Abstract
Anthrax toxin consists of three proteins (approx. 90 kDa each): lethal factor (LF); oedema factor (OF); and protective antigen (PA). The former two are enzymes that act when they reach the cytosol of a targeted cell. To enter the cytosol, however, which they do after being endocytosed into an acidic vesicle compartment, they require the third component, PA. PA (or rather its proteolytically generated fragment PA63) forms at low pH a heptameric β-barrel channel, (PA63)7, through which LF and OF are transported—a phenomenon we have demonstrated in planar phospholipid bilayers. It might appear that (PA63)7 simply forms a large hole through which LF and OF diffuse. However, LF and OF are folded proteins, much too large to fit through the approximately 15 Å diameter (PA63)7 β-barrel. This paper discusses how the (PA63)7 channel both participates in the unfolding of LF and OF and functions in their translocation as a proton–protein symporter.
Keywords: planar bilayer membranes, protein unfolding, lethal factor, voltage-driven transport
1. Introduction
Thirty years ago, Gill (1978) recognized a class of toxins that he described as the A–B model. The ‘A’ component of the toxin is an enzyme and is the ultimate cause of cell intoxication, but for it to reach its cytosolic target, it requires the ‘B’ component. How does this come about? After binding to a cell receptor (through the B moiety) and undergoing receptor-mediated endocytosis, the toxin arrives in an endocytotic vesicular compartment. From there, the B component facilitates the translocation of the A component into the cytosol. In some cases (e.g. cholera toxin and ricin), this is accomplished by co-opting a pre-existing translocation machine of the cell (Falnes & Sandvig 2000); in other cases, however (e.g. diphtheria toxin and botulinum neurotoxin), the toxin finds itself in an acidic vesicle, and it is from this environment that the B component promotes the translocation of the A component across the vesicle membrane (Falnes & Sandvig 2000). How does it do this? It turns out that under acidic conditions (below pH 6.5), the B portion of these toxins can form channels in planar phospholipid bilayer membranes (Hoch et al. 1985), and mutants of diphtheria toxin that are defective in this channel formation are likewise defective in cell intoxication (e.g. Falnes et al. 1992; Cabiaux et al. 1993; Silverman et al. 1994). It is therefore tempting to suggest that these channels form the route for the A moiety to cross the vesicle membrane. But despite this correlation between channel formation and intoxication, and despite that the A components of diphtheria toxin and botulinum neurotoxin are translocated across planar lipid bilayers in association with channel formation (Oh et al. 1999; Koriazova & Montal 2003), there is no direct evidence (with the exception of anthrax toxin, the subject of this paper) that these channels serve as a conduit for protein translocation. (Koriazova & Montal (2003) claimed that the blocking of the botulinum neurotoxin channel by the A chain is evidence for its translocation through that channel, but a more likely explanation is that the A chain blocks the channel from the trans side after it has been translocated across the membrane to that side.)
Anthrax toxin is also a member of the A–B class of toxins that takes the acidic vesicle route to intoxication. It differs from most members of this class in two respects: it has two A components instead of the traditional one, and the A components and the B component are completely separate protein entities, rather than being joined covalently in a single polypeptide or held together by non-covalent forces (for a review of anthrax toxin, see Young & Collier 2007). The two A components are oedema factor (OF; 89 kDa) and the benignly named lethal factor (LF; 90 kDa); the former is a calmodulin-dependent adenylate cyclase and the latter is a protease that cleaves mitogen-activated protein kinase kinases. The B component is protective antigen (PA; 83 kDa), so named for its use in vaccines to generate antibodies that protect against anthrax toxin. The pathway by which OF and LF gain access to the cytosol (reviewed by Young & Collier 2007) is as follows: (i) after PA83 binds to a receptor on the plasma membrane, a 20 kDa N-terminal piece (PA20) is cleaved from it by a furin-family protease, leaving the larger 63 kDa C-terminal piece (PA63) still attached to the receptor, (ii) PA63 self-assembles into a pre-pore heptamer (PA63)7, which has seven binding sites for LF and OF (but for steric reasons can accommodate only up to three), (iii) the complex of the pre-pore with its bound LFs and/or OFs is endocytosed and ends up in an acidic vesicle, and (iv) in this acidic environment, the pre-pore (PA63)7 undergoes a conformational change to form the (PA63)7 pore or channel—an extended 14-stranded β-barrel, which, as we shall see, serves as the conduit for the translocation of LF and OF into the cytosol.
