Abstract
Immunopathological mechanisms are speculated to underlie haemorrhagic fever with renal syndrome (HFRS) caused by Hantaviruses. CD4+CD25+ T regulatory cells (Tregs), a subset of CD4+ T cells, expressed high levels of CD25 and the forkhead box transcription factor P3 (FoxP3), plays an important role in the down-regulation of various immune responses. Therefore, we hypothesized that in patients with HFRS the immunopathology could be, at least in part, the result of an inefficient control of pathogenic effector T cells by Tregs. The number of Tregs was determined by flow cytometry according to their characteristic CD4+CD25high membrane phenotype. The functional characterization of Tregs was analysed by suppression of proliferation and secretion of cytokines by co-cultured effector CD4+CD25− T cells. FoxP3 mRNA level was assessed by quantitative real-time polymerase chain reaction. We observed that CD4+CD25high cells of patients with HFRS showed a conventional phenotype. Furthermore, acute-stage patients with HFRS exhibited significantly reduced numbers of peripheral Tregs compared with healthy donors, and marked improvement was observed in convalescent-phase patients. The frequency of Tregs was correlated positively with platelet count, and was correlated negatively with blood urea nitrogen, serum creatinine and serum aspartate aminotransferase. On the other hand, Tregs from both healthy individuals and patients with HFRS exhibited equal FoxP3 expression of mRNA, and their ability to suppress the proliferation and cytokine secretion of CD4+ effector T cells was unimpaired in HFRS patients.
Keywords: Hantavirus, immune regulation, T regulatory cells
Introduction
CD4+CD25+ T regulatory cells (Tregs), described as a minor population of CD4+ T cells, express high levels of CD25, play an important role in the control of immune reactivity against self-antigens and have the ability to inhibit chronic inflammatory responses [1,2]. Although studies have shown clearly that Tregs are produced mainly in the thymus and present in healthy individuals from birth, these cells can also be derived from mature naive CD4+CD25− T cells in the periphery under special stimulatory conditions [3–5]. The Tregs subset express constitutively a variety of cell surface molecules, such as CD25, CD45RBlow, CD62L and CTLA-4, as well as glucocorticoid-induced tumour necrosis factor receptor.
Glucocorticoid-induced tumour necrosis factor receptor associated more commonly with activated/memory cells [6–8]. The forkhead box/winged helix transcription factor P3 (FoxP3) has been identified as a key regulatory gene for the development and function of Tregs[9]. Spontaneous mutation of FoxP3 leads to widespread lymphocytosis and autoimmunity in mice and humans with immune deregulation, polyendocrinopathy, enteropathy and X-linked syndrome [10,11]. Although Tregs require activation by antigen exposure to initiate suppressive functions, the effector (suppression) phase is independent of antigen specificity [12,13]. Soluble cytokines and cell–cell contact-dependent mechanisms have been shown to contribute to the suppressive activities mediated by Tregs[14,15]. In humans, an alteration in the generation and development of the suppressive function of Tregs is often associated with autoimmune diseases [16–18].
Haemorrhagic fever with renal syndrome (HFRS), characterized by altered vascular permeability and acute thrombocytopenia, is a zoonosis caused by different species of Hantaviruses. More than 100 000 cases of HFRS are reported annually in Asia and Europe and most are reported in China [19]. Hantaviruses infect predominantly endothelial cells and macrophages, but infection has no direct cytopathic effect on these or other cells [20–22], indicating that direct viral cytotoxicity is not responsible for the pathology observed in humans. Several findings suggest that patients with HFRS are in a state of high-level cellular immune response, which may be involved in the development of inflammation and pathological lesions [23,24]. For example, in the early stages of HFRS, decreased activity of spontaneous suppressor T cells was concurrent with increased numbers of CD8+ cells and a reversed CD4 : CD8 ratio [25,26]. The strong Hantavirus-specific CD8+ T cells responses might be responsible for the damage of epithelial cell apoptosis during HFRS/Hantavirus pulmonary syndrome (HPS), which caused elevated levels of extracellular perforin and granzyme B [27,28]. Moreover, infection of dendritic cells with Hantavirus induced the release of the proinflammatory cytokines tumour necrosis factor-α and interferon (IFN)-α[29]. So far, there are still no studies of Tregs in HFRS patients. Only one study showed that the ratio of activated antigen (CD25)-positive lymphocytes of peripheral blood mononuclear cells (PBMC) in the acute phase of HFRS was higher than that in convalescent phase [26]. The finding has implied the possibility that Tregs play a role in the development of inflammation and pathological lesions.
In order to elucidate the role of Tregs in the pathogenesis of HFRS, we measured the percentage of Tregs in HFRS patients and investigated their suppressive activities in inhibiting proliferation and cytokine secretion of CD4+ effector T cells. Our data showed that Tregs numbers were reduced, but their suppressive function was intact during the acute stage of HFRS. In addition, marked improvement in the number of Tregs was observed in convalescent patients.
