Abstract
Magnetic resonance (MR) imaging studies have revealed fronto-temporal cortical gray matter volume reductions in schizophrenia. However, whether age- and sex-matched unmedicated schizotypal personality disorder (SPD) patients share some or all of the structural brain-imaging characteristics of schizophrenia patients has not been studied. We examined cortical gray/white matter volumes in a large sample of unmedicated schizophrenia-spectrum patients (n=79 SPD, n=57 schizophrenia) and 148 healthy controls. MR images were reoriented to standard position parallel to the anterior-posterior commissure line, segmented into gray and white matter tissue types, and assigned to Brodmann areas (BAs) using a postmortem-histological atlas. Group differences in regional volume of gray and white matter in the BAs was examined with MANOVA. Schizophrenia patients had reduced gray matter volume widely across the cortex but more marked in frontal and temporal lobes. SPD patients had reductions in the same regions but only about half that observed in schizophrenia and sparing in key regions including BA10. In schizophrenia, greater fronto-temporal volume loss was associated with greater negative symptom severity and in SPD, greater interpersonal and cognitive impairment. Overall, our findings suggest that increased prefrontal volume in BA10 and sparing of volume loss in temporal cortex (BAs 22 and 20) may be a protective factor in SPD which reduces vulnerability to psychosis.
Keywords: MRI, schizophrenia, schizotypal personality disorder, frontal lobe volume, temporal lobe volume, cingulate gyrus, negative symptoms, gray matter volume, white matter volume
1. Introduction
Numerous studies have reported structural brain imaging abnormalities in schizophrenia-spectrum disorders. Similar to schizophrenia, cerebrospinal fluid (CSF) is increased and cortical volumes are decreased in schizotypal personality disorder (SPD) (Buchsbaum et al 1997; Dickey et al 2000; Koo et al 2006). Temporal cortex volume reductions, particularly in the superior temporal gyrus (STG) have been consistently reported in schizophrenia (Davidson and Heinrichs 2003; Downhill et al 2001; Gur et al 2000; Hirayasu et al 2000; Shenton et al 2001; Wright et al 2000). Reduced temporal cortex volume has also been observed in SPD in STG (Dickey et al 1999; Kawasaki et al 2004; Takahashi et al 2006a), Heschl’s gyrus (Dickey et al 2002b), and middle and inferior temporal gyrus (Downhill et al 2001) in some but not all studies (Takahashi et al 2006b). Studies with schizophrenia patients and their relatives also suggest reduced medial temporal volume including the amygdala and/or hippocampal complex, but these findings have not been consistently observed in SPD (Dickey et al 2002a; Dickey et al 1999; Seidman et al 2002; Seidman et al 2003). One recent study reported reduced hippocampal volume in SPD (Dickey et al 2007). Taken together, these findings are consistent with a model of common temporal lobe abnormalities across the schizophrenia spectrum.
While SPD and schizophrenia patients appear to show some common temporal lobe abnormalities, SPD patients do not appear to show volumetric decreases in frontal cortex to the same extent as schizophrenia (Kawasaki et al 2004; Siever et al 1990; Siever and Davis 2004; Siever et al 2002; Suzuki et al 2005). Reductions in frontal volume are associated with the deficit-like symptoms of SPD, implying that patients with smaller frontal lobe volume are more likely to display traits such as asociality (Diwadkar et al 2006; Raine et al 1992; Siever et al 1993). We (Haznedar et al 2004) and others (Takahashi et al 2004) have reported that cingulate gray matter volume is relatively preserved in SPD compared with schizophrenia. Volume reductions in the parietal lobe have also been reported to show a spectrum pattern with more widespread gray matter reductions in schizophrenia than SPD (Zhou et al 2007). Although relatively fewer anatomical MRI studies have been conducted in SPD, they suggest that SPD patients show abnormalities in some, but not all of the brain regions implicated in schizophrenia.
Individuals with SPD share a broad range of similarities with schizophrenia patients in terms of genetics, neurobiology, and phenomenology (Siever and Davis 2004). These similarities may be essential for the pathogenesis of schizophrenia and related to the vulnerability to schizophrenia. However, additional pathological changes may be required for the development of overt and sustained psychosis. Siever and Davis (Siever and Davis 2004) suggest a model of schizophrenia-spectrum disorders which hypothesizes that shared deficits may reflect a common neurodevelopmentally based cortical pathology (e.g., temporal cortex). However, in contrast to patients with schizophrenia, SPD patients are hypothesized to have reduced vulnerability to psychosis due to protective factors which include greater prefrontal reserves. Factors such as capacity to recruit alternate prefrontal areas (e.g., BA10) to compensate for other dysfunctional areas during cognitive demands (Buchsbaum et al 2002) and less reduced frontal lobe volume when compared to schizophrenia (Suzuki et al 2005) may protect the SPD patients from chronic schizophrenia (Siever and Davis 2004).
Prior MRI work comparing SPD and schizophrenia patients, e.g., (Kawasaki et al 2004; Zhou et al 2007) has involved medicated patients or voxel-based morphometry which did not provide combined three-group contrasts, a comparison of the magnitude of volume change in SPD and schizophrenia, or statistical contrasts of different BAs or hemispheric differences. The main goal of this study was to use MRI to determine the extent to which cortical gray and white matter volume differs from normal in a large, unmedicated schizophrenia-spectrum sample. We examined gray and white matter volume within BAs of the entire cortex and the cingulate gyrus in three age- and sex-matched groups: healthy controls and unmedicated patients with SPD or schizophrenia. We hypothesized that there would be a distinction between schizotypy and schizophrenia in terms of underlying regional cortical volume; SPD patients would show less severe prefrontal and temporal cortex volume reductions than schizophrenia patients. We also explored the symptomatic correlates of fronto-temporal cortex volume in our large sample and hypothesized that in both groups, fronto-temporal volume reductions would be associated with greater symptom severity.
2. Method
2.1. Participants
We studied three age- and sex-matched groups: 79 individuals with schizotypal personality disorder, 57 patients with schizophrenia, and 148 healthy controls. The three groups did not significantly differ in age or sex. Demographic and clinical data are presented in Table-1.
Table 1.
Demographic and Clinical Characteristics of Schizophrenia Spectrum Groups and Healthy Controls
| Characteristic | Healthy Controls (n=148) | SPD patients (n=79) | Sz patients (n=57) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
| Age (years) | 35.6 | 13.0 | 17–65 | 37.9a | 10.1 | 19–62 | 34.8b | 14.6 | 17–65 |
| Male/female | 99/49 | 62/17 c | 42/15 d | ||||||
| Psychoactive Meds | N | % | N | % | N | % | |||
| Never medicated | - | - | 61 | 77% | 29 | 51% | |||
| Previously medicated | - | - | 18* | 23% | 28 | 49% | |||
| Clinical Symptoms: | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range |
| BPRS total score | 48.5 | 11.3 | 26–83 | ||||||
| Positive symptom factor | 21.4 | 6.4 | 10–37 | ||||||
| Negative symptom factor | 8.5 | 3.2 | 3–14 | ||||||
| Total number of DSM-III-R symptoms (of 9 possible) | 6.8 | 1.1 | 5–9 | ||||||
| Cognitive impairment factor | 1.5 | .8 | 0–5 | ||||||
| Interpersonal factor | 2.9 | .9 | 1–5 | ||||||
| Paranoid factor | 2.4 | .9 | 1–5 | ||||||
post-hoc t-test for HC vs. SPD group, t(225)=1.36, p=0.17.
HC vs. Sz group, t(203)=0.38, p=0.70; SPD vs. Sz group, t(134)=1.46, p=0.15.
HC vs. SPD group, t(225)=1.84, p=0.07. Note: ANOVAs reported in results section were also analyzed with sex as a covariate and they yielded same pattern of results in terms of group interactions.
HC vs. Sz group, t(203)=0.94, p=0.35; SPD vs Sz group, t(134)=0.65, p=0.52.
15 of the previously medicated SPD patients received a non-neuroleptic medication and 3 received neuroleptics. All SPD and schizophrenia patients were off psychoactive medications for a minimum of two weeks prior to their MRI scan.