The experiments discussed in this paper were all done on planar phospholipid bilayer membranes. After describing the structure and properties of the (PA63)7 channel and reviewing the data demonstrating that OF and LF can be translocated through it, I argue that the channel is not merely a large hole through which these molecules diffuse, but rather that it participates in their unfolding and functions as a proton–protein symporter. Although the basic findings that I present have been shown to apply to whole OF and LF (Krantz et al. 2006), most of the experiments have been done with LFN, the approximately 30 kDa 263-residue N-terminal portion of LF.
2. The (PA63)7 channel
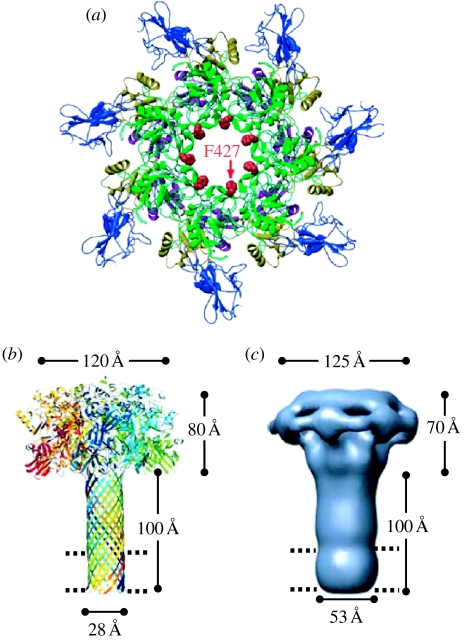
Although the structure of the (PA63)7 pre-pore (figure 1a) has been determined (Petosa et al. 1997), that of the (PA63)7 channel itself has not. Nevertheless, we have a very good idea of its overall architecture. The reaction of alternate cysteine-labelled residues with sulphydryl-specific reagents indicates that the channel is a β-barrel, approximately 100 Å long, with approximately 25 Å spanning the bilayer and 75 Å extending beyond it (Nassi et al. 2002). By analogy to the structure of the heptameric β-barrel α-haemolysin channel, which has been determined (Song et al. 1996), the (PA63)7 channel is thought to be a similar mushroom-like structure (although having a considerably longer stem), with the binding sites for OF and LF in the cap and residue F427 (discussed below) near the junction of the cap and the stem (figure 1b). The more recent, low-resolution, negatively stained EM image of the (PA63)7 channel (Katayama et al. 2008) is reassuringly consistent with this picture (figure 1c).
Figure 1.
Structure of the (PA63)7 pore (channel) and pre-pore. (a) Ribbons rendering of the pre-pore viewed axially. The phenylalanines at residue 427 are seen to face the lumen of the pre-pore. After conversion of the pre-pore to the pore, they continue to face the channel lumen, but, as evidenced from EPR spectra of spin label introduced uniformly at this site (Krantz et al. 2005), they have moved closer together; that is, the lumen diameter is smaller (adapted from Krantz et al. 2005). (b) Model of the mushroom-like (PA63)7 channel based on the crystal structure of monomeric PA, the crystal structure of the heptameric α-haemolysin channel and cysteine mutagenesis experiments that established the length of the β-barrel stem. The ring of phenylalanines at residue 427 (the ϕ-clamp) lies near the junction of the cap and the stem. The parallel dashed lines here and in (c) indicate the thickness of the hydrophobic interior of the phospholipid bilayer (adapted from Nguyen 2004, published with permission from Adenine Press, Inc. http://www.jbsdonline.com). (c) The structure of the (PA63)7 channel as obtained from negative-stain electron microscopy at a resolution of approximately 25 Å. The agreement of this structure with the model in (b) is gratifying (adapted from Katayama et al. 2008, adapted with permission from Macmillan Publishers Ltd; see http://www.nature.com/nsmb).
When added to planar lipid bilayer membranes, (PA63)7 forms channels with a conductance of approximately 55 pS in 100 mM KCl at pH 5.5 (Krantz et al. 2005); at picomolar concentrations, several thousand channels can be incorporated into the membrane. The channels are fairly cation selective (a fact that will become important in our discussion of protein translocation), but not exclusively so, exhibiting a finite permeability to Cl− (Blaustein & Finkelstein 1990a). They display some voltage-dependent gating (Finkelstein 1994), but this has no significant effect on the translocation phenomena described below.
