synthesize GABA in the brain (Kash et al., 1997) or overexpression of the GABA transporter which decreases the concentration of extracellular GABA (Zhao et al., 2003) has been shown to induce seizures. Seizures would appear to be the best explanation for the high frequency of sudden deaths in the DKO mice. Although no evidence of histopathology was found in the brains of the DKO mice, this is not an uncommon finding in seizures.
Based on the impaired secretion, it would be surprising if the global knockout of IA-2/IA-2ß did not result in a number of other still unrecognized abnormalities. In this context, it would be of particular interest to know whether the knockout of IA-2/IA-2ß decreases the content and/or release of neurotransmitters not only from the brain, but also from other peripheral organs containing neuroendocrine cells (e.g., adrenals, thyroid, thymus). Ongoing experiments on DKO mice are showing profound changes in the diurnal variation of blood pressure, heart rate and temperature (unpublished data). Thus, the IA-2/IA-2ß DKO mouse offers a rich variety of phenotypes that might be useful not only in identifying new hormones/neurotransmitters, but also for systematically studying the therapeutic efficacy of drugs on these various abnormalities. Several hundred proteins now are known to be associated with DCV and SV, some of which are structural (transmembrane) proteins (Takamori et al., 2006). Our findings with IA-2/IA-2ß thus raise the possibility that at the human level, mutations in one or more of these secretory vesicle structural proteins also might result in a decrease in the secretion of hormones and neurotransmitters and this, in turn, could lead to affective disorders.
Supplementary Material
Acknowledgement
This work was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health and in part by a Japan Society for the Promotion of Science Fellowship (T. N.). The authors thank Dr. Shinichiro Nakamura for his histology studies.
Nonstandard abbreviations used
- CTA
conditioned-taste aversion
- DA
dopamine
- DCV
dense core secretory vesicles
- DKO
double knockout
- FSH
follicular stimulating hormone
- 5-HT
serotonin
- islet-associated protein 2
IA-2
- LH
lutenizing hormone
- NE
norepinephrine
- KA
kainic acid
- KRB
Krebs-Ringer Bicarbonate
- PTP
protein tyrosine phosphatase
- PTZ
pentylenetetrazol
- SV
synaptic vesicles
- TST
tail suspension test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach P, Bonifacio E, Williams AJ, Ziegler AG, Gale EA, Bingley PJ. Autoantibodies to IA-2beta improve diabetes risk assessment in high-risk relatives. Diabetologia. 2008;51:488–492. doi: 10.1007/s00125-007-0912-9. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Schellinck HM, de la Riva C, Kendrick KM, Keverne EB. Changes in neurotransmitter release in the main olfactory bulb following an olfactory conditioning procedure in mice. Neuroscience. 1998;87:583–590. doi: 10.1016/s0306-4522(98)00182-1. [DOI] [PubMed] [Google Scholar]
- Chen L, Cagniard B, Mathews T, Jones S, Koh HC, Ding Y, Carvey PM, Ling Z, Kang UJ, Zhuang X. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J Biol Chem. 2005;280:21418–21426. doi: 10.1074/jbc.M413955200. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What's wrong with my mouse? : behavioral phenotyping of transgenic and knockout mice. Wiley-Liss; New York ; Chichester: 2000. [Google Scholar]