All patients met DSM-IV criteria and were off medication at the time of their MRI scans for a minimum of two weeks. All participants were screened for severe medical or neurological illness and head injury by comprehensive medical history, and laboratory tests taken by a physician. Healthy controls and SPD patients received an interview with a psychologist using the Structured Clinical Interview for DSM-III-R Axis I disorders (Spitzer et al 1992) and the Structured Interview for DSM-III-R Personality Disorders (Pfohl et al 1989). Schizophrenia patients were diagnosed using the Comprehensive Assessment for Symptoms and History (Andreasen et al 1992). Participants were excluded if they met lifetime criteria for substance dependence or abuse in the past six months, had taken any psychotropic medications in the last two weeks, or had a positive urine toxicology screen for drugs of abuse. Healthy controls had no personal history, nor a first degree relative with an Axis I or Axis II diagnosis.
The healthy controls and majority of the SPD patients (90%) were recruited through advertisement in local newspapers. The remaining SPD patients were recruited through referrals from psychiatric clinics at the Bronx Veterans Affairs Medical Center and Mount Sinai. Schizophrenia patients were recruited from inpatient and outpatient units at both of these hospitals. Participants provided written informed consent in accordance with Institutional Review Board guidelines. A subgroup of the healthy controls (n=24), SPD patients (n=12), and schizophrenia patients (n=26) were previously included in studies examining Brodmann areas using FDG-PET (Buchsbaum et al 2002), cingulate volume (Haznedar et al 2004) and whole temporal lobe volume (Downhill et al 2001).
All of the schizophrenia patients received the Brief Psychiatric Scale (BPRS) (Overall and Gorham 1962) within one week of their MRI scan. We report the total 18-item BPRS score, Positive and Negative Symptom Factor scores (Opler 1984). Since BPRS was not obtained for the SPD patients, we grouped their DSM symptoms into three composite scores: interpersonal, paranoid, and cognitive/perceptual based on our prior work (Bergman et al 1996; Mitropoulou et al 2002). These factors have been argued to be more sensitive than the BPRS in SPD patients and the interpersonal factor resembles the negative symptom score on the BPRS.
2.2 MR image collection
T1-weighted axial MRI scans were acquired with a 1.5-T Signa-5x system. The acquisition parameters were: repetition time=24 msec, echo time=5 msec, flip angle=40°, slice thickness=1.2 mm, pixel matrix=256×256, field of view=23 cm, and total slices=128. MRI scans were re-sectioned to standard Talairach-Tournoux (Talairach and Tournoux 1988) position.
2.3 Brodmann area measurement and tissue type quantification
Gray and white matter volumes within Brodmann areas and cingulate gyrus were analyzed on coronal MRI slices using our standard methods briefly described below and detailed elsewhere (Buchsbaum et al 2002; Hazlett et al 1998; Mitelman et al 2005a).
A digitized version of a histologically-based atlas (Perry et al 1991) which includes 33 coronal slice maps of Brodmann areas defined by microscopic examination of one entire postmortem brain was used to delineate the Brodmann areas. Coronal slices perpendicular to the anterior-posterior commissure line were reconstructed in a 256×256 pixel matrix. First, we determined the front (first slice containing the cortical ribbon) and back of the brain (last slice containing the cortical ribbon) and identified 33 evenly spaced slices such that the first slice began 1/34th of the distance from front to back. For each temporal lobe, we identified the slice with the anterior temporal pole and the most posterior extent of the Sylvian fissure and divided the interval into 13 equally spaced slices. The posterior extent was determined by following the main fissure posteriorly until it had no sulcal depth. The brain edge was obtained on the approximately circular 33 non-temporal slices and 26 (13 in each hemisphere) temporal slices by depositing points visually on the tips of the gyri and then fitting a spline curve to the points. Each slice was then divided into 20 radial sectors on each hemisphere surface and 10 midline sectors. Brodmann areas were then assessed for the gray matter, white matter, and CSF pixels within each sector (see later discussion of segmentation method); means were weighted according to the number of sectors in each region of interest and proportionally combined to obtain a single measure. Four Brodmann areas were combined (1-2-3-5) as delineated by Perry.
For gray matter and white matter quantification, the coronal images for each of the lobes were segmented into matter type by using cut-off values individually determined in each subject by examining the within-brain-edge histogram of axial MRI signal intensity values. Every subject had a clear point of rarity between gray and white matter. Validation of our gray/white matter segmentation approach has been reported (Mitelman et al 2003; Mitelman et al 2005a).
2.4 Statistical methods
We obtained volume data from 39 Brodmann areas (BAs: 1-2-3-5, 4,6,7a, 7b,8,9,10,11,12,17,18,19,20,21,22,23,24,25,27,28,29,30,31,32,34,35,36,37,38,39,40,41,42,43,44,45,46, and 47) identified by the Perry atlas (Figure-1). Subgroups of these variables developed on a theoretical and anatomical basis were entered into a series of mixed-design MANOVAs with diagnostic group (Healthy controls vs. SPD vs. Schizophrenia) as the between-group factor. Repeated measures included: region (e.g., orbital, dorsolateral), selected sets of Brodmann areas (nested within regions, e.g., orbital: BA11, BA12, BA47 and dorsolateral: BA44, BA45, BA46), hemisphere, and tissue type (gray, white matter). Group×Region and higher order interactions were examined to establish regional differences. Follow-up simple interactions were performed to identify the strongest sources of group interactions. We computed relative size as the ratio of area of ROI volume/total brain volume multiplied by 100. While ratio measures may not adequately correct for normal variation in intracranial volume, they are more commonly reported. Note that in reporting repeated-measures MANOVA, means for the main effect of diagnostic group and Group×Region interactions are collapsed across the BAs.
Figure 1. Delineation of cortical Brodmann areas. Upper left: Perry brain atlas for slice #5 showing frontal pole Brodmann areas (e.g., 9, 46, 45…) in raw drawing and with sectors added.
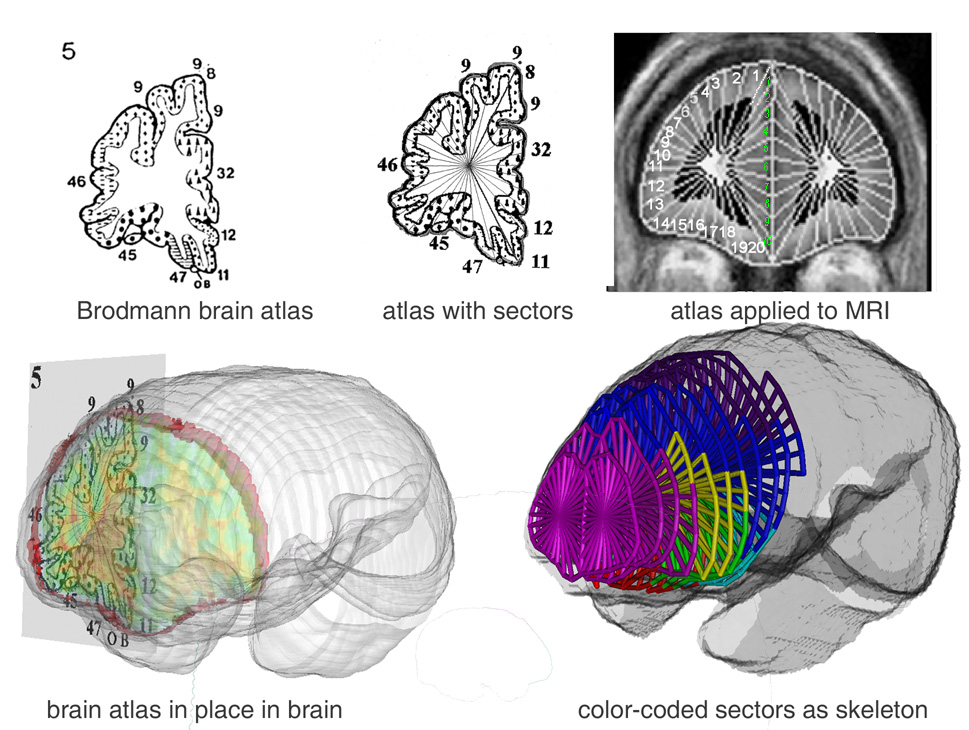
Upper right: Coronal brain edges are traced on individual MRI slices and set of sectors applied by computer algorithm, 20 sectors on the lateral and orbital surface (white numbers) and 10 in the medial portion (green numbers) for a total of 30. Bottom Left: Perry atlas in three-dimensional perspective within MRI surface. Gray and white matter volume within each Brodmann area is calculated. Bottom right: Diagram of frontal Brodmann areas as colored stick diagram: area 10 is magenta, area 9 is blue, area 46 yellow, as example.
