Abstract
Breast imaging is largely indicated for detection, diagnosis, and clinical management of breast cancer and for evaluation of the integrity of breast implants. In this work, a prospective view of techniques for breast cancer detection and diagnosis is provided based on an assessment of current trends. The potential role of emerging techniques that are under various stages of research and development is also addressed. It appears that the primary imaging tool for breast cancer screening in the next decade will be high-resolution, high-contrast, anatomical x-ray imaging with or without depth information. MRI and ultrasonography will have an increasingly important adjunctive role for imaging high-risk patients and women with dense breasts. Pilot studies with dedicated breast CT have demonstrated high-resolution three-dimensional imaging capabilities, but several technological barriers must be overcome before clinical adoption. Radionuclide based imaging techniques and x-ray imaging with intravenously injected contrast offer substantial potential as a diagnostic tools and for evaluation of suspicious lesions. Developing optical and electromagnetic imaging techniques hold significant potential for physiologic information and they are likely to be of most value when integrated with or adjunctively used with techniques that provide anatomic information. Experimental studies with breast specimens suggest that phase-sensitive x-ray imaging techniques can provide edge enhancement and contrast improvement but more research is needed to evaluate their potential role in clinical breast imaging. From the technological perspective, in addition to improvements within each modality, there is likely to be a trend towards multi-modality systems that combine anatomic with physiologic information. We are also likely to transition from a standardized screening, where all women undergo the same imaging exam (mammography), to selection of a screening modality or modalities based an individual-risk or other classification.
Keywords: breast cancer, mammography, digital mammography, tomosynthesis, CT, ultrasound, MRI, contrast agents, optical imaging
INTRODUCTION
Imaging of the breast is indicated almost exclusively for the detection, diagnosis, and clinical management of cancer and for the assessment of the integrity of breast implants. Commonly used imaging modalities include mammography, ultrasonography, magnetic resonance imaging (MRI), scintimammography, single photon emission computed tomography (SPECT), and positron emission tomography (PET). The goal of this work is to provide a prospective view of breast imaging, primarily for detection and diagnosis.
In addition to standard recommendations for imaging based screening, self breast examination (SBE) has long been recommended because it is not unusual for women to feel anatomic changes in the breast that turn out to be malignant. Clinical breast examination (CBE) is commonly practiced as a part of routine physical examination by health providers or when prompted by a concern arising from SBE. The scientific evidence is inconclusive on the effectiveness of SBE and CBE in reducing breast cancer mortality. However, a recent study demonstrated a modest improvement in sensitivity when combined with mammography.1
MAMMOGRAPHY
Mammography is essentially the only widely used imaging modality for breast cancer screening. Various forms of radiographic imaging of the breast have been used for nearly one hundred years but mammography with dedicated equipment and technique did not emerge as a screening tool until the mid-1960s.2 The importance of physical compression of the breast and the significant association between microcalcifications and breast carcinoma were recognized in the early 1950s by Leborgne.3 While Gershon-Cohen in the United States and Gros in Europe were strong advocates for breast cancer screening, the development of a low kVp, high mAs technique that can be performed in a reproducible manner in 1960 by Egan enabled an organized breast cancer screening program.4 In 1966, Compagnie Générale de Radiologie (CGR, France) in collaboration with Gros developed the first dedicated mammography unit. This unit featured a molybdenum anode with a nominal 0.7-mm focal spot. Subsequently, several system manufacturers developed dedicated mammography units that facilitated its widespread availability.
Several large randomized clinical trials have shown that mammography reduces mortality from breast cancer.5, 6, 7, 8, 9, 10 While clinical trials have been essential in establishing the efficacy of mammography as a screening tool, the critical role of physics in the evolution of mammography cannot be overemphasized. Key developments in mammography include the invention of sensitive high-resolution image-intensifying screens, improved films, low x-ray absorption cassettes, and dedicated mammography film processors that enabled major dose reduction.11 The development of an x-ray tube with molybdenum (Mo) target; molybdenum filter, and beryllium (Be) window, and with a small focal spot12 that was specifically designed for mammography represents another major technological breakthrough. The concept of the projection geometry in which the central ray grazes the chest wall and meets the image receptor close to the chest wall is unique to mammography, and it represents a crucial design element that enables maximum inclusion of breast tissue. The development of low-ratio, high primary transmission moving grids and subsequently cellular grids are also important contributors to the high image quality achieved by modern mammography systems.
Screen-film mammography has long been considered as a “gold standard” for breast cancer screening. In addition to its ability to provide adequate visualization of soft tissue abnormalities, its particular strength is the ability to depict subtle calcifications. While screen-film mammography is a powerful tool for initial detection and subsequent follow-up of suspicious lesions, it has certain inherent limitations which are difficult to overcome. The most important and widely acknowledged weaknesses of screen-film mammography are associated with its limited dynamic range, contrast characteristics, susceptibility to suboptimal film processing conditions, and granularity. It also presents significant limitations in detecting very subtle lesions, especially in the presence of dense glandular tissue. These limitations were well elucidated during the preliminary development stages of digital mammography.13 Additional investment to develop improved screen-film technology is unlikely because of the emergence of digital mammography that provides wide dynamic range and offers the convenience of digital image manipulation, communication, and archival.
The term “digital mammography” is used for any technology which employs a single or multiple detector assembly to capture an electronic image of the x-rays transmitted through the breast that can be displayed, stored, and communicated electronically.14 Digital mammography is now a standard tool for breast cancer imaging and is steadily replacing screen-film mammography as the preferred tool for screening. As of this writing, approximately 36% of all mammography units in the United States are digital,15 and considering the higher throughput of digital than screen-film, it is likely that more than 36% of the examinations are performed with digital mammography. The conversion to digital is likely to accelerate and within the next three to five years digital mammography will be the dominant modality. The zero-spatial frequency detective quantum efficiency DQE(0) of digital mammography systems is in the range of 0.45 to 0.65, which is substantially higher than ∼0.35 or lower for screen-film systems.16, 17, 18 Clinical trials comparing digital mammography with screen-film mammography in a screening population demonstrate equivalency for cancer detection,19, 20, 21 and digital mammography performed significantly better for pre- and perimenopausal women younger than 50 years with dense breasts.22
Current technological approaches for digital mammography that are in clinical use can be broadly classified into “fixed-detector” and “flexible-detector” installations. In a “fixed-detector” installation, the dedicated detector is hard-wired to the support column of the mammography system and is not removable. Examples of such “fixed-detector” installations include indirect conversion hydrogenated amorphous silicon (a-Si:H) based detectors and direct conversion amorphous selenium (a-Se) based detectors. In contrast, a “flexible-detector” installation uses the x-ray cassette holder of a mammography system to house the detector. An example of such a “flexible-detector” system is computed radiography (CR) technology. One advantage of the flexible-detector installation is the ease of conversion of an existing screen-film mammography system to a digital system.
An indirect conversion system uses an intermediary stage, typically a scintillator to convert the transmitted x-rays to light photons, followed by detection of the converted light photons using an optical sensor. The scintillator of choice for indirect conversion systems is CsI:Tl, which, due to its columnar structure, suppresses lateral light diffusion and, hence, results in better preservation of spatial resolution. Development of a-Si:H arrays was an important contributor for adaptation to large field of view (full-field) imaging.23 Physical characterization indicate a substantial improvement in DQE (∼0.55 at zero-spatial frequency) characteristics compared to screen-film mammography.18, 24
A direct conversion system does not use an intermediary stage and conversion is made from x-rays to electrons after interaction in a photoconductive layer, typically a-Se. While a-Se was used more than four decades ago in xeroradiography and xeromammography, the development of advanced charge readout methods enabled its adaptation to digital mammography.25, 26 The elimination of the intermediate scintillator layer with direct detection systems allows such systems to achieve high spatial resolution,27 and higher DQE characteristics (0.54 to 0.64 at zero-spatial frequency) compared to screen-film mammography.17 In addition, a slot-scan photon-counting digital mammography system that uses silicon strip detectors is in clinical use in some countries. This approach, in addition to providing efficient scatter rejection due to the slot-scan geometry,28, 29 substantially reduces electronic noise contribution to the acquired image resulting in improved detective quantum efficiency.30
Computed radiography (CR) uses a photostimulable phosphor plate typically consisting of BaF(Br,I):Eu2+ crystals on a suitable substrate in the form of a portable cassette. When exposed to x rays, f-centers are created in proportion to x-ray exposure and remain stable for several hours. This latent image is “read out” by stimulating the photostimulable phosphor plate with a laser beam, typically in a raster fashion, resulting in the emission of light photons in the ultraviolet-blue region. The emitted light is detected by a photosensor, typically a photomultiplier tube, to generate an electronic image. While CR has been in clinical use for radiographic applications for nearly three decades, the development of a dual-side readout that uses a transparent phosphor substrate and light collection from both sides of the photostimulable phosphor plate31 was an important contributor for improved DQE (∼0.54 at zero-spatial frequency) of such systems.16, 32 Outside the United States, CR based single-side read digital mammography systems using BaFI-, BaSrFBrI-, and BaF(Br,I)-photostimulable phosphor plates are also in clinical use.33 Recent reviews of technological approaches for digital mammography have been published.14, 34, 35
While there is no consensus on spatial resolution requirements in digital mammography, current research suggests that for indirect detection approaches, a low noise detector with smaller pixel size than that used in current practice may enhance visual perception of small objects such as microcalcifications.36 Recently, it was observed that noise had a more dominant effect than display resolution for the tasks of detecting microcalcifications and discrimination of masses.37
Research and development to improve digital mammography system performance characteristics and to develop new detector technologies, are in progress. In indirect-type a-Si:H based detectors, a recent important advancement is the development of a continuous photodiode design rather than the traditionally used discrete a-Si:H photodiode array.38 This would increase the fill factor, resulting in improved signal transfer characteristics and dose efficiency. Also, incorporation of compensation lines with a-Si:H based detectors has been shown to reduce correlated electronic noise arising from external and ambient sources.39 Methods to limit lateral diffusion of light within the scintillator either by laser etching columnar CsI: Tl40 or by infusion of crystalline CsI:Tl within a tungsten grid matrix41 hold promise for improved spatial resolution, but have not yet been translated to practice.
Recent research with direct-conversion a-Se based detectors have been focused mainly on understanding the temporal imaging characteristics (image lag) and ghosting properties.42, 43, 44 Knowledge of these properties will be essential for emerging techniques of digital breast tomosynthesis (DBT) and contrast enhanced digital mammography (CEDM) that require fast image acquisition and readout. Ongoing investigations are aimed at improving the gain of a-Se based detectors through avalanche multiplication process and in understanding its implication on noise.45, 46 The development of low-noise detectors with improved sensitivity would also be beneficial to the promising technique of DBT that requires low dose per projection view. Attempts to adapt materials such as gallium arsenide (GaAs), cadmium zinc telluride (CdZnTe), mercuric iodide (HgI2) and lead iodide (PbI2) that hold promise of improved quantum efficiency are yet to bear fruition due to challenges such as charge-traps, higher leakage current, and spatial nonuniformity.47, 48, 49, 50
In CR technology, some of the important advances include the development of columnar stimulable phosphor screens based on CsBr,51 development of linear readout (line scan) technology for faster readout of the phosphor,52 and integration of these approaches.53, 54 However, we are not aware of their transition to digital mammography as yet, but that could likely occur in the near future.
Another area of substantial interest in detector technology is the development of energy-resolving large-area photon-counting detectors, which has been challenging. While several approaches for photon-counting detectors have been proposed including scintillation detection using micro-channel plates55 and gaseous detectors, it appears that semiconductor-based direct-conversion photon-counting systems may be better suited for medical imaging.56 Some of the semiconductor materials that are considered promising for photon-counting detectors include Si, GaAs, CdTe, and CdZnTe, typically bonded to a two-dimensional readout with application specific integrated circuits, and are commonly referred to as hybrid detectors. Several collaborative research projects to develop hybrid detectors exist,57, 58 increasing the prospects for their availability in the next decade.
The advent of digital mammography has prompted reconsideration of several aspects of mammographic imaging that translated from screen-film imaging. These include anode material, x-ray spectra, technique factors, and radiation dose. In addition to the common target-filter combinations of Mo-Mo, Mo-Rh, and Rh-Rh, there is an increasing trend to use other combinations such as W–Rh, W–Ag, and W–Al with digital mammography. These alternative W-target techniques may allow for modest reduction in radiation dose with no apparent reduction in image quality.59, 60, 61 In addition, the exploration of techniques such as contrast-enhanced digital mammography (CEDM) and DBT that may require x-ray tubes with increased heat capacity and output also have an influence on the choice of anode material. In addition, radiation dose reduction with photon counting detectors,62 a-Si:H indirect conversion detectors,63, 64 and CR65 have also been reported. While phantom studies indicated the possibility of 50% dose reduction with digital mammography,66 studies with clinical backgrounds indicate that such a drastic dose reduction could adversely affect detection of microcalcifications and discrimination of masses substantially, and to a lesser extent adversely affect detection of masses.67, 68 Continuing technological improvements and technique refinements could lead to further dose reduction while maintaining image quality.
Extensive investigations on radiation dose to the breast and its dependence on breast composition, breast thickness, and x-ray spectral characteristics have been documented.69, 70, 71, 72 While it was believed that radiation dose to other organs including the uterus would be low during a mammography exam, until recently quantitative estimates were unavailable.73, 74 A Monte Carlo computational study with an anthropomorphic software phantom indicates that the dose to the uterus during the first trimester, where a women may be unaware of her pregnancy, is less than 0.03 μGy per imaged breast during a bilateral two-view mammography exam.75 The use of a lead-shield further reduces this dose by a factor of 2 to 7, suggesting that if it is clinically necessary for a woman in her first trimester of pregnancy to undergo mammography, then the radiation dose to the fetus would be minimal.75
The advantages of vigorous physical compression of the breast in terms of adequate tissue separation, x-ray scatter, and radiation dose, for producing good mammographic image quality are well known. Unfortunately, to this date, the scientific community has been unable to overcome the vigorous breast compression needs of mammography. In screen-film mammography, it is well established that the use of anti-scatter grids improves contrast for breasts of average and above average compressed thickness. However, the usefulness of anti-scatter grid is not firmly established for thin and mainly adipose breasts. Current practice is to perform mammography with the anti-scatter grid in place for all imaging needs, except for magnification views. In spite of its advantages, anti-scatter grids do suffer from deficiencies such as incomplete suppression of scattered radiation, reduction in primary radiation reaching the detector, and artifacts.29, 76, 77
With the advent of digital mammography that would allow for numerical scatter correction techniques, the possibility of imaging without the anti-scatter grid were studied and showed promise of improved performance.78, 79, 80 However, another study indicated that removal of the anti-scatter grid could provide a modest reduction of ∼8% in radiation dose to the subject.81 Although scatter correction for images acquired without the anti-scatter grid using numerical techniques have been reported,82, 83, 84 its impact on contrast, resolution, noise, and artifacts are yet to be studied. At present, digital mammography, with the exception of slot-scanning systems, uses anti-scatter grids for scatter reduction. The emergence of DBT where the use of an anti-scatter grid is impractical, could further advance the research on scatter correction algorithms that may translate to digital mammography in the future.
We will continue to observe increased transition to digital from screen-film mammography. Also, improvements in detector technology and transition to higher kVp techniques will provide for substantial reduction in radiation dose. The prospects of a large-area photon-counting detector with the ability to provide energy resolution appears brighter.
ULTRASONOGRAPHY
The role of B-mode ultrasound in breast imaging has been largely limited to applications such as distinguishing between cystic versus solid masses, evaluation of palpable masses, and for needle core biopsy of masses. In recent years, the number of indications has been greatly expanded and breast ultrasonography is now an essential modality in breast imaging. A center frequency above 10 MHz is now recommended for adequate spatial resolution by the American College of Radiology (ACR). B-mode imaging works well for the established indications of breast ultrasonography, but its imaging ability is limited by the beam direction that is fixed and perpendicular to the face of the transducer.
In compound ultrasonography, the beam is electronically steered for improved sampling of the anatomy that may lie parallel to the ultrasound beam, rendering a more complete image. Harmonic imaging techniques take advantage of the variation of the velocity of the ultrasonic wave through different tissues that produces harmonic frequencies. The processing of harmonic signals leads to better selection of signals by suppressing effects such as side-lobe artifacts, beam defocusing, and reverberations, resulting in improved images.85, 86, 87 Color Doppler and more recently sensitive power Doppler ultrasound has been used for further evaluation of the breast.
In 2002, Kolb et al. published a landmark article that showed improved sensitivity (97% versus 74%) when adjunctively used with mammography compared to physical examination with mammography.88 They also observed an improvement in sensitivity for dense breasts with ultrasound compared to mammography. In addition, they noted that the time taken to perform an ultrasound exam was comparable to that of a clinical breast exam. However, he also observed an increased in false positive rates with the use of ultrasound as an adjunct to mammography. In recently published results of an ACRIN trial that included 2637 women with heterogeneously dense breast in at least one quadrant observed that the diagnostic accuracy improved from 0.78 to 0.91 when ultrasound was adjunctively used with mammography.89 However, there was also a substantial decrease in positive predictive value with mammography plus ultrasound (11.2%) compared to mammography (22.6%) alone. Also, the median time to perform a bilateral ultrasound exam was 19 min. Hence, the improved diagnostic accuracy provided by adjunctive use of ultrasound to mammography has to be carefully weighed against the decrease in positive predictive value and exam duration. From the technological perspective, it can be inferred that there may be a need for a combined dual-modality mammography-ultrasound system. Even if ultrasound is not adapted for screening, such a system is likely to be of benefit for diagnostic evaluation as it provides for co-registered dual-modality images.
