Abstract
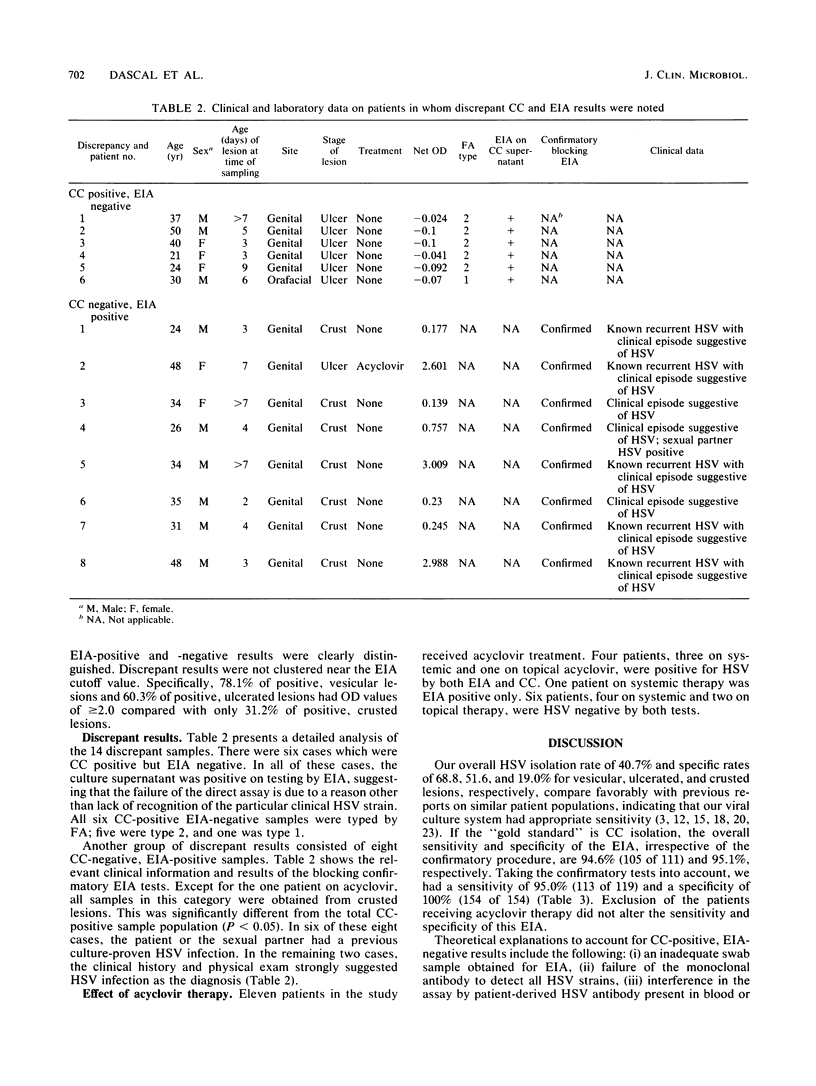
A commercial 4-h direct herpes simplex virus (HSV) antigen detection enzyme immunoassay (EIA) kit (Du Pont Herpchek) was evaluated by using 273 clinical specimens obtained in a hospital-based infectious disease practice. The EIA was compared with a standard culture method in which WI38 cells were inoculated within 20 min of sample collection. Cultures were observed for 2 weeks, and positive findings were confirmed by fluorescein-labeled monoclonal antibody (FA) staining. The values for the overall HSV detection rate were 40.7% by the standard culture method and 41.4% by EIA. In eight cases, the EIA was positive, while the culture method was negative; however, clinical data and confirmatory blocking EIA suggested that a true HSV infection was present. For six FA-confirmed, culture-positive samples, the direct EIA was negative; however, an EIA performed on the supernatants of these cultures was positive, suggesting that the failure of the EIA to detect these samples was not due to lack of strain specificity of the test. After confirmatory tests of standard culture and EIA discrepant results, the overall sensitivity of the test was 95.0% (113 of 119) and the specificity was 100% (154 of 154).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvin A. M., Hensleigh P. A., Prober C. G., Au D. S., Yasukawa L. L., Wittek A. E., Palumbo P. E., Paryani S. G., Yeager A. S. Failure of antepartum maternal cultures to predict the infant's risk of exposure to herpes simplex virus at delivery. N Engl J Med. 1986 Sep 25;315(13):796–800. doi: 10.1056/NEJM198609253151303. [DOI] [PubMed] [Google Scholar]
- Clayton A. L., Beckford U., Roberts C., Sutherland S., Druce A., Best J., Chantler S. Factors influencing the sensitivity of herpes simplex virus detection in clinical specimens in a simultaneous enzyme-linked immunosorbent assay using monoclonal antibodies. J Med Virol. 1985 Nov;17(3):275–282. doi: 10.1002/jmv.1890170309. [DOI] [PubMed] [Google Scholar]
- Clayton A. L., Roberts C., Godley M., Best J. M., Chantler S. M. Herpes simplex virus detection by ELISA: effect of enzyme amplification, nature of lesion sampled and specimen treatment. J Med Virol. 1986 Sep;20(1):89–97. doi: 10.1002/jmv.1890200111. [DOI] [PubMed] [Google Scholar]
- Dunkel E. C., Pavan-Langston D., Fitzpatrick K., Cukor G. Rapid detection of herpes simplex virus (HSV) antigen in human ocular infections. Curr Eye Res. 1988 Jul;7(7):661–666. doi: 10.3109/02713688809033194. [DOI] [PubMed] [Google Scholar]
- Gibbs R. S., Amstey M. S., Sweet R. L., Mead P. B., Sever J. L. Management of genital herpes infection in pregnancy. Obstet Gynecol. 1988 May;71(5):779–780. [PubMed] [Google Scholar]