For the multivariate analysis of variance (MANOVA), we report the multivariate (Wilks lambda) F from Statistica (StatSoft 2003). Fisher’s Least Significant Differences (LSD) tests were used to follow-up significant interaction effects with diagnostic group. We examine only the frontal lobe, cingulate, and lateral temporal lobe with nested MANOVA. This approach, which provided tests of hypothesized group differences, helps minimize Type I statistical error involved with t-tests for each area, group contrast, and hemisphere. In a further effort, we only examine significant main effects for diagnostic group and interactions between diagnostic group and gray/white and hemisphere or within-region Brodmann area effects. To examine symptom correlates of gray/white matter volume in prefrontal and temporal cortex, we used Pearson product-moment correlations. Lastly, because some studies have identified additional brain regions-of-interest, we report exploratory t-tests on every Brodmann area as a supplementary table only.
3. Results
We present the mean volumes and standard deviations for each of the Brodmann areas by group in a supplementary table (Table-2). Our hypothesis-driven MANOVA results are presented below.
3.1 Cingulate gyrus arch volume
A Group×Cingulate Brodmann area (BA25,24,31,23,29)×Matter type×Hemisphere MANOVA examined whether there were schizophrenia-spectrum differences in cingulate gyrus volume. Compared with healthy controls, both SPD and schizophrenia patients had significantly less gray matter volume (mean±SD: healthy controls=0.226± 0.024, SPD=0.215±0.018, schizophrenia=0.218±0.023), SPDs had significantly greater white matter volume and the two patient groups did not differ from each other (healthy controls=0.226±0.024, SPD=0.233±0.018, schizophrenia=0.230±0.023; Significant Group × Matter type interaction (F[2,281]=7.44, p=0.0007, Wilks; significant post-hoc comparisons were p<0.002).
Compared with healthy controls, the schizophrenia patients displayed the greatest reduction in cingulate volume in BA24 and SPD patients were intermediate, although none of the follow-up tests reached significance (Figure-2). The gray/white matter effect was largest for BA24, 31, and 23 (Figure-3). Follow-up tests revealed that both patient groups had significantly less gray and more white matter in BA31. SPD patients also showed reduced gray but increased white matter in BA24 and BA23 (all p<0.04, LSD). Schizophrenia patients had significantly reduced gray matter in BA31. White matter volume in BA24 was significantly greater in SPD than schizophrenia patients and this was the only cingulate region where the patient groups differed (p=0.002, LSD). The main effect of group (p=0.46) and none of the other interactions with group reached significance.
Figure 2.
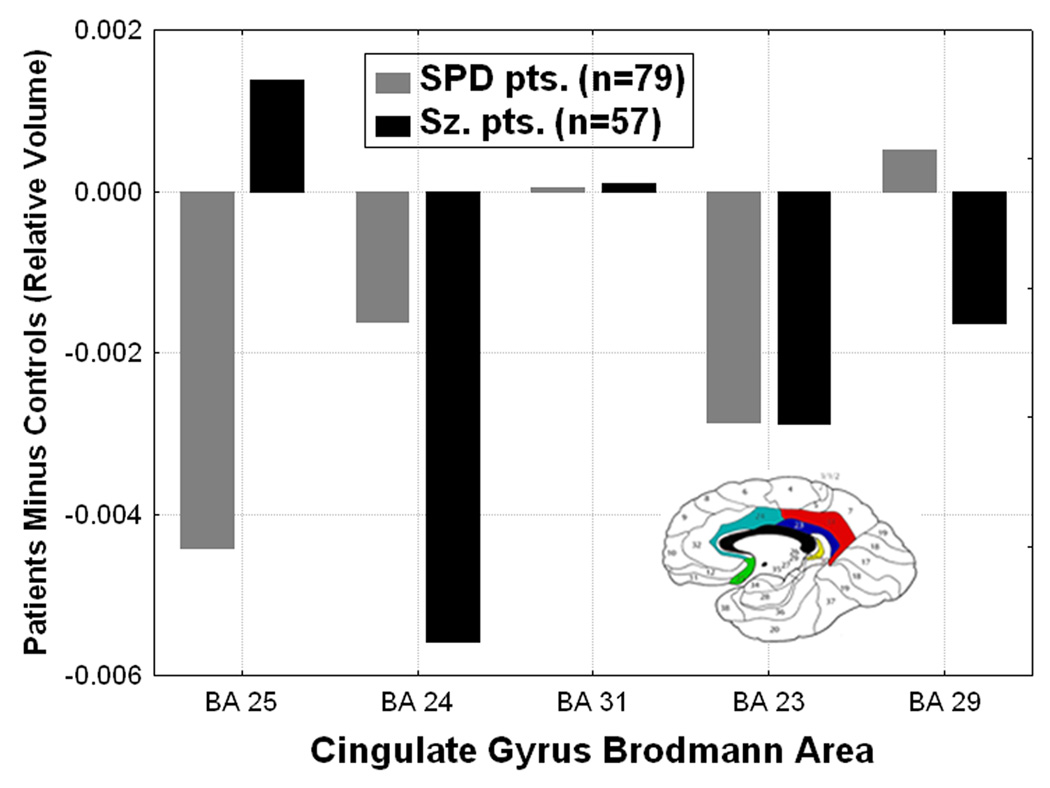
Differences from normal in cingulate gyrus Brodmann area volumes are shown for the schizophrenia spectrum groups. Compared with healthy controls, the schizophrenia patients showed the greatest cingulate volume reduction in BA 24 and SPD patients were intermediate (Group × Brodmann area interaction, (F[8,556]=2.92, p=0.003, Wilks). Y-axis shows 100×(roi volume/total brain volume) patients minus normals.
Figure 3.
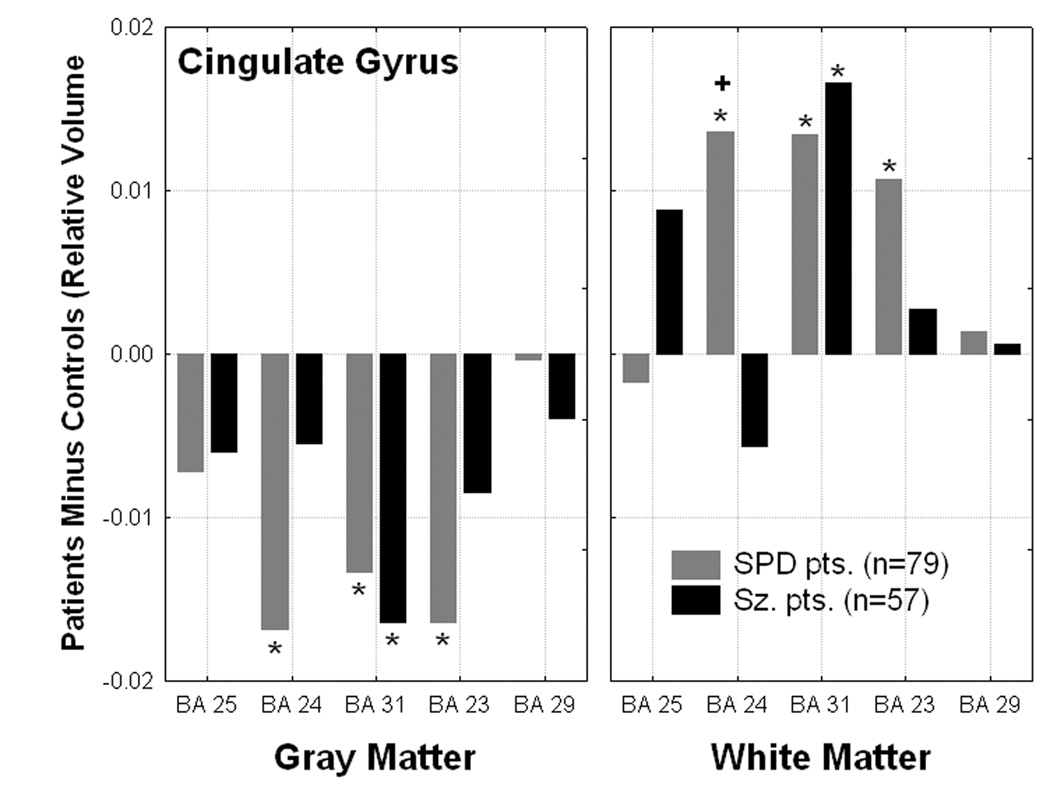
Differences from normal in gray/white matter volume in the cingulate gyrus showed significantly decreased gray and increased white matter volume in the schizophrenia-spectrum groups (Group × Matter type × Brodmann area interaction for the cingulate arch, F[8,556]=3.73, p=.0003, Wilks). *all p< 0.04, Fisher’s LSD). “+” denotes SPD>Sz patients, p=0.002, Fisher’s LSD test.