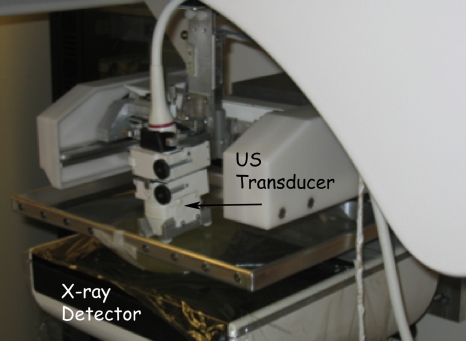
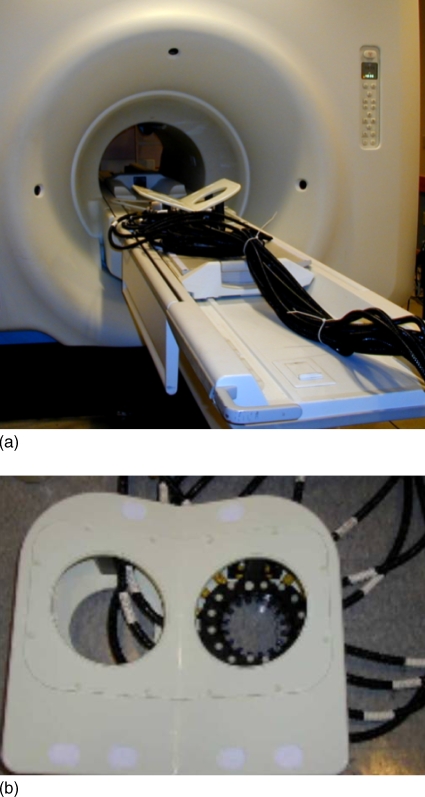
Development of a semi-automated scanning ultrasound device integrated with a digital breast tomosynthesis (DBT) system was recently reported.90 Figure 1 shows the combined DBT-US system. Performance characteristics of the DBT component indicates minimal in-plane blur and better management of artifacts with reconstruction using generalized filtered backprojection.91 Identification of compression paddles suitable for both x-ray and ultrasound imaging,92 as well as techniques to address coverage and motion issues during ultrasound scan93 have been described. Recently, development of an ultrasound computed tomography system that generates reflection, attenuation, and sound speed images has been reported.94 Spatial resolution measurements indicate in-plane resolutions of 0.5 and 4 mm in reflection and transmission modes, respectively. A pilot clinical study with 50 subjects indicate the ability to routinely detect masses greater than 15 mm in size.95
Figure 1.
Integrated dual-modality digital breast tomosynthesis and scanning ultrasound system. Provided by Paul L. Carson and Mitchell M. Goodsitt at the University of Michigan and Kai Thomenius at GE Global Research, Niskayuna, NY. This system was developed with support by NIH R01CA 91713 and Office of Naval Research grant MDA905-00-10041.
Technological improvements in transducer technology and signal processing have contributed to the extensive adoption of ultrasonography for diagnostic evaluation of the breast and for biopsy guidance. There is a possibility that ultrasound could have an adjunctive role to screening mammography for hetereogeneously dense breasts.
MAGNETIC RESONANCE IMAGING
In vitro measurements of relaxation times of tumors and normal tissue with nuclear magnetic resonance (NMR) was reported by Damadian in 1971.96 Subsequent to the development and application of local gradient fields that made imaging (NMR zeugmatography as proposed by Lauterbur97) feasible, Mansfield et al. reported on imaging with surgical breast tissue samples.98 Ross et al. and El Yousef et al. reported on some of the earliest in vivo breast MRI studies in the early 80’s.99, 100, 101 Development of a coil specifically designed for breast imaging was an important technological advancement.102 The development of gadolinium diethylene triamine pentaacetic acid (Gd-DTPA) contrast agent and its application to human imaging was an important milestone in the advancement of MRI.103, 104, 105, 106, 107, 108 Following the application of Gd-DTPA for contrast-enhanced breast MRI,109 initial interpretation criteria were based on morphology and contrast enhancement that were obtained with long acquisition times. However, this did not provide substantial contrast between tumor and proliferative changes. Hence, further research was focused on developing fast pulse sequences110, 111, 112, 113 that ushered the era of dynamic contrast enhancement studies. Another important advancement was the development of a bilateral breast coil that facilitated simultaneous imaging of both breasts. This allowed comparison of enhancement patterns between the breasts at reduced cost and time.
Currently dynamic contrast enhanced breast MRI is clinically used to provide volumetric three-dimensional (3-D) anatomical information and physiologic information that are indicative of increased vascular density and vascular permeability changes associated with angiogenesis.114 Clinical breast MRI studies have demonstrated its ability to provide accurate diagnosis, extent of disease and multi-centricity,115 and the ability to detect mammographically occult cancers in the contralateral breast.116, 117, 118, 119 Independent clinical trials for women at high-risk of hereditary breast cancer indicate increased sensitivity with breast MRI than mammography but with variable specificity.120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131 These studies have prompted the American Cancer Society to recommend the use of MRI as a adjunct to screening mammography for women with a lifetime risk of 20%–25% or greater.132 A recent meta analysis that included 44 prior studies indicate that breast MRI has a sensitivity of 0.9 and specificity of 0.72.133 Importantly, this analysis indicated that variability in specificity was due to cancer prevalence in the individual studies and due to interpretative criteria used to differentiate benign and malignant lesions.133 With the development of lexicons for breast MRI interpretation134 and standardization of procedures, variation in specificity is likely to be reduced. A recent two-part review addresses the current status of breast MRI.135, 136 Dynamic contrast enhanced MRI (DCE-MRI) is a very important tool for detection, diagnosis, and clinical management of breast cancer. However, it requires intravenous injection of Gd-contrast agent that entails some elevated risk.137, 138 Hence, by its nature contrast-enhanced MRI is likely to be limited to a subgroup of patients.
Another promising technique that has garnered substantial interest recently is proton magnetic resonance spectroscopy (MRS). This technique allows for quantitative characterization of total or composite choline concentration that has been shown to be elevated in malignant tumors compared to normal breast tissue.139 While initial works were focused on MRS,140, 141, 142, 143 recent research has been primarily on the use of MRS,144, 145, 146, 147 in part due to its higher sensitivity compared to MRS.148 MRS is a nonvasive technique that does not require contrast injection; however, most of current research has been on spectroscopic analysis of regions localized from prior DCE-MRI. Current studies have shown the correlation between DCE-MRI and MRS,149, 150 and demonstrated improved sensitivity and specificity when used as an adjunct to breast MRI.151, 152, 153 While there are interesting aspects of this technique, most of the studies were performed at 1.5 T with voxel volume of ∼1 cm3 indicating better suitability for large tumors. Since total choline concentration may be indicative of cell replication,154 MRS is being actively investigated and show promise for early determination of the effectiveness of neoadjuvant chemotherapy for locally advanced breast cancer.155, 156, 157, 158, 159 In addition, studies on MR spectroscopic imaging (MRSI) primarily to improve specificity have been reported.160, 161, 162 Importantly, one of the studies describe a method to improve signal-to-noise ratio and reduce acquisition time with sub-centimeter voxel volume.162 If this technique can evolve to the point of replacing contrast-enhanced MRI, then risks associated with contrast media and injection can be eliminated. Although this technique is in its infancy, the concept of a screening MR exam that provides high resolution morphological information with MRSI to provide physiologic information without contrast media is highly appealing. Recent reviews address the clinical prospects and challenges of MRS and allied techniques.163, 164, 165, 166
MRI already has a very important role in breast imaging as an adjunctive tool for screening and as a diagnostic tool for imaging lesions that are indeterminate from other modalities. MRI is also widely used for assessing the structural integrity of breast implants. Its role will continue to expand with the development of novel image acquisition techniques and technological improvements.
RADIONUCLIDE IMAGING
Radionuclide based imaging techniques such as scintimammography, single-photon emission computed tomography (SPECT), positron emission mammography (PEM), and positron emission tomography (PET) are additional imaging techniques that provide for physiologic information.
Scintimammography and SPECT typically use -sestamibi or -tetrofosmin for breast cancer imaging. Scintimammography is used for imaging mostly palpable lesions that were occult or indeterminate from other imaging modalities. For palpable mass, a meta-analysis indicates that the sensitivity and specificity were 87.8% and 87.5%, which is high.167 However, for nonpalpable lesions the sensitivity was reduced to 66.8%.167 In an another study, the sensitivity reduced from 74.2% for larger lesions to 48.2% for lesions smaller than 1 cm.168 New technological advances such as the development of dedicated cameras for scintimammography with improved spatial resolution has renewed interest in this field.169 Characterization of a dedicated scintimammography system was recently reported to achieve detection of ∼7 mm spheres bearing .170 A clinical study to evaluate its potential with 100 subjects scheduled to undergo biopsy of 2 cm or smaller masses indicated a substantial improvement in sensitivity for imaging lesions smaller than 1 cm.171 In an another study in which 94 high-risk subjects with normal mammographic exam were evaluated with a dedicated scintimammography camera using -sestamibi detected two malignancies, both of which were smaller than 1 cm.172 However, false-positives due to fibrocystic change, fibroadenoma, and fat necrosis were also observed. A study comparing dedicated scintimammography with conventional SPECT indicated no differences in sensitivity, but observed better performance for 1 cm or smaller lesions with the dedicated scintimammography system.173 Further improvements, such as development of a dual-head camera and with increased spatial resolution may improve sensitivity for smaller lesions.
The primary advantage of SPECT is its ability to provide 3-D information. In SPECT imaging, the development a dedicated system for breast imaging in a prone patient position with pendant breast geometry has been reported.174 This system uses a scanning trajectory that closely follows the breast contour. A more recent work described the development of a dedicated breast SPECT system using CdZnTe detectors.175 Integration of the dedicated SPECT system with a dedicated breast CT system to provide for co-registered dual-modality imaging is also in progress.176
For detection of primary tumors, -FDG-PET have been reported to have similar sensitivity as that of SPECT.177 A meta-analysis of whole body -FDG-PET that included 13 studies indicated an overall sensitivity of 89% and specificity of 80%.178 However, the mean tumor size in those studies ranged from 2 to 4 cm. Characterization of a dedicated breast PET system using four detector heads comprising of LYSO crystals coupled to position-sensitive photomultiplier tubes (PSPMTs) with multi-angle tomographic image acquisition capabilities was reported to achieve ∼2 mm spatial resolution.179 Development of PET detector modules with lutetium oxyorthosilicate (LSO) crystals coupled to PSPMTs,180 and more recently LSO coupled to position sensitive avalanche photodiodes with depth-of-interaction capabilities have been reported.181, 182 These systems can play an important role in monitoring effectiveness of therapeutic regimens.
To overcome the limited sensitivity and spatial resolution of whole-body PET systems, a PEM system was developed183, 184 and showed promising results in a pilot clinical study.185 Development of PEM systems using gadolinium orthosilicate (GSO) and yttrium-aluminium perovskite (YAP) scintillators have been reported.186, 187 A collaborative effort to develop a dedicated PEM system using cerium- and yttrium-doped lutetium orthosilicate (LYSO) scintillator elements coupled to avalanche photodiodes is also underway.188 These systems offer the potential of improved resolution and sensitivity. A pilot clinical study evaluating a prototype system using lutetium gadolinium oxyorthosilicate (LGSO) scintillator elements showed the ability to detect smaller lesions.189 All of the aforementioned dedicated PEM systems use stationary detector heads. A multi-institutional clinical study of a commercially available PEM system using translational detector heads that enrolled 94 subjects with known breast cancer or suspicious lesions showed high sensitivity and specificity and the ability to detect small lesions.190
A recent review address the clinical aspects and limitations of various radionuclide-based techniques for breast cancer imaging.191 With continued advancement of detector technology, radionuclide-based imaging systems dedicated to breast imaging are likely to play a significant role in therapeutic monitoring of known cancers and in diagnostic imaging of suspicious breast lesions.
INVESTIGATIONAL MODALITIES
Volumetric x-ray imaging techniques
While there have been considerable advances in mammography, there is one major inherent limitation in that the mammographic image reduces the three-dimensional anatomy of the breast into a two-dimensional image. This resulting superposition of normal breast structures, which causes a visually distracting mask, is often referred to as anatomical noise and has been shown to impair lesion detection.192, 193, 194 In addition, tissue superposition can mimic the presence of a lesion, resulting in increased recall rates and in increased biopsy rates, that add to the inconvenience and anxiety of the recalled subject. Hence, there is a need to develop techniques that provide depth information in breast x-ray imaging. Currently, stereoscopic digital mammography (SDM), digital breast tomosynthesis (DBT), and dedicated breast computed tomography (BCT) are three modalities that are being actively investigated.
Stereoscopic digital mammography
In stereoscopic digital mammography, two projection images spaced a few degrees apart are acquired with a digital mammography system. A dedicated stereoscopic workstation displays orthogonally polarized images and are viewed by the observer using passive cross-polarized glasses, so that each eye visualizes one image to provide depth perception. Studies evaluating the accuracy of depth perception indicate that 0.2 mm depth discrimination can be achieved using projection pair images acquired at an angular separation of ±6° and with geometric magnification.195, 196 Observer studies comparing standard monoscopic imaging with stereoscopic imaging using breast biopsy specimens indicate an improvement in detection of masses and microcalcifications, and an improved confidence for the estimation of likelihood of malignancy.197 Interim results from an ongoing prospective screening trial with high-risk women suggest that SDM reduces false-positive lesion detections by 39% and false-negative lesion detections by 46% compared to digital mammography, providing for significant improvements in sensitivity and specificity.198 However, the study used twice the dose of mammography. While contrast-detail experiments with a phantom indicates that ∼10% more radiation dose is needed for SDM compared to mammography,199 further research is needed to understand the dose requirements with clinical backgrounds. If ongoing and future clinical trials demonstrate its efficacy, it has the potential for quick adaptation due to the ready availability of digital mammography systems and dedicated stereoscopic displays.200
Digital breast tomosynthesis
Digital breast tomosynthesis (DBT), a technique that relies upon acquisition of multiple projection views over a limited angular range to reconstruct a volumetric image, has gained substantial interest primarily due to the availability of flat-panel detectors.91, 201, 202, 203 Subsequent to the landmark article in 1997 by Niklason et al. that demonstrated feasibility by modification of a digital mammography system,204 a similar system was used to study contrast-detail characteristics of several reconstruction algorithms.205, 206
Although in principle, implementation of DBT with a digital mammography system is straightforward, there are some design aspects that have to be considered. In a well-designed DBT system, the x-ray tube assembly has to avoid interference with the patient’s head during rotational movement and the rotational mechanics must provide for minimal vibration. Also, the x-ray beam collimation must adapt asymmetrically during the scan to ensure that the x-ray field does not extend marginally beyond the lateral edges of the detector. The acquisition of multiple frames in ∼10 s to minimize the potential for patient motion requires a detector that has rapid frame acquisition capability and with minimal image lag.
Current implementations of clinical prototype DBT systems use indirect conversion a-Si:H detectors with a 100-μm pixel pitch91 or direct conversion a-Se detectors.202, 207 Depending upon the manufacturer, a-Se based DBT system use a detector with 70-μm pixel pitch operated in a 2×2 binned mode202 or a detector with 85-μm pixel pitch operated in unbinned or 2×1 asymmetric binned mode.207, 208 Pixel binning approaches allow for faster readout of the detector, albeit at reduced resolution. As with digital mammography, the detector pixel pitch and the overall spatial resolution requirements for breast tomosynthesis are not well defined. After reconstruction, there is some degradation of in-plane (plane parallel to the detector) spatial resolution due to several factors including oblique x-ray incidence,209, 210 blur due to tube motion within the acquired projection view,208 mechanical vibration of the gantry, and reconstruction related blur such as that due to interpolation. There is considerable loss of spatial resolution along the depth-direction (perpendicular to the detector plane) due to limited angular sampling. Hence, there is a some concern about its ability to detect subtle structures such as amorphous microcalcifications.
Until recently, estimates of glandular breast dose during DBT were based on conversion factors for mammography. A Monte Carlo study provided conversion factors for DBT that showed variation in glandular dose with projection angle,211 facilitating the application towards advanced techniques such as tube-current modulation with changing projection angle, if needed. While the objective for a DBT acquisition is not to exceed the dose of a two-view mammogram, in a 15 projection acquisition for example, the detector receives substantially less signal per projection compared to a mammographic view. To some extent, this can be compensated by increasing the beam transmission though the breast with the use of W anode and higher kVp than in conventional mammography, as suggested by modeling and phantom studies,212, 213, 214 but may change as more experience is gained through clinical trials. Research in developing detectors with performance characteristics suitable for DBT seems well justified, in particular if higher kVp is used in the future for techniques such as contrast-enhanced DBT.
The adverse effects of scatter on projection imaging and computed tomography are well known. However, its adverse effects in DBT have not yet been quantified. Recently, comprehensive Monte Carlo based estimates of scatter and hence, scatter-to-primary ratio (SPR) was reported.215 SPR trends showed high dependence on compressed thickness and weak dependence on x-ray spectrum and breast glandularity, similar to mammography. Importantly, substantial spatial variation of SPR at oblique projections was observed. One interesting observation in that study215 was the increase in SPR observed at breast periphery, which was apparently due to the large component of x-ray scatter from the breast support plate compared to the primary x-ray component under the breast tissue. Conventional anti-scatter grids are not used in DBT because of the required increase in dose (bucky factor) and problems due to grid cut-off at oblique projection angles. While it may be possible to develop anti-scatter grids that are designed specifically for DBT, such designs have not yet materialized. Hence, accurate characterization of x-ray scatter during a DBT exam can be extremely useful in the development of computational techniques for scatter correction.
DBT reconstruction algorithms either use variants of back-projection technique or iterative techniques. Optimization of these reconstruction methods, quantification of their effect on resolution, noise, and artifacts as well as comparative analysis of these methods have been the subject of ongoing research.216, 217, 218, 219, 220, 221, 222 There are two important concerns with DBT, viz., its ability to provide adequate visualization of microcalcifications223 and “out-of-plane” artifacts that occur due to limited angular sampling. With regards to microcalcification visibility, a technique that could provide for improved morphology using back-projection or its variants was recently published.217 Also, improved image quality has been reported with an iterative algebraic reconstruction technique.218 Techniques specifically designed for suppressing artifacts are being actively investigated.219, 224, 225, 226
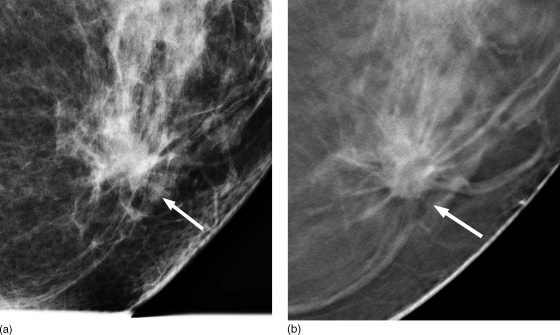
In spite of some challenges, DBT enables better separation of overlying tissues than mammography and it is likely to play an important role in breast imaging. Breast tomosynthesis has the potential of detecting occult lesions, particularly soft tissue abnormalities that can be extremely difficult to detect in the presence of dense glandular tissue. Currently, several clinical studies with human subjects are being conducted to evaluate the clinical potential of DBT. Early indications from ongoing clinical studies suggest that DBT show promise.223, 227 In a recently published study on their initial experience with DBT, most of the soft tissue abnormalities were subjectively rated as either equivalent or superior to mammography.223 Figure 2 shows a clinical case of a woman with 1.6 cm low-grade invasive ductal carcinoma with ductal carcinoma in situ (DCIS), where the carcinoma is better visualized with DBT (right) than with mammography (left). Interim results after accrual of 1957 women from an ongoing clinical trial indicate that DBT reduces recall rate to 4.4% compared to 7.5% with digital mammography, and unlike mammography does not appear to show dependence with breast density.227 With further refinements, breast tomosynthesis is likely to become a clinical tool for screening, particularly for women with dense breasts.