3. Protein translocation through the (PA63)7 channel
In all the experiments described in this paper, the membrane separates two aqueous solutions: the cis solution, to which (PA63)7 was added, and the opposite trans solution. The latter is taken as the reference solution, so that all voltages (V) are those of the cis solution with respect to that of the trans. Thus, a voltage of +20 mV means that the cis solution is at a potential of 20 mV positive with respect to the trans solution.
(a) Symmetric pH conditions: voltage-driven translocation
(i) Phenomenological description
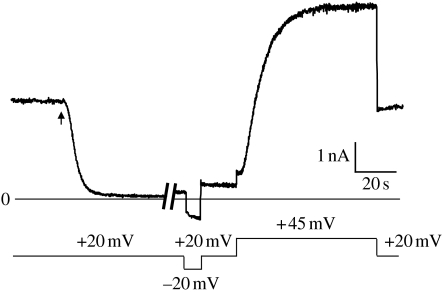
After a more-or-less steady-state conductance has been reached following the addition of (PA63)7 to one side of a membrane separating symmetric 0.1 M KCl solutions at pH 5.5, and with the voltage held at a small (e.g. 20 mV) positive value, the addition of nanomolar amounts of LFN to the cis solution produces a large rapid fall in conductance to a value of only a few per cent of the original level (figure 2). With the voltage held at +20 mV and LFN subsequently perfused out of the cis solution, the conductance remains at a low level, rising only slowly over time. If the voltage is then stepped from +20 to −20 mV, there is a very rapid increase in conductance followed by a slower rise, and when the voltage is stepped back to +20 mV, the conductance rapidly returns to a value only slightly larger than before (figure 2). On the other hand, if the voltage is stepped to +45 mV, the conductance rises over a period of several seconds, with S-shaped kinetics, to a value comparable to that which existed before the addition of LFN (figure 2). When the voltage is stepped back to +20 mV, the conductance remains at this level.
Figure 2.
The interaction of LFN with the (PA63)7 channel. After the (PA63)7-induced conductance had reached a more-or-less steady state, LFN was added (at the arrow) to the cis side to a concentration of 6 nM, resulting in a rapid fall in conductance. LFN (along with (PA63)7) was then perfused out of the cis compartment (during the approx. 4 min break in the record); the conductance increased only slightly over this time. When the voltage was stepped from +20 to −20 mV, there was a very rapid increase in conductance followed by a slower increase. When the voltage was stepped back to +20 mV, the conductance rapidly fell to a value somewhat larger than before. By contrast, when the voltage was stepped from +20 to +45 mV, there was an S-shaped rise of conductance to a value comparable to that before the addition of LFN, and it remained at that value when the voltage was stepped back from +45 to +20 mV.
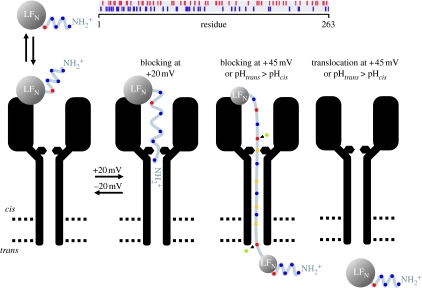
The explanation for the conductance changes shown in figure 2 is summarized in the cartoon in figure 3. The fall in conductance at +20 mV upon addition of LFN to the cis solution is caused by the entrance of its N-terminal end into the (PA63)7 channel, thereby blocking it (Zhang et al. 2004a). The blockage of ion flow through the channel is essentially total. This is manifested at the single-channel level by the drop of conductance from 55 pS to zero (Krantz et al. 2005), and at the macroscopic level by conductances that have fallen as low as 0.5 per cent of the original (PA63)7-induced conductance. This small, residual conductance can be attributed to the brief, infrequent unblocking of the channel at +20 mV seen at the single-channel level. (The degree of penetration of LFN into or through the channel at +20 mV, or at any other voltage, remains to be determined.) At −20 mV, the N-terminal end of LFN exits the channel to the cis side, thereby unblocking the channel, but it remains attached to the channel and only slowly dissociates from it. This slow dissociation is reflected in the slow increase in conductance at −20 mV as shown in figure 2. When the voltage is returned to +20 mV, the conductance falls back almost to the same low level existing before the voltage was stepped to −20 mV, as the LFN still attached to the channel reblocks it. On the other hand, when the voltage is stepped from +20 to +45 mV, LFN is translocated all the way through the channel and out into the trans solution (Zhang et al. 2004b); this is why the conductance does not fall back to near its previous low value when the voltage is stepped back to +20 mV—there is no longer any LFN attached to the channel that can block it. The rate of LFN translocation is both voltage and pH dependent: the larger the voltage, the faster the translocation rate (Zhang et al. 2004b); the higher the symmetric pH, the slower the translocation rate (Krantz et al. 2006).