3.2 Anterior, orbital, and dorsolateral prefrontal cortex volume
A Group×Rregion (Anterior: BA 8,9,10; Orbital: BA 11,12,47; Dorsolateral: BA 44,45,46)×Brodmann area×Matter type×Hemisphere MANOVA examined whether schizophrenia-spectrum differences existed within prefrontal cortex. The schizophrenia patients had significantly reduced overall gray (p=0.000013) but not white matter volume in prefrontal cortex (averaged across hemisphere and all BAs) compared with the controls and SPD patients (Figure-4). Compared with healthy controls, schizophrenia patients had reduced volume in the anterior region of the prefrontal cortex, especially BA10, (p=0.003, LSD test) while SPD patients showed greater-than-normal BA10 volume (Figure-5). In the right hemisphere, both patient groups showed reduced prefrontal volume while in the left hemisphere, schizophrenia but not SPD patients showed reduced volume, predominantly in the anterior prefrontal region (BA8, 9, and 10; Group×Region×Hemisphere interaction, F[4,560]=5.41, p=.0003, Group×Hemisphere interaction, F[2,281]=3.96, p=.02). Neither the main effect of group (p=0.15), nor any of the other interactions with group were significant.
Figure 4.
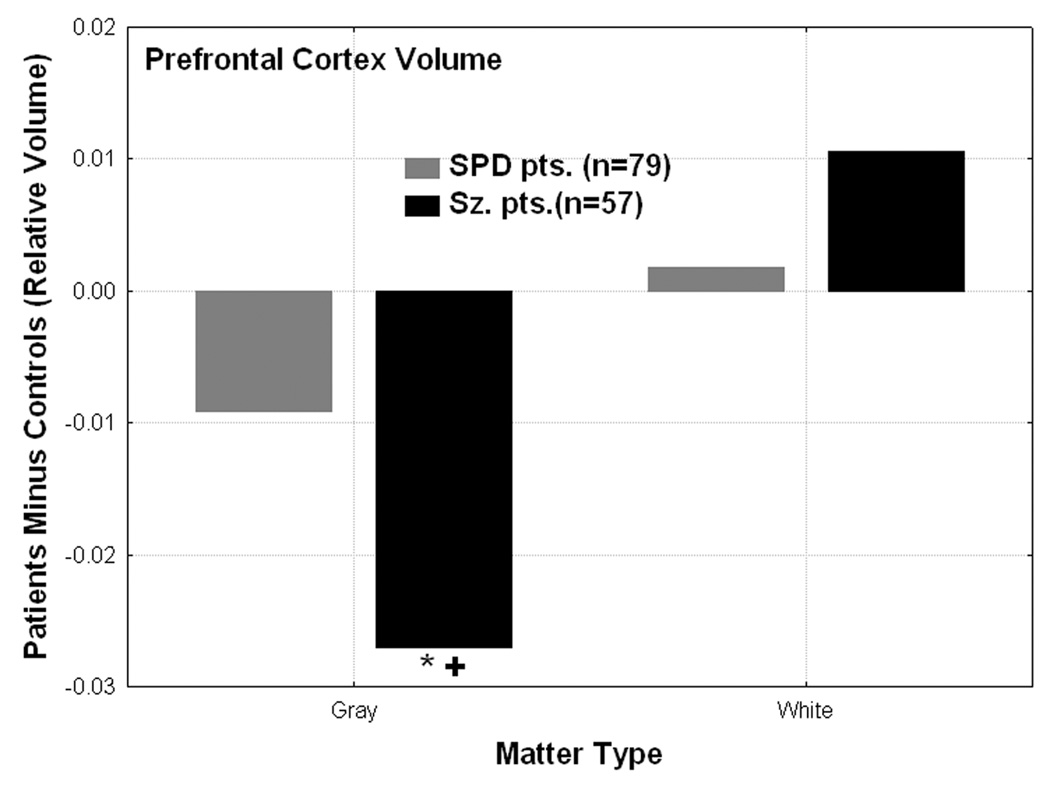
Gray and white matter prefrontal cortex volume differences from normal are shown for the schizophrenia-spectrum groups. The schizophrenia patients showed significantly reduced gray matter volume in prefrontal cortex (averaged across hemisphere and all 9 prefrontal cortex BAs which include BA 8, 9, 10, 11, 12, 47, 44, 45, 46) compared with the healthy controls (*N>Sz, p=0.00001, Fisher’s test) and SPD patients (“+” denotes SPD>Sz, p=0.009, Fisher’s LSD test). Group × Matter type interaction, F[2,281]=4.09, p=0.018).
Figure 5.
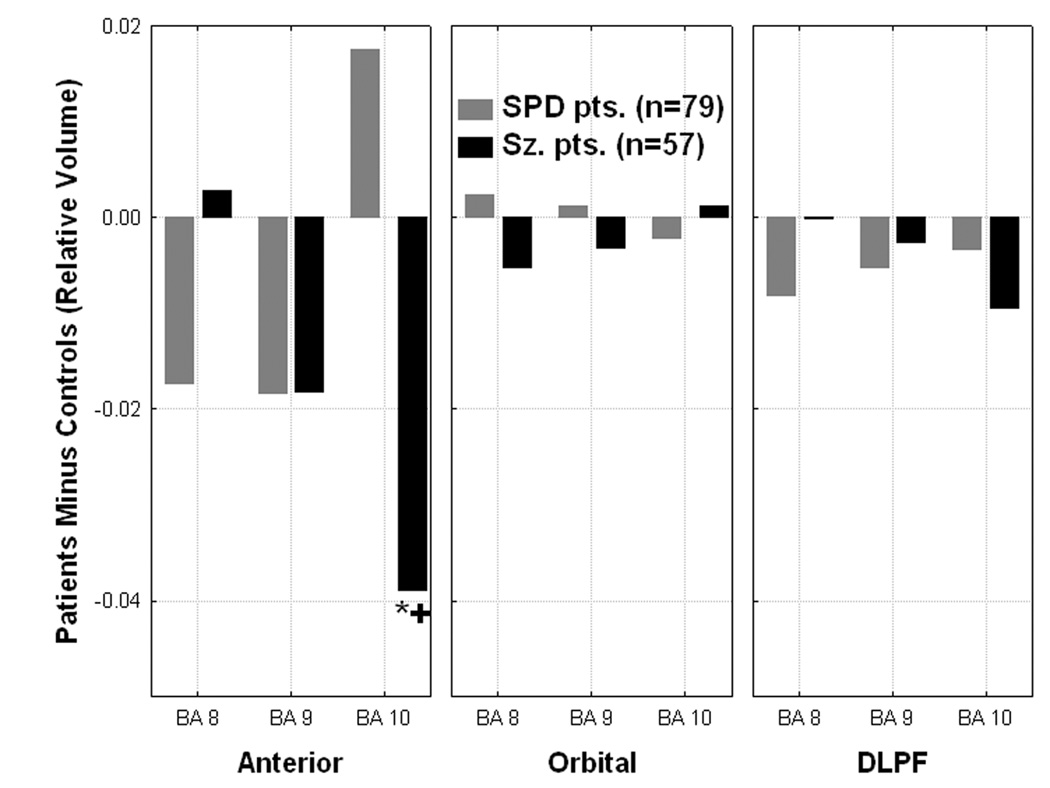
Significant Group × Region × Brodmann area interaction (F[8,556]=7.48, p=0.000001) is shown. *C>Sz patients, p=0.003, Fisher’s LSD test. +SPD>Sz patients, p=0.0001, Fisher’s LSD test. The Group × Region interaction (collapsed across BAs within each of the three regions: anterior, orbital, and dorsolateral) was also significant, F[4,560]=3.28, p=0.01.