Figure 2.
A clinical case showing 1.6 cm low-grade invasive ductal carcinoma with minor ductal carcinoma in situ (DCIS) component. Better visualization of the tumor is observed with DBT (right, B) compared to mammography (left, A). (Courtesy: Steven P. Poplack, Dartmouth Hitchcock Medical Center)
Dedicated breast computed tomography
Another technique that will overcome the tissue superposition problem is dedicated breast computed tomography (BCT), in which the subject is in a prone position and the pendant breast is imaged. The concept of a dedicated BCT system was envisioned in the 1970s228 and a system was built. Clinical trials using the dedicated BCT system229 and with a conventional CT scanner230 suggested that injected contrast media enhanced CT could differentiate malignant from benign lesions. However, it did not translate to clinical practice due to the limitations with the technology available at that time. Subsequently in the mid-1990s, images of surgical biopsy specimens acquired with a state-of-the-art scanner at that time were compared with specimen radiography images, and indicated that CT imaging can significantly improve the confidence to detect mass but was inferior for microcalcifications.231
In 2001, a landmark article by Boone et al. provided quantitative estimates of radiation dose from a dedicated BCT exam and image quality achieved with cadaveric breast.232 The development of high-frame-rate flat-panel detectors accelerated further exploration of this technique. Works addressing radiation dose to the imaged breast232, 233, 234 and other organs,235 x-ray scatter,236, 237 system design,238, 239 imaging trajectory,240, 241 image acquisition technique factors,242, 243, 244 resolution,245 noise,246 and artifacts 247 have been published.
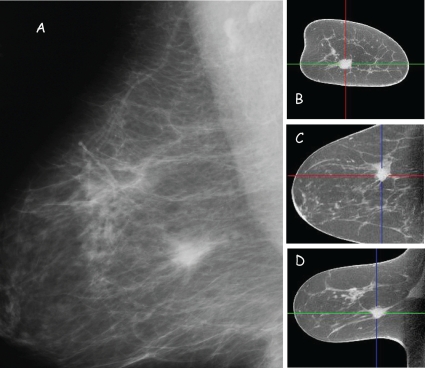
Independent studies with surgical mastectomy specimens248 and with limited number of subjects249 suggest that BCT provides for excellent anatomical detail and soft tissue lesion visualization. Figure 3 shows multi-planar reconstructions acquired with a clinical prototype dedicated BCT system and a MLO view projection image acquired with a digital mammography system. Excellent 3-D visualization of the anatomy and soft tissue lesion is observed. A recent study that compared visualization in BCT with lesions detected in screen-film mammography in 65 subjects, suggest that BCT was superior for visualization of masses but was inferior for visualization of microcalcifications.250
Figure 3.
Clinical images of a soft tissue lesion with digital mammography (A: MLO view) and multi-planar reconstructions (B: Coronal, C: Axial, and D: Sagittal views) with dedicated BCT. (Courtesy: Koning Corporation, West Henrietta, NY)
Some of the challenges with breast CT include simultaneous inclusion of medial and axillary aspects of the breast during the scan and adequate visualization of microcalcifications.250 Limitations in breast coverage with dedicated breast CT has been inferential, and as yet there have been no quantitative studies comparing tissue inclusion with mammography. Improved breast coverage can be potentially achieved through system design including novel table design251 and imaging trajectories,240, 241 and with appropriate patient positioning. Current prototype systems used in clinical studies utilize circular scan,250, 252 and we are aware of only one implementation using a non-circular trajectory for breast imaging with a laboratory prototype system.240 Further studies are needed to understand tissue coverage with dedicated breast CT for the various source-detector trajectories.
Initial computer simulation study and independently a receiver operating characteristic study with computer simulated BCT images suggested the possibility of detecting microcalcifications in the range of 175 to 200 μm.238, 253 However, experimental studies with phantoms suggest that visibility of microcalcifications may be limited to ∼300 μm with current technology.254, 255 Considering that these studies point to a noticeable improvement with increased dose or reduced breast size,254, 255 it is likely that image noise rather than resolution may be a dominant factor for limited visibility of microcalcifications. Hence, techniques for noise suppression246 may be beneficial. However, further research is needed to study its effect on artifacts, resolution, and lesion visualization. Additional improvements in microcalcification visualization can be potentially achieved with the use of high-resolution, low-noise detector technology such as photon-counting detectors256 or electron-multiplying CCDs,257 which are not yet available for large field of view imaging. A recent review article addresses the potential advantages of BCT and the challenges that need to be overcome for routine clinical use.258
While the role of BCT and its efficacy need to be ascertained through clinical studies, BCT has one major advantage in that it does not require physical compression of the breast, thus greatly alleviating patient discomfort. Ongoing clinical studies have shown exquisite anatomical detail and soft tissue lesion morphology. If visibility of microcalcifications is improved, breast CT allows for 3-D visualization of its distribution that could potentially improve specificity. While each of the aforementioned techniques that are targeted towards overcoming the tissue superposition problem are in various stages of research, an observer study with computer simulated images comparing digital mammography, DBT and BCT showed improved accuracy to detect masses,259 highlighting the potential advantage of DBT and BCT over digital mammography. Among the three techniques (SDM, DBT, and BCT) that are being investigated to provide depth information, the body of knowledge is too limited to obtain meaningful inferences about the superiority of one technique versus the other.
Contrast-enhanced x-ray imaging
While investigations into the applicability of digital subtraction angiography of the breast in the mid-1980s showed the potential for differential diagnosis for malignant and benign tumors,260, 261 the lack of appropriate imaging system was a major limitation. The advent of digital mammography has stimulated the exploration of quicker and cost-effective alternative for breast MRI such as intravenously injected iodinated contrast media enhanced imaging of the breast for angiogenesis imaging. Two approaches for contrast-enhanced digital mammography are being explored. In dual-energy K-edge subtraction, two images at different energies that straddle the K-edge of the contrast media are acquired after contrast administration and subtracted.262 In temporal subtraction, an image acquired prior to contrast administration is subtracted from the image(s) acquired after contrast administration.263 The former method reduces the likelihood of misregistration between the two images, while the latter is better suited for dynamic contrast enhancement studies and would require image processing for proper registration.
Multiple pilot clinical studies, each with a small number of subjects who had suspicious findings and underwent contrast-enhanced digital mammography, indicate enhancement of tumor and vasculature that are qualitatively similar to breast MRI.262, 263, 264, 265, 266 There have also been studies on optimization of the x-ray spectrum,267, 268 on determination of the minimum concentration of iodine required,267, 269 and on investigation of elements other than iodine that may provide for alternate contrast media.270, 271, 272
A pilot clinical study evaluating the potential of contrast enhanced DBT, showed characteristics that were concordant with digital mammography and breast MRI.273 Contrast-enhanced DBT is being actively investigated, particularly in terms of imaging technique optimization and quantification of iodine concentration.268, 274, 275 The role of contrast-enhanced conventional CT in management of breast cancer such as lymph node imaging, tumor staging, identifying tumor extent for breast conservative surgery, treatment planning for radiotherapy, and in monitoring of cancer therapy is well established. A pilot study with contrast-enhanced dedicated BCT showed enhancement of malignant lesions similar to MRI.250 One potential advantage of contrast-enhanced x-ray imaging is that signal intensity changes are typically linear with contrast agent uptake, unlike MRI.135 All of the aforementioned x-ray-based contrast-enhanced imaging techniques are in various stages of research.
While there are promising aspects, all of these techniques require intravenous injection, which entails a slightly elevated risk.137 While contrast media used with x-ray imaging are considered relatively safe, risks including allergic reactions and contrast nephropathy, though uncommon, are of concern. Hence, there is a need to develop contrast agents with minimal or no adverse effects. Further improvements in specificity are achievable with development of tumor-targeted agents276, 277 and with better understanding of the criteria for determining malignancy such as morphology, tumor enhancement due to agent uptake, and agent uptake kinetics.
Alternative modalities
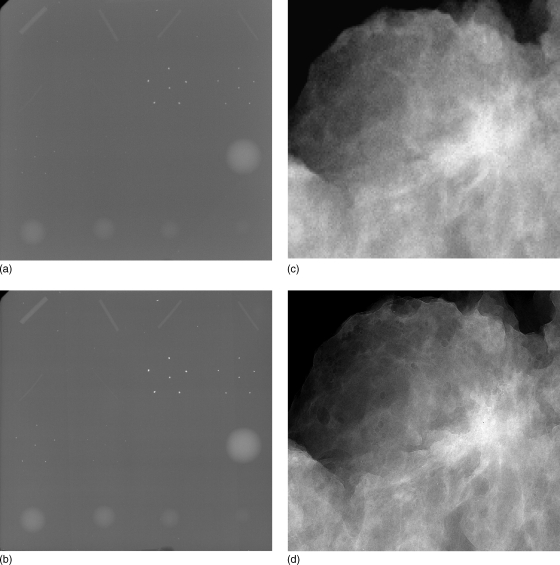
X-ray phase sensitive imaging such as in-line phase contrast x-ray imaging278, 279 and diffraction enhanced imaging280, 281, 282, 283 are currently being investigated. The theoretical basis and design considerations for phase contrast imaging284, 285 and method to retrieve the phase map from attenuation and phase contrast images286 have been well addressed. Development of a dual-detector system that uses two CR cassettes for simultaneous acquisition of conventional (attenuation) and phase contrast images, from which the phase map is retrieved was recently published.287 Figure 4 shows conventional (attenuation) and phase contrast images of ACR-recommended accreditation phantom and of a lumpectomy specimen acquired with a prototype system. Substantial improvement in contrast and edge-enhancement is observed. These techniques are at present limited to imaging specimens. Further research is needed to translate them to clinical use.
Figure 4.
Conventional (attenuation) images (A, C) and phase contrast images (B, D) of accreditation phantom (top row) and lumpectomy specimen (bottom row). All images were acquired at 40 kVp with same entrance exposure for attenuation and phase contrast images. These images were acquired using prototype systems developed at the University of Oklahoma (Hong Liu), in collaboration with University of Alabama at Birmingham (Xizeng Wu) and University of Iowa (Laurie L. Fajardo). (Courtesy: Hong Liu, University of Oklahoma)
Optical imaging of the breast such as diffuse optical tomography (DOT), diffuse optical imaging, and diffuse optical spectroscopy is being investigated as an adjunct technique. This emerging technique uses light in the near-infrared (NIR) regime to noninvasively image total hemoglobin, oxygen saturation, water content, optical scattering, and lipid concentration.288, 289, 290 While substantial variations in optical properties for normal tissues291 and tumors292 have been observed, a recent study indicates that normal and tumor tissues can be differentiated based on total hemoglobin, hemoglobin oxygen saturation, and other parameters.293 Leaky, densely packed vasculature associated with tumor angiogenesis could contribute to increased total hemoglobin, and increased metabolism in tumors could result in decreased oxygen saturation. NIRS has an inherent resolution of ∼4 to 6 mm because of the highly scattering nature of light. Ntziachristos et al.294 demonstrated that combining DOT with MRI anatomical maps can improve spatial resolution. More accurate quantification of NIRS parameters have been reported by incorporating structural priors from MRI during NIRS reconstruction.295 In order to obtain co-registered dual-modality images, investigators at Dartmouth have incorporated NIR spectroscopy instrumentation within an open-frame MR breast coil (Fig. 5). This combined approach has been used to demonstrate feasibility by imaging more than 25 women with both normal296 and disease indications from mammography.297 Correlation between indocyanine green enhancement and Gd-enhanced MRI,298 and its ability to differentiate cysts from solid tumors299 have also been reported. Ongoing investigations indicate that DOT in combination with MRI hold promise for early determination of the effectiveness of neoadjuvant chemotherapy.300, 301, 302 In addition to NIRS-MRI systems, development of DOT imaging systems in combination with DBT and ultrasound are also underway.303, 304, 305 A recent article provides an assessment of its future role in breast imaging.306
Figure 5.
Dual-modality MRI-NIR system with insert showing the NIR transmitter-receiver incorporated within the open breast coil. (Courtesy: Brian W. Pogue, Thayer School of Engineering, Dartmouth College)
Electrical impedance spectroscopy (EIS) and microwave imaging spectroscopy (MIS) are also being explored for potential use in breast cancer detection. A recent study suggests that EIS could potentially be used as a risk assessment tool for young women who are not routinely screened for breast cancer.307In vivo microwave imaging of women with normal mammographic exam suggests that dielectric properties are heterogeneous and correlate with radiographic density.308 A recent two-part series on measurements of ultra-wideband dielectric properties from normal and cancer tissue samples indicate substantial variations in dielectric properties for normal tissues, and that the contrast in dielectric properties between malignant and adipose tissues could be as high as 10:1, whereas that of malignant to fibroglandular tissue could be no more than 10%.309, 310 A pilot clinical study that evaluated EIS, MIS, and NIRS indicate substantial contrast difference between abnormal and normal breast tissue.311 A recent article addresses the status of these imaging modalities.312
CONCLUSION
Intense efforts are currently under way to improve the technological aspects of the aforementioned modalities. Those include advances in x-ray and gamma ray detector technology, MRI coil design, MRI pulse sequences, tomographic image reconstruction, signal processing, computational speed, and mechanical design. Based on current understanding and results from ongoing research, it appears that high-resolution, high-contrast, anatomical x-ray imaging either in 2-D (mammography) or with added depth information will be the primary screening modality in the next decade. However, MRI and ultrasound will have an increasingly important role for imaging high risk patients and for imaging women with dense breasts. With increasing field strength and improved homogeneity, MRS is likely to add value to dynamic contrast enhanced MRI studies. Contrast-enhanced x-ray imaging, scintimammography with dedicated cameras, dedicated SPECT, and dedicated PET offer great potential as diagnostic evaluation tools and for clinical management. While prototype BCT systems have been developed and pilot clinical studies demonstrate the ability to provide high-resolution 3-D anatomical information, there are some challenges that need to be addressed. NIR diffuse optics-based imaging and electromagnetic imaging techniques such as EIS and MIS could provide for physiologic information and is likely to be of most value when integrated with or adjunctively used with modalities that provide for anatomical structure such as x-ray, ultrasound, or MRI. At present, phase-sensitive x-ray imaging that provides for improved contrast and edge-enhancement has been demonstrated with specimens and would require substantial amount of translational research to bring it to clinical use. From the technological perspective, in addition to improvements with each modality, we are likely to observe an increasing trend towards multi-modality systems that combine the relative strengths of each modality.
Importantly, we are likely to observe a paradigm shift in the manner in which breast cancer screening will be performed in future. Breast cancer screening is currently performed in a standard manner for all women with mammography. However, this level of standardization is likely to be replaced by a screening program where the selection of an imaging modality or modalities would depend on an individual’s risk and other classifications. Already elements of this change can be observed with the recommendation by American Cancer Society to adjunctively use breast MRI for screening of high-risk subjects. This transformation to individual-risk or other classification based screening is likely to be of benefit to women. However this added layer, which can be referred to as pre-screening, does place an onus on the manner in which such classifications or risk assessments are performed and need to be carefully studied. In summary, ongoing research and recent advances indicate that the prospects of substantial improvements in early detection, accurate diagnosis, and improved monitoring of therapeutic response of breast cancer are highly promising.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Paul L. Carson and Dr. Mitchell M. Goodsitt at the University of Michigan, Dr. Hong Liu from the University of Oklahoma, Dr. Brian W. Pogue from Dartmouth College, Dr. Steven P. Poplack at Dartmouth Hitchcock Medical Center, and Koning Corporation for providing images. This work was partially supported by National Institutes of Health (NIH) Grant No. R01-EB002123 and Grant No. R01-EB004015 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the NIBIB. Mention of company names or products does not imply endorsement. The authors have collaborated in the past with GE Global Research Center.
References
- Oestreicher N., Lehman C. D., Seger D. J., Buist D. S., and White E., “The incremental contribution of clinical breast examination to invasive cancer detection in a mammography screening program,” AJR, Am. J. Roentgenol. 184(2), 428–432 (2005). [DOI] [PubMed] [Google Scholar]
- Bassett L. W. and Gold R. H., “The evolution of mammography,” AJR, Am. J. Roentgenol. 150(3), 493–498 (1988). [DOI] [PubMed] [Google Scholar]
- Leborgne R., “Diagnosis of tumors of the breast by simple roentgenography; calcifications in carcinomas,” Am. J. Roentgenol., Radium Ther. Nucl. Med. 65(1), 1–11 (1951). [PubMed] [Google Scholar]
- Egan R. L., “Experience with mammography in a tumor institution. Evaluation of 1,000 studies,” Radiology 75, 894–900 (1960). [DOI] [PubMed] [Google Scholar]
- Shapiro S., Strax P., and Venet L., “Periodic breast cancer screening in reducing mortality from breast cancer,” JAMA, J. Am. Med. Assoc. 215(11), 1777–1785 (1971). [PubMed] [Google Scholar]
- Thurfjell E. L. and Lindgren J. A., “Breast cancer survival rates with mammographic screening: similar favorable survival rates for women younger and those older than 50 years,” Radiology 201(2), 421–426 (1996). [DOI] [PubMed] [Google Scholar]
- Hendrick R. E., Smith R. A., J. H.RutledgeIII, and Smart C. R., “Benefit of screening mammography in women aged 40-49: a new meta-analysis of randomized controlled trials,” J Natl Cancer Inst Monogr 22, 87–92 (1997). [DOI] [PubMed] [Google Scholar]
- Tabar L., Vitak B., Chen H. H., Yen M. F., Duffy S. W., and Smith R. A., “Beyond randomized controlled trials: organized mammographic screening substantially reduces breast carcinoma mortality,” Cancer 91(9), 1724–1731 (2001). [DOI] [PubMed] [Google Scholar]
- The Swedish Organized Service Screening Evaluation Group, “Reduction in breast cancer mortality from organized service screening with mammography: 1. Further confirmation with extended data,” Cancer Epidemiol. Biomarkers Prev. 15(1), 45–51 (2006). [DOI] [PubMed] [Google Scholar]
- The Swedish Organized Service Screening Evaluation Group, “Reduction in breast cancer mortality from the organised service screening with mammography: 2. Validation with alternative analytic methods,” Cancer Epidemiol. Biomarkers Prev. 15(1), 52–56 (2006). [DOI] [PubMed] [Google Scholar]
- Price J. L. and Bler P. D., “The reduction of radiation and exposure time in mammography,” Br. J. Radiol. 43(508), 251–255 (1970). [DOI] [PubMed] [Google Scholar]
- Muntz E. P. and Logan W. W., “Focal spot size and scatter suppression in magnification mammography,” AJR, Am. J. Roentgenol. 133(3), 453–459 (1979). [DOI] [PubMed] [Google Scholar]
- Nishikawa R. M., Mawdsley G. E., Fenster A., and Yaffe M. J., “Scanned-projection digital mammography,” Med. Phys. 10.1118/1.596147 14(5), 717–727 (1987). [DOI] [PubMed] [Google Scholar]
- Karellas A., Vedantham S., and Suryanarayanan S., Digital Mammography Image Acquisition Technology, in RSNA Categorical Course in Diagnostic Radiology Physics: Advances in Breast Imaging—Physics, Technology and Clinical Applications, edited by Karellas A. and Giger M. L. (RSNA, Oak Brook, IL, 2004), p. 87–99. [Google Scholar]
- FDA US, MQSA Facility Score Card, January 2, 2008, Center for Devices and Radiological Health, http://www.fda.gov/CDRH/MAMMOGRAPHY/scorecard-statistics.html [Accessed: January 28, 2008].