Figure 3.
Cartoon of the interaction of LFN with the (PA63)7 channel and the effect of voltage and a pH gradient on this interaction. (The distribution of positively and negatively charged groups in the 263 residues of LFN is shown at the top.) The (PA63)7 channel is diagrammed as a long narrow stem, the bottom third of which crosses the bilayer (represented by the parallel dashed lines), opening up into a wide vestibule. The constriction near the junction of the vestibule with the stem is the ϕ-clamp formed by the seven F427s. At the top of the vestibule is a binding site for LFN; LFN is schematized as a folded structure with the N-terminal 26 residues disordered (Pannifer et al. 2001) and therefore able to enter the channel and block it at +20 mV once the N-terminal end has traversed the ϕ-clamp. The N-terminal end is driven out of the channel at −20 mV and thereby unblocks it, but remains attached to its binding site and only slowly dissociates from this site into the cis solution. (The degree of penetration of LFN into the channel at +20 mV has not yet been determined, and conceivably the N-terminal end may even traverse the entire channel and emerge into the trans solution.) At larger positive voltages (+45 mV), LFN unfolds with the aid of the ϕ-clamp (see text), and its extended structure is driven through the stem until ultimately the entire LFN protein has been translocated across the membrane. Before entering the cation-selective stem, the carboxyl groups on glutamates and aspartates pick up protons from the cis side and are thereby neutralized; thus, the LFN segment within the stem always bears a positive charge (or at worst is neutral) and is consequently driven by the electric field from the cis to the trans side. (The protonation of the aspartates and glutamates is indicated as occurring before they cross the ϕ-clamp, but it is possible that it occurs at some point after this in the stem.) As they exit the channel, these carboxyl groups release their protons into the trans solution, completing the co-transport of protons with the protein. Alternatively, instead of the translocation being driven by a step of voltage from +20 to +45 mV, it can be driven by raising the trans pH (see text). Blue circles, Arg+/His+/Lys+; green circles, H+; yellow circles, Glu0/Asp0; red circles, Glu−/Asp−. This figure is an elaboration of one in Zhang et al. (2004b).
The translocation of LFN through the (PA63)7 channel, described above, raises two questions that will concern us for the rest of this paper. (i) Since the entryway into the channel from the cis side narrows at some point to a diameter of approximately 12 Å (Blaustein & Finkelstein 1990b), and since the β-barrel itself has a diameter of approximately 15 Å (Krantz et al. 2004), just large enough to accommodate an α-helix with its side chains, what causes the folded LFN with its tertiary structure (Pannifer et al. 2001) to unfold? (ii) How can a positive voltage be driving LFN through the channel, when the net charge on LFN, even at pH 5.5, is negative? The answers to these questions reveal the special features of the (PA63)7 channel that make it a protein translocase, rather than an indiscriminate large hole.
(ii) Unfolding of LFN
Unfolding of LFN is promoted by low pH. In the pH range 6.5–5, LFN goes from its largely native, tertiary structure to a mostly molten globular state (M), with significant secondary structure and a minor component state (D) with diminished secondary structure (Krantz et al. 2004); even in its native state, the first 27 residues at its N terminus are disordered (Pannifer et al. 2001). From these facts, a preliminary crude picture of the channel-induced unfolding of LFN can be envisioned: initially, the disordered, positively charged N-terminal end is driven by a positive voltage into the channel, thereby blocking it. As the N-terminal residues proceed through the channel, driven by the voltage, LFN unwinds—either by the pulling of the M⇆D equilibrium to the right, if the D (unstructured) form is the translocated species, or directly, if the segments moving through the β-barrel are predominantly α-helical. A particular aspect of the (PA63)7 channel structure, which we now turn to, indicates that it participates directly in unwinding LFN from its molten globular state.
