Abstract
Apolipoprotein C-III (apoC-III) inhibits triglyceride hydrolysis and has been implicated in coronary artery disease. Through a genome-wide association study, we have found that about 5% of the Lancaster Amish are heterozygous carriers of a null mutation (R19X) in the gene encoding apoC-III (APOC3) and, as a result, express half the amount of apoC-III present in noncarriers. Mutation carriers compared to noncarriers had lower fasting and postprandial serum triglycerides, higher levels of HDL-cholesterol and lower levels of LDL-cholesterol. Subclinical atherosclerosis, as measured by coronary artery calcification, was less common in carriers than noncarriers, suggesting that lifelong deficiency of apoC-III has a cardioprotective effect.
Elevated plasma levels of low density lipoprotein cholesterol (LDL-C) and triglycerides (TG) are important contributors to premature coronary heart disease (CHD) (1-3), and genetic variants causing low LDL-C are associated with reduced risk of CHD (4). Recently, nonfasting TG was found to be an independent CHD risk factor (5, 6), in one study showing higher predictive power than fasting TG (FTG), the traditional measure, likely because of the atherogenic remnant lipoproteins generated during absorption and clearance of dietary fat (5).
To identify genetic factors contributing to FTG and post-prandial TG (ppTG) dietary response, we performed a single high fat feeding intervention and genome-wide association study (GWAS) in 809 Old Order Amish individuals as part of the Heredity and Phenotype Intervention (HAPI) Heart Study (7). Characteristics of these participants are shown in Table S1. These individuals were fed a milkshake containing 782 kcal/m2 body surface area with 77.6% of these calories from fat and had blood drawn for lipid levels 0, 1, 2, 3, 4 and 6 hours after the intervention. The Affymetrix GeneChip® Human Mapping 500K Array Set was used for genotyping leukocyte DNA from these 809 participants. Traits were normalized and analyses accounting for sex and sex-specific age and age2, body mass index (BMI) and relatedness among participants were performed as described in the Methods (8).
Results of the GWAS of FTG and ppTG (as estimated by the incremental area under the curve, iAUCTG (8)), transformed by their natural logarithm (ln), are shown in Table S2 and Figure S1. The strongest evidence for association with both ln-FTG (p = 3.8 × 10−14) and ln-iAUCTG (p = 2.8 × 10−10) occurred on chromosome 11q23 at single nucleotide polymorphism (SNP) rs10892151, which had a minor allele frequency (MAF) of 0.028 (A allele; Table S2). SNP rs10892151 is located within an intron of the DSCAML1 (Down syndrome cell adhesion molecule like 1) gene and also lies 823 kb away from the APOA1/C3/A4/A5 region, a cluster of more likely candidate genes given the established key roles of their products in lipid metabolism (9). SNP rs681524 (MAF = 0.062), 40 kb from the cluster, showed nominal association with ln-FTG (p = 1.1 × 10−5) and ln-iAUCTG (p = 0.004) and was moderately correlated with rs10892151 (D′ = 0.85, r2 = 0.31) (Figure S2).