- Fetterly K. A. and Schueler B. A., “Performance evaluation of a ‘dual-side read’ dedicated mammography computed radiography system,” Med. Phys. 10.1118/1.1584045 30(7), 1843–1854 (2003). [DOI] [PubMed] [Google Scholar]
- R. S.Saunders, Jr., Samei E., Jesneck J. L., and Lo J. Y., “Physical characterization of a prototype selenium-based full field digital mammography detector,” Med. Phys. 10.1118/1.1855033 32(2), 588–599 (2005). [DOI] [PubMed] [Google Scholar]
- Vedantham S., Karellas A., Suryanarayanan S., Albagli D., Han S., Tkaczyk E. J., Landberg C. E., Opsahl-Ong B., Granfors P. R., Levis I., D’Orsi C. J., and Hendrick R. E., “Full breast digital mammography with an amorphous silicon-based flat panel detector: Physical characteristics of a clinical prototype,” Med. Phys. 10.1118/1.598895 27(3), 558–567 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin J. M., D’Orsi C. J., Hendrick R. E., Moss L. J., Isaacs P. K., Karellas A., and Cutter G. R., “Clinical comparison of full-field digital mammography and screen-film mammography for detection of breast cancer,” AJR, Am. J. Roentgenol. 179(3), 671–677 (2002). [DOI] [PubMed] [Google Scholar]
- Skaane P., Hofvind S., and Skjennald A., “Randomized trial of screen-film versus full-field digital mammography with soft-copy reading in population-based screening program: Follow-up and final results of Oslo, II study,” Radiology 244(3), 708–717 (2007). [DOI] [PubMed] [Google Scholar]
- Pisano E. D., Gatsonis C., Hendrick E., Yaffe M., Baum J. K., Acharyya S., Conant E. F., Fajardo L. L., Bassett L., D’Orsi C., Jong R., and Rebner M., “Diagnostic performance of digital versus film mammography for breast-cancer screening,” N. Engl. J. Med. 10.1056/NEJMoa052911 353(17), 1773–1783 (2005). [DOI] [PubMed] [Google Scholar]
- Pisano E. D., Hendrick R. E., Yaffe M. J., Baum J. K., Acharyya S., Cormack J. B., Hanna L. A., Conant E. F., Fajardo L. L., Bassett L. W., D’Orsi C. J., Jong R. A., Rebner M., Tosteson A. N., and Gatsonis C. A., “Diagnostic accuracy of digital versus film mammography: Exploratory analysis of selected population subgroups in DMIST,” Radiology 246(2), 376–383 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonuk L. E., Boudry J., Huang W., McShan D. L., Morton E. J., Yorkston J., Longo M. J., and Street R. A., “Demonstration of megavoltage and diagnostic x-ray imaging with hydrogenated amorphous silicon arrays,” Med. Phys. 10.1118/1.596802 19(6), 1455–1466 (1992). [DOI] [PubMed] [Google Scholar]
- Suryanarayanan S., Karellas A., and Vedantham S., “Physical characteristics of a full-field digital mammography system,” Nucl. Instrum. Methods Phys. Res. A 10.1016/j.nima.2004.05.128 533(3), 560–570 (2004). [DOI] [Google Scholar]
- Rowlands J. A., Hunter D. M., and Araj N., “X-ray imaging using amorphous selenium: a photoinduced discharge readout method for digital mammography,” Med. Phys. 10.1118/1.596689 18(3), 421–431 (1991). [DOI] [PubMed] [Google Scholar]
- Zhao W., Law J., Waechter D., Huang Z., and Rowlands J. A., “Digital radiology using active matrix readout of amorphous selenium: detectors with high voltage protection,” Med. Phys. 10.1118/1.598229 25(4), 539–549 (1998). [DOI] [PubMed] [Google Scholar]
- Zhao W., Ji W. G., Debrie A., and Rowlands J. A., “Imaging performance of amorphous selenium based flat-panel detectors for digital mammography: Characterization of a small area prototype detector,” Med. Phys. 10.1118/1.1538233 30(2), 254–263 (2003). [DOI] [PubMed] [Google Scholar]
- Barnes G. T. and Brezovich I. A., “The design and performance of a scanning multiple slit assembly,” Med. Phys. 10.1118/1.594562 6(3), 197–204 (1979). [DOI] [PubMed] [Google Scholar]
- Boone J. M., Seibert J. A., Tang C. M., and Lane S. M., “Grid and slot scan scatter reduction in mammography: comparison by using Monte Carlo techniques,” Radiology 10.1148/radiol.2222010491 222(2), 519–527 (2002). [DOI] [PubMed] [Google Scholar]
- Aslund M., Cederstrom B., Lundqvist M., and Danielsson M., “Physical characterization of a scanning photon counting digital mammography system based on Si-strip detectors,” Med. Phys. 10.1118/1.2731032 34(6), 1918–1925 (2007). [DOI] [PubMed] [Google Scholar]
- Arakawa S., Itoh W., Kohda K., and Suzuki T., “Improvement of image quality in CR mammography by detection of emissions from dual sides of an imaging plate,” Proc. SPIE 10.1117/12.384536 3977, 590–600 (2000). [DOI] [Google Scholar]
- Seibert J. A., Boone J. M., Cooper V. N., and Lindfors K. K., “Cassette-based digital mammography,” Technol. Cancer Res. Treat. 3(5), 413–427 (2004). [DOI] [PubMed] [Google Scholar]
- Lawinski C., MacKenzie A., Cole H., Blake P., Honey I., and Pascoal A., Computed Radiography (CR) Systems for Mammography. A comparative technical report. MHRA 04107 (Medicines and Health Products Regulatory Agency (MHRA), London, 2004). [Google Scholar]
- Pisano E. D. and Yaffe M. J., “Digital mammography,” Radiology 10.1148/radiol.2342030897 234(2), 353–362 (2005). [DOI] [PubMed] [Google Scholar]
- Yaffe M. J. and Mainprize J. G., “Detectors for digital mammography,” Technol. Cancer Res. Treat. 3(4), 309–324 (2004). [DOI] [PubMed] [Google Scholar]
- Suryanarayanan S., Karellas A., Vedantham S., Sechopoulos I., and D’Orsi C. J., “Detection of simulated microcalcifications in a phantom with digital mammography: Effect of pixel size,” Radiology 10.1148/radiol.2441060977 244(1), 130–137 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- R. S.Saunders, Jr., Baker J. A., Delong D. M., Johnson J. P., and Samei E., “Does image quality matter¿ Impact of resolution and noise on mammographic task performance,” Med. Phys. 10.1118/1.2776253 34(10), 3971–3981 (2007). [DOI] [PubMed] [Google Scholar]
- Ei-Mohri Y., Antonuk L. E., Zhao Q., Wang Y., Li Y., Du H., and Sawant A., “Performance of a high fill factor, indirect detection prototype flat-panel imager for mammography,” Med. Phys. 10.1118/1.2403967 34(1), 315–327 (2007). [DOI] [PubMed] [Google Scholar]
- El-Mohri Y., Antonuk L. E., Zhao Q., Maolinbay M., Rong X., Jee K. W., Nassif S., and Cionca C., “A quantitative investigation of additive noise reduction for active matrix flat-panel imagers using compensation lines,” Med. Phys. 10.1118/1.1287053 27(8), 1855–1864 (2000). [DOI] [PubMed] [Google Scholar]
- Nagarkar V. V., Tipnis S. V., Gaysinskiy V. B., Miller S. R., Karellas A., and Vedantham S., “New design of a structured CsI(Tl) screen for digital mammography,” Proc. SPIE 10.1117/12.480249 5030, 541–546 (2003). [DOI] [Google Scholar]
- Sawant A., Antonuk L. E., El-Mohri Y., Zhao Q., Li Y., Su Z., Wang Y., Yamamoto J., Du H., Cunningham I., Klugerman M., and Shah K., “Segmented crystalline scintillators: an initial investigation of high quantum efficiency detectors for megavoltage x-ray imaging,” Med. Phys. 10.1118/1.2008407 32(10), 3067–3083 (2005). [DOI] [PubMed] [Google Scholar]
- Rau A. W., Bakueva L., and Rowlands J. A., “The x-ray time of flight method for investigation of ghosting in amorphous selenium-based flat panel medical x-ray imagers,” Med. Phys. 10.1118/1.2042248 32(10), 3160–3177 (2005). [DOI] [PubMed] [Google Scholar]
- Zhao W., DeCrescenzo G., Kasap S. O., and Rowlands J. A., “Ghosting caused by bulk charge trapping in direct conversion flat-panel detectors using amorphous selenium,” Med. Phys. 10.1118/1.1843353 32(2), 488–500 (2005). [DOI] [PubMed] [Google Scholar]
- Bloomquist A. K., Yaffe M. J., Mawdsley G. E., Hunter D. M., and Beideck D. J., “Lag and ghosting in a clinical flat-panel selenium digital mammography system,” Med. Phys. 10.1118/1.2218315 33(8), 2998–3005 (2006). [DOI] [PubMed] [Google Scholar]
- Hunt D. C., Tanioka K., and Rowlands J. A., “X-ray imaging using avalanche multiplication in amorphous selenium: investigation of intrinsic avalanche noise,” Med. Phys. 10.1118/1.2799494 34(12), 4654–4663 (2007). [DOI] [PubMed] [Google Scholar]
- Lee D. L., “Selenium detector with a grid for selenium charge gain,” Proc. SPIE 5745, 216–222 (2005). [Google Scholar]
- Mainprize J. G., Ford N. L., Yin S., Gordon E. E., Hamilton W. J., Tumer T. O., and Yaffe M. J., “A CdZnTe slot-scanned detector for digital mammography,” Med. Phys. 10.1118/1.1523932 29(12), 2767–2781 (2002). [DOI] [PubMed] [Google Scholar]
- Zentai G., Partain L. D., Pavlyuchkova R., Proano C. H., Schieber M. M., and Thomas J., “Dark current, sensitivity, and image lag comparison of mercuric iodide and lead iodide x-ray imagers,” Proc. SPIE 5541, 171–178 (2004). [Google Scholar]
- Su Z., Antonuk L. E., El-Mohri Y., Hu L., Du H., Sawant A., Li Y., Wang Y., Yamamoto J., and Zhao Q., “Systematic investigation of the signal properties of polycrystalline HgI2 detectors under mammographic, radiographic, fluoroscopic and radiotherapy irradiation conditions,” Phys. Med. Biol. 10.1088/0031-9155/50/12/012 50(12), 2907–2928 (2005). [DOI] [PubMed] [Google Scholar]
- Sakellaris T., Spyrou G., Tzanakos G., and Panayiotakis G., “Energy, angular and spatial distributions of primary electrons inside photoconducting materials for digital mammography: Monte Carlo simulation studies,” Phys. Med. Biol. 10.1088/0031-9155/52/21/007 52(21), 6439–6460 (2007). [DOI] [PubMed] [Google Scholar]
- Leblans P. J. R., Struye L., and Willems P., “New needle-crystalline CR detector,” Proc. SPIE 10.1117/12.430885 4320, 59–67 (2001). [DOI] [Google Scholar]
- Schaetzing R., Fasbender R., and Kersten P., “New high-speed scanning technique for computed radiography,” Proc. SPIE 10.1117/12.465595 4682, 511–520 (2002). [DOI] [Google Scholar]
- Rivetti S., Lanconelli N., Bertolini M., Borasi G., Acchiappati D., and Burani A., “Performance evaluation of a direct computed radiography system by means of physical characterization and contrast detail analysis,” Proc. SPIE 10.1117/12.710523 6510, 65104M (2007). [DOI] [Google Scholar]
- Herrmann C., Frankenberger J., Reiser G., and Lamotte J., “Optimization of a CR system comprising line-scanning and needle image plate technology with respect to examinations of extremities,” Proc. SPIE 10.1117/12.708368 6510, 65101B (2007). [DOI] [Google Scholar]
- Shikhaliev P. M., Xu T., Le H., and Molloi S., “Scanning-slit photon counting x-ray imaging system using a microchannel plate detector,” Med. Phys. 10.1118/1.1695651 31(5), 1061–1071 (2004). [DOI] [PubMed] [Google Scholar]
- Giersch J., “Medical quantum X-ray imaging with 2D detectors,” Nucl. Instrum. Methods Phys. Res. A 551, 125–138 (2005). [Google Scholar]
- Bech M., Bunk O., David C., Kraft P., Bronnimann C., Eikenberry E. F., and Pfeiffer F., “X-ray imaging with the PILATUS 100k detector,” Appl. Radiat. Isot. 66(4), 474–478 (2008). [DOI] [PubMed] [Google Scholar]
- Karg J., Niederlohner D., Giersch J., and Anton G., “Using the Medipix2 detector for energy weighting,” Nucl. Instrum. Methods Phys. Res. A 546, 306–311 (2005). [Google Scholar]
- Bernhardt P., Mertelmeier T., and Hoheisel M., “X-ray spectrum optimization of full-field digital mammography: simulation and phantom study,” Med. Phys. 10.1118/1.2351951 33(11), 4337–4349 (2006). [DOI] [PubMed] [Google Scholar]
- Toroi P., Zanca F., Young K. C., van Ongeval C., Marchal G., and Bosmans H., “Experimental investigation on the choice of the tungsten∕rhodium anode∕filter combination for an amorphous selenium-based digital mammography system,” Eur. Radiol. 17(9), 2368–2375 (2007). [DOI] [PubMed] [Google Scholar]
- Dance D. R., Thilander A. K., Sandborg M., Skinner C. L., Castellano I. A., and Carlsson G. A., “Influence of anode∕filter material and tube potential on contrast, signal-to-noise ratio and average absorbed dose in mammography: A Monte Carlo study,” Br. J. Radiol. 73(874), 1056–1067 (2000). [DOI] [PubMed] [Google Scholar]
- Heddson B., Ronnow K., Olsson M., and Miller D., “Digital versus screen-film mammography: A retrospective comparison in a population-based screening program,” Eur. J. Radiol. 64(3), 419–425 (2007). [DOI] [PubMed] [Google Scholar]
- Gennaro G. and di Maggio C., “Dose comparison between screen/film and full-field digital mammography,” Eur. Radiol. 16(11), 2559–2566 (2006). [DOI] [PubMed] [Google Scholar]
- Hermann K. P., Obenauer S., Marten K., Kehbel S., Fischer U., and Grabbe E., “Average glandular dose with amorphous silicon full-field digital mammography-Clinical results,” Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 174(6), 696–699 (2002). [DOI] [PubMed] [Google Scholar]
- Weigel S., Girnus R., Czwoydzinski J., Decker T., Spital S., and Heindel W., “Digital mammography screening: average glandular dose and first performance parameters,” Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 179(9), 892–895 (2007). [DOI] [PubMed] [Google Scholar]
- Gennaro G., Katz L., Souchay H., Alberelli C., and di Maggio C., “Are phantoms useful for predicting the potential of dose reduction in full-field digital mammography?,” Phys. Med. Biol. 10.1088/0031-9155/50/8/015 50(8), 1851–1870 (2005). [DOI] [PubMed] [Google Scholar]
- Samei E., R. S.Saunders, Jr., Baker J. A., and Delong D. M., “Digital mammography;: effects of reduced radiation dose on diagnostic performance,” Radiology 10.1148/radiol.2432061065 243(2), 396–404 (2007). [DOI] [PubMed] [Google Scholar]
- Ruschin M., Timberg P., Bath M., Hemdal B., Svahn T., Saunders R. S., Samei E., Andersson I., Mattsson S., Chakrabort D. P., and Tingber A., “Dose dependence of mass and microcalcification detection in digital mammography: Free response human observer studies,” Med. Phys. 10.1118/1.2405324 34(2), 400–407 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone J. M., “Normalized glandular dose (DgN) coefficients for arbitrary X-ray spectra in mammography: computer-fit values of Monte Carlo derived data,” Med. Phys. 10.1118/1.1472499 29(5), 869–875 (2002). [DOI] [PubMed] [Google Scholar]
- Dance D. R., “Monte Carlo calculation of conversion factors for the estimation of mean glandular breast dose,” Phys. Med. Biol. 10.1088/0031-9155/35/9/002 35(9), 1211–1219 (1990). [DOI] [PubMed] [Google Scholar]
- Hammerstein G. R., Miller D. W., White D. R., Masterson M. E., Woodard H. Q., and Laughlin J. S., “Absorbed radiation dose in mammography,” Radiology 130(2), 485–491 (1979). [DOI] [PubMed] [Google Scholar]
- Wu X., Gingold E. L., Barnes G. T., and Tucker D. M., “Normalized average glandular dose in molybdenum target-rhodium filter and rhodium target-rhodium filter mammography,” Radiology 193(1), 83–89 (1994). [DOI] [PubMed] [Google Scholar]
- Hatziioannou K. A., Psarrakos K., Molyvda-Athanasopoulou E., Kitis G., Papanastassiou E., Sofroniadis I., and Kimoundri O., “Dosimetric considerations in mammography,” Eur. Radiol. 10(7), 1193–1196 (2000). [DOI] [PubMed] [Google Scholar]
- Behrman R. H., Homer M. J., Yang W. T., and Whitman G. J., “Mammography and fetal dose,” Radiology 10.1148/radiol.2432060791 243(2), 605 (2007); [DOI] [PubMed] [Google Scholar]; Behrman R. H., Homer M. J., Yang W. T., and Whitman G. J., Radiology 10.1148/radiol.2432060791author reply 243(2), 605–606 (2007). [DOI] [PubMed] [Google Scholar]
- Sechopoulos I., Suryanarayanan S., Vedantham S., D’Orsi C. J., and Karellas A., “Radiation Dose to Organs and Tissues from Mammography: Monte Carlo and Phantom Study,” Radiology 246(2), 434–443 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett L. W., Farria D. M., Bansal S., Farquhar M. A., Wilcox P. A., and Feig S. A., “Reasons for failure of a mammography unit at clinical image review in the American College of Radiology Mammography Accreditation Program,” Radiology 215(3), 698–702 (2000). [DOI] [PubMed] [Google Scholar]
- Rezentes P. S., de Almeida A., and Barnes G. T., “Mammography grid performance,” Radiology 210(1), 227–232 (1999). [DOI] [PubMed] [Google Scholar]