Materials and methods
Patients and specimens
In this study, 76 individuals with HFRS (24 females and 32 males, age 20–50 years, mean age 40 ± 11·0 years) were analysed. All patients were confirmed serologically HFRS by enzyme-linked immunosorbent assay (ELISA) and/or an immunofluorescence test for specific immunoglobulin (Ig)M and IgG. The patients were hospitalized in the acute phase of illness, between 2 and 12 days (mean 8 days) after the onset of HFRS. We obtained medical records of clinical symptoms and signs and the following laboratory values from each patient with HFRS; haemoglobin, haematocrit, platelet count, leucocyte count, blood urea nitrogen (BUN), serum creatinine, serum albumin level, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) levels, proteinuria and 24-h urine protein amount. Hypotension was defined as systolic pressure below 90 mmHg. Thrombocytopenia was defined as a platelet count below 100 000/l. Acute renal failure was defined as a serum creatinine over 2 mg/dl. Abnormal liver function was defined as an AST or ALT over 80 IU/l. Forty-seven patients were in febrile, hypotensive or oliguric phase, and 29 entered polyuric phase. Abnormal liver function developed in 54 (71·1%) patients. Haemorrhage manifestation developed in 28 (36·8%) patients. Laboratory findings at admission are listed in Table 1.
Table 1.
Laboratory findings in 76 patients with haemorrhagic fever with renal syndrome (HFRS) at admission.
| Mean ± s.d. | Range | Reference values | |
|---|---|---|---|
| Hb (g/dl) | 12·1 ± 2·7 | 8·1–21·9 | 12–16 |
| Hct (%) | 41·5 ± 6·4 | 24·1–62·8 | 34–49 |
| WBC (×103/l) | 14·7 ± 10·9 | 3·8–58·2 | 5–10 |
| Plt (×103/l) | 71 ± 62 | 10–314 | 150–450 |
| BUN (mg/dl) | 57·8 ± 36·1 | 5·1–101·9 | 8–23 |
| Scr (mg/dl) | 5·6 ± 3·7 | 0·7–12·1 | 0·5–1·2 |
| AST (IU/l) | 155 ± 102 | 18–870 | 13–36 |
| ALT (IU/l) | 74 ± 79 | 11–414 | 5–33 |
Hb, haemoglobin; Hct, haematocrit; WBC, white blood cell; Plt, platelet; BUN, blood urea nitrogen; Scr, serum creatinine; AST, aspartate aminotransferase; ALT, alanine aminotransferase; s.d., standard deviation.
Acute blood samples were drawn at the time of hospitalization. Convalescent-phase samples were drawn 1 month after recovery from the disease. Thirty healthy donors (10 females and 20 males, age 18–52 years, mean age 40 ± 1·2 years) were included. Ethical approval was obtained and informed consent was obtained from all patients.
Flow cytometry analysis
Three-colour flow cytometry analysis was performed to quantify Tregs in human peripheral blood. For immunostaining, phycoerythrin, fluorescein isothiocyanate and peridinin chlorophyll protein conjugated monoclonal antibodies against CD4 (clone: L200), CD25 (clone: M-A251), FoxP3 (clone: 206D), CTLA-4 (clone: BNI3), CD45RO (clone: UCHL1), CD62L (clone: IM1231), CD69 (clone: CH-4) and CD95 (clone: DX2) were purchased from BD Biosciences (La Jolla, CA, USA). All antibodies were used at concentrations titrated for optimal staining. Briefly, a sample of peripheral blood was incubated in the dark for 30 min, washed with phosphate-buffered saline (PBS) twice and analysed in a FACSCalibur (Becton Dickinson). Intracellular staining of CTLA-4 and FoxP3 was performed by using fixation and permeabilization buffers according to the manufacturer's instructions, followed by visualization with streptavidin antibody. Suitable isotype controls were performed. To estimate the absolute numbers of Tregs, 200 µl of CD4- and CD25-labelled blood were added to 800 µl sheath fluid and 100 µl of FlowCount Fluorospheres (Beckman/Coulter Inc., Fullerton, CA, USA) counting beads and vortexed gently for 20 s. The number of cells in the chosen gate was assessed by counting the number of events in the gate on two occasions, taking the average value and then multiplying by the number of counting spheres/µl and the dilution factor. Flow cytometric analysis was performed on a FACSCalibur cytometer. Data processing was accomplished with CELLQuest software (Becton Dickinson).
Isolation and purification of T cell subsets
The PBMCs were separated by Ficoll density centrifugation. CD4+ T cells were isolated through negative selection by removing all other cell types after 15-min incubation with a mixture of biotin-conjugated antibodies. The resulting population was, on average, 95% and CD4+ was separated further into CD25+ and CD25− fractions by CD25 microbeads (Miltenyi Biotec, Milburn, CA, USA). A modified version of the protocol for positive selection was used to separate CD4+CD25high cells that have been described as highly enriched in Tregs[30]; CD4+ cells were incubated first with 5 µl of CD25 beads/107 cells and underwent three consecutive simple positive selections (autoMACS; Miltenyi Biotec) to obtain the CD25high population. The initial negative fraction was reincubated with CD25 beads at 20 µl/107 cells and underwent ‘sensitive depletion’ (an autoMAC option) to obtain the CD25− subset. The purity of the separated CD4+CD25high cells and CD4+CD25− cells subsets was confirmed by flow cytometry.