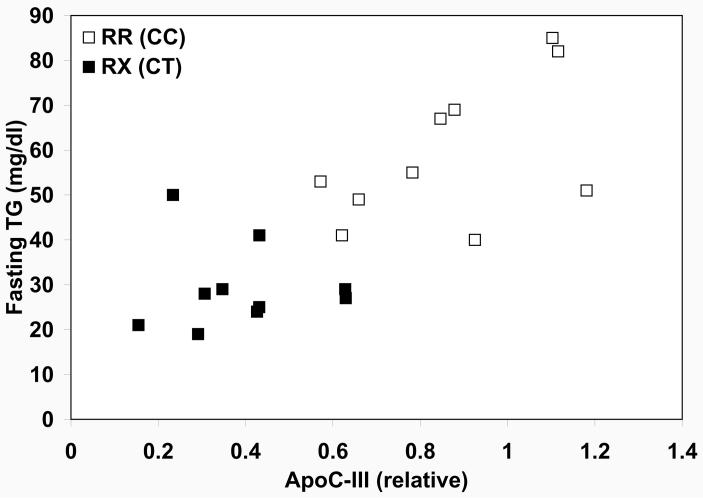
Rs10892151 A carriers evidenced markedly lower FTG and ppTG than non-carriers (Table S3), consistent with effects of knocking out the APOC3 gene in mice (10), leading to the hypothesis that SNP rs10892151 tagged a loss-of-function mutation in APOC3. Sequencing of the coding region of APOC3 revealed a C✧ T substitution at the terminal nucleotide of exon 2, the 55th nucleotide from the ATG start codon, resulting in the substitution of a premature stop codon for an arginine residue at amino acid position 19 (R19X). This position is located in the signal peptide of the protein, normally cleaved prior to the secretion of the mature 79 amino acid apoC-III peptide (11). Thus a complete lack of production of apoC-III from alleles containing this mutation would be predicted. Moreover, the location of the premature stop codon in the mRNA transcript of the mutated gene meets the criteria for nonsense-mediated mRNA, in which certain mRNA transcripts with premature stop codons are degraded rather than translated into protein (12, 13). Indeed, in a sample of 20 study participants (10 carriers of the 19X allele, RX [CT], and 10 non-carriers RR [CC]) comprising four two-generation families and one pair of siblings, apoC-III protein levels in R19X carriers were approximately half of that in their non-carrier relatives (39% vs. 87% of pooled serum control level, p = 0.0002, Figure 1). ApoC-III levels were highly correlated with ln-FTG levels (partial correlation coefficient r = 0.71, p = 0.0002) (Figure 1, non-transformed FTG shown).
Figure 1.
Triglyceride levels as a function of apoC-III protein levels stratified by APOC3 R19X genotype in 20 individuals. Filled squares indicate individuals carrying the 19X allele and open squares indicate non-carriers.
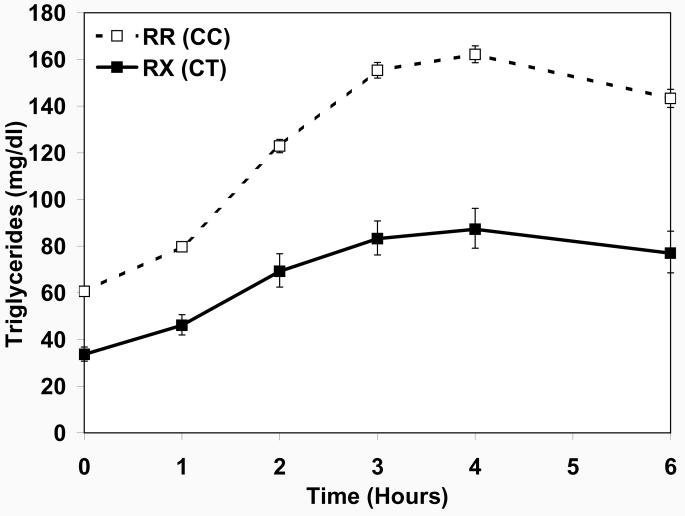
The newly discovered R19X mutation was in strong linkage disequilibrium with (i.e., highly correlated with) the most highly associated GWAS SNP rs10892151 (D′ = 1, r2 = 0.85) (Figure S2). Pedigree and haplotype analysis were consistent with a single copy of the mutated allele having entered the population prior to 1800 (supporting online text and Figures S3 and S4). Evaluation of the association of this novel R19X mutation with ln-FTG and ln-ppTG in the 802 of 809 HAPI Heart GWAS subjects successfully genotyped revealed similar associations to those identified with rs10892151 (Figure 2 and Table S4). R19X heterozygotes had significantly reduced TG levels compared to their RR counterparts at all six time points, with a median FTG (interquartile range, IQR) of 31 (25 – 48) vs. 57 (42 – 81) mg/dl (p = 4.1 × 10−13) and iAUCTG median of 214 (154-338 ) vs. 410 (276 – 608) mg/dl × 6-hr (p = 2.0 × 10−10). Nominal association (p < 0.05) of the mutation with ln-TG at 2, 3 and 4 hours as well as ln-tAUC, ln-iAUC and ln-maximum TG (ln-MAXTG) and ln-incremental maximum TG (ln-iMAXTG) persisted after controlling for lnFTG. The segregation of the R19X mutation with hypoTG and blunted ppTG response in a representative family is shown in Figure S5.
Figure 2.