- Chakraborty D. P., “The effect of the antiscatter grid on full-field digital mammography phantom images,” J. Digit Imaging 12(1), 12–22 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D. P., “Effect of the antiscatter grid and target/filters in full-field digital mammography,” Proc. SPIE 3659, 878–885 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldkamp W. J., Thijssen M. A., and Karssemeijer N., “The value of scatter removal by a grid in full field digital mammography,” Med. Phys. 10.1118/1.1584044 30(7), 1712–1718 (2003). [DOI] [PubMed] [Google Scholar]
- Gennaro G., Katz L., Souchay H., Klausz R., Alberelli C., and di Maggio C., “Grid removal and impact on population dose in full-field digital mammography,” Med. Phys. 10.1118/1.2426402 34(2), 547–555 (2007). [DOI] [PubMed] [Google Scholar]
- Baydush A. H., and C. E.Floyd, Jr., “Improved image quality in digital mammography with image processing,” Med. Phys. 10.1118/1.599016 27(7), 1503–1508 (2000). [DOI] [PubMed] [Google Scholar]
- Nykanen K. and Siltanen S., “X-ray scattering in full-field digital mammography,” Med. Phys. 10.1118/1.1584160 30(7), 1864–1873 (2003). [DOI] [PubMed] [Google Scholar]
- Trotter D. E. G., Tkaczyk J. E., Kaufhold J., Claus B. E. H., and Eberhard J. W., “Thickness-dependent scatter correction algorithm for digital mammography,” Proc. SPIE 10.1117/12.465591 4682, 469–478 (2002). [DOI] [Google Scholar]
- Sehgal C. M., Weinstein S. P., Arger P. H., and Conant E. F., “A review of breast ultrasound,” Journal of mammary gland biology and neoplasia 11(2), 113–123 (2006). [DOI] [PubMed] [Google Scholar]
- Weinstein S. P., Conant E. F., and Sehgal C., “Technical advances in breast ultrasound imaging,” Semin Ultrasound CT MR 27(4), 273–283 (2006). [DOI] [PubMed] [Google Scholar]
- Forsberg F., Piccoli C. W., Merton D. A., Palazzo J. J., and Hall A. L., “Breast lesions: imaging with contrast-enhanced subharmonic US—initial experience,” Radiology 244(3), 718–726 (2007). [DOI] [PubMed] [Google Scholar]
- Kolb T. M., Lichy J., and Newhouse J. H., “Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations,” Radiology 10.1148/radiol.2251011667 225(1), 165–175 (2002). [DOI] [PubMed] [Google Scholar]
- Berg W. A., Blume J. D., Cormack J. B., Mendelson E. B., Lehrer D., Bohm-Velez M., Pisano E. D., Jong R. A., Evans W. P., Morton M. J., Mahoney M. C., Larsen L. H., Barr R. G., Farria D. M., Marques H. S., and Boparai K., “Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer,” JAMA, J. Am. Med. Assoc. 299(18), 2151–2163 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A., Carson P. L., Eberhard J., Goodsitt M. M., Thomenius K., Lokhandwalla M., Buckley D., Roubidoux M. A., Helvie M. A., Booi R. C., LeCarpentier G. L., Erkamp R. Q., Chan H. P., Fowlkes J. B., Thomas J. A., and Landberg C. E., “Combination of digital mammography with semi-automated 3D breast ultrasound,” Technol. Cancer Res. Treat. 3(4), 325–334 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard J. W., Staudinger P., Smolenski J., Ding J., Schmitz A., McCoy J., Rumsey M., Al-Khalidy A., Ross W., Landberg C. E., Claus B. E., Carson P., Goodsitt M. M., Chan H. P., Roubidoux M., Thomas J. A., and Osland J., “High-speed large angle mammography tomosynthesis system,” Proc. SPIE 10.1117/12.653298 6142, 61420C (2006). [DOI] [Google Scholar]
- Booi R. C., Krucker J. F., Goodsitt M. M., O’Donnell M., Kapur A., LeCarpentier G. L., Roubidoux M. A., Fowlkes J. B., and Carson P. L., “Evaluating thin compression paddles for mammographically compatible ultrasound,” Ultrasound Med. Biol. 33(3), 472–482 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S. P., Goodsitt M. M., Roubidoux M. A., Booi R. C., LeCarpentier G. L., Thomenius C. R., Thomenius K. E., Chalek C. L., and Carson P. L., “Automated ultrasound scanning on a dual-modality breast imaging system: coverage and motion issues and solutions,” J. Ultrasound Med. 26(5), 645–655 (2007). [DOI] [PubMed] [Google Scholar]
- Duric N., Littrup P., Babkin A., Chambers D., Azevedo S., Pevzner R., Tokarev M., Holsapple E., Rama O., and Duncan R., “Development of ultrasound tomography for breast imaging: technical assessment,” Med. Phys. 10.1118/1.1897463 32(5), 1375–1386 (2005). [DOI] [PubMed] [Google Scholar]
- Duric N., Littrup P., Poulo L., Babkin A., Pevzner R., Holsapple E., Rama O., and Glide C., “Detection of breast cancer with ultrasound tomography: First result with the Computed Ultrasound Risk Evaluation (CURE) prototype,” Med. Phys. 10.1118/1.2432161 34(2), 773–785 (2007). [DOI] [PubMed] [Google Scholar]
- Damadian R., “Tumor detection by nuclear magnetic resonance,” Science 10.1126/science.171.3976.1151 171(976), 1151–1153 (1971). [DOI] [PubMed] [Google Scholar]
- Lauterbur P. C., “Image formation by induced local interactions: Examples employing nuclear magnetic resonance,” Nature (London) 10.1038/242190a0 242, 190–191 (1973). [DOI] [PubMed] [Google Scholar]
- Mansfield P., Morris P. G., Ordidge R., Coupland R. E., Bishop H. M., and Blamey R. W., “Carcinoma of the breast imaged by nuclear magnetic resonance (NMR),” Br. J. Radiol. 52(615), 242–243 (1979). [DOI] [PubMed] [Google Scholar]
- Ross R. J., Thompson J. S., Kim K., and Bailey R. A., “Nuclear magnetic resonance imaging and evaluation of human breast tissue: preliminary clinical trials,” Radiology 143(1), 195–205 (1982). [DOI] [PubMed] [Google Scholar]
- El Yousef S. J., Duchesneau R. H., Alfidi R. J., Haaga J. R., Bryan P. J., and LiPuma J. P., “Magnetic resonance imaging of the breast. Work in progress,” Radiology 150(3), 761–766 (1984). [DOI] [PubMed] [Google Scholar]
- El Yousef S. J., Alfidi R. J., Duchesneau R. H., Hubay C. A., Haaga J. R., Bryan P. J., LiPuma J. P., and Ament A. E., “Initial experience with nuclear magnetic resonance (NMR) imaging of the human breast,” J. Comput. Assist. Tomogr. 7(2), 215–218 (1983). [DOI] [PubMed] [Google Scholar]
- Stelling C. B., Wang P. C., Lieber A., Mattingly S. S., Griffen W. O., and Powell D. E., “Prototype coil for magnetic resonance imaging of the female breast. Work in progress,” Radiology 154(2), 457–462 (1985). [DOI] [PubMed] [Google Scholar]
- Brasch R. C., Weinmann H. J., and Wesbey G. E., “Contrast-enhanced NMR imaging: animal studies using gadolinium-DTPA complex,” AJR, Am. J. Roentgenol. 142(3), 625–630 (1984). [DOI] [PubMed] [Google Scholar]
- Carr D. H., Brown J., Bydder G. M., Steiner R. E., Weinmann H. J., Speck U., Hall A. S., and Young I. R., “Gadolinium-DTPA as a contrast agent in MRI: Initial clinical experience in 20 patients,” AJR, Am. J. Roentgenol. 143(2), 215–224 (1984). [DOI] [PubMed] [Google Scholar]
- Carr D. H., Brown J., Bydder G. M., Weinmann H. J., Speck U., Thomas D. J., and Young I. R., “Intravenous chelated gadolinium as a contrast agent in NMR imaging of cerebral tumours,” Lancet 1(8375), 484–486 (1984). [DOI] [PubMed] [Google Scholar]
- Laniado M., Weinmann H. J., Schorner W., Felix R., and Speck U., “First use of GdDTPA∕dimeglumine in man,” Physiol. Chem. Phys. Med. NMR 16(2), 157–165 (1984). [PubMed] [Google Scholar]
- Weinmann H. J., Brasch R. C., Press W. R., and Wesbey G. E., “Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent,” AJR, Am. J. Roentgenol. 142(3), 619–624 (1984). [DOI] [PubMed] [Google Scholar]
- Weinmann H. J., Laniado M., and Mutzel W., “Pharmacokinetics of GdDTPA∕dimeglumine after intravenous injection into healthy volunteers,” Physiol. Chem. Phys. Med. NMR 16(2), 167–172 (1984). [PubMed] [Google Scholar]
- Heywang S. H., Hahn D., Schmidt H., Krischke I., Eiermann W., Bassermann R., and Lissner J., “MR imaging of the breast using gadolinium-DTPA,” J. Comput. Assist. Tomogr. 10(2), 199–204 (1986). [DOI] [PubMed] [Google Scholar]
- Kaiser W. A. and Zeitler E., “MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Preliminary observations,” Radiology 170(3 Pt 1), 681–686 (1989). [DOI] [PubMed] [Google Scholar]
- Frahm J., Haase A., and Matthaei D., “Rapid NMR imaging of dynamic processes using the FLASH technique,” Magn. Reson. Med. 3(2), 321–327 (1986). [DOI] [PubMed] [Google Scholar]
- Frahm J., Haase A., and Matthaei D., “Rapid three-dimensional MR imaging using the FLASH technique,” J. Comput. Assist. Tomogr. 10(2), 363–368 (1986). [DOI] [PubMed] [Google Scholar]
- Haase A., Matthaei D., Hanicke W., and Frahm J., “Dynamic digital subtraction imaging using fast low-angle shot MR movie sequence,” Radiology 160(2), 537–541 (1986). [DOI] [PubMed] [Google Scholar]
- Folkman J. and Klagsbrun M., “Angiogenic factors,” Science 10.1126/science.2432664 235(4787), 442–447 (1987). [DOI] [PubMed] [Google Scholar]
- Flanagan F. L., Murray J. G., Gilligan P., Stack J. P., and Ennis J. T., “Digital subtraction in Gd-DTPA enhanced imaging of the breast,” Clin. Radiol. 50(12), 848–854 (1995). [DOI] [PubMed] [Google Scholar]
- Lehman C. D., Gatsonis C., Kuhl C. K., Hendrick R. E., Pisano E. D., Hanna L., Peacock S., Smazal S. F., Maki D. D., Julian T. B., DePeri E. R., Bluemke D. A., and Schnall M. D., “MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer,” N. Engl. J. Med. 356(13), 1295–1303 (2007). [DOI] [PubMed] [Google Scholar]
- Liberman L., Morris E. A., Kim C. M., Kaplan J. B., Abramson A. F., Menell J. H., Van Zee K. J., and Dershaw D. D., “MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer,” AJR, Am. J. Roentgenol. 180(2), 333–341 (2003). [DOI] [PubMed] [Google Scholar]
- Lee S. G., Orel S. G., Woo I. J., Cruz-Jove E., Putt M. E., Solin L. J., Czerniecki B. J., and Schnall M. D., “MR imaging screening of the contralateral breast in patients with newly diagnosed breast cancer: Preliminary results,” Radiology 226(3), 773–778 (2003). [DOI] [PubMed] [Google Scholar]
- Lehman C. D., Blume J. D., Thickman D., Bluemke D. A., Pisano E., Kuhl C., Julian T. B., Hylton N., Weatherall P., O’Loughlin M., Schnitt S. J., Gatsonics C., and Schnall M. D., “Added cancer yield of MRI in screening the contralateral breast of women recently diagnosed with breast cancer: Results from the International Breast Magnetic Resonance Consortium (IBMC) trial,” J. Surg. Oncol. 10.1002/jso.20350 92(1), 9–15 (2005); [DOI] [PubMed] [Google Scholar]; Lehman C. D., Blume J. D., Thickman D., Bluemke D. A., Pisano E., Kuhl C., Julian T. B., Hylton N., Weatherall P., O’Loughlin M., Schnitt S. J., Gatsonics C., and Schnall M. D., J. Surg. Oncol. discussion 92(1), 15–16 (2005). [DOI] [PubMed] [Google Scholar]
- Warner E., Plewes D. B., Shumak R. S., Catzavelos G. C., Di Prospero L. S., Yaffe M. J., Goel V., Ramsay E., Chart P. L., Cole D. E., Taylor G. A., Cutrara M., Samuels T. H., Murphy J. P., Murphy J. M., and Narod S. A., “Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer,” J. Clin. Oncol. 19(15), 3524–3531 (2001). [DOI] [PubMed] [Google Scholar]
- Stoutjesdijk M. J., Boetes C., Jager G. J., Beex L., Bult P., Hendriks J. H., Laheij R. J., Massuger L., van Die L. E., Wobbes T., and Barentsz J. O., “Magnetic resonance imaging and mammography in women with a hereditary risk of breast cancer,” J. Natl. Cancer Inst. 93(14), 1095–1102 (2001). [DOI] [PubMed] [Google Scholar]
- Kuhl C. K., Schrading S., Leutner C. C., Morakkabati-Spitz N., Wardelmann E., Fimmers R., Kuhn W., and Schild H. H., “Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer,” J. Clin. Oncol. 23(33), 8469–8476 (2005). [DOI] [PubMed] [Google Scholar]
- Kuhl C. K., Schmutzler R. K., Leutner C. C., Kempe A., Wardelmann E., Hocke A., Maringa M., Pfeifer U., Krebs D., and Schild H. H., “Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: Preliminary results,” Radiology 215(1), 267–279 (2000). [DOI] [PubMed] [Google Scholar]
- Lehman C. D., Blume J. D., Weatherall P., Thickman D., Hylton N., Warner E., Pisano E., Schnitt S. J., Gatsonis C., Schnall M., DeAngelis G. A., Stomper P., Rosen E. L., O’Loughlin M., Harms S., and Bluemke D. A., “Screening women at high risk for breast cancer with mammography and magnetic resonance imaging,” Cancer 103(9), 1898–1905 (2005). [DOI] [PubMed] [Google Scholar]
- Liberman L., Morris E. A., Benton C. L., Abramson A. F., and Dershaw D. D., “Probably benign lesions at breast magnetic resonance imaging: Preliminary experience in high-risk women,” Cancer 98(2), 377–388 (2003). [DOI] [PubMed] [Google Scholar]
- Liberman L., Morris E. A., Dershaw D. D., Abramson A. F., and Tan L. K., “Ductal enhancement on MR imaging of the breast,” AJR, Am. J. Roentgenol. 181(2), 519–525 (2003). [DOI] [PubMed] [Google Scholar]
- Tilanus-Linthorst M. M., Obdeijn I. M., Bartels K. C., de Koning H. J., and Oudkerk M., “First experiences in screening women at high risk for breast cancer with MR imaging,” Breast Cancer Res. Treat. 63(1), 53–60 (2000). [DOI] [PubMed] [Google Scholar]
- Morris E. A., Liberman L., Ballon D. J., Robson M., Abramson A. F., Heerdt A., and Dershaw D. D., “MRI of occult breast carcinoma in a high-risk population,” AJR, Am. J. Roentgenol. 181(3), 619–626 (2003). [DOI] [PubMed] [Google Scholar]
- Kriege M., Brekelmans C. T., Boetes C., Besnard P. E., Zonderland H. M., Obdeijn I. M., Manoliu R. A., Kok T., Peterse H., Tilanus-Linthorst M. M., Muller S. H., Meijer S., Oosterwijk J. C., Beex L. V., Tollenaar R. A., de Koning H. J., Rutgers E. J., and Klijn J. G., “Efficacy of MRI and mammography for breast-cancer screening in women with a familiar or genetic predisposition,” N. Engl. J. Med. 10.1056/NEJMoa031759 351(5), 427–437 (2004). [DOI] [PubMed] [Google Scholar]
- Leach M. O., Boggis C. R., Dixon A. K., Easton D. F., Eeles R. A., Evans D. G., Gilbert F. J., Griebsch I., Hoff R. J., Kessar P., Lakhani S. R., Moss S. M., Nerurkar A., Padhani A. R., Pointon L. J., Thompson D., and Waren R. M., “Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: A prospective multicentre cohort study (MARIBS),” Lancet 10.1016/S0140-6736(05)66481-1 365(9473), 1769–1778 (2005). [DOI] [PubMed] [Google Scholar]
- Sardanelli F., Podo F., D’Agnolo G., Verdecchia A., Santaquilani M., Musumeci R., Trecate G., Manoukian S., Morassut S., de Giacomi C., Federico M., Cortesi L., Corcione S., Cirillo S., Marra V., Cilotti A., Di Maggio C., Fausto A., Preda L., Zuiani C., Contegiacomo A., Orlacchio A., Calabrese M., Bonomo L., Di Cesare E., Tonutti M., Panizza P., and Del Maschio A., “Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): Interim results,” Radiology 242(3), 698–715 (2007). [DOI] [PubMed] [Google Scholar]
- Saslow D., Boetes C., Burke W., Harms S., Leach M. O., Lehman C. D., Morris E., Pisano E., Schnall M., Sener S., Smith R. A., Warner E., Yaffe M., Andrews K. S., and Russell C. A., “American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography,” Ca-Cancer J. Clin. 57(2), 75–89 (2007). [DOI] [PubMed] [Google Scholar]
- Peters N. H., Borel Rinkes I. H., Zuithoff N. P., Mali W. P., Moons K. G., and Peeters P. H., “Meta-analysis of MR imaging in the diagnosis of breast lesions,” Radiology 246(1), 116–124 (2008). [DOI] [PubMed] [Google Scholar]
- ACR, ACR Breast Imaging Reporting and Data Systems (BI-RADS): Breast Imaging Atlas (American College of Radiology, Reston, VA, 2003). [Google Scholar]
- Kuhl C., “The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice,” Radiology 10.1148/radiol.2442051620 244(2), 356–378 (2007). [DOI] [PubMed] [Google Scholar]
- Kuhl C. K., “Current status of breast MR imaging. Part 2. Clinical applications,” Radiology 244(3), 672–691 (2007). [DOI] [PubMed] [Google Scholar]
- Bellin M. F., Jakobsen J. A., Tomassin I., Thomsen H. S., Morcos S. K., Thomsen H. S., Morcos S. K., Almen T., Aspelin P., Bellin M. F., Clauss W., Flaten H., Grenier N., Idee J. M., Jakobsen J. A., Krestin G. P., Stacul F., and Webb J. A., “Contrast medium extravasation injury: Guidelines for prevention and management,” Eur. Radiol. 12(11), 2807–2812 (2002). [DOI] [PubMed] [Google Scholar]
- FDA, US Public Health Advisory: Update on Magnetic Resonance Imaging (MRI) Contrast Agents Containing Gadolinium and Nephrogenic Fibrosing Dermopathy, Date created: December 22, 2006; updated May 23, 2007. http://www.fda.gov/Cder/Drug/advisory/gadolinium_agents_20061222.htm [Accessed: April 29, 2008].