Real-time quantitative polymerase chain reaction
The FoxP3 mRNA expression was quantified by real-time polymerase chain reaction (PCR) using ABI PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA, USA). The human housekeeping gene β-actin primers and probe set was used as a reference for sample normalization. Total RNA isolated from CD4+CD25high T cell was reverse-transcribed into cDNA by using primed random hexamer. The primer set for FoxP3 was 5′-TTCGAAGAGCCAGAGGACTT-3′ and 5′-GCTGCTCCAGAGACTGTACC-3′. The probe for FoxP3 was 5′-FAM-CTCAAGCACTGCCAGGCGGACCATC-TAMRA-3′. The primer set for β-actin was 5′-ATCTGCTGGAAGGTGGACAGCGA-3′ and 5′-CCCAGCACAATGAAGATCAAGATCAT-3′. The probe for β-actin was 5′-FAM-TGAGCGCA AGTACTCCGTGTGGATCGGCG-TAMRA-3′. The primers and probes used in the real-time PCR were ordered from Sangon (Shanghai, China) and designed not to amplify genomic DNA. Standard curves were generated from serial dilutions of purified plasmid DNA encoding the respective genes with a linear regression R greater than 0·99 and used to quantify mRNA copy numbers for each sample. The amplification protocol used was described as follows: 1 µl of synthesized cDNA product was added subsequently into PCR mix containing 25 µl of TaqMan 2× PCR master mix (Applied Biosystems), 30 pmol human FoxP3 primer with 10 pmol probe and 2·5 µl β-actin primer/probe set, and distilled water was added to make a total reaction volume of 50 µl. The PCR was programmed as an initial incubation for 10 min at 95°C followed by 40 thermal cycles of 15 s at 95°C and 1 min at 60°C. The normalized values in each sample were calculated as the relative quantity of FoxP3 mRNA expression divided by the relative quantity of β-actin mRNA expression. All reactions were confirmed by at least one additional independent run.
Proliferation assay
To assess the proliferative response of purified CD4+CD25high and CD4+CD25− T cells, co-cultures were established in 96-well U-bottomed plates incubated with 0·5 µg/ml anti-CD3 monoclonal antibody (clone: UCHT1) overnight at 4°C, and washed. CD4+CD25− T cells (responders) and CD4+CD25high T cells (suppressor) (104 cells/well) were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) in different responder/suppressor ratios (0:1, 1:1, 1:1/2, 1:1/4, 1:1/8, 1:0). To every well, 1·0 × 104 irradiated (2500 rads) PBMC were added as antigen-presenting cells and all cells were cultured in a final volume of 200 µl. All tests were conducted in triplicate. Cell cultures were then incubated at 37°C for 4 days and supernatants were obtained for cytokine measurements before being pulsed with 1 µCi [3H]-thymidine per well for the final 18 h of incubation. Plates were harvested onto nylon filters using the Betaplate system and radioactivity was quantified using a Betaplate counter (Beckman Instruments, Fullerton, CA, USA). Results are expressed in counts per minute (cpm) as the mean of triplicate cultures ± standard deviation. Percentage suppression was calculated using the formula: (1 − cpm in presence of CD4+CD25+ T cells/cpm in absence of CD4+CD25+ T cells) × 100%.
Measurement of cytokine production
The supernatants that were removed before addition of [3H]-thymidine were diluted for measurement of cytokine concentration by ELISA (R&D kits, Minneapolis, MN, USA). Briefly, microtitre plates pre-coated with capturing monoclonal antibodies were blocked with 2% bovine serum albumin (BSA)/PBS. After washing, samples and controls were added at 50 µl per well and incubated for 2 h with a biotinylated detecting antibody (50 µl per well) in 2% BSA/PBS/Tween-20. Plates were washed and incubated for 30 min with streptavidin-conjugated horseradish peroxidase. Next, 100 µl of 0·0125% tetramethylbenzidine and 0·008% H2O2 in citrate buffer was used as substrate. A standard curve was performed for each plate and used to calculate the absolute concentrations of cytokines.
Statistical analysis
Normally distributed data sets were analysed by Student's t-test, paired t-test, analysis of variance and linear regression and correlation analysis (using Primer for Biostatistics). The Wilcoxon two-sample test and Kruskall–Wallis test were used for data sets that were not distributed normally (using SAS, SAS Institute Inc., Cary, NC, USA). P = 0·05 was considered significant.
Results
Defining the human Tregs population
The Tregs were identified as CD4+CD25high T cells by selecting those CD4+ cells whose CD25 expression exceeded the level of CD25 positivity seen on the CD4 negative population [31] (Fig. 1a). To characterize further the CD4+CD25high T cells, different levels of expressions of the memory marker CD45RO, inhibitory receptor CTLA-4, death receptor CD95, early activation marker CD69 and homing receptor CD62L were compared among the CD4+CD25–, CD4+CD25int and CD4+CD25high subsets (Fig. 1b). The highest percentage of CD45RO+, CTLA-4+, CD95+ or CD62L+ cells was detected in the CD4+CD25high subsets, and the percentages were 91% ± 2·4% (range: 89–95%), 87% ± 3·4% (range: 84–92%), 82% ± 4·1% (range: 79–86%) and 84% ± 3·1% (range: 81–88%) respectively. CD69 was not expressed. In agreement with previous reports, the expression levels of all of these markers were unchanged on the CD4+CD25high T cells from either healthy controls or patients with HFRS, thereby allowing identification of these cells as Tregs. The terms CD4+CD25high T cells and Tregs will be used synonymously throughout the study.
Fig. 1.
