Abstract
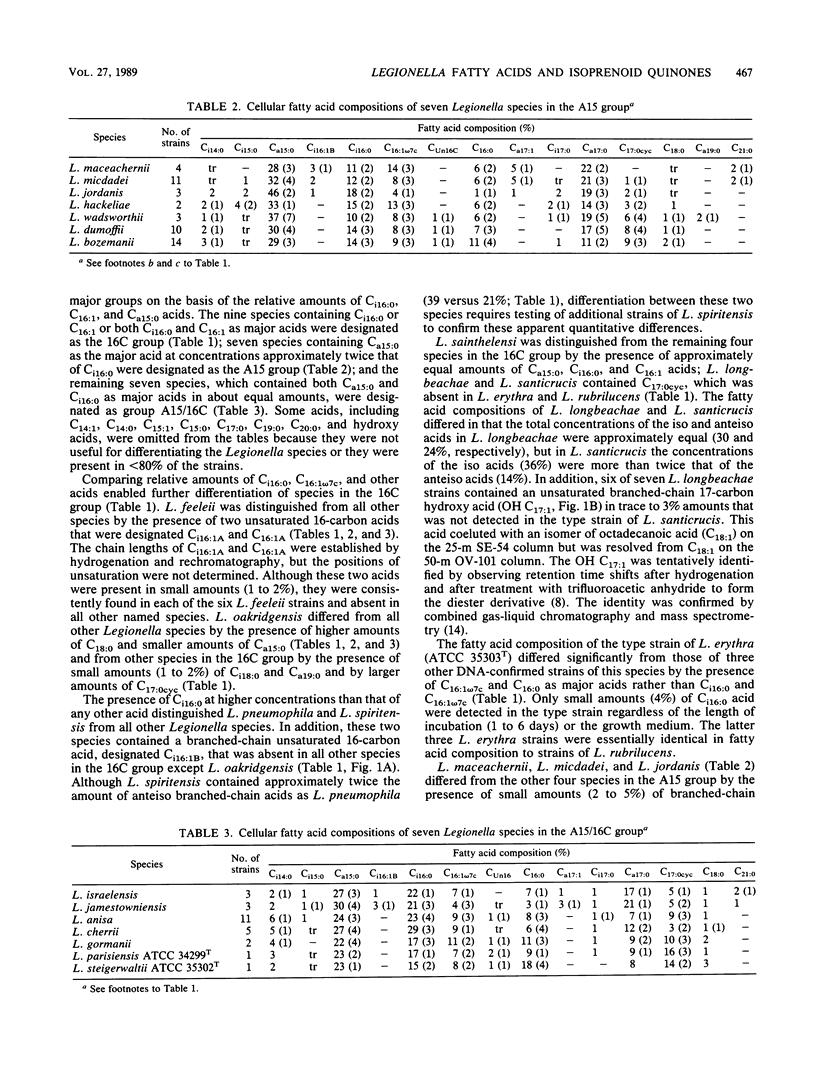
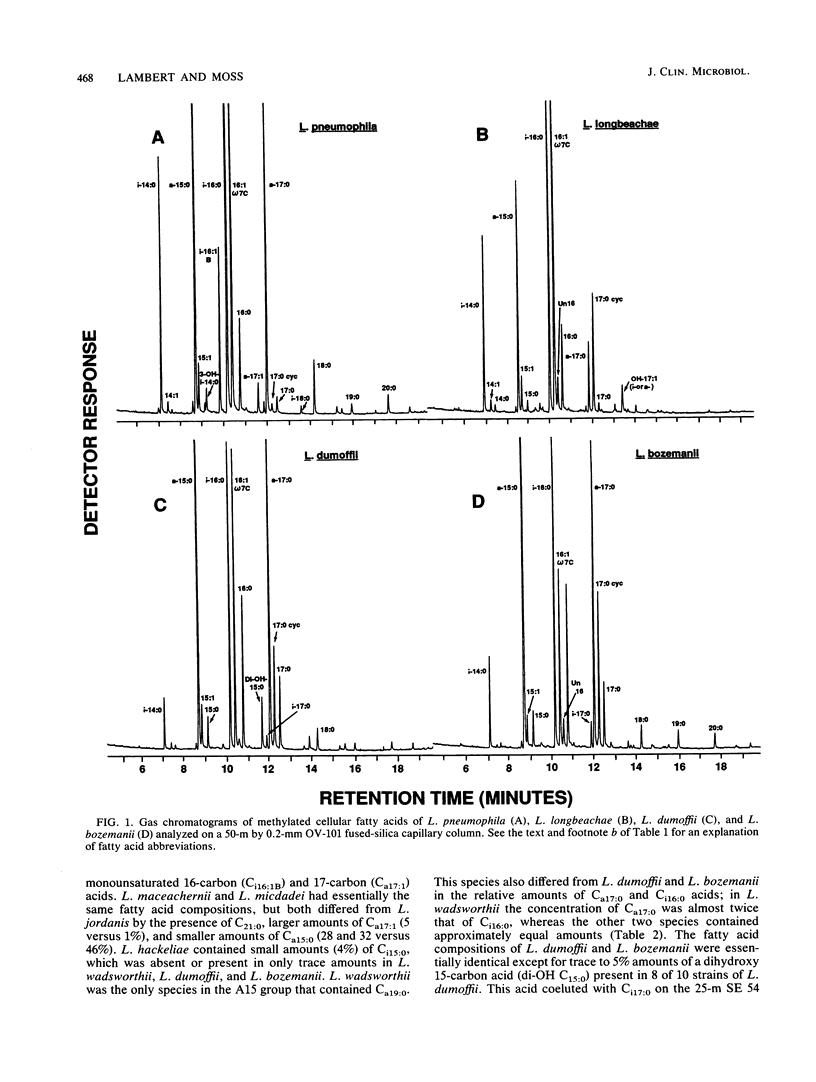
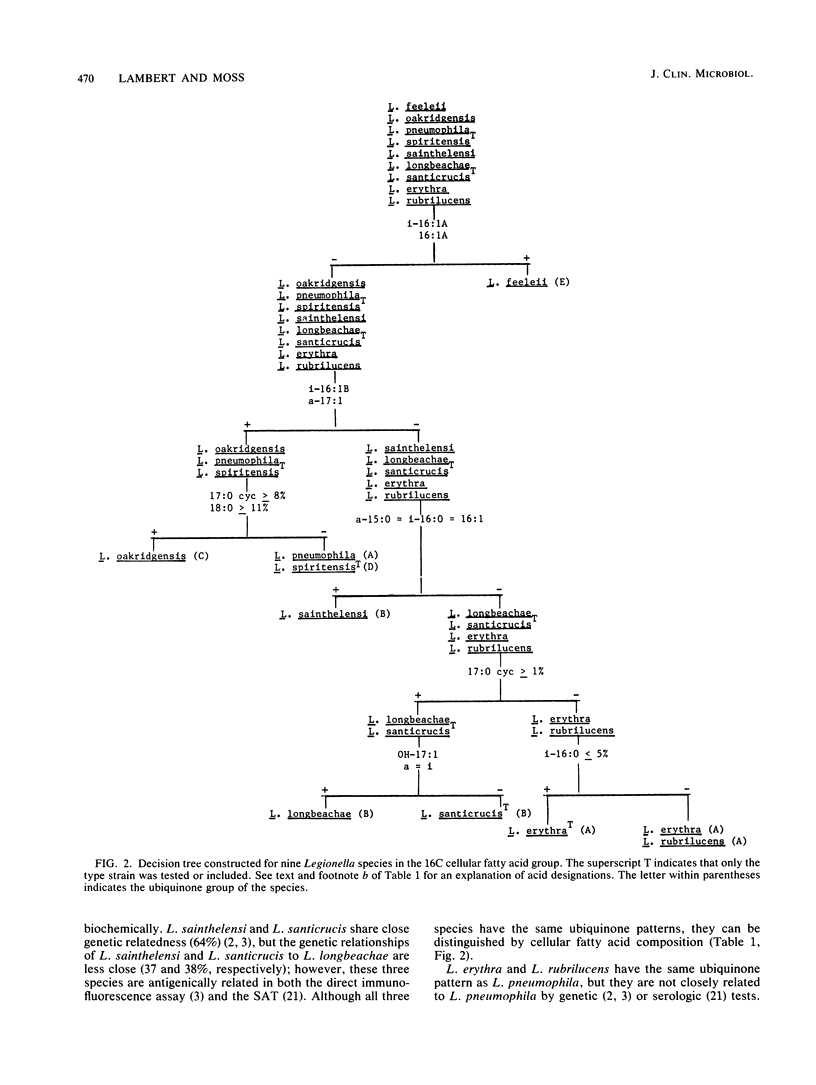
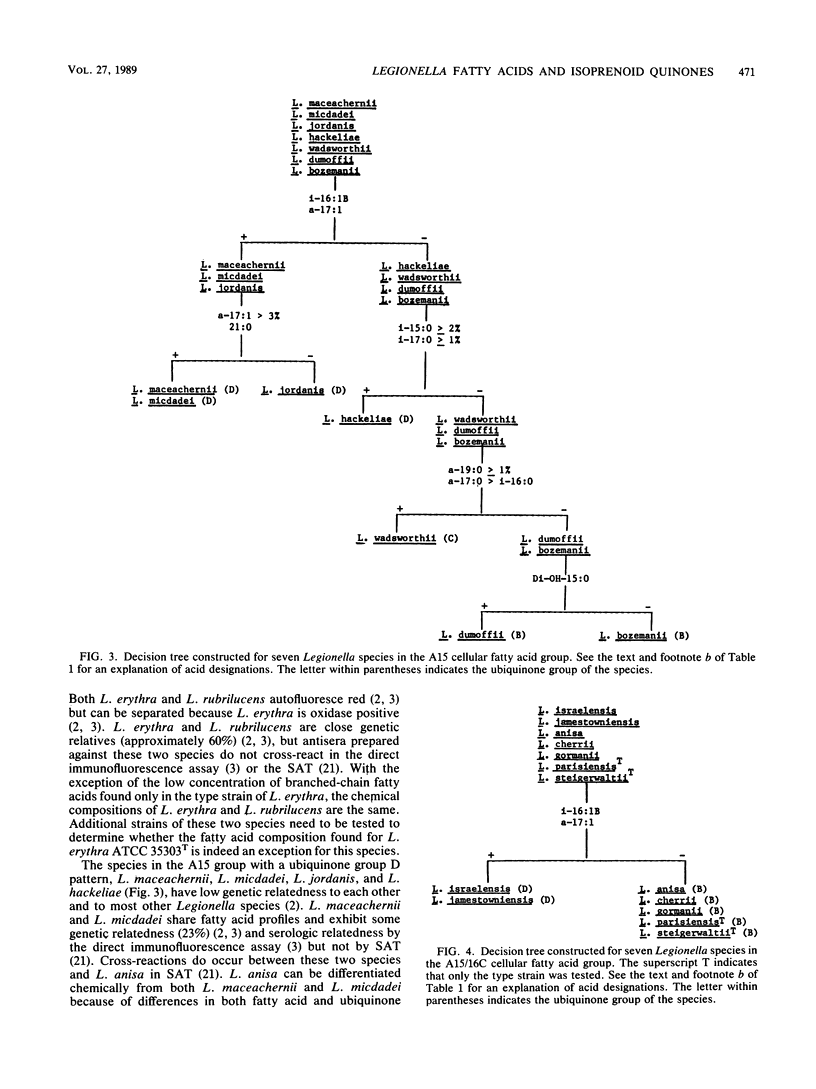
The cellular fatty acid compositions and ubiquinone contents of 182 Legionella strains representing 23 species were determined by capillary gas-liquid chromatography and reverse-phase high-performance liquid chromatography, respectively. Except for the type strain of Legionella erythra (ATCC 35303T), all Legionella species contained large (40 to 90%) amounts of branched-chain fatty acids and only trace to small (less than 0.5 to 5%) amounts of ester-linked hydroxy acids. The 23 species were placed in three major fatty acid groups on the basis of differences in the relative amounts of 14-methylpentadecanoic (Ci16:0), hexadecanoic (C16:1), and 12-methyltetradecanoic (Ca15:0) acids. All Legionella species contained ubiquinones with 9 to 14 isoprene units in the side chains and were divided into five different ubiquinone groups. The species were further differentiated into 16 groups on the basis of qualitative and quantitative differences in their fatty acid compositions and ubiquinone contents. Both of these chemical characteristics can be used to distinguish Legionella species from other gram-negative bacteria and rapidly and accurately identify suspected isolates before serologic and other tests are done.