- Gribbestad I. S., Sitter B., Lundgren S., Krane J., and Axelson D., “Metabolite composition in breast tumors examined by proton nuclear magnetic resonance spectroscopy,” Anticancer Res. 19(3A), 1737–1746 (1999). [PubMed] [Google Scholar]
- Barzilai A., Horowitz A., Geier A., and Degani H., “Phosphate metabolites and steroid hormone receptors of benign and malignant breast tumors. A nuclear magnetic resonance study,” Cancer 67(11), 2919–2925 (1991). [DOI] [PubMed] [Google Scholar]
- Cohen J. S., “Phospholipid and energy metabolism of cancer cells monitored by 31P magnetic resonance spectroscopy: possible clinical significance,” Mayo Clin. Proc. 63(12), 1199–1207 (1988). [DOI] [PubMed] [Google Scholar]
- Kalra R., Wade K. E., Hands L., Styles P., Camplejohn R., Greenall M., Adams G. E., Harris A. L., and Radda G. K., “Phosphomonoester is associated with proliferation in human breast cancer: a 31P MRS study,” Br. J. Cancer 67(5), 1145–1153 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. M. and Park J. H., “Human in-vivo 31P MR spectroscopy of benign and malignant breast tumors,” Korean J Radiol 2(2), 80–86 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvistad K. A., Bakken I. J., Gribbestad I. S., Ehrnholm B., Lundgren S., Fjosne H. E., and Haraldseth O., “Characterization of neoplastic and normal human breast tissues with in vivo (1)H MR spectroscopy,” J. Magn. Reson Imaging 10(2), 159–164 (1999). [DOI] [PubMed] [Google Scholar]
- Roebuck J. R., Cecil K. M., Schnall M. D., and Lenkinski R. E., “Human breast lesions: characterization with proton MR spectroscopy,” Radiology 209(1), 269–275 (1998). [DOI] [PubMed] [Google Scholar]
- Gribbestad I. S., Singstad T. E., Nilsen G., Fjosne H. E., Engan T., Haugen O. A., and Rinck P. A., “In vivo 1H MRS of normal breast and breast tumors using a dedicated double breast coil,” J. Magn. Reson Imaging 8(6), 1191–1197 (1998). [DOI] [PubMed] [Google Scholar]
- Yeung D. K., Cheung H. S., and Tse G. M., “Human breast lesions: characterization with contrast-enhanced in vivo proton MR spectroscopy—initial results,” Radiology 220(1), 40–46 (2001). [DOI] [PubMed] [Google Scholar]
- Katz-Brull R., Lavin P. T., and Lenkinski R. E., “Clinical utility of proton magnetic resonance spectroscopy in characterizing breast lesions,” J. Natl. Cancer Inst. 94(16), 1197–1203 (2002). [DOI] [PubMed] [Google Scholar]
- Baek H. M., Yu H. J., Chen J. H., Nalcioglu O., and Su M. Y., “Quantitative correlation between (1)H MRS and dynamic contrast-enhanced MRI of human breast cancer,” Magn. Reson. Imaging 26(4), 523–531 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M. Y., Baik H. M., Yu H. J., Chen J. H., Mehta R. S., and Nalcioglu O., “Comparison of choline and pharmacokinetic parameters in breast cancer measured by MR spectroscopic imaging and dynamic contrast enhanced MRI,” Technol. Cancer Res. Treat. 5(4), 401–410 (2006). [PubMed] [Google Scholar]
- Meisamy S., Bolan P. J., Baker E. H., Pollema M. G., Le C. T., Kelcz F., Lechner M. C., Luikens B. A., Carlson R. A., Brandt K. R., Amrami K. K., Nelson M. T., Everson L. I., Emory T. H., Tuttle T. M., Yee D., and Garwood M., “Adding in vivo quantitative 1H MR spectroscopy to improve diagnostic accuracy of breast MR imaging: Preliminary results of observer performance study at 4.0 T,” Radiology 236(2), 465–475 (2005). [DOI] [PubMed] [Google Scholar]
- Huang W., Fisher P. R., Dulaimy K., Tudorica L. A., O’Hea B., and Button T. M., “Detection of breast malignancy: Diagnostic MR protocol for improved specificity,” Radiology 232(2), 585–591 (2004). [DOI] [PubMed] [Google Scholar]
- Bartella L., Thakur S. B., Morris E. A., Dershaw D. D., Huang W., Chough E., Cruz M. C., and Liberman L., “Enhancing nonmass lesions in the breast: Evaluation with proton (1H) MR spectroscopy,” Radiology 10.1148/radiol.2451061639 245(1), 80–87 (2007). [DOI] [PubMed] [Google Scholar]
- Aboagye E. O. and Bhujwalla Z. M., “Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells,” Cancer Res. 59(1), 80–84 (1999). [PubMed] [Google Scholar]
- Kumar M., Jagannathan N. R., Seenu V., Dwivedi S. N., Julka P. K., and Rath G. K., “Monitoring the therapeutic response of locally advanced breast cancer patients: Sequential in vivo proton MR spectroscopy study,” J. Magn. Reson Imaging 24(2), 325–332 (2006). [DOI] [PubMed] [Google Scholar]
- Jagannathan N. R., Kumar M., Seenu V., Coshic O., Dwivedi S. N., Julka P. K., Srivastava A., and Rath G. K., “Evaluation of total choline from in-vivo volume localized proton MR spectroscopy and its response to neoadjuvant chemotherapy in locally advanced breast cancer,” Br. J. Cancer 84(8), 1016–1022 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek H. M., Chen J. H., Nalcioglu O., and Su M. Y., “Proton MR spectroscopy for monitoring early treatment response of breast cancer to neo-adjuvant chemotherapy,” Ann. Oncol. 19(5), 1022–1024 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton D. J., Chaturvedi A., Hubbard A., Lind M. J., Lowry M., Maraveyas A., Pickles M. D., Tozer D. J., and Turnbull L. W., “Neoadjuvant chemotherapy in breast cancer: Early response prediction with quantitative MR imaging and spectroscopy,” Br. J. Cancer 94(3), 427–435 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisamy S., Bolan P. J., Baker E. H., Bliss R. L., Gulbahce E., Everson L. I., Nelson M. T., Emory T. H., Tuttle T. M., Yee D., and Garwood M., “Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo (1)H MR spectroscopy--a pilot study at 4 T,” Radiology 233(2), 424–431 (2004). [DOI] [PubMed] [Google Scholar]
- Jacobs M. A., Barker P. B., Bottomley P. A., Bhujwalla Z., and Bluemke D. A., “Proton magnetic resonance spectroscopic imaging of human breast cancer: a preliminary study,” J. Magn. Reson Imaging 19(1), 68–75 (2004). [DOI] [PubMed] [Google Scholar]
- Jacobs M. A., Ouwerkerk R., Wolff A. C., Stearns V., Bottomley P. A., Barker P. B., Argani P., Khouri N., Davidson N. E., Bhujwalla Z. M., and Bluemke D. A., “Multiparametric and multinuclear magnetic resonance imaging of human breast cancer: current applications,” Technol. Cancer Res. Treat. 3(6), 543–550 (2004). [DOI] [PubMed] [Google Scholar]
- Hu J., Yu Y., Kou Z., Huang W., Jiang Q., Xuan Y., Li T., Sehgal V., Blake C., Haacke E. M., and Soulen R. L., “A high spatial resolution (1)H magnetic resonance spectroscopic imaging technique for breast cancer with a short echo time,” Magn. Reson. Imaging 26(3), 360–366 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadin I. S., McIntosh A., Meisamy S., Corum C., Snyder A. L., Powell N. J., Nelson M. T., Yee D., Garwood M., and Bolan P. J., “Metabolite quantification and high-field MRS in breast cancer,” NMR Biomed. (to be published). [DOI] [PMC free article] [PubMed]
- Tse G. M., Yeung D. K., King A. D., Cheung H. S., and Yang W. T., “In vivo proton magnetic resonance spectroscopy of breast lesions: An update,” Breast Cancer Res. Treat. 104(3), 249–255 (2007). [DOI] [PubMed] [Google Scholar]
- Stanwell P. and Mountford C., “In vivo proton MR spectroscopy of the breast,” Radiographics 27(Suppl 1), S253–S266 (2007). [DOI] [PubMed] [Google Scholar]
- Wolbarst A. B. and Hendee W. R., “Evolving and experimental technologies in medical imaging,” Radiology 238(1), 16–39 (2006). [DOI] [PubMed] [Google Scholar]
- Liberman M., Sampalis F., Mulder D. S., and Sampalis J. S., “Breast cancer diagnosis by scintimammography: a meta-analysis and review of the literature,” Breast Cancer Res. Treat. 80(1), 115–126 (2003). [DOI] [PubMed] [Google Scholar]
- Khalkhali I., Villanueva-Meyer J., Edell S. L., Connolly J. L., Schnitt S. J., Baum J. K., Houlihan M. J., Jenkins R. M., and Haber S. B., “Diagnostic accuracy of 99mTc-sestamibi breast imaging: multicenter trial results,” J. Nucl. Med. 41(12), 1973–1979 (2000). [PubMed] [Google Scholar]
- Hruska C. B., O’Connor M. K., and Collins D. A., “Comparison of small field of view gamma camera systems for scintimammography,” Nucl. Med. Commun. 10.1097/00006231-200505000-00008 26(5), 441–445 (2005). [DOI] [PubMed] [Google Scholar]
- Mueller B., O’Conner M. K., Blevis I., Rhodes D. J., Smith R., Collins D. A., and Phillips S. W., “Evaluation of a small cadmium zinc telluride detector for scintimammography,” J. Nucl. Med. 44(4), 602–609 (2003). [PubMed] [Google Scholar]
- O’Connor M. K., Phillips S. W., Hruska C. B., Rhodes D. J., and Collins D. A., “Molecular breast imaging: advantages and limitations of a scintimammographic technique in patients with small breast tumors,” Breast J. 10.1111/j.1524-4741.2006.00356.x 13(1), 3–11 (2007). [DOI] [PubMed] [Google Scholar]
- Brem R. F., Rapelyea J. A., Zisman G., Mohtashemi K., Raub J., Teal C. B., Majewski S., and Welch B. L., “Occult breast cancer: scintimammography with high-resolution breast-specific gamma camera in women at high risk for breast cancer,” Radiology 237(1), 274–280 (2005). [DOI] [PubMed] [Google Scholar]
- Spanu A., Cottu P., Manca A., Chessa F., Sanna D., and Madeddu G., “Scintimammography with dedicated breast camera in unifocal and multifocal∕multicentric primary breast cancer detection: a comparative study with SPECT,” Int. J. Oncol. 31(2), 369–377 (2007). [PubMed] [Google Scholar]
- Tornai M. P., Bowsher J. E., Archer C. N., Peter J., Jaszczak R. J., MacDonald L. R., Patt B. E., and Iwanczyk J. S., “A 3D gantry single photon emission tomograph with hemispherical coverage for dedicated breast imaging,” Nucl. Instrum. Methods Phys. Res. A 10.1016/S0168-9002(02)01906-X 497(1), 157–167 (2003). [DOI] [Google Scholar]
- Brzymialkiewicz C. N., Tornai M. P., McKinley R. L., and Bowsher J. E., “Evaluation of fully 3-D emission mammotomography with a compact cadmium zinc telluride detector,” IEEE Trans. Med. Imaging 10.1109/TMI.2005.852501 24(7), 868–877 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw M. L., McKinley R. L., Samei E., Archer C. N., and Tornai M. P., “Initial x-ray design considerations for application specific emission and transmission tomography (ASETT) of the breast,” Proceedings of the Annual Meeting of the Society of Nuclear Medicine, New Orleans, Louisiana, J. Nucl. Med. 44(5), 287P (2003).
- Yutani K., Shiba E., Kusuoka H., Tatsumi M., Uehara T., Taguchi T., Takai S. I., and Nishimura T., “Comparison of FDG-PET with MIBI-SPECT in the detection of breast cancer and axillary lymph node metastasis,” J. Comput. Assist. Tomogr. 24(2), 274–280 (2000). [DOI] [PubMed] [Google Scholar]
- Smith M. F., Majewski S., Weisenberger A. G., Kieper D. A., Raylman R. R., and Turkington T. G., “Analysis of factors affecting positron emission mammography (PEM) image formation.,” IEEE Trans. Nucl. Sci. 10.1109/TNS.2002.807942 50, 53–59 (2003). [DOI] [Google Scholar]
- Raylman R. R., Majewski S., Smith M. F., Proffitt J., Hammond W., Srinivasan A., McKisson J., Popov V., Weisenberger A., Judy C. O., Kross B., Ramasubramanian S., Banta L. E., Kinahan P. E., and Champley K., “The positron emission mammography∕tomography breast imaging and biopsy system (PEM∕PET): Design, construction and phantom-based measurements,” Phys. Med. Biol. 10.1088/0031-9155/53/3/009 53(3), 637–653 (2008). [DOI] [PubMed] [Google Scholar]
- Doshi N. K., Shao Y., Silverman R. W., and Cherry S. R., “Design and evaluation of an LSO PET detector for breast cancer imaging,” Med. Phys. 10.1118/1.599019 27(7), 1535–1543 (2000). [DOI] [PubMed] [Google Scholar]
- Dokhale P. A., Silverman R. W., Shah K. S., Grazioso R., Farrell R., Glodo J., McClish M. A., Entine G., Tran V. H., and Cherry S. R., “Performance measurements of a depth-encoding PET detector module based on position-sensitive avalanche photodiode read-out,” Phys. Med. Biol. 10.1088/0031-9155/49/18/007 49(18), 4293–4304 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang J., Olcott P. D., Chinn G., Foudray A. M., and Levine C. S., “Study of the performance of a novel 1 mm resolution dual-panel PET camera design dedicated to breast cancer imaging using Monte Carlo simulation,” Med. Phys. 10.1118/1.2409480 34(2), 689–702 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Murthy K., Weinberg I. N., and Mako F., “Feasibility study for positron emission mammography,” Med. Phys. 10.1118/1.597169 21(4), 529–538 (1994). [DOI] [PubMed] [Google Scholar]
- Murthy K., Aznar M., Bergman A. M., Thompson C. J., Robar J. L., Lisbona R., Loutfi A., and Gagnon J. H., “Positron emission mammographic instrument: initial results,” Radiology 215(1), 280–285 (2000). [DOI] [PubMed] [Google Scholar]
- Murthy K., Aznar M., Thompson C. J., Loutfi A., Lisbona R., and Gagnon J. H., “Results of preliminary clinical trials of the positron emission mammography system PEM-I: A dedicated breast imaging system producing glucose metabolic images using FDG,” J. Nucl. Med. 41(11), 1851–1858 (2000). [PubMed] [Google Scholar]
- Raylman R. R., Majewski S., Wojcik R., Weisenberger A. G., Kross B., Popov V., and Bishop H. A., “The potential role of positron emission mammography for detection of breast cancer. A phantom study,” Med. Phys. 10.1118/1.1287439 27(8), 1943–1954 (2000). [DOI] [PubMed] [Google Scholar]
- Motta A., Guerra A. D., Belcari N., Moehrs S., Panetta D., Righi S., and Valentini D., “Fast 3D-EM reconstruction using Planograms for stationary planar positron emission mammography camera,” Comput. Med. Imaging Graph. 29(8), 587–596 (2005). [DOI] [PubMed] [Google Scholar]
- Amaral P., Carriço B., Ferreira M., Moura R., Ortigão C., Rodrigues P., Da Silva J. C., Trindade A., and Varela J., “Performance and quality control of clear-PEM detector modules,” Nucl. Instrum. Methods Phys. Res. A 580(2), 1123–1126 (2007). [Google Scholar]
- Rosen E. L., Turkington T. G., Soo M. S., Baker J. A., and Coleman R. E., “Detection of primary breast carcinoma with a dedicated, large-field-of-view FDG PET mammography device: Initial experience,” Radiology 234(2), 527–534 (2005). [DOI] [PubMed] [Google Scholar]
- Berg W. A., Weinberg I. N., Narayanan D., Lobrano M. E., Ross E., Amodei L., Tafra L., Adler L. P., Uddo J., W.Stein, 3rd, and Levine E. A., “High-resolution fluorodeoxyglucose positron emission tomography with compression (‘positron emission mammography’) is highly accurate in depicting primary breast cancer,” Breast J. 10.1111/j.1075-122X.2006.00269.x 12(4), 309–323 (2006). [DOI] [PubMed] [Google Scholar]
- Benard F. and Turcotte E., “Imaging in breast cancer: Single-photon computed tomography and positron-emission tomography,” Breast Cancer Res 7(4), 153–162 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. E., Jacobson F. L., and Judy P. F., “Human observer detection experiments with mammograms and power-low noise,” Med. Phys. 10.1118/1.1355308 28(4), 419–437 (2001). [DOI] [PubMed] [Google Scholar]
- Burgess A. E., Li X., and Abbey C. K., “Visual signal detectability with two noise components: Anomalous masking effects,” J. Opt. Soc. Am. A 14(9), 2420–2442 (1997). [DOI] [PubMed] [Google Scholar]
- Chakraborty D. P. and Kundel H. L., “Anomalous nodule visibility effects in mammography images,” Proc. SPIE 10.1117/12.431174 4324, 68–76 (2001). [DOI] [Google Scholar]
- Chan H. P., Goodsitt M. M., Hadjiiski L. M., Bailey J. E., Klein K., Darner K. L., and Sahiner B., “Effects of magnification and zooming on depth perception in digital stereomammography: An observer performance study,” Phys. Med. Biol. 10.1088/0031-9155/48/22/007 48(22), 3721–3734 (2003). [DOI] [PubMed] [Google Scholar]
- Goodsitt M. M., Chan H. P., Darner K. L., and Hadjiiski L. M., “The effects of stereo shift angle, geometric magnification and display zoom on depth measurements in digital stereomammography,” Med. Phys. 10.1118/1.1517615 29(11), 2725–2734 (2002). [DOI] [PubMed] [Google Scholar]
- Chan H. P., Goodsitt M. M., Helvie M. A., Hadjiiski L. M., Lydick J. T., Roubidoux M. A., Bailey J. E., Nees A., Blane C. E., and Sahiner B., “ROC study of the effect of stereoscopic imaging on assessment of breast lesions,” Med. Phys. 10.1118/1.1870172 32(4), 1001–1009 (2005). [DOI] [PubMed] [Google Scholar]
- Getty D. J.et al. , “Improved accuracy of lesion detection in breast cancer screening with stereoscopic digital mammography [abstract],” in 93rd Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA). RSNA Scientific Assembly and Annual Meeting Program2007, pp.381–382.