Triglycerides levels before and during the high fat challenge by R19X APOC3 genotype. Shown are covariate-adjusted geometric means with 95% confidence intervals. Filled squares indicate individuals carrying the 19X allele and open squares indicate non-carriers.
R19X carriers had significantly higher HDL-C levels (mean ± SD: 67 ± 17 vs. 55 ± 14 mg/dl, p = 9.0 × 10−7) and lower total cholesterol (191 ± 35 vs. 209 ± 46 mg/dl, p = 0.02) and LDL-C (116 ± 32 vs. 140 ± 43 mg/dl, p = 0.001) levels (Table 1) than non-carriers. There were no differences in BMI (p = 0.98) or waist circumference (p = 0.50), and fasting glucose and insulin were similar between R19X carriers and non-carriers (p = 0.53 and 0.72 respectively). R19X carriers had significantly lower levels of non-HDL (p = 0.0005), very low density lipoprotein (VLDL, 1.5 × 10−10), VLDL3 (p = 2.8 × 10−10), intermediate density lipoprotein (IDL, p = 8.8 × 10−15), real LDL (p = 0.01), and remnant lipoprotein cholesterol (p = 3.0 × 10−20), and higher levels of both HDL2 (p = 7.0 × 10−6) and HDL3 (p = 3.0 × 10−5) cholesterol. Based upon the mutation's effect on TG, VLDL cholesterol levels increased significantly less in carriers than non-carriers at 4 hours after the high fat challenge (p = 0.0009) (Table 1).
Table 1.
Morphometric and metabolic characteristics and lipid subfractions by APOC3 R19X genotype (all analyses adjusted for sex- and sex-specific age and age2 and all analyses except BMI and waist adjusted for BMI) in the HAPI Heart Study. Variables analyzed directly are presented as mean ± SD. Variables ln-transformed for normalization prior to analysis are presented as median (interquartile range). All values are fasting unless otherwise indicated.
| Trait | RR (CC) | RX (CT) | p-value | |
|---|---|---|---|---|
| N | 763 | 39 | -- | |
| Age | 44 ± 14 | 42 ± 12 | 0.66 | |
| Sex (%M:%F) | 55:45 | 56:44 | -- | |
| BMI (kg/m2) | 26.6 ± 4.4 | 26.6 ± 4.1 | 0.98 | |
| Waist (cm) | 87 ± 11 | 89 ± 12 | 0.50 | |
| SBP (mm Hg) | 121 ± 14 | 119 ± 14 | 0.67 | |
| DBP (mm Hg) | 77 ± 9 | 77 ± 9 | 0.55 | |
| Glucose (mg/dl) | 86 ± 9 | 87 ± 4 | 0.53 | |
| Insulin (μU/ml) | 8.1 (6.5 – 10.2) | 8.8 (6.3 – 12.0) | 0.72 | |
| Total Cholesterol (mg/dl) | 209 ± 46 | 191 ± 35 | 0.02 | |
| Triglycerides (mg/dl) | 57 (42 – 81) | 31 (25 – 48) | 4.1 × 10−13 | |
| HDL-C (mg/dl) | 55.1 ± 13.9 | 67.1 ± 17.3 | 9.0 × 10−7 | |
| HDL2-C (mg/dl) | 13 (10 – 18) | 18 (14 – 27) | 7.0 × 10−6 | |
| HDL3-C (mg/dl) | 41.1 ± 7.4 | 46.8 ± 9.2 | 3.0 × 10−5 | |
| Non-HDL Cholesterol (mg/dl) | 159 ± 43 | 132 ± 29 | 0.0005 | |
| LDL-C (mg/dl) | 140 ± 43 | 116 ± 32 | 0.001 | |
| Real LDL-C (mg/dl) | 125 ± 36 | 109 ± 25 | 0.01 | |
| VLDL-C (mg/dl) | 16 (14 – 20) | 12 (12 – 14) | 1.5 × 10−10 | |
| 4 Hour VLDL-C change (mg/dl) | 3.3 ± 2.8 | 1.8 ± 2.0 | 0.0009 | |
| Remnant Lipoprotein-C (mg/dl) | 17 (11 – 26) | 6 (4 – 10) | 3.0 × 10−20 | |
| IDL-C (mg/dl) | 8 (3 – 14) | 1 (1 – 2) | 8.8 × 10−15 | |
| VLDL3-C (mg/dl) | 9 (7 – 11) | 6 (6 – 7) | 2.8 × 10−10 | |
Abbreviations: BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol: VLDL-C: very low density lipoprotein cholesterol; IDL-C: intermediate density lipoprotein cholesterol
The hypothesis that a mutation conferring such a favorable lipid profile would be cardioprotective was evaluated in the Amish Family Calcification Study (14), which includes 335 of the HAPI Heart fat challenge participants along with 698 additional Amish individuals, all of whom underwent electron beam computed tomography (EBCT) to quantify coronary artery calcification (CAC), a subclinical measure of coronary atherosclerosis. A standard lipid panel was also obtained. The effect of the APOC3 R19X mutation on decreased FTG and increased HDL-C levels replicated in the 698 non-overlapping AFCS participants (p = 1.9 × 10−22 and p = 1.3 × 10−14 respectively; p = 2.9 × 10−29 and 1.3 × 10−17 in the full AFCS). LDL-C levels tended to be lower in R19X carriers than in non-carriers in the non-overlapping subset, and the difference reached statistical significance in the full AFCS (p = 0.01) (Table S5).