- Gleaves C. A., Wilson D. J., Wold A. D., Smith T. F. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J Clin Microbiol. 1985 Jan;21(1):29–32. doi: 10.1128/jcm.21.1.29-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. C., Corey L., McDougall J. K., Tolentino E., Nowinski R. C. Monoclonal antibodies to herpes simplex viruses: use in antigenic typing and rapid diagnosis. J Infect Dis. 1983 May;147(5):829–837. doi: 10.1093/infdis/147.5.829. [DOI] [PubMed] [Google Scholar]
- Hayden F. G., Sorensen A. S., Bateman J. A. Comparison of the Immulok cultureset kit and virus isolation for detection of herpes simplex virus in clinical specimens. J Clin Microbiol. 1983 Jul;18(1):222–224. doi: 10.1128/jcm.18.1.222-224.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntoon C. J., House R. F., Jr, Smith T. F. Recovery of viruses from three transport media incorporated into culturettes. Arch Pathol Lab Med. 1981 Aug;105(8):436–437. [PubMed] [Google Scholar]
- Jackson J. B., Balfour H. H., Jr Practical diagnostic testing for human immunodeficiency virus. Clin Microbiol Rev. 1988 Jan;1(1):124–138. doi: 10.1128/cmr.1.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. B., Leavitt R. W., Richards D. F. Comparison of the Scott Selecticult-HSV kit with conventional culture and direct immunoperoxidase staining for detection of herpes simplex virus in cultures of clinical specimens. J Clin Microbiol. 1985 Mar;21(3):438–441. doi: 10.1128/jcm.21.3.438-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty W. E., Krofft S., Remington M., Giddings R., Winter C., Cent A., Corey L. Diagnosis of herpes simplex virus by direct immunofluorescence and viral isolation from samples of external genital lesions in a high-prevalence population. J Clin Microbiol. 1987 Feb;25(2):323–326. doi: 10.1128/jcm.25.2.323-326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo D. R., Brennan T., Egbertson S. H., Moore D. F. Rapid herpes simplex virus detection in clinical samples submitted to a state virology laboratory. J Clin Microbiol. 1985 May;21(5):768–771. doi: 10.1128/jcm.21.5.768-771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. A., Smith T. F. Evaluation of an enzyme-linked immunosorbent assay for the detection of herpes simplex virus antigen. J Clin Microbiol. 1984 Jun;19(6):730–732. doi: 10.1128/jcm.19.6.730-732.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerurkar L. S., Namba M., Brashears G., Jacob A. J., Lee Y. J., Sever J. L. Rapid detection of herpes simplex virus in clinical specimens by use of a capture biotin-streptavidin enzyme-linked immunosorbent assay. J Clin Microbiol. 1984 Jul;20(1):109–114. doi: 10.1128/jcm.20.1.109-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. E., Magliolo R. A., Stehlik M. L., Whiteman P. A., Faro S., Rogers T. E. Retrospective evaluation of the isolation and identification of herpes simplex virus with Cultureset and human fibroblasts. J Clin Microbiol. 