- Maidment A. D., Bakic P. R., and Albert M., “Effects of quantum noise and binocular summation on dose requirements in stereoradiography,” Med. Phys. 10.1118/1.1621869 30(12), 3061–3071 (2003). [DOI] [PubMed] [Google Scholar]
- Getty D. J. and Green P. J., “Clinical medical applications for stereoscopic 3D displays,” J. Soc. Inf. Disp. 10.1889/1.2749323 15(6), 377–384 (2007). [DOI] [Google Scholar]
- Bissonnette M., Hansroul M., Masson E., Savard S., Cadieux S., Warmoes P., Gravel D., Agopyan J., Polischuk B., Haerer W., Mertelmeier T., Lo J. Y., Chen Y., Dobbins JTr., Jesneck J. L., and Singh S., “Digital breast tomosynthesis using an amorphous selenium flat panel detector,” Proc. SPIE 10.1117/12.601622 5745, 529–540 (2005). [DOI] [Google Scholar]
- Ren B., Ruth C., Stein J., Smith A., Shaw I., and Jing Z., “Design and performance of the prototype full field breast tomosynthesis system with selenium based flat panel detector,” Proc. SPIE 10.1117/12.595833 5745, 550–561 (2005). [DOI] [Google Scholar]
- Maidment A. D., Ullberg C., Lindman K., Adelöw L., Egerström J., Eklund M., Francke T., Jordung U., Kristoffersson T., Lindqvist L., Marchal D., Olla H., Penton E., Rantanen J., Solokov S., Weber N., and Westerberg H., “Evaluation of a photon-counting breast tomosynthesis imaging system,” Proc. SPIE 10.1117/12.654651 6142, 61420B (2006). [DOI] [Google Scholar]
- Niklason L. T., Christian B. T., Niklason L. E., Kopans D. B., Castleberry D. E., OpsahlOng B. H., Landberg C. E., Slanetz P. J., Giardino A. A., Moore R., Albagli D., DeJule M. C., Fitzgerald P. F., Fobare D. F., Giambattista B. W., Kwasnick R. F., Liu J. Q., Lubowski S. J., Possin G. E., Richotte J. F., Wei C. Y., and Wirth R. F., “Digital Tomosynthesis in breast imaging,” Radiology 205(2), 399–406 (1997). [DOI] [PubMed] [Google Scholar]
- Suryanarayanan S., Karellas A., Vedantham S., Baker S. P., Glick S. J., D’Orsi C. J., and Webber R. L., “Evaluation of linear and nonlinear tomosynthetic reconstruction methods in digital mammography,” Acad. Radiol. 8(3), 219–224 (2001). [DOI] [PubMed] [Google Scholar]
- Suryanarayanan S., Karellas A., Vedantham S., Glick S. J., D’Orsi C. J., Baker S. P., and Webber R. L., “Comparison of tomosynthesis methods used with digital mammography,” Acad. Radiol. 7(12), 1085–1097 (2000). [DOI] [PubMed] [Google Scholar]
- Zhao W., Zhao B., Fisher P. R., Warmoes P., Mertelmeier T., and Orman J., “Optimization of detector operation and imaging geometry for breast tomosynthesis,” Proc. SPIE 10.1117/12.713718 6510, 65101M (2007). [DOI] [Google Scholar]
- Zhao B. and Zhao W., “Imaging performance of an amorphous selenium digital mammography detector in a breast tomosynthesis system,” Med. Phys. 10.1118/1.2903425 35(5), 1978–1987 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano A., Kyprianou I. S., Jennings R. J., and Sempau J., “Anisotropic imaging performance in breast tomosynthesis,” Med. Phys. 10.1118/1.2779943 34(11), 4076–4091 (2007). [DOI] [PubMed] [Google Scholar]
- Mainprize J. G., Bloomquist A. K., Kempston M. P., and Yaffe M. J., “Resolution at oblique incidence angles of a flat panel imager for breast tomosynthesis,” Med. Phys. 10.1118/1.2241994 33(9), 3159–3164 (2006). [DOI] [PubMed] [Google Scholar]
- Sechopoulos I., Suryanarayanan S., Vedantham S., D’Orsi C., and Karellas A., “Computation of the glandular radiation dose in digital tomosynthesis of the breast,” Med. Phys. 10.1118/1.2400836 34(1), 221–232 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Zhao B., and Zhao W., “A computer simulation platform for the optimization of a breast tomosynthesis system,” Med. Phys. 10.1118/1.2558160 34(3), 1098–1109 (2007). [DOI] [PubMed] [Google Scholar]
- Glick S. J. and Gong X., “Optimal spectra for indirect detector breast tomosynthesis,” Proc. SPIE 10.1117/12.649547 6142, 61421L (2006). [DOI] [Google Scholar]
- Wu T., Liu B., Moore R., and Kopans D. B., “Optimal acquisition techniques for digital breast tomosynthesis screening,” Proc. SPIE 10.1117/12.652289 6142, 61425E (2006). [DOI] [Google Scholar]
- Sechopoulos I., Suryanarayanan S., Vedantham S., D’Orsi C. J., and Karellas A., “Scatter radiation in digital tomosynthesis of the breast,” Med. Phys. 10.1118/1.2428404 34(2), 564–576 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann F., Meyer H., Diekmann S., Puong S., Muller S., Bick U., and Rogalla P., “Thick slices from tomosynthesis data sets: Phantom study for the evaluation of different algorithms,” J. Digit Imaging (to be published). [DOI] [PMC free article] [PubMed]
- Chen Y., Lo J. Y., and J. T.Dobbins, 3rd, “Importance of point-by-point back projection correction for isocentric motion in digital breast tomosynthesis: Relevance to morphology of structures such as microcalcifications,” Med. Phys. 10.1118/1.2776256 34(10), 3885–3892 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chan H. P., Sahiner B., Wei J., Goodsitt M. M., Hadjiiski L. M., Ge J., and Zhou C., “A comparative study of limited-angle cone-beam reconstruction methods for breast tomosynthesis,” Med. Phys. 10.1118/1.2237543 33(10), 3781–3795 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Moore R. H., and Kopans D. B., “Voting strategy for artifact reduction in digital breast tomosynthesis,” Med. Phys. 10.1118/1.2207127 33(7), 2461–2471 (2006). [DOI] [PubMed] [Google Scholar]
- Rakowski J. T. and Dennis M. J., “A comparison of reconstruction algorithms for C-arm mammography tomosynthesis,” Med. Phys. 10.1118/1.2219090 33(8), 3018–3032 (2006). [DOI] [PubMed] [Google Scholar]
- Mertelmeier T., Orman J., Haerer W., and Dudam M. K., “Optimizing filtered backprojection reconstruction for a breast tomosynthesis prototype device,” Proc. SPIE 10.1117/12.651380 6142, 61420F (2006). [DOI] [Google Scholar]
- Chen Y., Lo J. Y., Baker J. A., and Dobbins J. Tr., “Gaussian frequency blending algorithm with matrix inversion tomosynthesis (MITS) and filtered back projection (FBP) for better digital breast tomosynthesis reconstruction,” Proc. SPIE 10.1117/12.652264 6142, 61420E (2006). [DOI] [Google Scholar]
- Poplack S. P., Tosteson T. D., Kogel C. A., and Nagy H. M., “Digital breast tomosynthesis: initial experience in 98 women with abnormal digital screening mammography,” AJR, Am. J. Roentgenol. 10.2214/AJR.07.2231 189(3), 616–623 (2007). [DOI] [PubMed] [Google Scholar]
- Badea C., Kolitsi Z., and Pallikarakis N., “A wavelet-based method for removal of out-of-plane structures in digital tomosynthesis,” Comput. Med. Imaging Graph. 10.1016/S0895-6111(98)00037-8 22(4), 309–315 (1998). [DOI] [PubMed] [Google Scholar]
- Kolitsi Z., Panayiotakis G., and Pallikarakis N., “A method for selective removal of out-of-plane structures in digital tomosynthesis,” Med. Phys. 10.1118/1.597060 20(1), 47–50 (1993). [DOI] [PubMed] [Google Scholar]
- Ge J., Chan H. P., Zhang Y., Sahiner B., Wei J., and Hadjiiski L. M., “Digital tomosynthesis mammography: An interplane artifact reduction method for microcalcifications on reconstructed slices based on 3D geometrical information,” in 92nd Scientific Assembly and Annual Meeting of the RSNA. RSNA Scientific Assembly and Annual Meeting Program 2006, p. 230.
- Moore R. H.et al. , “Initial callback rates for conventional and digital breast tomosynthesis mammography comparison in the screening setting,” in 93rd Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA). Scientific Assembly and Annual Meeting Program 2007, p. 381.
- Redington R. W. and J. L.Henkes, Jr., “Mammography,” U.S. Patent, No. 3,973,126, General Electric Co., Shenectady, NY, August 3, 1976.
- Chang C. H., Sibala J. L., Fritz S. L., S. J.Dwyer, 3rd, Templeton A. W., Lin F., and Jewell W. R., “Computed tomography in detection and diagnosis of breast cancer,” Cancer 46(4 Suppl), 939–946 (1980). [DOI] [PubMed] [Google Scholar]
- Chang C. H., Nesbit D. E., Fisher D. R., Fritz S. L., S. J.Dwyer, 3rd, Templeton A. W., Lin F., and Jewell W. R., “Computed tomographic mammography using a conventional body scanner,” AJR, Am. J. Roentgenol. 138(3), 553–558 (1982). [DOI] [PubMed] [Google Scholar]
- Raptopoulos V., Baum J. K., Hochman M., Karellas A., Houlihan M. J., and D’Orsi C. J., “High resolution CT mammography of surgical biopsy specimens,” J. Comput. Assist. Tomogr. 10.1097/00004728-199603000-00003 20(2), 179–184 (1996). [DOI] [PubMed] [Google Scholar]
- Boone J. M., Nelson T. R., Lindfors K. K., and Seibert J. A., “Dedicated breast CT: radiation dose and image quality evaluation,” Radiology 10.1148/radiol.2213010334 221(3), 657–667 (2001). [DOI] [PubMed] [Google Scholar]
- Boone J. M., Shah N., and Nelson T. R., “A comprehensive analysis of DgN(CT) coefficients for pendant-geometry cone-beam breast computed tomography,” Med. Phys. 10.1118/1.1636571 31(2), 226–235 (2004). [DOI] [PubMed] [Google Scholar]
- Thacker S. C. and Glick S. J., “Normalized glandular dose (DgN) coefficients for flat-panel CT breast imaging,” Phys. Med. Biol. 10.1088/0031-9155/49/24/003 49(24), 5433–5444 (2004). [DOI] [PubMed] [Google Scholar]
- Sechopoulos I., Vedantham S., Suryanarayanan S., D’Orsi C. J., and Karellas A., “Monte Carlo and phantom study of the radiation dose to the body from dedicated CT of the breast,” Radiology 247(1), 98–105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan A. L., Boone J. M., and Shah N., “Evaluation of x-ray scatter properties in a dedicated cone-beam breast CT scanner,” Med. Phys. 10.1118/1.1954908 32(9), 2967–2975 (2005). [DOI] [PubMed] [Google Scholar]
- Liu B., Glick S. J., and Groiselle C., “Characterization of scatter radiation in cone beam CT mammography,” Proc. SPIE 5745, 818–827 (2005). [Google Scholar]
- Chen B. and Ning R., “Cone-beam volume CT breast imaging: feasibility study,” Med. Phys. 10.1118/1.1461843 29(5), 755–770 (2002). [DOI] [PubMed] [Google Scholar]
- Boone J. M., Breast CT: Its prospect for breast cancer screening and diagnosis, in Advances in breast imaging: Physics, Technology and Clinical Applications, Categorical Course in Diagnostic Radiology Physics, edited by Karellas A. and Giger M. L. (Radiological Society of North America (RSNA), Oak Brook, IL, 2004). [Google Scholar]
- McKinley R. L., Bryzmialkiewicz C. N., Madhav P., and Tornai M. P., “Investigation of cone-beam acquisitions implemented using a novel dedicated mammotomography system with unique arbitrary orbit capability,” Proc. SPIE 10.1117/12.596089 5745, 609–617 (2005). [DOI] [Google Scholar]
- Zeng K., Yu H., Fajardo L. L., and Wang G., “Cone-beam mammo-computed tomography from data along two tilting arcs,” Med. Phys. 10.1118/1.2336510 33(10), 3621–3633 (2006). [DOI] [PubMed] [Google Scholar]
- Boone J. M., Kwan A. L., Seibert J. A., Shah N., Lindfors K. K., and Nelson T. R., “Technique factors and their relationship to radiation dose in pendant geometry breast CT,” Med. Phys. 10.1118/1.2128126 32(12), 3767–3776 (2005). [DOI] [PubMed] [Google Scholar]
- Glick S. J., Thacker S., Gong X., and Liu B., “Evaluating the impact of X-ray spectral shape on image quality in flat-panel CT breast imaging,” Med. Phys. 10.1118/1.2388574 34(1), 5–24 (2007). [DOI] [PubMed] [Google Scholar]
- McKinley R. L., Tornai M. P., Samei E., and Bradshaw M. L., “Simulation study of a quasi-monochromatic beam for x-ray computed mammotomography,” Med. Phys. 10.1118/1.1668371 31(4), 800–813 (2004). [DOI] [PubMed] [Google Scholar]
- Kwan A. L., Boone J. M., Yang K., and Huang S. Y., “Evaluation of the spatial resolution characteristics of a cone-beam breast CT scanner,” Med. Phys. 10.1118/1.2400830 34(1), 275–281 (2007). [DOI] [PubMed] [Google Scholar]
- Zhong J., Ning R., and Conover D., “Image denoising based on multiscale singularity detection for cone beam CT breast imaging,” IEEE Trans. Med. Imaging 10.1109/TMI.2004.826944 23(6), 696–703 (2004). [DOI] [PubMed] [Google Scholar]
- Altunbas M. C., Shaw C. C., Chen L., Lai C., Liu X., Han T., and Wang T., “A post-reconstruction method to correct cupping artifacts in cone beam breast computed tomography,” Med. Phys. 10.1118/1.2748106 34(7), 3109–3118 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. T., Carkaci S., Chen L., Lai C. J., Sahin A., Whitman G. J., and Shaw C. C., “Dedicated cone-beam breast CT: Feasibility study with surgical mastectomy specimens,” AJR, Am. J. Roentgenol. 189(6), 1312–1315 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone J. M., Kwan A. L., Yang K., Burkett G. W., Lindfors K. K., and Nelson T. R., “Computed tomography for imaging the breast,” Journal of mammary gland biology and neoplasia 11(2), 103–111 (2006). [DOI] [PubMed] [Google Scholar]
- Lindfors K. K., Boone J. M., Nelson T. R., Yang K., Kwan A. L., and Miller D. F., “Dedicated breast CT: Initial clinical experience,” Radiology 10.1148/radiol.2463070410 246(3), '725–733 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty D. J., Madhav P., McKinley R. L., and Tornai M. P., “Investigating novel patient bed designs for use in a hybrid dual modality dedicated 3D breast imaging system,” Proc. SPIE 10.1117/12.713764 6510, 65101H (2007). [DOI] [Google Scholar]
- Ning R., Conover D., Yu Y., Zhang Y., Cai W., Betancourt-Benitez R., and Lu X., “A novel cone beam breast CT scanner: system evaluation,” Proc. SPIE 10.1117/12.710340 6510, 651030 (2007). [DOI] [Google Scholar]
- Gong X., Vedula A. A., and Glick S. J., “Microcalcification detection using cone-beam CT mammography with a flat-panel imager,” Phys. Med. Biol. 10.1088/0031-9155/49/11/005 49(11), 2183–2195 (2004). [DOI] [PubMed] [Google Scholar]
- Lai C. J., Shaw C. C., Chen L., Altunbas M. C., Liu X., Han T., Wang T., Yang W. T., Whitman G. J., and Tu S. J., “Visibility of microcalcification in cone beam breast CT: Effects of X-ray tube voltage and radiation dose,” Med. Phys. 10.1118/1.2745921 34(7), 2995–3004 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan A. L., Yang K., Lindfors K. K., Nelson T. R., and Boone J. M., “Visualization of micro-calcifications in a prototype breast CT [abstract],” Med. Phys. 33, 1990 (2006). [Google Scholar]
- Shikhaliev P. M., “Tilted angle CZT detector for photon counting∕energy weighting x-ray and CT imaging,” Phys. Med. Biol. 10.1088/0031-9155/51/17/010 51(17), 4267–4287 (2006). [DOI] [PubMed] [Google Scholar]
- Rudin S., Kuhls A. T., Yadava G. K., Josan G. C., Wu Y., Chityala R. N., Rangwala H. S., Ionita N. C., Hoffmann K. R., and Bednarek D. R., “New light-amplifier-based detector designs for high spatial resolution and high sensitivity CBCT mammography and fluoroscopy,” Proc. SPIE 10.1117/12.649501 6142, 61421R (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick S. J., “Breast CT,” Annu. Rev. Biomed. Eng. 10.1146/annurev.bioeng.9.060906.151924 9, 501–526 (2007). [DOI] [PubMed] [Google Scholar]
- Gong X., Glick S. J., Liu B., Vedula A. A., and Thacker S., “A computer simulation study comparing lesion detection accuracy with digital mammography, breast tomosynthesis, and cone-beam CT breast imaging,” Med. Phys. 10.1118/1.2174127 33(4), 1041–1052 (2006). [DOI] [PubMed] [Google Scholar]