Among AFCS participants, according to evidence-based clinical guidelines set by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP-III) (15), LDL-C levels, the primary CHD prevention target, were more likely to be optimal (<100 mg/dl) in R19X carriers (OR = 2.24 [95% CI: 1.07 – 4.66]) than in non-carriers (Table 2), though the difference did not quite reach statistical significance (p = 0.07). More strikingly, High HDL-C (>60 mg/dl), considered by the ATP-III to be cardioprotective enough to cancel out one additional CHD risk factor (15), was much more common in R19X carriers than in non-carriers (OR = 7.0 [95% CI: 3.3 – 14.8], p = 9.1 × 10−6). Notably, ATP-III-defined low HDL-C (<40 mg/dl) was absent in the carriers but present in 13.5% of non-carriers (Fisher's exact p = 4.2 × 10−4). All R19X carriers had FTG in the normal (<150 mg/dl) range (maximum = 77 mg/dl), while only 83.6% of non-carriers had normal FTG (Fisher's exact p = 0.0004). Among AFCS participants in the baseline age range (30 – 74 years) of the Framingham Heart Study, mutation carriers had significantly lower ln-transformed 10 year Framingham CHD risk (16) (risk ratio = 0.68 [95% CI: 0.58 – 0.79] , p = 3.9 × 10−7) (Table 2 and Figure S6).
Table 2.
National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP-III) protective lipid levels, Framingham 10 year coronary heart disease (CHD) risk and coronary artery calcification (CAC) as a function of APOC3 R19X Genotype in the Amish Family Calcification Study. Dichotomous traits analyzed by generalized estimating equations (GEE) adjusted for age, sex and sibship as described in the methods (8), except triglycerides, which contains a zero cell. Framingham 10-year CHD risk analyzed as a natural log-transformed quantitative trait with variance components (adjusted for sex and sex-specific age and age2) as described in the methods (8).