In looking at the structure of the pre-pore, one is struck by the ring of seven hydrophobic residues, phenylalanines, lining the lumen at position 427 (figure 1a). Upon conversion of the pre-pore to the pore, these residues end up near the junction of the mushroom cap with its stem, creating a narrow aromatic iris facing the lumen of an otherwise hydrophilic-lined pore (Krantz et al. 2005). That these phenylalanines are absolutely conserved in homologous toxins (Krantz et al. 2005) indicates that they perform some essential function in toxin action, which is indeed the case as we shall now see.
If the bulky phenylalanine residue at position 427 is replaced by the small alanine residue, then, as expected, the single-channel conductance (a reflection of the rate of movement of K+ through the channel) is increased (Krantz et al. 2005); by contrast, and totally unexpectedly, the rate of voltage-driven translocation of LFN is reduced (at a given voltage) by approximately a factor of six (Krantz et al. 2006; A. Finkelstein 2007, unpublished results). How might this be explained? If the limiting step in translocation of LFN is its rate of movement through the approximately 100 Å β-barrel, then the rate of translocation of any of its segments will be proportional to the probability that some part(s) of the segment resides at the residue 427 iris; that is, there is an energy well there for a portion of each segment, but the rate of its exit from the well is much more rapid than the subsequent rate of movement through the β-barrel. We have speculated that the well formed by the seven phenylalanines, which we have called the ‘ϕ-clamp’, is a hydrophobic well that binds hydrophobic sequences presented by the protein being translocated as it unfolds (Krantz et al. 2005). Given, however, that the replacement of phenylalanine by other hydrophobic residues still leads to a reduction of the rate of voltage-driven translocation (by approx. a factor of two to six; Krantz et al. 2006; unpublished results), it appears that not only are hydrophobic residues bound, but also positively charged residues (lysines and arginines), through their interaction with the π-electron clouds of the phenylalanines. In either case, this pause of a segment at the ϕ-clamp stabilizes the segment(s) behind it in an unfolded state, and thereby actively assists in the unfolding of the protein.
(iii) Proton–protein symporter
From my description of the voltage-driven translocation of LFN through the (PA63)7 channel by positive voltages, it would be natural for the reader to assume that LFN bears a net positive charge. Unfortunately, this is not the case; even at pH 5.5, LFN bears a net negative charge (approx. −6). Both the second law of thermodynamics and the laws of electrostatics require that the translocated species have a net positive charge, and the simplest mechanism to achieve this is for the carboxyl groups of the aspartic and glutamic residues to be largely neutralized, i.e. protonated. Thus, these carboxyls on LFN must pick up protons from the cis solution, at some point during their entrance to the channel, and then discharge them into the trans solution as they exit the channel. In other words, the (PA63)7 channel functions as a proton–protein symporter. At all times, the portion of LFN that lies within the channel bears a net positive charge.
At pH 5.5, a carboxyl on LFN spends approximately 97 per cent of its time in its ionized, negatively charged form. Why then should its minority, protonated neutral form be the one that enters the channel? I would argue that the channel's significant cation selectivity (although not ideal) implies that it strongly disfavours anion entry into it. Indeed, if only one , which is essentially not titratable, is introduced at most positions in LFN (through the reaction of a cysteine-modified residue at those positions with 2-sulphonatoethyl methanethiosulphonate (MTS-ES)), LFN translocation does not occur (S. Juris 2006, unpublished results). Interestingly, and also consistent with the importance of the channel's cation selectivity for translocation, is the observation that if five of its six negatively charged residues (three aspartates and three glutamates) are mutated to serines, thereby rendering the channel relatively non-selective, LFN can no longer be voltage driven through the channel (D. Anderson 2008, personal communication). We now see that lowering the pH symmetrically promotes LFN translocation (Krantz et al. 2006) not only because it produces a loss of its native structure (Krantz et al. 2004), and thereby makes it easier to unfold, but also because it raises the fraction of time that its carboxyl groups spend in the neutral, protonated state.