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner D. J. Classification of the legionellae. Semin Respir Infect. 1987 Dec;2(4):190–205. [PubMed] [Google Scholar]
- Brenner D. J., Steigerwalt A. G., McDade J. E. Classification of the Legionnaires' disease bacterium: Legionella pneumophila, genus novum, species nova, of the family Legionellaceae, familia nova. Ann Intern Med. 1979 Apr;90(4):656–658. doi: 10.7326/0003-4819-90-4-656. [DOI] [PubMed] [Google Scholar]
- Edelstein P. H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981 Sep;14(3):298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr D. E., Bibb W. F., Moss C. W. Isoprenoid quinones of the genus Legionella. J Clin Microbiol. 1982 Jun;15(6):1044–1048. doi: 10.1128/jcm.15.6.1044-1048.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. A., Moss C. W. Comparison of the effects of acid and base hydrolyses on hydroxy and cyclopropane fatty acids in bacteria. J Clin Microbiol. 1983 Dec;18(6):1370–1377. doi: 10.1128/jcm.18.6.1370-1377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry W. R. Dihydroxy and monohydroxy fatty acids in Legionella pneumophila. J Bacteriol. 1981 Aug;147(2):373–381. doi: 10.1128/jb.147.2.373-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade J. E., Shepard C. C., Fraser D. W., Tsai T. R., Redus M. A., Dowdle W. R. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977 Dec 1;297(22):1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Bibb W. F., Karr D. E., Guerrant G. O., Lambert M. A. Cellular fatty acid composition and ubiquinone content of Legionella feeleii sp. nov. J Clin Microbiol. 1983 Oct;18(4):917–919. doi: 10.1128/jcm.18.4.917-919.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W. Gas-liquid chromatography as an analytical tool in microbiology. J Chromatogr. 1981 Jan 9;203:337–347. doi: 10.1016/s0021-9673(00)80305-2. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Guerrant G. O. Separation of bacterial ubiquinones by reverse-phase high-pressure liquid chromatography. J Clin Microbiol. 1983 Jul;18(1):15–17. doi: 10.1128/jcm.18.1.15-17.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasculle A. W., Feeley J. C., Gibson R. J., Cordes L. G., Myerowitz R. L., Patton C. M., Gorman G. W., Carmack C. L., Ezzell J. W., Dowling J. N. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J Infect Dis. 1980 Jun;141(6):727–732. doi: 10.1093/infdis/141.6.727. [DOI] [PubMed] [Google Scholar]