| Parameter | RR (CC) | RX (CT) | OR (95% CI) | p |
|---|---|---|---|---|
| Optimal LDL-C (< 100 mg/dl) | 120 / 969 (12.4%) | 14 / 59 (23.7%) | 2.2 (1.1 – 4.7) | 0.07 |
| High HDL-C (≥ 60 mg/dl) | 362 / 973 (37.2%) | 45 / 59 (76.3%) | 7.0 (3.3 – 14.8) | 9.1 × 10−6 |
| Normal Triglycerides (<150 mg/dl) | 840 / 973 (86.3%) | 59 / 59 (100.0%) | -- | 0.0004* |
| Framingham 10 year CHD risk† | Median = 5.2% | Median = 3.8% | Risk Ratio: 0.68 (0.58 – 0.79) |
3.9 × 10−7 |
| Any CAC | 526 / 974 (54.0%) | 20 / 59 (33.9%) | 0.35 (0.21 – 0.60) | 0.002 |
| CAC score > 100 Agatston Units | 283 / 974 (29.1%) | 9 / 59 (15.3%) | 0.40 (0.18 – 0.85) | 0.01 |
| CAC score ≥ 75th MESA Percentile‡ | 270 / 753 (35.9%) | 10 / 52 (19.2%) | 0.38 (0.19 – 0.77) | 0.003 |
Fisher's exact test
Included only individuals age 30 – 74 years to match baseline ages in the Framingham Heart Study
Included only individuals age 45 – 84 years to match baseline ages in the Multi-Ethnic Study of Atherosclerosis
Consistent with their protective lipid profile, R19X carriers were significantly less likely than non-carriers to have any detectable CAC (OR = 0.35 [95% CI: 0.21 – 0.60], p = 0.002) or CAC scores greater than 100 Agatston units (OR = 0.40 [95% CI: 0.18 – 0.85], p = 0.01) (Figure S7). CAC scores of 1 – 100 were previously associated with a four-fold and scores greater than 100 with a seven-fold increased risk of coronary events in comparison with individuals with no detectable calcification in the population-based Multi-Ethnic Study of Atherosclerosis (MESA) after adjustment for standard risk factors (17). Among AFCS participants in the age range (45 – 84 years) of the MESA study, R19X carriers were significantly less likely to have a score at or above their respective MESA-derived (18)ethnicity-, sex- and age-specific 75th percentile (OR = 0.38 [95% CI: 0.19 – 0.77], p = 0.003) (Table 2), a level associated in MESA after adjustment for traditional risk factors with an eight-fold risk of CHD events in comparison with the lower two quartiles (19). Analysis of the quantitative trait ln(CAC score + 1), which approximated a normal distribution after adjustment for age and sex (Figure S8), yielded a significant negative association between CAC quantity and being a carrier (βR19X = −0.84 ± 0.30 [e.g., mean ratio = 0.43, 95% CI: 0.24 – 0.78], p = 0.005) (Table S5).
ApoC-III, secreted from the liver and to a lesser extent by the intestines, is a component of both HDL and apoB-containing lipoprotein particles (9, 20), impairs catabolism and hepatic uptake of apoB-containing lipoproteins (21, 22), appears to enhance the catabolism of HDL particles (23), enhances monocyte adhesion to vascular endothelial cells (24), and activates inflammatory signaling pathways (25). Along with the observed association of elevated apoC-III concentration with increased CHD risk (26), the roles of apoC-III in lipoprotein metabolism would predict that apoC-III deficiency would decrease atherothrombotic tendency.
Previous reports of confirmed apoC-III deficiency in humans were complicated by the prothrombotic effects of accompanying apoA-I and/or apoA-IV deficiency (27-30), small sample sizes and/or structurally abnormal apoC-III (31)(32). The current report of a favorable lipid profile and reduced subclinical coronary artery atherosclerosis in R19X null mutation carriers provides strong evidence in a relatively large number of individuals that apoC-III deficiency (by approximately 50% of normal levels) is indeed cardioprotective.
Indirectly lowered APOC3 expression is one mechanism of the lipid-lowering effect of fibrates(33), and the use of several other lipid-lowering agents, including statins, thiazolidinediones, ezetimibe, niacin, fish oil and weight loss, has also been associated with decreases in apoC-III levels (reviewed in (20)). That a naturally occurring null mutation in APOC3 confers a favorable lipid profile and apparent cardioprotection and does not result in any obvious detrimental effect raises the possibility that therapies aimed specifically at downregulating apoC-III expression will be clinically efficacious and safe in reducing the morbidity and mortality associated with CHD.