(b) Asymmetric pH conditions: translocation driven by pH gradients
Up until this point, we have been considering LFN translocation through the (PA63)7 channel driven by a voltage difference (ΔV) across the membrane under symmetric pH conditions. But it is equally possible to drive LFN through the channel by a ΔpH across the membrane, cis pH low/trans pH high (figure 4), with ΔV=0. (Although the record in figure 4 was obtained at ΔV=+20 mV, translocation can be driven with essentially ΔV=0; Krantz et al. 2006; a small ΔV is necessary only to allow currents to be observed.) This is the more traditional situation for a proton-driven symport system, where the gradient of the chemical component of the electrochemical potential of is the driving force, rather than the symmetric pH situation where the gradient of the electrical component of is the driving force. From a thermodynamic viewpoint, the two situations are equivalent, but what about mechanistically? In the ΔpH=0 case, the mechanism of translocation is a ΔV operating on an always positively charged piece of LFN within the channel. What is the mechanism by which a ΔpH drives translocation when ΔV=0?
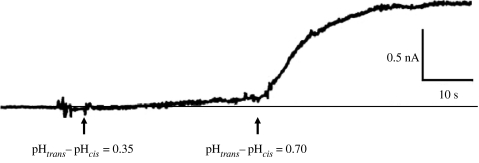
Figure 4.
LFN translocation being driven by a pH gradient. Prior to the start of the record, the (PA63)7-induced conductance was reduced over 30-fold by the addition (at symmetric pH 5.5 and +20 mV) of LFN to the cis solution, after which LFN was then perfused out of that solution. We see that raising the trans pH to 5.85 induced a slow rate of rise in conductance, reflecting the translocation of LFN, and raising it further to 6.2 caused a much greater increase in the rate of LFN translocation (adapted from Krantz et al. 2006).
An indication of what this might be is that there is no ostensible pKa for a putative residue, or residues, that is being titrated. Thus, incremental elevations of the trans pH from 5.5 to 8.8 continue to produce increases in the rate of LFN translocation, with no end in sight (Krantz et al. 2006). To explain this, we have proposed a charge-state Brownian ratchet mechanism that hinges on the cation selectivity of the (PA63)7 channel (Krantz et al. 2006). To enter the cation-selective portion of the channel, aspartate and glutamate residues have, for the most part, to be protonated (neutralized). If the pH on the cis side is lower than that on the trans side, then the probability of an aspartate or glutamate being in its neutralized form is greater on the cis side than on the trans side, and hence the rate of their entry from the cis side is greater than that from the trans side. In this way, the random Brownian motion of an LFN segment within the channel is biased towards the higher pH trans side. Thus, the (PA63)7 symporter effects protein translocation through two Brownian ratchets operating in tandem: the ϕ-clamp ratchet, which promotes the unfolding of the protein, and the charge-state ratchet, which biases the entry rates of the protein into the channel (figure 3).
The idea that transporters and pumps are in many ways channel-like has gained credence over the last few years, particularly as structural data have become available; indeed, this is the theme of the present symposium. The general picture that emerges is that of a channel selective for small non-electrolytes and ions, with subtle conformational changes induced by their binding reflected in the opening and closing of gates. In that context, the (PA63)7 proton–protein symporter we have been considering is quite different. There are no conformational changes or gates involved in its transport of proteins. Rather, what we have here is a relatively static cation-selective channel. But owing to this cation selectivity, the aspartates and glutamates on substrate proteins must be protonated in order for them to enter the channel. Consequently, protein transport is obligatorily coupled to protons, and, as a result, protein transport can be driven across a membrane by an electrochemical gradient of protons across that membrane. Another unusual feature of this system is that although protein transport is obligatorily coupled to proton transport, protons, being cations, can traverse the channel without having to be associated with protein transport. In transporter terminology, there is a proton leakage pathway, or the possibility of proton slippage in the transport cycle.
In addition to its role in promoting unfolding, the ϕ-clamp plays a prominent part in proton-gradient-driven translocation. Replacement of the phenylalanines at position 427 with alanines results in a much larger reduction in the rate of ΔpH-driven translocation than of ΔV-driven translocation (Krantz et al. 2006). What might explain this? We feel that the iris formed at 427 by phenylalanine, and other large hydrophobic residues (e.g. tryptophan, tyrosine, leucine), can bind residues passing through it tightly enough to form a hydrophobic seal that preserves the ΔpH across it, and hence the driving force for translocation. With alanine instead of phenylalanine, the seal is not tight and the pH gradient can be partially dissipated. The leakiness of the seal with the F427A mutant is evidenced by the flickering and incomplete block of conductance by LFN (Krantz et al. 2005).