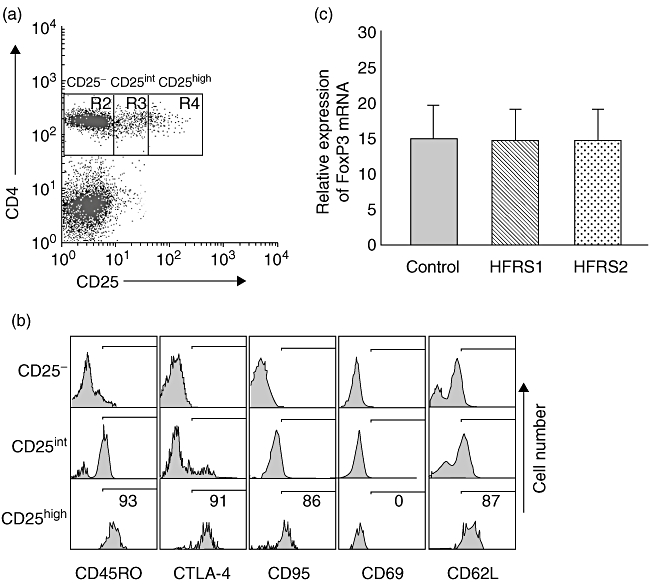
Defining the human T regulatory cell (Treg) population. (a) Tregs were identified as CD4+CD25high T cells by selecting those CD4-positive cells whose CD25 expression exceeded the level of CD25 positivity seen on the CD4-negative population. (b) CD4+CD25high T cells from either healthy subjects or patients expressed the same levels of CTLA4, CD45RO, CD95 or CD62L; CD69 was not expressed. Results are representative of 20 separate experiments performed on patients with haemorrhagic fever with renal syndrome (HFRS) and healthy subjects. (c) CD4+CD25high T cells isolated from HFRS1 (n = 20), HFRS2 (n = 20) and healthy controls (n = 20) exhibited equal forkhead box P3 (FoxP3)-expression levels.
mRNA expression of FoxP3 in Tregs from HFRS patients
In our study, CD4+CD25high T cells were highly purified using a modified protocol (described in Materials and methods). Optimization of this bead-based method allowed us to isolate CD4+CD25high T cells rapidly and the purity of the separated CD4+CD25high and CD4+CD25− cell subsets was confirmed to be > 90% by flow cytometry. Previous studies suggested that FoxP3 is expressed selectively in Tregs, so we also used real-time revese transcription (RT)–PCR to quantify the expression levels of FoxP3 to confirm further that the purity of CD4+CD25high cells isolated magnetically from both HFRS and healthy individuals are uniform. We found that CD4+CD25high T cells isolated from acute-stage HFRS patients (HFRS1, n = 20), convalescent-phase patients (HFRS2, n = 20) and healthy individuals (control, n = 20) exhibited equal FoxP3 expression levels (Fig. 1c). Thus, we confirmed that the gated CD4+CD25high T cells are indeed Tregs and not activated CD25+ T cells.
Circulating Tregs numbers are reduced in acute-stage HFRS patients
The proportions and numbers of CD4+CD25high T cells in HFRS1 (n = 76), HFRS2 (n = 65) and healthy controls (n = 30) are shown in Table 2 and Fig. 2. The percentage of CD4+CD25high T cells in the CD4+ population was reduced in HFRS1 [1·7% ± 0·4% (range: 1·4–3·5%)] compared with healthy controls [2·9% ± 0·4% (range: 1·8–4·3%)]. The total number of circulating CD4+CD25high T cells was also lower in HFRS1 [26·1 ± 3·8 cells/µl (range: 20·1–34·5 cells/µl)] compared with healthy controls [39·9 ± 6·2 cells/µl (range: 24·7–46·2 cells/µl)]. The proportions and numbers of CD4+CD25high T cells in the acute phase of illness, between 2 and 12 days (mean 10 days) after the onset of HFRS, were not different (Fig. 2a and b). Interestingly, there was a significant increase in the percentage and numbers of CD4+CD25high T cells in HFRS2 [2·8% ± 0·6% (range: 1·9–4·5%), 39·6 ± 6·3 cells/µl (range: 22·1–48·4) respectively] (Fig. 2c and d). The percentage of CD4+CD25+ T cells in the CD4+ population was higher in HFRS1 [28·7% ± 7·4% (range: 20·3–34·1%)] compared with HFRS2 [21·6% ± 3·5% (range: 19·1–24·2%)] and healthy controls [20·5% ± 3·9% (range: 18·7–24·6%)]. The total number of circulating CD4+CD25+ T cells was also higher in HFRS1 [435·9 ± 12·1 cells/µl (range: 401·5–512·3 cells/µl)] compared with HFRS2 [301·5 ± 11·4 (range: 253·1–361·4 cells/µl)] and healthy controls [289·1 ± 12·1 cells/µl (range: 231·6–312·4 cells/µl)].
Table 2.
Circulating cell populations in patients with haemorrhagic fever with renal syndrome (HFRS) at initial sampling (HFRS 1), follow-up (HFRS 2) and healthy controls.
| Surface marker | HFRS1 | HFRS2 | Control |
|---|---|---|---|
| CD4+CD25high T (%CD4+) | 1·7% ± 0·4%*,** | 2·8% ± 0·6% | 2·9% ± 0·4% |
| CD4+CD25+ T (%CD4+) | 28·7% ± 7·4%*,** | 21·6% ± 3·5% | 20·5% ± 3·9% |
| Number of CD4+CD25high T (cells/µl) | 26·1 ± 3·8*,** | 39·6 ± 6·3 | 39·9 ± 6·2 |
| Number of CD4+CD25+ T (cells/µl) | 435·9 ± 12·1*,** | 301·5 ± 11·4 | 289·1 ± 12·1 |
P < 0·01. P-values refer to comparisons between the patients with HFRS and controls.
P < 0·01. P-values refer to comparisons between HFRS1 and HFRS2.
Fig. 2.