Supplementary Material
Acknowledgments
34. We gratefully acknowledge our Amish liaisons, field workers and clinic staff and the extraordinary cooperation and support of the Amish community without which these studies would not have been possible. We thank Drs. Richa Agarwala and Alejandro Schäffer for their assistance in pedigree construction and Kathy Ryan for data management. This work was supported by NIH grants R01 HL088119, R01 AR046838, U01 HL72515, R01 AG18728, U01 HL084756, and the University of Maryland General Clinical Research Center, Grant M01 RR 16500, University of Maryland Clinical Nutrition Research Unit Grant P30 DK072488, the Johns Hopkins University General Clinical Research Center, Grant M01 RR 000052, General Clinical Research Centers Program, National Center for Research Resources (NCRR), NIH, and the Baltimore Veterans Administration Geriatric Research and Education Clinical Center (GRECC) and the Paul Beeson Faculty Scholars in Aging Program.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/cgi/content/full/322/5908/1702. Their manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References
- 1.Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG. J. Clin. Invest. 1973;52:1544. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarwar N, et al. Circulation. 2007;115:450. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 3.Miller M, et al. J. Am. Coll. Cardiol. 2008;51:724. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. N. Engl. J. Med. 2006;354:1264. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 5.Bansal S, et al. JAMA. 2007;298:309. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. JAMA. 2007;298:299. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell BD, et al. Am. Heart J. 2008;155:823. doi: 10.1016/j.ahj.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Materials and methods are available as supporting material on Science Online
- 9.Havel RJ, Kane JP. Structure and Metabolism of Plasma Lipoproteins. ed. 8th. McGraw-Hill; New York: 2005. chap. 114. [Google Scholar]
- 10.Maeda N, et al. J. Biol. Chem. 1994;269:23610. [PubMed] [Google Scholar]
- 11.Hospattankar AV, Brewer HB, Jr, Ronan R, Fairwell T. FEBS Lett. 1986;197:67. doi: 10.1016/0014-5793(86)80300-3. [DOI] [PubMed] [Google Scholar]
- 12.Isken O, Maquat LE. Genes Dev. 2007;21:1833. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- 13.Nagy E, Maquat LE. Trends Biochem. Sci. 1998;23:198. doi: 10.1016/s0968-0004(98)01208-0. [DOI] [PubMed] [Google Scholar]
- 14.Post W, et al. Circulation. 2007;115:717. doi: 10.1161/CIRCULATIONAHA.106.637512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.JAMA. 2001;285:2486. [Google Scholar]
- 16.Wilson PW, et al. Circulation. 1998;97:1837. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.Detrano R, et al. N. Engl. J. Med. 2008;358:1336. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 18.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Circulation. 2006;113:30. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 19.Folsom AR, et al. Arch. Intern. Med. 2008;168:1333. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ooi EM, Barrett PH, Chan DC, Watts GF. Clin. Sci. (Lond) 2008;114:611. doi: 10.1042/CS20070308. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg HN, et al. J. Clin. Invest. 1986;78:1287. doi: 10.1172/JCI112713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clavey V, Lestavel-Delattre S, Copin C, Bard JM, Fruchart JC. Arterioscler. Thromb. Vasc. Biol. 1995;15:963. doi: 10.1161/01.atv.15.7.963. [DOI] [PubMed] [Google Scholar]
- 23.Chan DC, Nguyen MN, Watts GF, Barrett PH. J. Clin. Endocrinol. Metab. 2008;93:557. doi: 10.1210/jc.2006-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawakami A, et al. Circulation. 2006;113:691. doi: 10.1161/CIRCULATIONAHA.105.591743. [DOI] [PubMed] [Google Scholar]
- 25.Libby P. Circ. Res. 2007;100:299. doi: 10.1161/01.RES.0000259393.89870.58. [DOI] [PubMed] [Google Scholar]
- 26.Sacks FM, et al. Circulation. 2000;102:1886. doi: 10.1161/01.cir.102.16.1886. [DOI] [PubMed] [Google Scholar]
- 27.Norum RA, et al. N. Engl. J. Med. 1982;306:1513. doi: 10.1056/NEJM198206243062503. [DOI] [PubMed] [Google Scholar]
- 28.Karathanasis SK, Norum RA, Zannis VI, Breslow JL. Nature. 1983;301:718. doi: 10.1038/301718a0. [DOI] [PubMed] [Google Scholar]
- 29.Karathanasis SK, Ferris E, Haddad IA. Proc. Natl. Acad. Sci. U. S. A. 1987;84:7198. doi: 10.1073/pnas.84.20.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ordovas JM, Cassidy DK, Civeira F, Bisgaier CL, Schaefer EJ. J. Biol. Chem. 1989;264:16339. [PubMed] [Google Scholar]
- 31.von Eckardstein A, et al. J. Clin. Invest. 1991;87:1724. doi: 10.1172/JCI115190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, et al. J. Lipid Res. 2000;41:1760. [PubMed] [Google Scholar]
- 33.Auwerx J, Schoonjans K, Fruchart JC, Staels B. Atherosclerosis. 1996;124(Suppl):S29. doi: 10.1016/0021-9150(96)05854-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.