The mechanism we have proposed for ΔpH-driven translocation is dependent on there being titratable negatively charged residues in the translocated substrate. It appears, however, that this may not be the entire story. In translocation experiments with synthetic polypeptides that have no aspartates or glutamates, and in which the C-terminal carboxyl group is blocked, we still observe stimulation of translocation when the trans pH is raised (unpublished results). This suggests that, in this instance, the titration of residues within the (PA63)7 channel is promoting polypeptide translocation. What these residues are (perhaps the histidines at residues 304 and 310) and the mechanism driving this translocation (perhaps the creation or modification of an internal electric field) remains to be determined.
One final point. There is no question that protein translocation through the (PA63)7 channel in planar phospholipid bilayers is biologically relevant. Although I have confined the presentation here to the translocation of LFN, essentially the same basic results are obtained with whole LF and OF (Krantz et al. 2006). The effect on ΔpH-driven translocation when the phenylalanine at 427 is replaced by alanine, which is much greater than its effect on ΔV-driven translocation, corresponds to the effect this mutation has on cell intoxication. This, along with the fact that large voltages (above 50 mV) are required to achieve, at best, very slow translocation of whole LF and OF across planar bilayers, whereas even at low voltages a pH gradient produces a significant rate of translocation (Krantz et al. 2006), indicates that it is the ΔpH across the acidic endosome's membrane that drives these proteins into the cytosol, rather than a presumptive large voltage difference across that membrane.
Acknowledgments
I thank Drs Stephen Juris and Damon Anderson for permission to cite some of their unpublished results, and Mr Daniel Basilio for his help in making the figures. The work from my laboratory described in this paper was supported by grant no. GM29210 from the National Institutes of Health.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Membrane transport in flux: the ambiguous interface between channels and pumps’.
References
- Blaustein R.O., Finkelstein A. Voltage-dependent block of anthrax toxin channels in planar phospholipid bilayer membranes by symmetric tetraalkylammonium ions: effects on macroscopic conductance. J. Gen. Physiol. 1990a;96:905–919. doi: 10.1085/jgp.96.5.905. doi:10.1085/jgp.96.5.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein R.O., Finkelstein A. Diffusion limitation in the block by symmetric tetraalkylammonium ions of anthrax toxin channels in planar phospholipid bilayer membranes. J. Gen. Physiol. 1990b;96:943–957. doi: 10.1085/jgp.96.5.943. doi:10.1085/jgp.96.5.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabiaux V., Mindell J., Collier R.J. Membrane translocation and channel-forming activities of diphtheria toxin are blocked by replacing isoleucine 364 with lysine. Infect. Immun. 1993;61:2200–2202. doi: 10.1128/iai.61.5.2200-2202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falnes P.O., Sandvig K. Penetration of protein toxins into cells. Curr. Opin. Cell Biol. 2000;12:407–413. doi: 10.1016/s0955-0674(00)00109-5. doi:10.1016/S0955-0674(00)00109-5 [DOI] [PubMed] [Google Scholar]
- Falnes P.O., Madshas I.H., Sandvig K., Olsnes S. Replacement of negative by positive charges in the presumed membrane-inserted part of diphtheria toxin B fragment. Effect on membrane translocation and on formation of cation channels. J. Biol. Chem. 1992;267:12 284–12 290. [PubMed] [Google Scholar]
- Finkelstein A. The channel formed in planar lipid bilayers by the protective antigen component of anthrax toxin. Toxicology. 1994;87:29–41. doi: 10.1016/0300-483x(94)90153-8. doi:10.1016/0300-483X(94)90153-8 [DOI] [PubMed] [Google Scholar]
- Gill D.M. Seven toxic peptides that cross cell membranes. In: Jeljaszewicz J., Wadstrom T., editors. Bacterial toxins and cell membranes. Academic Press; New York, NY: 1978. pp. 291–332. [Google Scholar]