- Watt A. C., Ackerman L. V., Shetty P. C., Burke M., Flynn M., Grodsinsky C., Fine G., and Wilderman S., “Differentiation between benign and malignant disease of the breast using digital subtraction angiography of the breast,” Cancer 56(6), 1287–1292 (1985). [DOI] [PubMed] [Google Scholar]
- Watt A. C., Ackerman L. V., Windham J. P., Shetty P. C., Burke M. W., Flynn M. J., Grodinsky C., Fine G., and Wilderman S. J., “Breast lesions: Differential diagnosis using digital subtraction angiography,” Radiology 159(1), 39–42 (1986). [DOI] [PubMed] [Google Scholar]
- Lewin J. M., Isaacs P. K., Vance V., and Larke F. J., “Dual-energy contrast-enhanced digital subtraction mammography: feasibility,” Radiology 10.1148/radiol.2291021276 229(1), 261–268 (2003). [DOI] [PubMed] [Google Scholar]
- Jong R. A., Yaffe M. J., Skarpathiotakis M., Shumak R. S., Danjoux N. M., Gunesekara A., and Plewes D. B., “Contrast-enhanced digital mammography: Initial clinical experience,” Radiology 10.1148/radiol.2283020961 228(3), 842–850 (2003). [DOI] [PubMed] [Google Scholar]
- Diekmann F., Diekmann S., Jeunehomme F., Muller S., Hamm B., and Bick U., “Digital mammography using iodine-based contrast media: Initial clinical experience with dynamic contrast medium enhancement,” Invest. Radiol. 10.1097/01.rli.0000167421.83203.4e 40(7), 397–404 (2005). [DOI] [PubMed] [Google Scholar]
- Diekmann F., Diekmann S., Taupitz M., Bick U., Winzer K. J., Huttner C., Muller S., Jeunehomme F., and Hamm B., “Use of iodine-based contrast media in digital full-field mammography—initial experience,” Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 175(3), 342–345 (2003). [DOI] [PubMed] [Google Scholar]
- Dromain C., Balleyguier C., Muller S., Mathieu M. C., Rochard F., Opolon P., and Sigal R., “Evaluation of tumor angiogenesis of breast carcinoma using contrast-enhanced digital mammography,” AJR, Am. J. Roentgenol. 187(5), W528–537 (2006). [DOI] [PubMed] [Google Scholar]
- Skarpathiotakis M., Yaffe M. J., Bloomquist A. K., Rico D., Muller S., Rick A., and Jeunehomme F., “Development of contrast digital mammography,” Med. Phys. 10.1118/1.1510128 29(10), 2419–2426 (2002). [DOI] [PubMed] [Google Scholar]
- Saito M., “Dual-energy approach to contrast-enhanced mammography using the balanced filter method: spectral optimization and preliminary phantom measurement,” Med. Phys. 10.1118/1.2790841 34(11), 4236–4246 (2007). [DOI] [PubMed] [Google Scholar]
- Baldelli P., Bravin A., Di Maggio C., Gennaro G., Sarnelli A., Taibi A., and Gambaccini M., “Evaluation of the minimum iodine concentration for contrast-enhanced subtraction mammography,” Phys. Med. Biol. 10.1088/0031-9155/51/17/008 51(17), 4233–4251 (2006). [DOI] [PubMed] [Google Scholar]
- Diekmann F., Sommer A., Lawaczeck R., Diekmann S., Pietsch H., Speck U., Hamm B., and Bick U., “Contrast-to-noise ratios of different elements in digital mammography: evaluation of their potential as new contrast agents,” Invest. Radiol. 10.1097/01.rli.0000258682.99546.9f 42(5), 319–325 (2007). [DOI] [PubMed] [Google Scholar]
- Lawaczeck R., Diekmann F., Diekmann S., Hamm B., Bick U., Press W. R., Schirmer H., Schon K., and Weinmann H. J., “New contrast media designed for x-ray energy subtraction imaging in digital mammography,” Invest. Radiol. 38(9), 602–608 (2003). [DOI] [PubMed] [Google Scholar]
- Suryanarayanan S., Sechopoulos I., Vedantham S., and Karellas A., “Feasibility of low dose x-ray contrast enhanced digital mammography with gold nanoparticles,” Med. Phys. 34(6), 2360 (2007). [Google Scholar]
- Chen S. C., Carton A. K., Albert M., Conant E. F., Schnall M. D., and Maidment A. D., “Initial clinical experience with contrast-enhanced digital breast tomosynthesis,” Acad. Radiol. 14(2), 229–238 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puong S., Bouchevreau X., Duchateau N., Iordache R., and Muller S., “Optimization of beam parameters and iodine quantification in dual-energy contrast enhanced digital breast tomosynthesis,” Proc. SPIE 10.1117/12.770148 6913, 69130Z (2008). [DOI] [Google Scholar]
- Saunders R., Samei E., Badea C., Yuan H., Ghaghada K., Qi Y., Hedlund L. W., and Mukundan S., “Optimization of dual energy contrast enhanced breast tomosynthesis for improved mammographic lesion detection and diagnosis,” Proc. SPIE 10.1117/12.772042 6913, 69130Y (2008). [DOI] [Google Scholar]
- Badea C. T., Samei E., Ghaghada K., Saunders R., Yuan H., Qi Y., Hedlund L. W., and Mukundan S., “Utility of a prototype liposomal contrast agent for x-ray imaging of breast cancer: A proof of concept using micro-CT in small animals,” Proc. SPIE 10.1117/12.772307 6913, 691303 (2008). [DOI] [Google Scholar]
- Simon G. H., Fu Y., Berejnoi K., Fournier L. S., Lucidi V., Yeh B., Shames D. M., and Brasch R. C., “Initial computed tomography imaging experience using a new macromolecular iodinated contrast medium in experimental breast cancer,” Invest. Radiol. 40(9), 614–620 (2005). [DOI] [PubMed] [Google Scholar]
- Wilkins S. W., Gureyev T. E., Gao D., Pogany A., and Stevenson A. W., “Phase-contrast imaging using polychromatic hard X-rays,” Nature (London) 10.1038/384335a0 384, 335–338 (1996). [DOI] [Google Scholar]
- Suzuki Y., Yagi N., and Uesugi K., “X-ray refraction-enhanced imaging and a method for phase retrieval for a simple object,” J. Synchrotron Radiat. 10.1107/S090904950200554X 9(Pt 3), 160–165 (2002). [DOI] [PubMed] [Google Scholar]
- Chapman D., Thomlinson W., Johnston R. E., Washburn D., Pisano E., Gmur N., Zhong Z., Menk R., Arfelli F., and Sayers D., “Diffraction enhanced x-ray imaging,” Phys. Med. Biol. 10.1088/0031-9155/42/11/001 42(11), 2015–2025 (1997). [DOI] [PubMed] [Google Scholar]
- Hasnah M. O., Zhong Z., Oltulu O., Pisano E., Johnston R. E., Sayers D., Thomlinson W., and Chapman D., “Diffraction enhanced imaging contrast mechanisms in breast cancer specimens,” Med. Phys. 10.1118/1.1507782 29(10), 2216–2221 (2002). [DOI] [PubMed] [Google Scholar]
- Pisano E. D., Johnston R. E., Chapman D., Geradts J., Iacocca M. V., Livasy C. A., Washburn D. B., Sayers D. E., Zhong Z., Kiss M. Z., and Thomlinson W. C., “Human breast cancer specimens: diffraction-enhanced imaging with histologic correlation—improved conspicuity of lesion detail compared with digital radiography,” Radiology 214(3), 895–901 (2000). [DOI] [PubMed] [Google Scholar]
- Fiedler S., Bravin A., Keyrilainen J., Fernandez M., Suortti P., Thomlinson W., Tenhunen M., Virkkunen P., and Karjalainen-Lindsberg M., “Imaging lobular breast carcinoma: comparison of synchrotron radiation DEI-CT technique with clinical CT, mammography and histology,” Phys. Med. Biol. 10.1088/0031-9155/49/2/001 49(2), 175–188 (2004). [DOI] [PubMed] [Google Scholar]
- Wu X. and Liu H., “A new theory of phase-contrast x-ray imaging based on Wigner distributions,” Med. Phys. 10.1118/1.1776672 31(9), 2378–2384 (2004). [DOI] [PubMed] [Google Scholar]
- Wu X. and Liu H., “Clinical implementation of x-ray phase-contrast imaging: theoretical foundations and design considerations,” Med. Phys. 10.1118/1.1593836 30(8), 2169–2179 (2003). [DOI] [PubMed] [Google Scholar]
- Wu X. and Liu H., “Clarification of aspects in in-line phase-sensitive x-ray imaging,” Med. Phys. 10.1118/1.2431475 34(2), 737–743 (2007). [DOI] [PubMed] [Google Scholar]
- Meng F., Yan A., Zhou G., Wu X., and Liu H., “Development of a dual-detector X-ray imaging system for phase retrieval study,” Nucl. Instrum. Methods Phys. Res. B 254(2), 300–306 (2007). [Google Scholar]
- Corlu A., Choe R., Durduran T., Lee K., Schweiger M., Arridge S. R., Hillman E. M. C., and Yodh A. G., “Diffuse optical tomography with spectral constraints and wavelength optimization,” Appl. Opt. 10.1364/AO.44.002082 44(11), 2082–2093 (2005). [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Pogue B. W., Brooksby B., Jiang S., Dehghani H., Kogel C., Wells W. A., Poplack S., and Paulsen K. D., “Near-infrared characterization of breast tumors in-vivo using spectrally-constrained reconstruction,” Technol. Cancer Res. Treat. 4(5), 513–526 (2005). [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Pogue B. W., Jiang S., Dehghani H., and Paulsen K. D., “Spectrally constrained chromophore and scattering near-infrared tomography provides quantitative and robust reconstruction,” Appl. Opt. 10.1364/AO.44.001858 44(10), 1858–1869 (2005). [DOI] [PubMed] [Google Scholar]
- Shah N., Cerussi A. E., Jakubowski D., Hsiang D., Butler J., and Tromberg B. J., “Spatial variations in optical and physiological properties of healthy breast tissue,” J. Biomed. Opt. 10.1117/1.1695560 9(3), 534–540 (2004). [DOI] [PubMed] [Google Scholar]
- van Veen R. L., Sterenborg H. J., Marinelli A. W., and Menke-Pluymers M., “Intraoperatively assessed optical properties of malignant and healthy breast tissue used to determine the optimum wavelength of contrast for optical mammography,” J. Biomed. Opt. 10.1117/1.1803547 9(6), 1129–1136 (2004). [DOI] [PubMed] [Google Scholar]
- Zhou C., Choe R., Shah N., Durduran T., Yu G., Durkin A., Hsiang D., Mehta R., Butler J., Cerussi A., Tromberg B. J., and Yodh A. G., “Diffuse optical monitoring of blood flow and oxygenation in human breast cancer during early stages of neoadjuvant chemotherapy,” J. Biomed. Opt. 10.1117/1.2798595 12(5), 051903 (2007). [DOI] [PubMed] [Google Scholar]
- Ntziachristos V., Yodh A. G., Schnall M. D., and Chance B., “MRI-guided diffuse optical spectroscopy of malignant and benign breast lesions,” Neoplasia 10.1038/sj.neo.7900244 4(4), 347–354 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooksby B., Jiang S., Dehghani H., Pogue B. W., Paulsen K. D., Weaver J. B., Kogel C., and Poplack S. P., “Combining near-infrared tomography and magnetic resonance imaging to study in vivo breast tissue: Implementation of a Laplacian-type regularization to incorporate magnetic resonance structure,” J. Biomed. Opt. 10.1117/1.2098627 10(5), 051504 (2005). [DOI] [PubMed] [Google Scholar]
- Brooksby B., Pogue B. W., Jiang S., Dehghani H., Srinivasan S., Kogel C., Tosteson T. D., Weaver J., Poplack S. P., and Paulsen K. D., “Imaging breast adipose and fibroglandular tissue molecular signatures by using hybrid MRI-guided near-infrared spectral tomography,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0509636103 103(23), 8828–8833 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. M., Pogue B. W., Jiang S., Dehghani H., Wang X., Paulsen K. D., Wells W. A., Forero J., Kogel C., Weaver J. B., Poplack S. P., and Kaufman P. A., “Image-guided optical spectroscopy provides molecular-specific information in vivo: MRI-guided spectroscopy of breast cancer hemoglobin, water, and scatterer size,” Opt. Lett. 10.1364/OL.32.000933 32(8), 933–935 (2007). [DOI] [PubMed] [Google Scholar]
- Ntziachristos V., Yodh A. G., Schnall M., and Chance B., “Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.040570597 97(6), 2767–2772 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Zhang Q., Bartlett M., Schutz L., Fajardo L. L., and Jiang H., “Differentiation of cysts from solid tumors in the breast with diffuse optical tomography,” Acad. Radiol. 11(1), 53–60 (2004). [DOI] [PubMed] [Google Scholar]
- Tromberg B. J., Cerussi A., Shah N., Compton M., Durkin A., Hsiang D., Butler J., and Mehta R., “Imaging in breast cancer: diffuse optics in breast cancer: detecting tumors in pre-menopausal women and monitoring neoadjuvant chemotherapy,” Breast Cancer Research 7(6), 279–285 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N., Gibbs J., Wolverton D., Cerussi A., Hylton N., and Tromberg B. J., “Combined diffuse optical spectroscopy and contrast-enhanced magnetic resonance imaging for monitoring breast cancer neoadjuvant chemotherapy: a case study,” J. Biomed. Opt. 10.1117/1.2070147 10(5), 051503 (2005). [DOI] [PubMed] [Google Scholar]
- Jakubowski D. B., Cerussi A. E., Bevilacqua F., Shah N., Hsiang D., Butler J., and Tromberg B. J., “Monitoring neoadjuvant chemotherapy in breast cancer using quantitative diffuse optical spectroscopy: A case study,” J. Biomed. Opt. 10.1117/1.1629681 9(1), 230–238 (2004). [DOI] [PubMed] [Google Scholar]
- Boverman G., Fang Q., Carp S. A., Miller E. L., Brooks D. H., Selb J., Moore R. H., Kopans D. B., and Boas D. A., “Spatio-temporal imaging of the hemoglobin in the compressed breast with diffuse optical tomography,” Phys. Med. Biol. 10.1088/0031-9155/52/12/018 52(12), 3619–3641 (2007). [DOI] [PubMed] [Google Scholar]
- Li C., Zhao H., Anderson B., and Jiang H., “Multispectral breast imaging using a ten-wavelength, 64×64 source∕detector channels silicon photodiode-based diffuse optical tomography system,” Med. Phys. 10.1118/1.2171508 33(3), 627–636 (2006). [DOI] [PubMed] [Google Scholar]
- Zhu Q., Chen N., and Kurtzman S. H., “Imaging tumor angiogenesis by use of combined near-infrared diffusive light and ultrasound,” Opt. Lett. 10.1364/OL.28.000337 28, 337–339 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromberg B. J., Pogue B. W., Paulsen K. D., Yodh A. G., Boas D. A., and Cerussi A. E., “Assessing the future of diffuse optical imaging technologies for breast cancer management,” Med. Phys. 10.1118/1.2919078 35(6), 2443–2451 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojadinovic A., Nissan A., Shriver C. D., Mittendorf E. A., Akin M. D., Dickerson V., Lenington S., Platt L. D., Stavros T., Goldstein S. R., Moskovitz O., Gallimidi Z., Fields S. I., Yeshaya A., Allweis T. M., Manassa R., Pappo T., Ginor R. X., D’Agostino R. B., and Gur D., “Electrical impedance scanning as a new breast cancer risk stratification tool for young women,” J. Surg. Oncol. 97(2), 112–120 (2008). [DOI] [PubMed] [Google Scholar]
- Meaney P. M., Fanning M. W., Raynolds T., Fox C. J., Fang Q., Kogel C. A., Poplack S. P., and Paulsen K. D., “Initial clinical experience with microwave breast imaging in women with normal mammography,” Acad. Radiol. 14(2), 207–218 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik M., McCartney L., Popovic D., Watkins C. B., Lindstrom M. J., Harter J., Sewall S., Magliocco A., Booske J. H., Okoniewski M., and Hagness S. C., “A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries,” Phys. Med. Biol. 10.1088/0031-9155/52/10/001 52(10), 2637–2656 (2007). [DOI] [PubMed] [Google Scholar]
- Lazebnik M., Popovic D., McCartney L., Watkins C. B., Lindstrom M. J., Harter J., Sewall S., Ogilvie T., Magliocco A., Breslin T. M., Temple W., Mew D., Booske J. H., Okoniewski M., and Hagness S. C., “A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries,” Phys. Med. Biol. 10.1088/0031-9155/52/20/002 52(20), 6093–6115 (2007). [DOI] [PubMed] [Google Scholar]
- Poplack S. P., Tosteson T. D., Wells W. A., Pogue B. W., Meaney P. M., Hartov A., Kogel C. A., Soho S. K., Gibson J. J., and Paulsen K. D., “Electromagnetic breast imaging: results of a pilot study in women with abnormal mammograms,” Radiology 10.1148/radiol.2432060286 243(2), 350–359 (2007). [DOI] [PubMed] [Google Scholar]
- Brenner R. J. and Parisky Y., “Alternative breast-imaging approaches,” Radiol. Clin. North Am. 45(5), 907–923 (2007). [DOI] [PubMed] [Google Scholar]