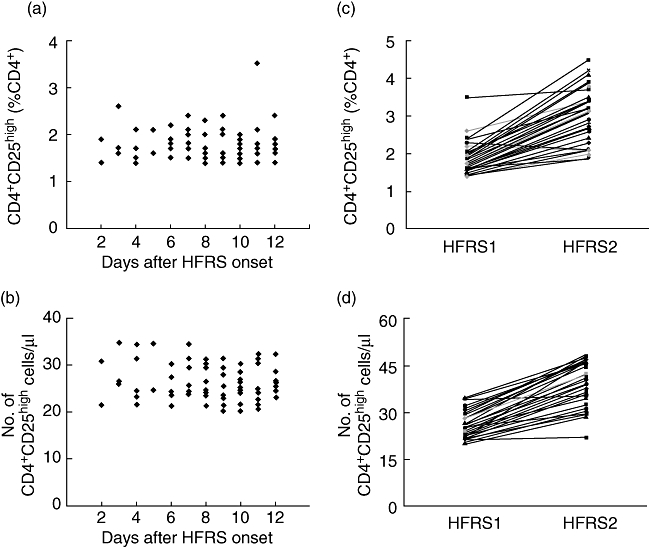
The proportions and numbers of CD4+CD25high T cells in haemorrhagic fever with renal syndrome (HFRS). (a,b) The proportions and numbers of CD4+CD25high T cells in the acute phase of illness between days 2 and 12 (mean 10 days) after the onset of HFRS were not different. (c,d) The proportions and numbers of CD4+CD25high T cells were increased significantly in HFRS2.
Decreased frequency of CD4+CD25+FoxP3+ T cells in HFRS patients
To determine further the change in the number of Tregs, we used intracellular staining for FoxP3 protein and to estimate the percentage of CD4+CD25+FoxP3+ T cells in CD4+ and CD4+CD25+ T cells from HFRS1 (n = 76), HFRS2 (n = 65) and healthy controls (n = 30) using flow cytometry. Figure 3 (a,b) shows representative fluorescence activated cell sorter (FACS) pictures from a single HFRS patient and healthy control. As expected, during acute stage the percentage of CD4+CD25+FoxP3+ T cells in CD4+ T cells [1·6% ± 0·4% (range: 1·4–2·1%)] or CD4+CD25+ T cells [16·5% ± 4·2% (range: 12·1–19·2%)] from patients with HFRS was decreased significantly compared with healthy controls [2·3% ± 0·4%, 24·7% ± 4·7% respectively (range: 2·1–2·7%, 20·3–27·6% respectively)]. After treatment, the percentage of CD4+CD25+FoxP3+ T cells in CD4+ T cells [2·2% ± 0·7% (range: 1·9–2·8%)] and CD4+CD25+ T cells [25·4% ± 4·7% (range: 20·1–29·9%)] were elevated significantly in convalescent phase patients (Fig. 3c and d). This showed that only a subset of the CD4+CD25+ T cell population may be CD4+ Tregs.
Fig. 3.
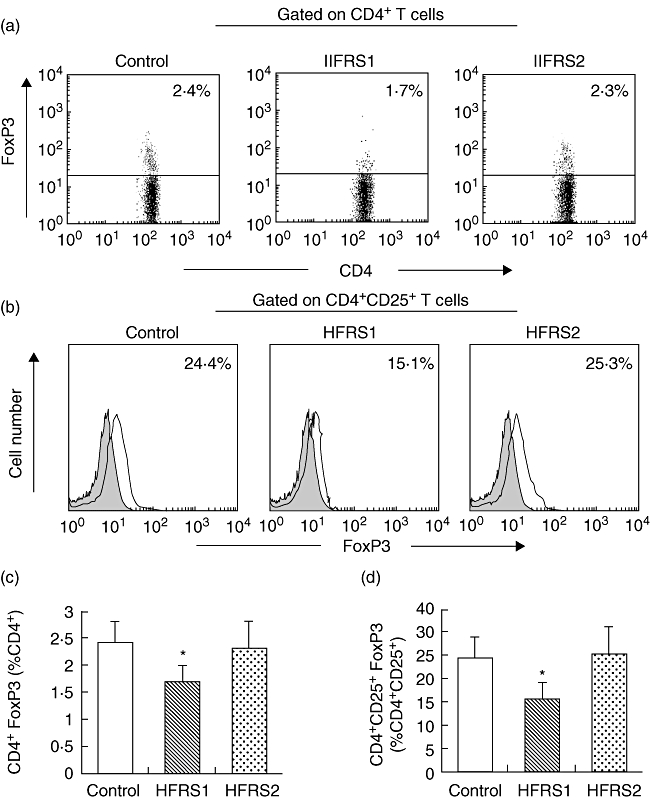
The percentage of CD4+ forkhead box P3 (FoxP3+) T cells in CD4+ and CD4+CD25+FoxP3+ T cells in CD4+CD25+ T cells from haemorrhagic fever with renal syndrome 1 (HFRS1) (n = 76) was decreased significantly compared with healthy controls (n = 30), but there was a significant increase in HFRS2 (n = 65). *P < 0·01. (a,b) Representative fluorescence activated cell sorter pictures from a single HFRS patient and a healthy donor. Filled histograms represent staining with secondary antibody alone. (c) Averaged percentage of CD4+FoxP3+ cells in CD4+ T cell subsets from HFRS1, HFRS2 and healthy controls by intracellular staining for FoxP3 protein. (d) Averaged percentage of CD4+CD25+FoxP3+ cells in CD4+CD25+ T cell subsets from HFRS1, HFRS2 and healthy controls by standard deviation; *P < 0·01.
Correlation of laboratory values with frequency of Tregs
The mean platelet count, BUN and serum creatinine of patients with HFRS are shown in Table 1. The frequency of Tregs was correlated positively with platelet count (r = 0·515, P < 0·001) and correlated negatively with BUN (r = −0·472, P < 0·001), serum creatinine (r = −0·379, P < 0·001) and AST (r = −0·263, P = 0·002).