- Hoch D.H., Romero-Mira M., Ehrlich B.E., Finkelstein A., DasGupta B.R., Simpson L.L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers. Relevance to translocation of proteins across membranes. Proc. Natl Acad. Sci. USA. 1985;82:1692–1696. doi: 10.1073/pnas.82.6.1692. doi:10.1073/pnas.82.6.1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H., Janowiak B.E., Brzozowski M., Juryck J., Falke S., Gogol E.P., Collier R.J., Fisher M.T. GroEL as a molecular scaffold for structural analysis of the anthrax toxin pore. Nat. Struct. Biol. 2008;15:754–760. doi: 10.1038/nsmb.1442. doi:10.1038/nsmb.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriazova L.K., Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat. Struct. Biol. 2003;10:13–18. doi: 10.1038/nsb879. doi:10.1038/nsb879 [DOI] [PubMed] [Google Scholar]
- Krantz B.A., Trivedi A.D., Cunningham K., Christensen K.A., Collier R.J. Acid-induced unfolding of the amino-terminal domains of the lethal and edema factors of anthrax toxin. J. Mol. Biol. 2004;344:739–756. doi: 10.1016/j.jmb.2004.09.067. doi:10.1016/j.jmb.2004.09.067 [DOI] [PubMed] [Google Scholar]
- Krantz B.A., Melnyk R.A., Zhang S., Juris S.J., Lacy D.B., Wu Z., Finkelstein A., Collier R.J. A phenylalanine clamp catalyzes protein translocation through the anthrax pore. Science. 2005;309:777–781. doi: 10.1126/science.1113380. doi:10.1126/science.1113380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz B.A., Finkelstein A., Collier R.J. Protein translocation through the anthrax toxin transmembrane pore is driven by a proton gradient. J. Mol. Biol. 2006;355:968–979. doi: 10.1016/j.jmb.2005.11.030. doi:10.1016/j.jmb.2005.11.030 [DOI] [PubMed] [Google Scholar]
- Nassi S., Collier R.J., Finkelstein A. PA63 channel of anthrax toxin: an extended β-barrel. Biochemistry. 2002;41:1445–1450. doi: 10.1021/bi0119518. doi:10.1021/bi0119518 [DOI] [PubMed] [Google Scholar]
- Nguyen T.L. Three-dimensional model of the pore form of anthrax protective antigen. Structure and biological implications. J. Biomol. Struct. Dyn. 2004;22:253–265. doi: 10.1080/07391102.2004.10531226. [DOI] [PubMed] [Google Scholar]
- Oh K.J., Senzel L., Collier R.J., Finkelstein A. Translocation of the catalytic domain of diphtheria toxin across planar phospholipid bilayers by its own T domain. Proc. Natl Acad. Sci. USA. 1999;96:8467–8470. doi: 10.1073/pnas.96.15.8467. doi:10.1073/pnas.96.15.8467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannifer A.D., et al. Crystal structure of the anthrax lethal factor. Nature. 2001;414:229–233. doi: 10.1038/n35101998. doi:10.1038/n35101998 [DOI] [PubMed] [Google Scholar]
- Petosa C., Collier R.J., Klimpel K.R., Leppla S.H., Liddington R.C. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385:833–838. doi: 10.1038/385833a0. doi:10.1038/385833a0 [DOI] [PubMed] [Google Scholar]
- Silverman J.A., Mindell J.A., Finkelstein A., Shen W.H., Collier R.J. Mutational analysis of the helical hairpin region of diphtheria toxin's transmembrane domain. J. Biol. Chem. 1994;269:22 524–22 532. [PubMed] [Google Scholar]
- Song L., Hobaugh M.R., Shustak C., Cheley S., Bayley H., Gouaux J.E. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. doi:10.1126/science.274.5294.1859 [DOI] [PubMed] [Google Scholar]
- Young J.A.T., Collier R.J. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. doi:10.1146/annurev.biochem.75.103004.142728 [DOI] [PubMed] [Google Scholar]
- Zhang S., Finkelstein A., Collier R.J. Evidence that translocation of anthrax toxin's lethal factor is initiated by entry of its N terminus into the protective antigen channel. Proc. Natl Acad. Sci. USA. 2004a;101:16 756–16 761. doi: 10.1073/pnas.0405754101. doi:10.1073/pnas.0405754101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Udho E., Wu Z., Collier R.J., Finkelstein A. Protein translocation through anthrax toxin channels formed in planar lipid bilayers. Biophys. J. 2004b;87:3842–3849. doi: 10.1529/biophysj.104.050864. doi:10.1529/biophysj.104.050864 [DOI] [PMC free article] [PubMed] [Google Scholar]