3.3 Temporal lobe (BA22, 21, and 20) volume
A Group×Region(superior: BA22, middle: BA21, and inferior temporal gyrus: BA20)×Matter type×Hemisphere MANOVA was conducted to examine group differences in the temporal cortex. There was a highly significant main effect of group which indicated a spectrum pattern with Healthy controls>SPD>Schizophrenia patients for average relative temporal lobe volume (F[2,281]=5.14, p=0.006; Controls: mean=0.659±0.042, SPD patients: mean=0.649±0.044, Schizophrenia patients: mean=0.638±0.048). This spectrum pattern was found in the left hemisphere but in the right hemisphere, the schizophrenia patients showed reduced volume and the SPD patients were similar to normal (Group×Hemisphere interaction, F[2,281]=10.60, p=0.00004). A Group×Matter type interaction indicated a spectrum pattern for temporal lobe gray matter with C>SPD>Sz and the opposite Sz>SPD>C for white matter (F[2,281]=12.67, p=0.000005). Compared with controls, both patient groups showed reduced gray matter volume in the middle temporal gyrus, only the schizophrenia patients showed reduced gray matter volume in superior and inferior temporal gyrus, and SPD patients showed increased white matter volume in the inferior temporal gyrus (Figure-6-top).
Figure 6 (TOP).
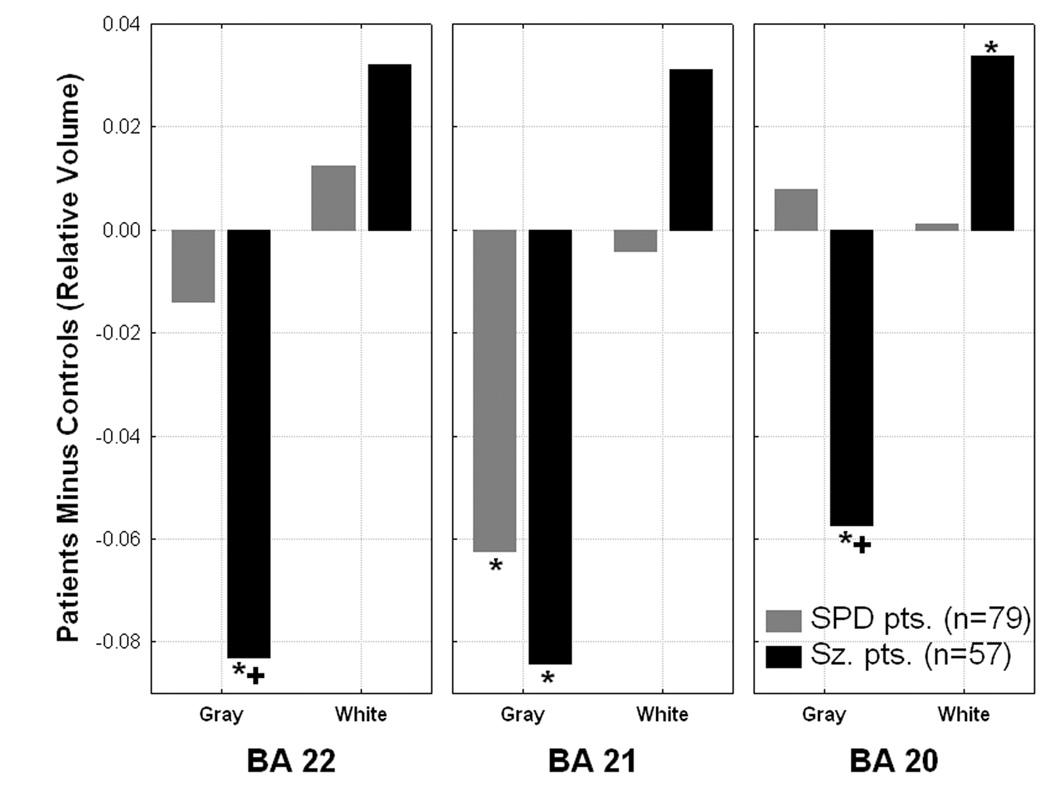
A significant Group × Brodmann area × Matter type interaction (F[4,560]=5.89, p=0.0001) was observed in the temporal lobe. *significantly different from healthy controls, all p values between 0.04-0.000001, 1SPD>Sz patients, both p<0.0005, Fisher’s LSD test.
In the superior temporal gyrus, the schizophrenia patients (p=0.03, LSD test) but not the SPD patients showed significantly reduced volume in the left hemisphere compared to normal. In the middle temporal gyrus, both patient groups showed significantly reduced left (both p<0.013) but not right volume. In the inferior temporal gyrus there were no group differences in laterality (Figure-6-bottom).
Figure 6 (BOTTOM).
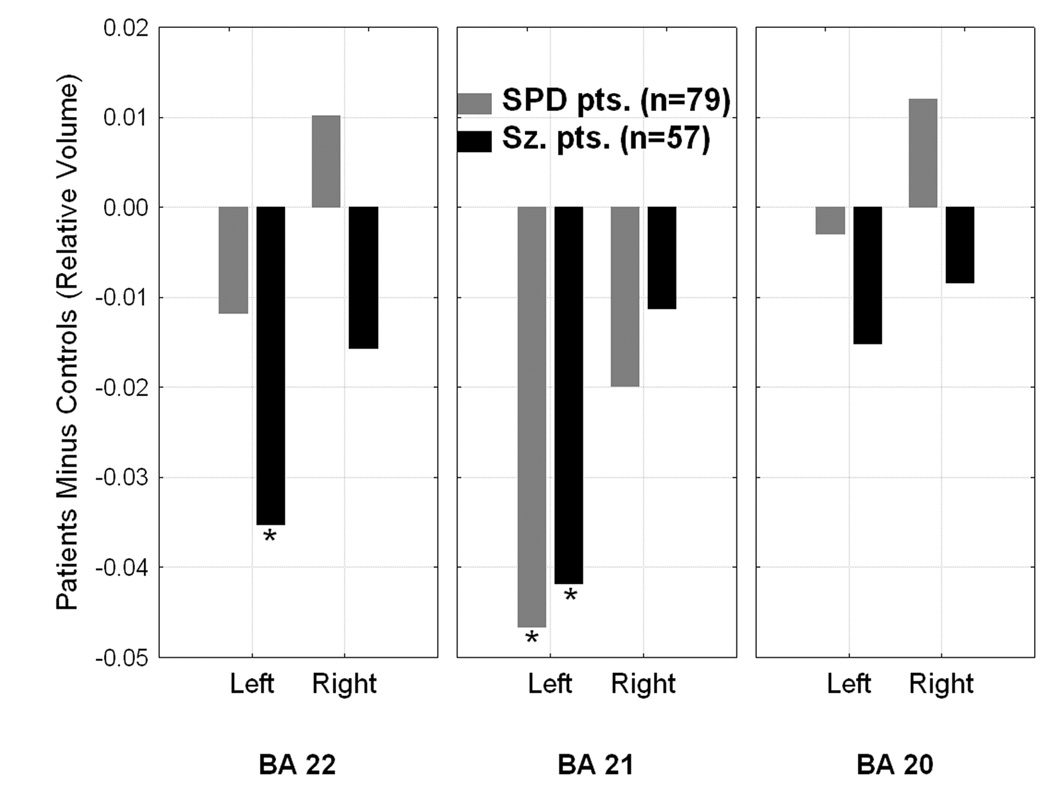
Group × Brodmann area × Hemisphere interaction, F[4,560]=2.79, p=0.026. *significantly different from healthy controls, all p values<0.04, Fisher’s LSD test. A Group×Brodmann area interaction (collapsed across gray/white matter; F[4,560]=8.09, p=0.000002) indicated that the schizophrenia patients had smaller superior and middle temporal gyrus volume compared with healthy controls (p<0.031 and p<0.025, respectively) while SPD patients only had reduced middle temporal gyrus volume (p<0.002).
3.4 Absolute brain volume
The three groups did not differ on total brain volume (Controls: mean=1216cm3±SD=125; SPD patients: mean =1205cm3±125, schizophrenia patients: mean= 1214cm3±100, F[2,281]=0.20, p=0.82). Total brain volume was determined by adding the volume of gray and white matter for all Brodmann regions across the 33 coronal slices.