Regulatory function of Tregs from HFRS patients
Quantitative analysis of the regulatory function of CD4+CD25high T cells was performed by co-culture with autologous T responder cells at different ratios. The assay was repeated for all subjects. CD4+CD25− (responder) cells from HFRS1, HFRS2 and healthy controls exhibited a similar strong proliferation to soluble anti-CD3 stimulus, while CD4+CD25high (suppressor) cells were anergic to this stimulation (data not shown). When CD4+CD25high regulatory T cells were co-cultured with autologous responder cells at different ratios (responder : suppressor ratios: 0:1, 1:1, 1:1/2, 1:1/4, 1:1/8 and 1:0), a dose-dependent suppression of proliferation was observed in both patients and healthy controls (Fig. 4a). In HFRS1, HFRS2 and healthy controls, CD4+CD25high regulatory T cells suppressed CD4+CD25− responder T cell proliferation consistently. Increasing the ratio of responder : suppressor T cells resulted in less suppression. No significant differences were detected between patients and healthy controls under the conditions we tested (Fig. 4a).
Fig. 4.
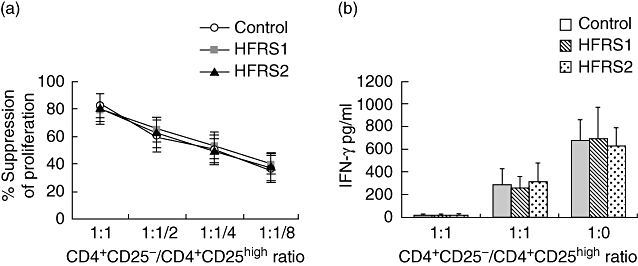
T regulatory cells (Tregs) from patients with haemorrhagic fever with renal syndrome (HFRS) maintain their regulatory function. (a) CD4+CD25high T cells from HFRS1 (n = 15), HFRS2 (n = 15) and healthy controls (n = 15) exhibited equal suppressor activity at different ratios of responder/suppressor T cells. (b) Supernatants from CD4+CD25high T cells or CD4+CD25− T cells cultured alone or from co-culture of both populations at a 1 : 1 ratio were evaluated at day 4. Data represent 20 different experiments. *P < 0·01.
The Tregs suppress the production of cytokine
Besides potently suppressing the proliferation of effector CD4+CD25− T cells, Tregs can also suppress the production of proinflammatory cytokines. Therefore, it is important to determine whether there is any defect in suppressing cytokine productions from T helper cells by Tregs from HFRS patients. We analysed the concentrations of cytokines in the supernatants obtained from the co-culture of CD4+CD25high T cells and CD4+CD25− T cells. As shown in Fig. 4b, CD4+CD25− T cells cultured alone produced large amounts of IFN-γ from HFRS1, HFRS2 and healthy controls. Supernatants from cultures of CD4+CD25high T cells alone with antigenpresenting cells contained few IFN-γ. Co-culture of CD4+CD25high T cells with CD4+CD25− T cells at a 1:1 ratio resulted in significant inhibition of IFN-γ secretion in the culture supernatants from healthy controls and patients with HFRS. This suggests that CD4+CD25high T cells from HFRS1, HFRS2 and healthy controls are effective in suppressing cytokine production IFN-γ in a similar degree.
Discussion
The Tregs may influence the immune response to infectious pathogens [32,33]. The relationship between Tregs and infections is complex and remain poorly understood, although the role of Tregs responses in controlling tissue damaging inflammatory reactions has been described in several human infections as well as in numerous model infection systems in mice. So far, little is known about the properties of Tregs in patients with HFRS.
Our data demonstrate that the number of Tregs in acute-stage HFRS patients is decreased significantly. When patients’ condition stabilized following therapy, the number of Tregs in the patients’ peripheral blood returned to normal value by the time we obtained the second blood sample. Our finding is different from that of the only other study to investigate CD4+CD25+ cells in HFRS. Huang et al. found that the ratio of CD4+CD25+ lymphocytes in the acute phase of HFRS was higher than that in convalescent phase [26]. The explanation for the discrepancy is that in their study CD4+CD25+ cells contained CD4+CD25int cells and CD4+CD25high cells and they did not distinguish Tregs from activated CD4+CD25+ cells. In our study, the proportions and numbers of CD4+CD25+ cells, which contained CD4+CD25int cells and CD4+CD25high cells, was also higher in HFRS1. CD25 is a marker for Tregs in humans, but this molecule also represents an activation marker of effector T cells. Studies have shown that in human peripheral blood, regulatory activity is associated with the small fraction of CD4+ T cells expressing the CD25 bright phenotype and most of them were CTLA4-positive and expressed CD45RO, CD95 or CD62L and CD69 was not expressed [6,31]. The best marker for distinction is FoxP3, because it can distinguish Tregs from T cells without regulatory function that are also present in the CD4+CD25+ T cell pool. To control our CD25-based gating, we used real-time RT–PCR to quantify the levels of FoxP3 within CD4+CD25high T cells. All patients had a typical CD4+CD25high T cell population, displaying similar phenotype. Most importantly, we found no difference on the FoxP3 mRNA expression levels of this population between controls and patients, thereby allowing identification of these cells as Tregs.