- Cryan JF, O'Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci U S A. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado PL, Miller HL, Salomon RM, Licinio J, Krystal JH, Moreno FA, Heninger GR, Charney DS. Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol Psychiatry. 1999;46:212–220. doi: 10.1016/s0006-3223(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Dere E, De Souza-Silva MA, Spieler RE, Lin JS, Ohtsu H, Haas HL, Huston JP. Changes in motoric, exploratory and emotional behaviours and neuronal acetylcholine content and 5-HT turnover in histidine decarboxylase-KO mice. Eur J Neurosci. 2004;20:1051–1058. doi: 10.1111/j.1460-9568.2004.03546.x. [DOI] [PubMed] [Google Scholar]
- Fykse EM, Fonnum F. Uptake of gamma-aminobutyric acid by a synaptic vesicle fraction isolated from rat brain. J Neurochem. 1988;50:1237–1242. doi: 10.1111/j.1471-4159.1988.tb10599.x. [DOI] [PubMed] [Google Scholar]
- Harashima S, Clark A, Christie MR, Notkins AL. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proc Natl Acad Sci U S A. 2005;102:8704–8709. doi: 10.1073/pnas.0408887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima SI, Harashima C, Nishimura T, Hu Y, Notkins AL. Overexpression of the autoantigen IA-2 puts beta cells into a pre-apoptotic state: autoantigen-induced, but non-autoimmune-mediated, tissue destruction. Clin Exp Immunol. 2007;150:49–60. doi: 10.1111/j.1365-2249.2007.03455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y. Centrifugal precipitation chromatography: principle, apparatus, and optimization of key parameters for protein fractionation by ammonium sulfate precipitation. Anal Biochem. 2000;277:143–153. doi: 10.1006/abio.1999.4365. [DOI] [PubMed] [Google Scholar]
- Kash SF, Johnson RS, Tecott LH, Noebels JL, Mayfield RD, Hanahan D, Baekkeskov S. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubosaki A, Gross S, Miura J, Saeki K, Zhu M, Nakamura S, Hendriks W, Notkins AL. Targeted disruption of the IA-2beta gene causes glucose intolerance and impairs insulin secretion but does not prevent the development of diabetes in NOD mice. Diabetes. 2004;53:1684–1691. doi: 10.2337/diabetes.53.7.1684. [DOI] [PubMed] [Google Scholar]
- Kubosaki A, Nakamura S, Clark A, Morris JF, Notkins AL. Disruption of the transmembrane dense core vesicle proteins IA-2 and IA-2beta causes female infertility. Endocrinology. 2006;147:811–815. doi: 10.1210/en.2005-0638. [DOI] [PubMed] [Google Scholar]
- Kubosaki A, Nakamura S, Notkins AL. Dense core vesicle proteins IA-2 and IA-2beta: metabolic alterations in double knockout mice. Diabetes. 2005;54(Suppl 2):S46–51. doi: 10.2337/diabetes.54.suppl_2.s46. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Bensoula AN, Filali M. Rotorod sensorimotor learning in cerebellar mutant mice. Neurosci Res. 1995;22:423–426. doi: 10.1016/0168-0102(95)00916-h. [DOI] [PubMed] [Google Scholar]
- Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994;13:505–514. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- Lan MS, Wasserfall C, Maclaren NK, Notkins AL. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1996;93:6367–6370. doi: 10.1073/pnas.93.13.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter EH, Tsumura H, Serreze DV, Chapman HD, Rabin DU, Lan MS, Notkins AL. Mapping to chromosomes 1 and 12 of mouse homologs of human protein tyrosine phosphatase, receptor-type, related genes encoding pancreatic beta cell autoantigens. Mamm Genome. 1997;8:949–950. doi: 10.1007/s003359900619. [DOI] [PubMed] [Google Scholar]
- Linetska MV, Storchak LG, Himmelreich NH. Phenylarsine oxide inhibits alpha-latrotoxin-stimulated [3H]GABA release from rat brain synaptosomes. Neurochem Int. 2003;42:583–590. doi: 10.1016/s0197-0186(02)00158-4. [DOI] [PubMed] [Google Scholar]