In order to examine whether the three groups differed in absolute volume for the four lobes, we conducted a Group×Lobe (frontal, parietal, temporal, occipital)×Matter type×Hemisphere MANOVA. Brodmann areas included for each lobe were as follows: frontal lobe: BA8,9,10,11,12,32,44,45,46, and 47; parietal lobe: BA7,39, and 40; temporal lobe: BA20,21,22,27,28,30,35,36,37,38,41, and 42; and occipital lobe: BA17,18, and 19. The groups did not differ in overall absolute volume (collapsed across all lobes, matter type, and hemisphere), F[2, 281]=1.27, p=0.28. Compared with the healthy controls, the schizophrenia patients showed significantly reduced gray matter volume in all four lobes, however, the temporal lobe reduction (p<0.004, LSD test) was most striking and more prominent in the left hemisphere (Group×Lobe interaction (F[6,558]=2,41, p=0.026; Group×Lobe×Hemisphere interaction, F[6,558]=2.49, p=0.02). Schizophrenia patients showed small increases in white matter in all four lobes compared with healthy controls. In contrast, SPD patients showed more mild gray matter reductions in frontal, parietal, and temporal cortex, and none in occipital cortex (Group×Lobe×Matter type interaction, F[6,558]=4.12, p<0.0005; Figure-7). The Group×Matter type interaction confirmed a spectrum pattern showing controls had greater overall gray matter volume averaged across the four lobes, schizophrenia patients had the least, and SPD patients were intermediate, while the pattern was reversed for white matter volume with schizophrenia patients having the greatest white matter volume (F[2,281]=9.74, p=0.00008). A Group×Hemisphere interaction indicated that overall volume shrinkage was marked in both the left and right hemispheres in schizophrenia while SPD patients were intermediate between healthy controls and schizophrenia patients in the left hemisphere, but similar to normal in the right (F[2,281]=8.37, p<0.0003). A MANOVA on relative volume for the four lobes produced the same pattern of significant group differences (e.g., Group×Lobe×Matter type interaction, F[6,558]=4.20, p<0.0004).
Figure 7.
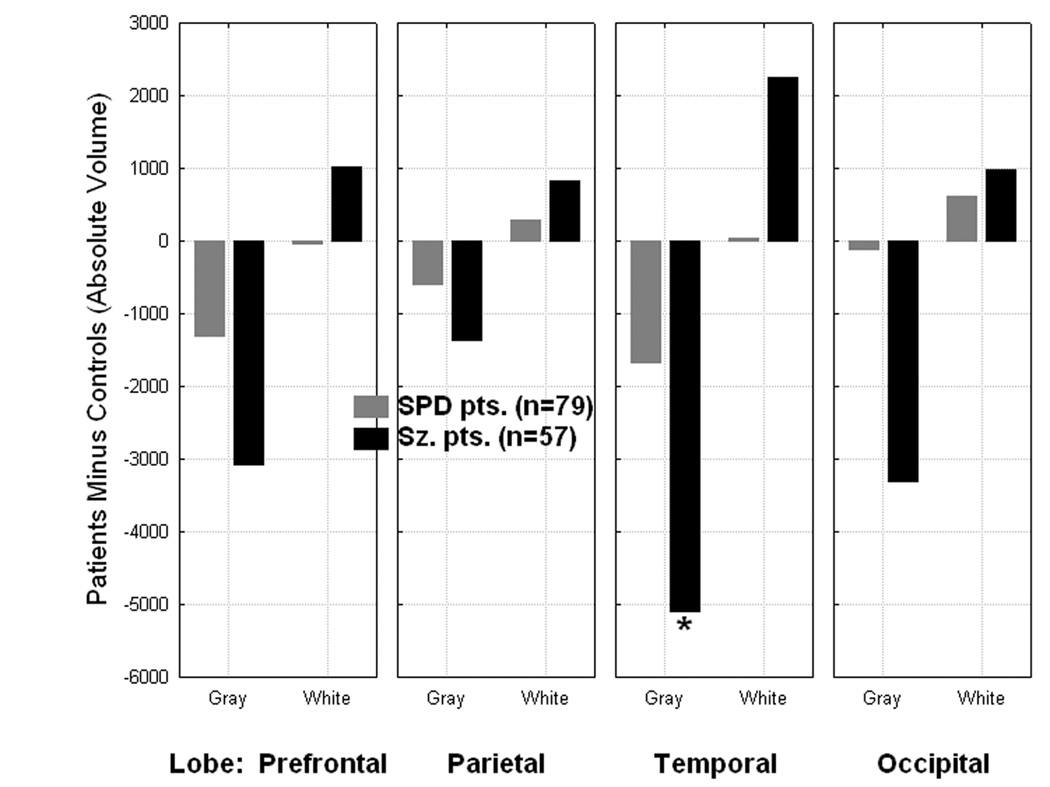
Group × Lobe × Matter type interaction, F[6, 558]=4.12, p=0.00047, *p=0.0036, Fisher’s LSD test.
3.5 Symptom correlates
To minimize the number of tests, we conducted volume vs. clinical symptom correlations with only the seven BAs in prefrontal and temporal cortex where we found significant between-group volume differences (i.e. significant interactions with Group: BA24, 8, 9, 10, 22, 21, and 20).
Among the schizophrenia patients, smaller gray and larger white matter volume in fronto-temporal BAs was associated with greater severity of BPRS negative symptoms (Table-3). In contrast, individual differences in positive symptom severity was generally not associated with cortical volume.
Table 3.
BPRS Symptom correlates of gray and white matter volume in schizophrenia patients.
| Positive Symptoms | Negative Symptoms | |||
|---|---|---|---|---|
| Matter: | Matter: | |||
| G | W | G | W | |
| BA 24 | ns | ns | ns | ns |
| BA 8 | ns | −0.30* | −0.32* | 0.41** |
| BA 9 | ns | ns | −0.30* | ns |
| BA 10 | ns | ns | −0.37* | ns |
| BA 22 | ns | ns | −0.47** | 0.37* |
| BA 21 | ns | ns | −0.51** | 0.43** |
| BA 20 | ns | ns | −0.41** | 0 .35* |
p<0.05, Pearson correlation
p<0.0018, Bonferroni corrected for p<0.05.
Only two of the seven BAs examined showed significant correlations in the SPD group. Smaller gray matter volume in BA24 was associated with greater interpersonal impairment (r=−0.31, p=.007). Smaller white matter volume in BA22 (STG) was associated with greater cognitive impairment (r=−0.25, p=.026).
4. Discussion
The present study indicates the schizophrenia patients had reduced gray matter volume widely across the cortex but more profoundly in the frontal and temporal lobes. The SPD patients similarly had reduced volume in the frontal and temporal lobes only and the decrease was about half that observed in schizophrenia. Thus, for the frontotemporal deficit, the schizophrenia-spectrum concept is supported with SPD patients intermediate between healthy controls and schizophrenia patients. This spectrum finding suggests that SPD probably represents a milder form of disease along the schizophrenia continuum which is consistent with the review of Dickey et al. (Dickey et al 2002a) and other morphometric studies involving SPD and schizophrenia patients (Kawasaki et al 2004; Suzuki et al 2005; Takahashi et al 2006b).
However, detailed analyses of each Brodmann area have suggested additional possible variations (Buchsbaum et al 2002). Four potential possibilities of normal-SPD-schizophrenia differences may be evaluated: a spectrum for the brain region with SPD intermediate between normal and schizophrenia, a sparing of the region with SPD the same as normal, a protective factor with SPD larger than normal, and a commonality with SPD and schizophrenia showing identical values. The whole prefrontal cortex, anterior cingulate (BA24) and left temporal lobe (BA21) are all examples of this spectrum relationship. Left temporal lobe BA22 shows very much smaller-than-normal volumes in schizophrenia patients than SPD patients, indicating the sparing pattern. Polar frontal lobe, BA10 is an example of a possibly protective or compensatory area with total volume (averaged over gray and white matter) decreases in schizophrenia and increases in SPD patients. Yet, the middle temporal gyrus (BA21) and posterior cingulate (BA31) are areas where common gray matter deficits in SPD and schizophrenia are seen. Taken together, this variety of patterns is consistent with a multiple gene model in which several deficits produce schizophrenia, fewer deficits produce SPD, and some protective or modulatory factors ameliorate full development of schizophrenia in SPD. This model originated from the hypothesis that schizophrenia results from the cumulative impact of multiple common small-effect, genetic variants, interacting with environmental exposures to exceed a biological threshold (Gottesman 1982).