Functional analysis demonstrated that Tregs from patients with HFRS maintain their regulatory function. In our experiments, they can suppress the proliferation and cytokine secretion of CD4+ effector T cells in a manner equally efficient to those from healthy controls. Proliferation was reduced drastically upon the addition of Treg cells. Co-culture is currently accepted methods for assessment Tregs function in vitro[8]. In agreement with our data, Putnam et al.[34] also reported that stimulation with soluble anti-CD3 plus soluble anti-CD28 or low doses of plate-bound anti-CD3 resulted in similar suppression of CD4+CD25high T cells and CD4+CD25− T responder cells co-cultures in type 1 diabetic and normal subjects. Huan et al. found that FoxP3 message and protein expression levels in peripheral Tregs were related quantitatively to a reduction in functional suppression of Tregs[35]. Therefore, the intact regulatory function of Tregs in HFRS patients may be related to normal FoxP3 message expression levels. Based on these results, we conclude that Tregs are fully functional in acute HFRS. We suggest that the immunopathology in HFRS is not caused by defective Treg function.
The balance between regulatory and effector functions determines the outcome of disease. In general, regulatory Treg responses benefit the host by reducing the collateral tissue damage that usually accompanies immune responses to infections and which in some circumstances are fully responsible for any lesions observed. Under some circumstances the Treg response is also considered as detrimental to the host, because a prompt and strong Treg response may impair protective immune response to infectious agents. The outcome of such immune blunting may be that the agent is more able to persist which, in some infections, such as hepatitis C and hepatitis B infections in humans [36–38], can result in chronic tissue damage. Studies showed that HFRS is due to immunologically mediated capillary leakage in target organs. Increased numbers of CD8+ cells and the release of proinflammatory cytokines have been implicated in the HFRS disease process [24,39]. Terajima et al. hypothesize that the protection of endothelial cells may be overwhelmed by excess amounts of activated CD8+ T cells, which may be the mechanism of capillary leakage in HPS and HFRS patients [40]. A reduced Treg frequency may result in insufficient suppression of the effector functions of activated T cells and failure to terminate the immune responses, which predispose to the development of immunopathology in HFRS. In contrast, increased numbers of Tregs may play an important role in limiting immunopathology in the natural rodent reservoirs of Hantaviruses, which make infected hosts experience few or no pathological changes, and the host rodent can remain infected persistently for life [41,42]. The mechanisms causing haemorrhage are likely to vary among viruses. Lühn found Treg frequencies and also Treg : effector T cell ratios were increased in patients with acute infection and a relative rise of Treg : effector T cell ratios is beneficial for disease [43]. The value of Treg responses in controlling tissue damaging inflammatory reactions has also been described in other infections. In the infections of cornea of the eye with HSV, the chronic inflammatory reaction appears to be caused by an immunopathological response to the pathogen. Suvas et al.[44] have shown that the severity of the lesion is influenced beneficially by FoxP3 Tregs because animals depleted of such cells develop more severe disease and are susceptible to lower doses of infection. In the current study, we failed to explain why Tregs are decreased in the peripheral blood of acute-stage HFRS patients. A possibility is that Treg generation is decreased. Tregs are produced mainly in the thymus but can also be induced in the periphery under adequate conditions. In HFRS patients multiple factors include Hantaviruses; other cells or cytokines may impair their immediate environment for their differentiation and lead possibly to suppress their generation transiently in the periphery. Another possibility is that circulating Tregs may be recruited rapidly to infected organs and/or into lymph nodes, but current evidence suggests that the major site of disease pathogenesis is in the blood. Therefore, further studies are needed.
The present data imply that reduced circulating Tregs may be associated with the pathogenesis of HFRS. Reversible numbers and intact function of Tregs in the peripheral blood of HFRS patients contribute presumably to the disease course. Manipulating strategically the Treg responses to achieve a favourable balanced relationship between the host and the infecting agent may prove to have great clinical benefit.
Acknowledgments
This study was supported by the Foundation of Health Department of Heilongjiang (project number: 2007-212), Office of Education of Heilongjiang (project number: 11531160) and Innovation of Science and Technology of Harbin Youth (project number: 2008RFQXS008). We thank Professor Wang Wei Zhi (Department of Neurology, the Second Affiliated Hospital of Harbin Medical University) for providing purified plasmid DNA encoding the genes of β-actin and FoxP3.