- Lu J, Li Q, Xie H, Chen ZJ, Borovitskaya AE, Maclaren NK, Notkins AL, Lan MS. Identification of a second transmembrane protein tyrosine phosphatase, IA-2beta, as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37-kDa tryptic fragment. Proc Natl Acad Sci U S A. 1996;93:2307–2311. doi: 10.1073/pnas.93.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistrelli G, Toma S, Isacchi A. Substitution of two variant residues in the protein tyrosine phosphatase-like PTP35/IA-2 sequence reconstitutes catalytic activity. Biochem Biophys Res Commun. 1996;227:581–588. doi: 10.1006/bbrc.1996.1549. [DOI] [PubMed] [Google Scholar]
- Martin P, Massol J, Colin JN, Lacomblez L, Puech AJ. Antidepressant profile of bupropion and three metabolites in mice. Pharmacopsychiatry. 1990;23:187–194. doi: 10.1055/s-2007-1014505. [DOI] [PubMed] [Google Scholar]
- Mziaut H, Trajkovski M, Kersting S, Ehninger A, Altkruger A, Lemaitre RP, Schmidt D, Saeger HD, Lee MS, Drechsel DN, Muller S, Solimena M. Synergy of glucose and growth hormone signalling in islet cells through ICA512 and STAT5. Nat Cell Biol. 2006;8:435–445. doi: 10.1038/ncb1395. [DOI] [PubMed] [Google Scholar]
- Notkins AL. New predictors of disease. Molecules called predictive autoantibodies appear in the blood years before people show symptoms of various disorders. Tests that detected these molecules could warn of the need to take preventive action. Sci Am. 2007;296:72–79. [PubMed] [Google Scholar]
- Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108:1247–1252. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani N, Nawashiro H, Fukui S, Ooigawa H, Ohsumi A, Toyooka T, Shima K, Gomi H, Brenner M. Enhanced hippocampal neurodegeneration after traumatic or kainate excitotoxicity in GFAP-null mice. J Clin Neurosci. 2006;13:934–938. doi: 10.1016/j.jocn.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Pennanen L, Welzl H, D'Adamo P, Nitsch RM, Gotz J. Accelerated extinction of conditioned taste aversion in P301L tau transgenic mice. Neurobiol Dis. 2004;15:500–509. doi: 10.1016/j.nbd.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Roberts C, Roberts GA, Lobner K, Bearzatto M, Clark A, Bonifacio E, Christie MR. Expression of the protein tyrosine phosphatase-like protein IA-2 during pancreatic islet development. J Histochem Cytochem. 2001;49:767–776. doi: 10.1177/002215540104900610. [DOI] [PubMed] [Google Scholar]
- Saeki K, Xie J, Notkins AL. Genomic structure of mouse IA-2: comparison with its human homologue. Diabetologia. 2000;43:1429–1434. doi: 10.1007/s001250051550. [DOI] [PubMed] [Google Scholar]
- Saeki K, Zhu M, Kubosaki A, Xie J, Lan MS, Notkins AL. Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes. 2002;51:1842–1850. doi: 10.2337/diabetes.51.6.1842. [DOI] [PubMed] [Google Scholar]
- Solimena M, Dirkx R, Jr., Hermel JM, Pleasic-Williams S, Shapiro JA, Caron L, Rabin DU. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J. 1996;15:2102–2114. [PMC free article] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Turner TJ, Goldin SM. Multiple components of synaptosomal [3H]-gamma-aminobutyric acid release resolved by a rapid superfusion system. Biochemistry. 1989;28:586–593. doi: 10.1021/bi00428a026. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Takei K, Marek KL, Midyett K, Sudhof TC, De Camilli P, Jahn R. Synaptotagmin: a membrane constituent of neuropeptide-containing large dense-core vesicles. J Neurosci. 1993;13:3895–3903. doi: 10.1523/JNEUROSCI.13-09-03895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmeier C, Hutton JC. Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. J Biol Chem. 1996;271:18161–18170. doi: 10.1074/jbc.271.30.18161. [DOI] [PubMed] [Google Scholar]
- Zhao WJ, Ma YH, Fei J, Mei ZT, Guo LH. Increase in drug-induced seizure susceptibility of transgenic mice overexpressing GABA transporter-1. Acta Pharmacol Sin. 2003;24:991–995. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.