1985 Aug;22(2):255–258. doi: 10.1128/jcm.22.2.255-258.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouletty P., Chomel J. J., Thouvenot D., Catalan F., Rabillon V., Kadouche J. Detection of herpes simplex virus in direct specimens by immunofluorescence assay using a monoclonal antibody. J Clin Microbiol. 1987 May;25(5):958–959. doi: 10.1128/jcm.25.5.958-959.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober C. G., Hensleigh P. A., Boucher F. D., Yasukawa L. L., Au D. S., Arvin A. M. Use of routine viral cultures at delivery to identify neonates exposed to herpes simplex virus. N Engl J Med. 1988 Apr 7;318(14):887–891. doi: 10.1056/NEJM198804073181404. [DOI] [PubMed] [Google Scholar]
- Salmon V. C., Turner R. B., Speranza M. J., Overall J. C., Jr Rapid detection of herpes simplex virus in clinical specimens by centrifugation and immunoperoxidase staining. J Clin Microbiol. 1986 Apr;23(4):683–686. doi: 10.1128/jcm.23.4.683-686.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Dennis J., Devlin V., Gallo D., Mills J. Comparison of direct immunofluorescence and direct immunoperoxidase procedures for detection of herpes simplex virus antigen in lesion specimens. J Clin Microbiol. 1983 Aug;18(2):445–448. doi: 10.1128/jcm.18.2.445-448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell D. L., Horn S. A., Dilbeck P. W. Comparison of Cultureset and Bartels Immunodiagnostics with conventional tissue culture for isolation and identification of herpes simplex virus. J Clin Microbiol. 1984 May;19(5):705–706. doi: 10.1128/jcm.19.5.705-706.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Rogers R. E., Katz B. P., Brickler J. F., Lineback P. L., Van der Pol B., Jones R. B. Diagnosis of chlamydial infection in women attending antenatal and gynecologic clinics. J Clin Microbiol. 1987 May;25(5):868–872. doi: 10.1128/jcm.25.5.868-872.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. C., Judson F. N., Barnes R. C., Gruninger R. P., Ryan R. W., Steingrimsson O. Multicenter comparative evaluation of two rapid microscopic methods and culture for detection of Chlamydia trachomatis in patient specimens. J Clin Microbiol. 1988 Feb;26(2):167–170. doi: 10.1128/jcm.26.2.167-170.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warford A. L., Eveland W. G., Strong C. A., Levy R. A., Rekrut K. A. Enhanced virus isolation by use of the transporter for a regional laboratory. J Clin Microbiol. 1984 Apr;19(4):561–562. doi: 10.1128/jcm.19.4.561-562.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Kim H. W., Clem T., Wyatt R. G., Kalica A. R., Chanock R. M., Kapikian A. Z. Enzyme-linked immunosorbent assay (ELISA) for detection of human reovirus-like agent of infantile gastroenteritis. Lancet. 1977 Aug 6;2(8032):263–267. doi: 10.1016/s0140-6736(77)90951-5. [DOI] [PubMed] [Google Scholar]