In the temporal lobe, patients with schizophrenia show reduced volume in both left hemisphere BA22 and 21, while SPD patients show small reductions in BA22 but full reduction in BA21. Dickey (Dickey et al 1999) found about a 10% decrease (0.03 for relative data) for BA22 in SPD but did not report schizophrenia data; our SPD decrease in the current study was 0.02 for BA22 but 0.08 for BA21. In our earlier study with a much smaller SPD sample (n=13 overlapping with current sample), larger decreases were actually seen for SPD than patients with schizophrenia for both BA22 and 21. Our overall pattern of temporal lobe volume decreases being less widespread in SPD than schizophrenia patients, in comparison with healthy controls is consistent with other studies examining these three groups, e.g., (Kawasaki et al 2004; Takahashi et al 2006b). However, our finding of a shared volume reduction in SPD and schizophrenia patients in middle temporal gyrus is inconsistent with a voxel-based study that reported shared volume reduction in the left superior but not middle temporal gyrus (Kawasaki et al 2004). Differences in tracing (Dickey and Downhill studies visually traced only the mid-portion of the temporal lobe and identified the superior temporal gyrus), Brodman area identification methods (we used a stereotaxic method for separating areas 22 and 21, somewhat more analgous to voxel-basedmorphometry) methods of gray-white segmentation (threshold method used here) , and symptom severity may be important. Both the Kawasaki and Takahashi studies used a clinic-based SPD sample taking low-dose antipsychotics while we studied an unmedicated community sample suggesting these samples may differ in illness severity.
In the prefrontal cortex, the schizophrenia patients showed overall volume loss in gray but not white matter while the SPD patients did not differ from normal. This effect was most marked in BA10 where the schizophrenia patients had smaller volume compared with the healthy controls while SPD patients had greater-than-normal volume (although not significantly more). This may result from some variation sparing SPD patients from the full development of schizophrenia, an adaptive response to symptoms of the schizophrenia spectrum, or an associated developmental anomaly among other possibilities. Consistent with our findings, Suzuki et al (2005) demonstrated that the prefrontal volumes were largely preserved in SPD in contrast to widespread prefrontal involvement in schizophrenia. It is of interest that in our prior FDG-PET study (Buchsbaum et al 2002) using a task involving verbal working memory in a subgroup of subjects from this study, metabolic rates in BA10 were distinctly higher in SPD patients compared with healthy controls while schizophrenia patients were lowest and this was determined in the same way as in our present study.
In contrast to the prefrontal and temporal cortex findings where the schizophrenia patients showed more gray matter volume loss, the SPD patients showed greater differences from normal in cingulate gyrus gray matter. SPD patients had reduced gray matter volume primarily in BA24, 31, and 23 compared to normal while schizophrenia patients showed a reduction in only BA31. However, because SPD patients showed significantly greater white matter volume in these same Brodmann areas, the net volume loss in these areas was greater in schizophrenia than SPD. In other words, averaged across all the cingulate BAs, both the SPD and schizophrenia patients had reduced gray matter volume, but the SPD patients had greater-than-normal white matter volume. In a separate sample of chronic patients with schizophrenia, we also observed the largest gray matter reduction in BA24 and 31 (Mitelman et al 2005b) and others have also found posterior cingulate volume reduction, (Zhou et al 2005) consistent with the current results. We previously reported no normal-SPD group differences (Haznedar et al 1997) in the cingulate in a subgroup (n=12) of the SPD patients from the current study which suggests that large sample sizes are important for detecting subtle group differences. We have discussed elsewhere in detail (Mitelman et al 2005b) that abnormalities in the posterior cingulate and BA31 may involve thought disorder and verbal memory. Social anhedonia, important in schizophrenia, is also prominent in SPD patients (Horan et al 2007). A factor analytic study of the Beck and FDG-PET linked BA31 with anhedonia in unipolar patients (Dunn et al 2002).
The schizophrenia-spectrum fronto-temporal volume abnormalities we observed were related to symptom severity. In patients with schizophrenia, negative symptom severity of the illness as assessed with the BPRS was associated with reduced frontopolar (BA8,9,10) and temporal lobe gray matter volume and increased white matter. Among the SPD patients, smaller BA24 gray matter volume was associated with greater interpersonal impairment, while smaller STG white matter volume was associated with greater cognitive impairment. Previous MRI work reported smaller STG volume was associated with more positive symptoms in schizophrenia but that no significant correlations emerged among the SPD patients (Takahashi et al 2006a). Other work indicates prefrontal and temporal gray matter volume is unrelated to various clinical symptoms in schizophrenia while reduced white matter volume in these regions is associated with negative symptoms (Sanfilipo et al 2000). Differences in patient characteristics may explain these differing findings.
This study has a large size with attendant power to detect small differences, an important aspect when comparing SPD and normal groups. However, due to the range of diagnoses, the same symptom severity estimation scales were not available on both SPD patients and schizophrenics. Family history data would have been potentially informative, but was not collected in a formal structured genetic interview, and so was not included in the analyses. Past treatment with neuroleptics may have had some morphological effect. All of the patients in our study were off medication at the time of their MRI scan. While 77% of the SPD patients had never previously been medicated, 51% of the schizophrenia patients met this criterion. Thus, chronic effects of medication could have been more pronounced in the schizophrenia group, although the proportion difference is not large. Smaller middle temporal gyrus gray matter volumes in first-episode patients with schizophrenia but not affective psychosis (Kuroki et al 2006) suggest that our finding of a reduction in this same region in both SPD and schizophrenia is not due to prior medication treatment, instead it is a schizophrenia-spectrum specific abnormality. Future work comparing never-medicated and previously-medicated schizophrenia-spectrum patients is needed to fully address the issue of medication effects. Recently, a lack of correlation between duration of untreated psychosis and cingulate or frontal subregion volume was found (Takahashi et al 2007) but the left superior planum temporale volume was negatively correlated. Hospitalization is presumably more common in the schizophrenia group, so the effects of the inpatient environment on cortical size cannot be ruled out.
Our data analysis tends to reveal gray matter deficits associated with white matter increases. Since each Brodmann area is expressed as relative to whole brain, widespread mm3 decreases in gray matter might be reflected in relative white matter increases and since we found gray matter decrease in schizophrenia across all four lobes when mm3 data was analyzed, this must contribute to the relative white matter increase. However, the white matter increase in patients was also widespread when absolute mm3 data was analyzed. Thus there are both global tissue findings as well as regional ones: both BA8 and BA10 break the gray-down/white-up rule. A detailed analysis with adjustment of regional volumes for brain size estimated from body size may help to address this question.
In conclusion, our findings support the concept that SPD is in the schizophrenia spectrum and the frontal and temporal volume decreases are generally midway between normal and schizophrenia values. Our results suggest that increased volume in prefrontal cortex (BA10) and sparing of volume loss in temporal cortex (BA22 and BA20) may be a protective factor in SPD which reduces vulnerability to psychosis.