- Tang P. W., Toma S., MacMillan L. G. Legionella oakridgensis: laboratory diagnosis of a human infection. J Clin Microbiol. 1985 Mar;21(3):462–463. doi: 10.1128/jcm.21.3.462-463.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker W. L., Benson R. F., Staneck J. L., Vincent S. R., Mayberry W. R., Brenner D. J., Wilkinson H. W. Legionella cincinnatiensis sp. nov. isolated from a patient with pneumonia. J Clin Microbiol. 1988 Mar;26(3):418–420. doi: 10.1128/jcm.26.3.418-420.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker W. L., Plikaytis B. B., Wilkinson H. W. Identification of 22 Legionella species and 33 serogroups with the slide agglutination test. J Clin Microbiol. 1985 May;21(5):779–782. doi: 10.1128/jcm.21.5.779-782.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker W. L., Wilkinson H. W., Benson R. F. Comparison of slide agglutination test and direct immunofluorescence assay for identification of Legionella isolates. J Clin Microbiol. 1983 Nov;18(5):1113–1118. doi: 10.1128/jcm.18.5.1113-1118.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H. W., Thacker W. L., Benson R. F., Polt S. S., Brookings E., Mayberry W. R., Brenner D. J., Gilley R. G., Kirklin J. K. Legionella birminghamensis sp. nov. isolated from a cardiac transplant recipient. J Clin Microbiol. 1987 Nov;25(11):2120–2122. doi: 10.1128/jcm.25.11.2120-2122.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