References
- 1.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundgren A, Suri-Payer E, Enarsson K, Svennerholm A-M, Lundin BS. Helicobacter pylori-specific CD4+CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun. 2003;71:1755–62. doi: 10.1128/IAI.71.4.1755-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang S, Alard P, Zhao Y, et al. Conversion of CD4+CD25− cells into CD4+CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J Exp Med. 2005;201:127–37. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kretschmer K, Apostolou I, Hawiger D, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 5.Vukmanovic-Stejic M, Zhang Y, Cook JE, et al. Human CD4+CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–33. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McHugh RS, Whitters MJ, Piccirillo CA. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 8.Baecher-Allan C, Wolf E, Hafler DA. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+CD25+ T cells. Clin Immunol. 2005;115:10–8. doi: 10.1016/j.clim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 10.Khattri R, Cox T, Yasayko SA, et al. An essential role for Scurfin in CD4+CD25+ regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 11.Chang X, Zheng P, Liu Y. Foxp3: a genetic link between immunodeficiency and autoimmune diseases. Autoimmun Rev. 2006;5:399–402. doi: 10.1016/j.autrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 13.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 14.Von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Ono M, Setoguchi R. Foxp3+CD25+CD4+ natural regulatory T cells in dominant selftolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu MF, Wang CR, Fung LL, et al. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59:198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 17.Cao D, Malmstrom V, Baecher-Allan C, et al. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–23. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 18.Chi LJ, Wang HB, Wang WZ. Impairment of circulating CD4+CD25+ regulatory T cells in patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Periph Nerv Syst. 2008;13:54–63. doi: 10.1111/j.1529-8027.2008.00158.x. [DOI] [PubMed] [Google Scholar]
- 19.Bai X, Huang C. Study farther on hemorrhagic fever with renal syndrome. Chin J Infect Dis. 2002;20:197–8. [Google Scholar]
- 20.Khaiboullina SF, Netski DM, Krumpe P, Jeor SCSt. Effects of tumor necrosis factor alpha on Sin Nombre virus infection in vitro. J Virol. 2000;74:11966–71. doi: 10.1128/jvi.74.24.11966-11971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardestam J, Klingström J, Mattsson K, Lundkvist Å. HFRS causing hantaviruses do not induce apoptosis in confluent Vero E6 and A-549 cells. J Med Virol. 2005;76:234–40. doi: 10.1002/jmv.20347. [DOI] [PubMed] [Google Scholar]
- 22.Rowe RK, Pekosz A. Bidirectional virus secretion and nonciliated cell tropism following Andes virus infection of primary airway epithelial cell cultures. J Virol. 2006;80:1087–97. doi: 10.1128/JVI.80.3.1087-1097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khaiboullina SF, Jeor SCSt. Hantavirus immunology. Viral Immunol. 2002;15:609–25. doi: 10.1089/088282402320914548. [DOI] [PubMed] [Google Scholar]
- 24.Markotic A, Dasic G, Gagro A, et al. Role of peripheral blood mononuclear cell (PBMC) phenotype changes in the pathogenesis of haemorrhagic fever with renal syndrome (HFRS) Clin Exp Immunol. 1999;115:329–34. doi: 10.1046/j.1365-2249.1999.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mustonen J, Helin H, Pietila K, et al. Renal biopsy findings and clinicopathologic correlations in nephropathia epidemica. Clin Nephrol. 1994;41:121–6. [PubMed] [Google Scholar]
- 26.Huang C, Jin B, Wang M, et al. Hemorrhagic fever with renal syndrome: relationship between pathogenesis and cellular immunity. J Infect Dis. 1994;169:868–70. doi: 10.1093/infdis/169.4.868. [DOI] [PubMed] [Google Scholar]
- 27.Klingström J, Hardestam J, Stoltz M, et al. Loss of cell membrane integrity in puumala hantavirus-infected patients correlates with levels of epithelial cell apoptosis and perforin. J Virol. 2006;16:8279–82. doi: 10.1128/JVI.00742-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilpatrick ED, Terajima M, Koster FT, et al. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J Immunol. 2004;5:3297–304. doi: 10.4049/jimmunol.172.5.3297. [DOI] [PubMed] [Google Scholar]
- 29.Raftery MJ, Kraus AA, Ulrich R, et al. Hantavirus infection of dendritic cells. JVI. 2002:10724–33. doi: 10.1128/JVI.76.21.10724-10733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J Immunol. 2002;169:6210–17. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 31.Baecher-Allan C, Brown JA, Freeman GJ, et al. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 32.Rouse BT, Suvas S. Regulatory cells and infectious agents: détentes cordiale and contraire. J Immunol. 2004;173:2211–15. doi: 10.4049/jimmunol.173.4.2211. [DOI] [PubMed] [Google Scholar]
- 33.Baker CAR, Clark R, Ventura F, et al. Peripheral CD4 loss of regulatory T cells is associated with persistent viraemia in chronic HIV infection. Clin Exp Immunol. 2007;147:533–9. doi: 10.1111/j.1365-2249.2006.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putnam AL, Vendrame F, Dotta F, et al. CD4+CD25high regulatory T cells in human autoimmune diabetes. J Autoimmun. 2005;24:55–62. doi: 10.1016/j.jaut.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Huan J, Culbertson N, Spencer L, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3 regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–11. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 37.Stoop JN, von der Molen RG, Kuipers EJ, et al. Inhibition of viral replication reduces regulatory T cells and enhances the antiviral immune response in chronic hepatitis B. Virology. 2007;361:141–8. doi: 10.1016/j.virol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Bolacchi F, Sinistro A, Ciaprini C, et al. Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin Exp Immunol. 2006;144:188–96. doi: 10.1111/j.1365-2249.2006.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mori M, Rothman AL, Kurane I, et al. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J Infect Dis. 1999;179:295–302. doi: 10.1086/314597. [DOI] [PubMed] [Google Scholar]
- 40.Terajima M, Hayasaka D, Maeda K, et al. Immunopathogenesis of hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome: do CD8+ T cells trigger capillary leakage in viral hemorrhagic fevers? Immunol Lett. 2007;113:117–20. doi: 10.1016/j.imlet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Easterbrook JD, Zink MC, Klein SL. Regulatory T cells enhance persistence of the zoonotic pathogen Seoul virus in its reservoir host. Proc Natl Acad Sci USA. 2007;104:15502–7. doi: 10.1073/pnas.0707453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schountz T, Prescott J, Cogswell AC, et al. Regulatory T cell-like responses in deer mice persistently infected with Sin Nombre virus. Proc Natl Acad Sci USA. 2007;104:15496–501. doi: 10.1073/pnas.0707454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lühn K, Simmons CP, Moran E, et al. Increased frequencies of CD4+ CD25(high) regulatory T cells in acute dengue infection. J Exp Med. 2007;204:979–85. doi: 10.1084/jem.20061381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suvas S, Azkur AK, BS K, et al. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesion. J Immunol. 2004;172:4123–32. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]