Supplementary Material
Acknowledgment
This work was supported in part by an Independent Investigator Award from NARSAD (Dr. Hazlett), NIMH grants (Dr. Hazlett: MH073911; Dr. Buchsbaum: MH40071, MH56489, and MH60023; Dr. Siever: MH56606), a VA Merit Award (Dr. Siever), a grant from the National Center for Research Resources (M01-RR00071) awarded to the General Clinical Research Center, Mount Sinai School of Medicine, and a MIRECC VISN3 award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen N, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH): An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Bergman AJ, Harvey PD, Mitropoulou V, Aronson A, Marder D, Silverman J, et al. The factor structure of schizotypal symptoms in a clinical population. Schizophr Bull. 1996;22:501–509. doi: 10.1093/schbul/22.3.501. [DOI] [PubMed] [Google Scholar]
- Buchsbaum M, Yang S, Hazlett E, Siegel B, Germans M, Haznedar M, et al. Ventricular volume and asymmetry in schizotypal personality disorder and schizophrenia assessed with magnetic resonance imaging. Schizophr Res. 1997;27:45–53. doi: 10.1016/S0920-9964(97)00087-X. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Nenadic I, Hazlett EA, Spiegel-Cohen J, Fleischman MB, Akhavan A, et al. Differential metabolic rates in prefrontal and temporal Brodmann areas in schizophrenia and schizotypal personality disorder. Schizophr Res. 2002;54:141–150. doi: 10.1016/s0920-9964(01)00361-9. [DOI] [PubMed] [Google Scholar]
- Davidson LL, Heinrichs RW. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: a meta-analysis. Psychiatry Res. 2003;122:69–87. doi: 10.1016/s0925-4927(02)00118-x. [DOI] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Shenton ME. The brain in schizotypal personality disorder: a review of structural MRI and CT findings. Harv Rev Psychiatry. 2000a;10:1–15. doi: 10.1080/10673220216201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Frumin M, Niznikiewicz MA, Hirayasu Y, et al. Smaller left Heschl's gyrus volume in patients with schizotypal personality disorder. The American journal of psychiatry. 2002b;159:1521–1527. doi: 10.1176/appi.ajp.159.9.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, et al. Schizotypal personality disorder and MRI abnormalities of temporal lobe gray matter. Biol Psychiatry. 1999;45:1393–1402. doi: 10.1016/s0006-3223(99)00030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Xu ML, Seidman LJ, Voglmaier MM, Niznikiewicz MA, et al. MRI abnormalities of the hippocampus and cavum septi pellucidi in females with schizotypal personality disorder. Schizophr Res. 2007;89:49–58. doi: 10.1016/j.schres.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, Shenton ME, Hirayasu Y, Fischer I, Voglmaier MM, Niznikiewicz MA, et al. Large CSF volume not attributable to ventricular volume in schizotypal personality disorder. Am J Psychiatry. 2000;157:48–54. doi: 10.1176/ajp.157.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Progress in neuro-psychopharmacology & biological psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Downhill JE, Buchsbaum MS, Hazlett EA, Barth S, Lees Roitman S, Nunn M, et al. Temporal lobe volume determined by magnetic resonance imaging in schizotypal personality disorder and schizophrenia. Schizophr Res. 2001;48:187–199. doi: 10.1016/s0920-9964(00)00131-6. [DOI] [PubMed] [Google Scholar]
- Dunn RT, Kimbrell TA, Ketter TA, Frye MA, Willis MW, Luckenbaugh DA, et al. Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol Psychiatry. 2002;51:387–399. doi: 10.1016/s0006-3223(01)01244-6. [DOI] [PubMed] [Google Scholar]
- Gottesman I, Shields J, Hanson DR. Schizophrenia, the epigenetic puzzle. New York: Cambridge University Press; 1982. [Google Scholar]
- Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, et al. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Haznedar MM, Singer MB, Schnur DB, Jimenez EA, et al. Prefrontal cortex glucose metabolism and startle eyeblink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology. 1998;35:186–198. [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, Shihabuddin L, New A, Siever LJ. Cingulate gyrus volume and metabolism in the schizophrenia spectrum. Schizophr Res. 2004;71:249–262. doi: 10.1016/j.schres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Metzger M, Solimando A, Spiegel-Cohen J, Hollander E. Anterior cingulate gyrus volume and glucose metabolism in autistic disorder. Am J Psychiatry. 1997;154:1047–1050. doi: 10.1176/ajp.154.8.1047. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Brown SA, Blanchard JJ. Social anhedonia and schizotypy: the contribution of individual differences in affective traits, stress, and coping. Psychiatry Res. 2007;149:147–156. doi: 10.1016/j.psychres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Nohara S, Hagino H, Takahashi T, Matsui M, et al. Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. European archives of psychiatry and clinical neuroscience. 2004;254:406–414. doi: 10.1007/s00406-004-0522-1. [DOI] [PubMed] [Google Scholar]
- Koo MS, Dickey CC, Park HJ, Kubicki M, Ji NY, Bouix S, et al. Smaller neocortical gray matter and larger sulcal cerebrospinal fluid volumes in neuroleptic-naive women with schizotypal personality disorder. Arch Gen Psychiatry. 2006;63:1090–1100. doi: 10.1001/archpsyc.63.10.1090. [DOI] [PubMed] [Google Scholar]
- Kuroki N, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Ersner-Hershfield H, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. The American journal of psychiatry. 2006;163:2103–2110. doi: 10.1176/appi.ajp.163.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman S, Shihabuddin L, Brickman A, Hazlett E, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry. 2003;160:2154–2168. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage. 2005a;27:753–770. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005b;72:91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Maldari LA, Moriarty PJ, New AS, Silverman JM, et al. Neuropsychological performance in schizotypal personality disorder: evidence regarding diagnostic specificity. Biol Psychiatry. 2002;52:1175–1182. doi: 10.1016/s0006-3223(02)01426-9. [DOI] [PubMed] [Google Scholar]
- Opler LA, Kay SR, Rosado V, Lindenmayer J. Positive and negative syndromes in chronic schizophrenic inpatients. Jounal of Nervous and Mental Disease. 1984;172:317–325. doi: 10.1097/00005053-198406000-00002. [DOI] [PubMed] [Google Scholar]
- Overall J, Gorham D. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Perry R, Oakley A, Perry E. Coronal brain map and dissection guide: Localization of Brodman areas in coronal sections. 1991 [Google Scholar]
- Pfohl B, Blum N, M Z. Structured Interview for DSM-III-R Personality (SIDP-R) Iowa City: University of Iowa; 1989. [Google Scholar]
- Raine A, Sheard C, Reynolds GP, Lencz T. Prefrontal structural and functional deficits associated with individual differences in schizotypal personality. Schizophr Res. 1992;7:237–247. doi: 10.1016/0920-9964(92)90018-z. [DOI] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, et al. Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry. 2000;57:471–480. doi: 10.1001/archpsyc.57.5.471. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N, et al. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry. 2002;59:839–849. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Pantelis C, Keshavan MS, Faraone SV, Goldstein JM, Horton NJ, et al. A review and new report of medial temporal lobe dysfunction as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric family study of the parahippocampal gyrus. Schizophr Bull. 2003;29:803–830. doi: 10.1093/oxfordjournals.schbul.a007048. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever L, Kalus O, Keefe R. The boundaries of schizophrenia. Psychiatry Clinics of North America. 1993;15:217–244. [PubMed] [Google Scholar]
- Siever L, Silverman J, Horvath T, Klar H, Coccaro E, Keefe R, et al. Increased morbid risk for schizophrenia-related disorders in relatives of schizotypal personality disordered patients. Arch Gen Psychiatry. 1990;47:634–640. doi: 10.1001/archpsyc.1990.01810190034005. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Koenigsberg HW, Harvey P, Mitropoulou V, Laruelle M, Abi-Dargham A, et al. Cognitive and brain function in schizotypal personality disorder. Schizophr Res. 2002;54:157–167. doi: 10.1016/s0920-9964(01)00363-2. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- StatSoft I. Statistica. 6.0 ed. Tulsa, OK: 2003. www.statsoft.com. [Google Scholar]
- Suzuki M, Zhou SY, Takahashi T, Hagino H, Kawasaki Y, Niu L, et al. Differential contributions of prefrontal and temporolimbic pathology to mechanisms of psychosis. Brain. 2005;128:2109–2122. doi: 10.1093/brain/awh554. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Tanino R, Zhou SY, Hagino H, Niu L, et al. Volume reduction of the left planum temporale gray matter associated with long duration of untreated psychosis in schizophrenia: A preliminary report. Psychiatry Res. 2007;154:209–219. doi: 10.1016/j.pscychresns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Zhou SY, Hagino H, Kawasaki Y, Yamashita I, et al. Lack of normal gender differences of the perigenual cingulate gyrus in schizophrenia spectrum disorders. A magnetic resonance imaging study. European archives of psychiatry and clinical neuroscience. 2004;254:273–280. doi: 10.1007/s00406-004-0491-4. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Zhou SY, Tanino R, Hagino H, Kawasaki Y, et al. Morphologic alterations of the parcellated superior temporal gyrus in schizophrenia spectrum. Schizophr Res. 2006a;83:131–143. doi: 10.1016/j.schres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Zhou SY, Tanino R, Hagino H, Niu L, et al. Temporal lobe gray matter in schizophrenia spectrum: a volumetric MRI study of the fusiform gyrus, parahippocampal gyrus, and middle and inferior temporal gyri. Schizophr Res. 2006b;87:116–126. doi: 10.1016/j.schres.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Matsui M, et al. Volumetric analysis of sulci/gyri-defined in vivo frontal lobe regions in schizophrenia: Precentral gyrus, cingulate gyrus, and prefrontal region. Psychiatry Res. 2005;139:127–139. doi: 10.1016/j.pscychresns.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Suzuki M, Takahashi T, Hagino H, Kawasaki Y, Matsui M, et al. Parietal lobe volume deficits in schizophrenia spectrum disorders. Schizophr Res. 2007;89:35–48. doi: 10.1016/j.schres.2006.08.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


